Abstract
Toxic heavy metal lead enters in the environment due to industrial and anthropogenic activity threatens ecosystems and public health. Natural garlic extract (GE) exhibits antioxidant properties and various applications against several ailments. Therefore, this study scrutinized the protective effects of tocopherol polyethylene glycol succinate-coated garlic selenium (TPGS-GSNP) against lead acetate (LA) toxicity in rabbits. Sixty-four mature male rabbits were involved and divided into 8 equal groups. They received distilled water (negative control; T1), 30 mg/kg bw of LA (positive control; T2), 800 mg/kg bw of GE (T3), GE + LA (T4), 1 mg/kg bw of TPGS-Selenium (T5), TPGS-S + LA (T6), 1 mg/kg bw of TPGS-GSNP (T7), and TPGS-GSNP + LA (T8). Consequently, treatments were administered three times a week for 12 weeks. Following the treatment period, serum oxidant-antioxidant, protein, and lipid profiles, liver and kidney function, histopathological findings of the adrenal, liver, and kidneys, femur bone marrow chromosomal aberrations, and mitotic activity were collected and analysed. LA exposure showed significant reductions in antioxidant levels, organ weights, and mitotic activity while increasing oxidative stress, corticosteroid levels, and chromosomal aberrations. Importantly, TPGS-GSNP administration significantly improved these markers compared to the LA group. In addition, histological analysis revealed structural improvements of the studied organs in the TPGS-GSNP group compared to the LA group, which displayed high cellular necrotic and degenerative changes. In conclusion, synthetic TPGS-GSNP demonstrated higher protective efficacy against LA-induced toxicity compared to natural GE or selenium alone. However, more future studies could be conducted to explore the potential of TPGS-GSNP as an anticancer or immunomodulatory agent.
Similar content being viewed by others
Introduction
In addition to its presence in the environment as a heavy metal, lead is also used to create corrosion- and acid-resistant polymers for use in the construction and pharmaceutical sectors, as well as in paints, ceramics, hair dyes, water pipes, and cosmetics [30]. Moreover, high quantities of dust and soil in residential areas close to smelters, high-density landfills, and refineries result in lead exposure. Consuming foods grown in lead-rich soils, such as fruits, grains, and vegetables, can also expose one to lead [15]. That is why lead is considered one of the main toxic contaminants in the environment and can be the result of industrial activity and car exhaust [20].
Inhalation and oral absorption are the two primary routes for absorbing inorganic lead compounds. After absorption, lead is transported throughout the body, particularly in the bloodstream, where it can accumulate in bones and harm a number of organs, including the liver, immune system, heart, kidney, gonads, and nervous system [13].
Enzymatic poisons, such as lead compounds, are nephrotoxic, immunotoxic, carcinogenic, and neurotoxic [39]. The liver and kidneys may undergo permanent functional and structural alterations as a result of chronic exposure [24]. Moreover, lead causes damage to the adrenal gland cells in the fascia and glomeruli regions, which in turn causes the mitochondria to degenerate and the cytoplasm to vacuolate [38].
Furthermore, it has been reported that long-term occupational exposure to lead increases the risk of atherosclerosis in addition to dyslipidemia, hormonal depression, and hypercholesterolemia [24]. According to [6, 29], exposure may affect tryptophan metabolism, blood lipid profiles, lipoprotein levels, and xenobiotic deoxygenation. Numerous enzymatic processes are disrupted by lead’s affinity for attaching to electron-donating groups (protease, glutathione, sulfhydryl groups, etc.) [40]. As a result, lead reduces the effectiveness of the body’s antioxidant defense system, produces reactive oxygen species, interferes with a number of vital elements, damages cell membranes, and prevents the activity of antioxidant enzymes (which depend on sulfhydryl groups) [42].
Additionally, lead can cause cellular lipid peroxidation and declined antioxidant enzyme activity, which causes an excess generation of reactive oxygen species (ROS) and oxidative stress (OS) [22]. The impact of lead exposure on cellular membranes, DNA, and antioxidant defense mechanisms are all part of lead-induced toxicity caused by OS mechanisms. Different targets, including the lungs, blood vessels, testes, sperm, liver, and brain, are impacted depending on the dosage of lead exposure [26]. Lead buildup inside of cells has a negative impact on mitochondria, changing how they normally operate by causing OS. The reduction in cellular defense mechanisms is mostly caused by intracellular accumulation of lead, which extremely affects the normal function of mitochondria due to the induction of OS [57].
As one of the necessary trace elements, selenium plays a crucial role in redox enzymes, which enhance the activity of the glutathione peroxidase enzymes and protect the body's cells and tissues from free radical damage. Heart disease, immunological problems, male infertility, and a higher risk of cancer are all linked to dietary selenium deficiency. When compared with other organic and inorganic selenium compounds, selenium nanoparticles (NPs) have greater biocompatibility, bioactivity, low oxygen concentration, superior antioxidant activity, and disease-preventing efficacy [32]. According to [28], selenium NPs have antioxidant, antibacterial, anti-inflammatory, anti-microbial, and anti-mutagenic properties. In the splenocytes of tumour-bearing mice, selenium NPs elevated the cytokines IFN- and IL-12 and induced delayed hypersensitivity [50].
Allium sativum, also known as garlic, is a member of the Allium genus and has a wide range of medicinal uses. More than 200 chemical substances are found in garlic, including sulfur-containing volatile oils that contain allicin, allin, ajone, allinase, peroxidase, and myrosinase [11]. Garlic is thought to have a number of biological effects, including the induction of endogenous antioxidants in tissue organs, stimulation of immune response, improved foreign substance detoxification, antimicrobial, antidiabetic, hypolipidemic, inhibition of lipid peroxidation, attenuation of urea and creatinine levels, and promotion of antioxidant enzyme activities [5, 10, 47, 58]. According to [10], in fresh garlic extract allinase converts alliin into allicin (allyl 2- propenethiosulfinate or diallyl thiosulfinate). Also, it contains gamma-l-glutamyl-S-alkyl-l-cysteine and 1-propenyl allyl thiosulfonate, allyl methyl thiosulfonate, and other bioactive substances.
Plants contain the powerful antioxidant vitamin E. Its structural components include an isoprenoid side chain and a chromanol ring. Tocopherols and tocotrienols, which can be further classified as alpha-, beta-, gamma-, and delta-isomers depending on where the side chains are located on the chromanol ring, are together referred to as vitamin E [2]. The most prevalent vitamin E isomer in the body is alpha-tocopherol [14]. The hydroxyl group on the chromanol ring, which quickly donates hydrogen to decrease the free radicals, is what gives vitamin E its strong antioxidant effect [44]. According to both in vivo and in vitro studies, tocotrienol has a greater antioxidant impact than tocopherol for three reasons: (1) it is more evenly distributed throughout the lipid membrane; (2) it interacts with free radicals more because of the double bonds on its isoprenoid side chain; and (3) it has a higher redox cycling efficiency [43].
In recent decades, nanotechnology has transformed and improved medical science and provided opportunities to improve the health sector [19]. A broad class NPs with at least one dimension less than 100 nm having different physical and chemical characteristics has been studied extensively for their capacity to deliver medications in the ideal dosage range, frequently leading to higher therapeutic efficiency of the medications and diminished side effects [41, 55]. Selenium is a crucial trace element essential for growth, health, and reproduction in the diet. Selenium NPs, a new source of selenium, have significant environmental, sustainability, and nutritional benefits due to their decreased toxicity and gradual release of selenium after intake. In this regard, it is reported that selenium NPs in combined spirulina platensis or spirulina alga are affected for the treatment of heat-stress rabbits [9, 16]. Moreover, against the cadmium-induce toxicity, selenium NPs showed potential hepatoprotective and nephroprotective efficacy in rabbits [53]. However, not much literature is available regarding the protective effects of selenium NPs against toxic metals effect in rabbits, i.e., lead. Therefore, this study is an attempt to prepare synthetic TPGS-GSNP by loading known natural antioxidants, namely ethanolic extract of garlic, selenium, and alpha-tocopherol, into nanomaterial with physical properties that qualify it to enter the cells to form chelates with the accumulated lead (self-induced) in rabbit’s tissues exposed to lead toxicity.
Materials and methods
Preparation of lead acetate
By dissolving 30 g of lead acetate (LA, Thomas Baker, India) in one litre of physiological saline (30 mg/mL), a solution was prepared. Male rabbits were treated orally with LA (1 mL of stock solution/ Kg of body weight or 30 mg/kg) for 12 weeks [3].
Preparation of TPGS-S
TPGS-S were prepared by liquefying 10 mg of solid d-α-Tocopherol Polyethylene Glycol 1000 Succinate (TPGS, CAS NO: 9002-96-4, Sigma Aldrich, Darmstadt, Germany) at 50 °C, then dissolved in 500 mL of distilled water. Three hundred and 46 mg of sodium selenite (CAS NO: 10102-18-8 and CAS NO: 9002-96-4, Sigma Aldrich, Darmstadt, Germany) was dissolved in 50 mL of distilled water to prepare a 40 mM solution. Then, dilutions of 1 and 5 mM were prepared by using the stock solution. TPGS-S was prepared by mixing 5 mL of sodium selenite solution with 5 mL of TPGS solution under a magnetic stirrer at the speed of 600 rpm [36].
Synthesis of TPGS-GSNPs
Ethanolic extract of garlic (GE) was prepared using the Soxhlet apparatus [21]. GE-loaded TPGS-GS NPs were prepared using the thin-film rehydration method [54]. Briefly, NPs were synthesized at TPGS-S: GE ratio of 100:1 to 1:1 by mixing the stock solution of 10% (w/v) of GE with each TPGS-S solution concentration (0.01–1.00% w/v) as required, and evaporated at 50 °C. A fresh ascorbic acid solution was also prepared. The TPGS solution was magnetically mixed at 600 rpm, and then 5 mL of ascorbic acid was added dropwise with stirring to half speed. Then, 10 mL of de-ionized distilled water was added to complete the final volume of 25 mL. The solution gradually changed from colourless to deep orange-red and reacted for 24 h to ensure uniform nanosized SeNPs in high yield. The whole experiment was done at room temperature. Characterization of TPGS-GSNPs was done by utilizing UV–visible spectroscopy (Metertech SP-8001 Taiwan), Scanning Electron Microscope (SEM-Tescan Vega III, Czech), and Fourier-transform infrared spectroscopy (Shimadzu-8400s, Japan).
Animal ethics and welfare
The National and International Animal Welfare Standards were followed when caring for the animals used in this investigation. The study was carried out after receiving approval from (blinded for review University's) ethics and policy committee. Before the trial began, 64 adult rabbits (weighing between 1500 and 2000 g) were acclimated to the animal house setting. A properly ventilated room with a temperature of 22 ± 2 °C and a light/dark (12:12). Ad libitum standard chow and water were utilized to host the rabbits.
Experimental design
The male rabbits were allocated to eight equal groups at random (8 each). By oral gavage, the negative control (T1 group) received distilled water (1.0 mL/kg BW), LA; the positive control (T2 group) received LA (30 mg/kg BW) [3], GE (T3 group) received GE (22.13 mg/kg BW) [31], GE + LA (T4 group) received the combination of GE and LA, TPGS-S (T5 group) received TPGS-S (22.13 mg/kg BW), TPGS-S + LA (T6 group) received combination of TPGS-S and LA, TPGS-GSNP (T7 group) received TPGS-GSNP (22.13 mg/kg BW), and TPGS-GSNP + LA (T8 group) received combination of TPGS-GSNP and LA. All groups were treated three times per week for 12 weeks.
At the end of the experiment, male rabbits were weighed, anaesthetized, euthanized, and blood samples were collected by cardiac puncture to obtain blood sera. According to [15, 20], the biomarkers of toxicity were selected to evaluate the oxidant-antioxidant, protein and lipid profiles, and liver and kidney functions. The sera were used for assessment the serum activity of CAT, SOD, and GPx, as well as the serum levels of corticosteroids, MDA, total GSH, reduced GSH, albumin, globulin, total protein, albumin: globulin ratio, TNf-alpha, ALP, ALT, AST, bilirubin, urea nitrogen, creatinine, BUN: creatinine ratio, total cholesterol, HDL-c, LDL-c, and VLDL-c. The adrenal gland, liver, and kidneys were dislocated and weighted to calculate their relative weights (g/100 BW). Sample tissues from the adrenal gland, liver, and kidneys were also obtained and fixed in formalin buffer solution for histopathological examination. Femur bone marrow was excised to determine chromosomal aberrations and mitotic index.
Assessment of serum corticosteroids and TNf-alpha levels
Rabbit cortisol and TNf-alpha ELISA kits (Sunlong, China) were employed. Protocol steps were based on the kit manufacturer’s procedures.
Biochemical assays
Assessment of serum concentration of MDA, CAT, SOD, GPx, total GSH, reduced GSH, albumin, globulin, total protein, albumin: globulin ratio, ALP, ALT, AST, bilirubin, urea nitrogen, creatinine, BUN: creatinine ratio, total cholesterol, HDL-c, LDL-c, and VLDL-c were employed according to the manufacturer’s kit protocols (SOLARBIO, China).
Histological slide preparation of adrenal gland, liver, and kidneys
The microscopic slides were prepared at 5 µM of thickness and hematoxylin–eosin-stained according to [34].
Semi-quantitative analysis
In this work, the histopathological changes of the liver, kidneys, and adrenal glands were assessed semi-quantitatively using the structural alterations of such histological patterns, including necrosis, degeneration, inflammation, cellular debris, and blood vessels. From each group, three slides from each tissue sample obtained from eight rabbits with comparable lesions were compared. Three separate pathologists blindly evaluated tissue specimens. A random score was used to determine the quantification of each parameter: 0 for absenteeism, 1 for present in 0–25%, 2 for present in 26–50%, 3 for present in 51–75%, and 4 for present in 76–100% of the lesion. In carefully chosen lesions, morphological study of the investigated organs has been conducted [45].
Procedure of morphological measurements
The tissue was embedded in paraffin, sectioned, and stained as soon as it was fixed in 10% neutral formal solution. A thorough morphological analysis was carried out at the Department of Pathology, College of Veterinary Medicine, University of Al-Qadisiyah. For every lesion under analysis, a thorough description of the cellular component and structural characteristics of the tissue was done. After that, these parameters were subjected to a semi-quantitative analysis so that the experimental groups could be compared. The following criteria were applied to the description of the morphology and histology: (1) presence and severity of necrosis; (2) presence and severity of degeneration; (3) number and integrity of blood vessels; (4) presence and distribution of inflammatory reaction; (5) presence and distribution of cellular debris; and (6) presence and distribution of colloid materials. The morphological assessment was performed in three different thin sections taken respectively from the midway of the organ. Finally, the presence and distribution of inflammatory cells were taken as an index for the degree of inflammation.
Data analysis and expression
The results were expressed to each of the studied categories as absent (score 0; no presence of any of the considered parameters), scarcely present (score 1; parameter detected on 0–25% of the lesions), low present (score 2; parameter found in 26–50% of lesions), moderately present (score 3; parameter found in 51–75% of lesions), and intensively present (score 4; parameter found in 76–100% of lesions). Accordingly, the numerical comparison was done for the mean ± standard error (M ± SE).
Determine chromosomal aberrations
At the end of the two periods after 6 and 12 weeks, the femur bone marrow of the sacrificed male rabbits was excised to determine chromosomal aberrations as described by Savage [52] and mitotic index according to [46].
Statistical analysis
The results were displayed as M ± SE. The data were compared by one-way analysis of variance (ANOVA 1), followed by Newman- Keuls test to find out the significant differences between groups (p < 0.05). The statistical analysis was performed using GraphPad Prism V5 (USA) [12].
Results
Characterization of TPGS-GSNP
In the presence of sodium selenite and GE at pH = 9, the color of the solution was changed to reddish brown, which indicates the synthesis of selenium NPs in the colloidal solution, which was found to be dependent on reaction time or concentration of plant extract. Moreover, UV–VIS spectroscopy of TPGS-GSNP shows two separate peaks at 275 nm and 380 nm, Fig. 1A. Meanwhile, the FTIR spectrum of the TPGS-GSNP revealed that conjugated double bonds cause the band to move from 3820.08 cm−1 to 3890.26 cm−1. The OH stretching frequency is found at 3920.98, 3820.08, and 3685.72 cm−1, and the C–H stretching alkane is found at 2960.98 cm−1. The strong C=O stretching secondary amide is found at 1644.33 cm−1. A powerful N–O stretching nitro compound was detected between 1426.09 and 1248.57 cm−1. The OH stretching frequency is found for the C=C stretching cyclic alkene at 3920.98, 3720.72, and 1644.33 cm−1. The peaks at 1080.09 and 1017.87 cm−1 validate C–O and C–C stretching vibrations, as well as C–O–H and C–O–C bending vibrations. At the same time, C-X stretching results in a band at 880.88 and 810.78 cm−1. The bands at 595.45 and 540.09 cm−1 were caused by C–N–C bending in amines, Fig. 1B. On the other hand, SEM results found that the TPGS-GSNP has a diameter of ~ 100 nm, Fig. 1C.
Relative organ’s weight
As illustrated in Table 1, relative organ weights (g/100 g of body weight) of the liver, kidney, and adrenal gland were significantly increased in LA-exposed rabbits without treatment (T2 group) and those treated with GE (T4 group) and TPGS-S (T6 group) compared to control rabbits (T1 group). In contrast, those treated with TPGS-GS (T8 group) showed insignificant differences compared with the control (T1 group) and those not exposed to LA-treated rabbits with GE (T3 group) and TPGS-S (T5 group).
Serum biochemical profile
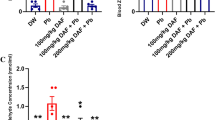
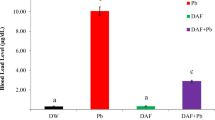
Figure 2 shows the serum concentrations of corticosteroids, TNf-α, and MDA have significantly increased in LA-exposed rabbits, either untreated (T2 group) or treated with GE (T4 group) and with TPGS-S (T6 group) in comparison to control (T1 group) and non-toxic induced rabbits and treated with GE and TPGS-S (T3 and T5 groups, respectively). In contrast, those treated with a combination of TPGS-GSNP and LA (T8 group) showed no significant difference compared to control rabbits (T1 group) and non-toxic treated with TPGS-GSNP (T7 group). In contrast, serum CAT, SOD, GPx, total GSH, and rGSH levels, Fig. 2, were significantly lower in LA-induced toxic untreated rabbits (T2 group) and LA-induced treated with GE or TPGS-S rabbits (T3 and T5 groups, respectively) compared to control (T1 group), non-toxic treated rabbits with GE, TPGS-S, and TPGS-GSNP (T3, T5, T7 groups, respectively), and LA- induced toxic rabbits treated with TPGS-GSNP (T8 group).
Serum concentration of oxidants (corticosteroids; nmole/L, TNFα; pg/mL, and MDA; nmole/L) and antioxidant (CAT; pg/L, SOD; U/L, GPx; U/L, total GSH; ng/L, and reduced GSH; ng/L) in male rabbits treated for 12 weeks with distilled water (T1), lead acetate; LA (T2), garlic extract; GE (T3), GE and LA (T4), tocopherol polyethylene glycol succinate-selenium; TPGS-S (T5), TPGS-S and LA (T6), TPGS coated garlic selenium nanoparticles; TPGS-GSNP (T7), and TPGS-GSNP and LA (T8). The values were of 8 observations, presented as M ± SE. The values were statistically analyzed using ANOVA -1 and Newman- Keuls test. The different letters denote significant differences (p > 0.05)
Figure 3 shows that serum levels of T-c, LDL-c, and VLDL-c were significantly increased, and serum level of HDL-c was significantly decreased in LA-induced rabbits (T2, T4, and T6 groups) compared to control (T1 group) and non-toxic induced rabbits (T3, T5, and T7 Groups). The results show that the TPGS-GSNP treatment (T8 group) significantly lessened the increased levels of these parameters compared to those treated with GE and TPGS-S (T4 and T6 groups, respectively).
Serum protein profile (concentration of total protein; g/dL, albumine; g/dL, globuline; g/dL, and albumine: globuline ratio) in male rabbits treated for 12 weeks with distilled water (T1), lead acetate; LA (T2), garlic extract; GE (T3), GE and LA (T4), tocopherol polyethylene glycol succinate-selenium; TPGS-S (T5), TPGS-S and LA (T6), TPGS coated garlic selenium nanoparticles; TPGS-GSNP (T7), and TPGS-GSNP and LA (T8). The values were of 8 observations, presented as M ± SE. The values were statistically analyzed using ANOVA-1 and Newman- Keuls test. The different letters denote significant differences (p > 0.05)
Moreover, Fig. 4 also illustrates that serum activities of ALT, AST, and ALP were significantly increased in LA-induced rabbits (T2, T4, and T6 groups) than in control rabbits (T1 group), whereas those non-toxic induced and treated with GE, TPGS-S or TPGS-GSNP (T3, T5, and T7 respectively) showed significant decrease than control rabbits.
Serum lipid profile (concentration of total cholesterol; mg/dL, HDL-c; mg/dL, LDL-c; mg/dL; and VLDL-c; mg/dL) in male rabbits treated for 12 weeks with distilled water (T1), lead acetate; LA (T2), garlic extract; GE (T3), GE and LA (T4), tocopherol polyethylene glycol succinate-selenium; TPGS-S (T5), TPGS-S and LA (T6), TPGS coated garlic selenium nanoparticles; TPGS-GSNP (T7), and TPGS-GSNP and LA (T8). The values were of 8 observations, presented as M ± SE. The values were statistically analyzed using ANOVA-1 and Newman- Keuls test. The different letters denote significant differences (p > 0.05)
Treatment of non-toxic rabbits with TPGS-S and TPGS-GSNP (T5 and T7 groups, respectively) resulted in significantly higher serum total protein levels than control (T1 group) and non-toxic rabbits treated with GE (T3 group), while LA-induced rabbits (T2, T4, T6, and T8 groups) showed significantly lower levels than previous groups. Still, they showed insignificant differences between each other. Serum albumin levels of control (T1 group) and intact rabbits treated with GE, TPGS-S, and TPGS-GSNP (T3, T5, and T7, respectively) exhibited insignificant differences between each other. Whereas LA-induced rabbits treated with TPGS-S and TPGS-GSNP (T6 and T8 groups, respectively) exhibited significantly increased than those untreated (T2 group) or those treated with GE (T4 group). When comparing any two corresponding groups (T1 vs. T2, T3 vs. T4, T5 vs. T6, and T7 vs. T8 groups), the serum globulin levels and albumin: globulin ratio of non-toxic induced rabbits were significantly higher than LA-induced rabbits, Fig. 5.
Liver function tests (serum concentration of ALT; IU/L, AST; IU/L, and ALP; IU/L) in male rabbits treated for 12 weeks with distilled water (T1), lead acetate; LA (T2), garlic extract; GE (T3), GE and LA (T4), tocopherol polyethylene glycol succinate-selenium; TPGS-S (T5), TPGS-S and LA (T6), TPGS coated garlic selenium nanoparticles; TPGS-GSNP (T7), and TPGS-GSNP and LA (T8). The values were of 8 observations, presented as M ± SE. The values were statistically analyzed using ANOVA-1 and Newman- Keuls test. The different letters denote significant differences (p > 0.05)
Serum levels of bilirubin, urea nitrogen, and creatinine in T1, T3, T5, and T7 groups (non-toxic untreated rabbits and those treated with GE, TPGS-S, and TPGS-GSNP, respectively) showed insignificant differences between each other, which were significantly lower than corresponding LA exposed rabbits, either untreated (T2 group) or treated with GE (T4 group), TPGS-S (T6 group), or TPGS-GSNP (T8 group). When compared to LA-exposed rabbits, only a significant decrease was shown in T8 group rabbits, among others. In contrast, the urea nitrogen: creatinine ratio of non-toxic rabbits was only insignificant in T7 group (treated with TPGS-GSNP) in comparison to control (T1), whereas those of T3 and T5 (treated with GE and TPGS-S, respectively) were significantly higher than control. When comparing the LA-exposed groups, only the ratio of T8 group rabbits was significantly lower among others, Fig. 6.
Kidney function tests (serum concentration of bilirubin; mg/dL, blood ura nitrogen; mg/dL, creatinine; mg/dL, and BUN:creatinine ratio) in male rabbits treated for 12 weeks with distilled water (T1), lead acetate; LA (T2), garlic extract; GE (T3), GE and LA (T4), tocopherol polyethylene glycol succinate-selenium; TPGS-S (T5), TPGS-S and LA (T6), TPGS coated garlic selenium nanoparticles; TPGS-GSNP (T7), and TPGS-GSNP and LA (T8). The values were of 8 observations, presented as M ± SE. The values were statistically analyzed using ANOVA-1 and Newman- Keuls test. The different letters denote significant difference (p > 0.05)
Chromosomal aberrations
As presented in Table 2, the percentage of chromosomal aberrations (Ch. fragment, Ch. ring, Ch. deletion, and Ch. acentric), hypoploidy, polyploidy, and dienteric chromosome. In non-induced toxic rabbits treated with GE (T3 group), TPGS-S (T5 group), and TPGS-GSNP (T7 group), as well as LA-induced toxic rabbits treated with TPGS-GSNP (T8 group) revealed insignificant differences compared to control rabbits (T1 group). The table also showed that the percentage of chromosomal aberrations in LA-induced toxic rabbits treated with GE (T4 group) and TPGS-S (T6 group) significantly decreased than LA-induced toxic untreated rabbits (T2 group).
Cytogenic type
Table 3 illustrates the results of the cytogenic types in terms of chromosomal aberration (%), mitotic index (%), and micronuclei (%). All the cytogenic types percentages of T3, T5, T7, and T8 groups (non-induced toxic rabbits treated with GE, TPGS-S, TPGS-GSNP, and LA-induced toxic rabbits treated with TPGS-GSNP, respectively) showed insignificant differences compared to control rabbits (T1 group). The table also showed that the cytogenic types percentage in LA-induced toxic rabbits treated with GE (T4 group) and TPGS-S (T6 group) significantly decreased than LA-induced toxic untreated rabbits (T2 group).
Histopathological findings
Liver
Normal liver architectures were noticed in the sections from control (Fig. 7a), GE-treated (Fig. 7b), TPGS-S treated (Fig. 7c), and TPGS-GSNP treated (Fig. 7d) male rabbits. In the LA-induced toxic male rabbits (Fig. 8a), the liver section showed definite lesions of hepatocytes necrosis, deposition of eosinophilic exudate with profuse oedema, hepatocytes showed vacuolar degeneration with tiny and large droplets, hydropic degeneration, local hyperemia, considerable sinusoidal contraction, and pyknotic nuclei of some hepatocytes, as well as extravasated RBCs. Although most liver parenchyma seems to be normal in LA-induced toxic rabbits treated with GE (Fig. 8b), TPGS-S (Fig. 8c), and TPGS-GSNP (Fig. 8d), some histopathological changes were noticed, such as infiltration of some inflammatory cells near the portal vein and hepatic sinusoidal dilatation.
a–d Micrographs (H&E, × 40) from the livers of male rabbits after 12 weeks of treatment. a with distilled water (C), b with garlic extract (GE), c with tocopherol polyethylene glycol-succinate-selenium (TPGS-S), and d with tocopherol polyethylene glycol-succinate coated with garlic selenium nanoparticles (TPGS-GSNP). The micrographs show normal architecture, with hepatocytes radiating from the central vein
a–d Micrographs (H&E, × 40) from the livers of male rabbits after 12 weeks of treatment. a with lead acetate (LA), b with combination of GE and LA (GSLA), c with combination of tocopherol polyethylene glycol-succinate-selenium and LA (TPGS-SLA), and d with combination of tocopherol polyethylene glycol-succinate coated with garlic selenium nanoparticles and LA (TPGS-GSNPLA). LA: shows severe necrosis of hepatocytes (N), deposition of eosinophils exudate (E), and infiltration of lymphocytes were obvious. GELA and TPGSSLA: show lower degree of pathological markers. TPGS-GSNPLA: shows normally radiated hepatocyte cords, infiltration of some inflammatory cells (yellow arrows) near the portal vein, and hepatic sinusoidal dilatation
Kidneys
When compared with the control section (Fig. 9a), the histopathological sections from kidneys of GE treated (Fig. 9b), TPGS-S treated (Fig. 9c), and TPGS-GSNP treated (Fig. 9d) males showed normal glomeruli. Still, there is a deposition of eosinophilic exudate in the proximal tubule’s lumen. In contrast, LA-induced toxic males (Fig. 10a) revealed necrosis of the glomeruli, moderate parenchymatous tubular degeneration, predominantly in the distal tubules, and deposition of eosinophilic exudate in the proximal and distal tubule’s lumen. Hydropic and vacuolar degeneration was also noticed, but the severe degree was characterized by tubular epithelial cell desquamation in almost all cells. In the same context, histological sections from kidneys of LA-induced toxic males treated with GE (Fig. 10b), TPGS-S (Fig. 10c), and TPGS-GSNP (Fig. 10d) showed normal glomeruli. However, some of the proximal tubules are suffering from sloughing, and some of the distal tubules are engorged with eosinophilic materials.
a–d Micrographs (H&E, × 40) from the kidneys of male rabbits after 12 weeks of treatment. a with distilled water (C), b with garlic extract (GE), c with tocopherol polyethylene glycol-succinate-selenium (TPGS-S), and d with tocopherol polyethylene glycol-succinate coated with garlic selenium nanoparticles (TPGS-GSNP). The micrographs show normal glomeruli and renal tubules (proximal and distal). GE, TPGS-S, TPGS-GSNP sections show normal glomeruli, but there is a deposition of eosinophilic exudate in the lumen of the proximal tubule (yellow arrow)
a–d Micrographs (H&E, × 40) from the kidneys of male rabbits after 12 weeks of treatment. a with lead acetate (LA), b with combination of GE and LA (GSLA), c with combination of tocopherol polyethylene glycol-succinate-selenium and LA (TPGS-SLA), and d with combination of tocopherol polyethylene glycol-succinate coated with garlic selenium nanoparticles and LA (TPGS-GSNPLA). LA: shows necrosis of the glomeruli (yellow arrows), sloughing of the epithelial layer of the renal tubules (black arrow), thickening of the wall of the renal tubules and deposition of eosinophil exudate (blue arrow). GELA, TPGSSLA, and TPGS-GSNPLA show normal glomeruli (yellow arrows), but some of the renal tubules suffer from sloughing (blue arrows)
Adrenal gland
Histological sections obtained from adrenal glands of control (Fig. 11a), GE treated (Fig. 11b), TPGS-S treated (Fig. 11c), and TPGS-GSNP treated (Fig. 11d) males showed normal histological features (cortex and inner layer medulla). In comparison, images obtained from LA-induced toxic males (Fig. 12a) showed variable changes ranging from hypereosinophilia, pyknotic nuclei, congested blood vessels, and hemorrhage. Moreover, degeneration and inflammation were very clear in both the cortex and medulla of this group. LA-induced toxic males treated with GE (Fig. 12b), TPGS-S (Fig. 12c), and TPGS-GSNP (Fig. 12d) and regular cells in all zones.
a–d Micrographs (H&E, × 40) from the adrenal glands of male rabbits after 12 weeks of treatment. a with distilled water (C), b with garlic extract (GE), c with tocopherol polyethylene glycol-succinate-selenium (TPGS-S), and d with tocopherol polyethylene glycol-succinate coated with garlic selenium nanoparticles (TPGS-GSNP). The micrographs show normal histological features (cortex and inner layer medulla). GE, TPGS-S, and TPGS-GSNP sections show regular cells in all zones with dilated sinusoids in zona reticularis
a–d Micrographs (H&E, × 40) from the adrenal glands of male rabbits after 12 weeks of treatment. a with lead acetate (LA), b with combination of GE and LA (GSLA), c with combination of tocopherol polyethylene glycol-succinate-selenium and LA (TPGS-SLA), and d with combination of tocopherol polyethylene glycol-succinate coated with garlic selenium nanoparticles and LA (TPGS-GSNPLA). LA: shows changes in different regions, including cellular debris necrosis of cortical cells. GELA, TPGS-SLA, and TPGS-GSNPLA: show light vaculation in zona glomerulosa (ZG), minimal apoptosis in zona fasciculata (ZF) and lipofuscin pigments in (ZR)
Semi-quantitative analysis
When histological sections from the livers, kidneys, and adrenal glands were subjected to a semi-quantitative analysis, there were notable variations in the experimental groups' scores for each of the histopathological features, which included necrosis, degeneration, inflammatory response, and the presence of cellular debris. Liver (Table 4), kidney (Table 5), and adrenal glands (Table 6) features in the LA-induced toxic groups (T2, T4, T6, and T8) were significantly higher (p > 0.05) than those of the corresponding non-toxic groups (T1, T3, T5, and T7). In the comparison of the LA-toxic groups with each other, the group treated with LA (T8) had the greatest score (p > 0.05), followed by the groups treated with GE and TPGS-S had the lower scores (T4 and T6, respectively). TPGS-GSNP had the lowest score (p > 0.05) among LA toxic groups.
Discussion
The biosynthesized TPGS-GSNP shows maximum absorbance at two regions, i.e., 275 and 380 nm. The obtained results are in accordance with absorbance data available in the literature, which define that the extinction peak may shorten, broaden, or split into two at various wavelengths as dispersed particles agglomerate or destabilize in the solutions [1, 51]. The FTIR spectrum indicates that the bioactive compounds in garlic extract that have NO-nitro and CO- CO-carboxyl groups are involved in the reduction and capping of TPGS-GSNP. Similar [4] also reported the same result by comparing the FTIR spectrums of garlic clove extract and its SeNPs. Also, the SEM investigation revealed round lobes that resemble aggregated nanostructures in a regular pattern. The uniformity in SEM spectra describes the single structural morphology of produced TPGS-GSNP with particle sizes ranging below ~ 100 nm.
Currently used chelating agents for LA toxicity are unable to remove the intracellular lead, and therefore, they are unable to mitigate the associated side effects. This study is an attempt to investigate using encapsulated nanoparticles (TPGS-GSNP) to deliver antioxidants into cells for improved treatment. Many studies have been devoted to investigating the effects of natural herbs and their constituents, such as GE, selenium, and α-tocopherol, as alternative treatments for many disturbances resulting from exposure to heavy metals, including LA [26].
Currently, the study has detected that prolonged exposure of male rabbits to LA, in the current study, developed oxidative stress (OS) associated with dyslipidemia, characterized by high serum levels of cholesterol-containing compounds such as HDL, LDL, and VLDL, also hypoproteinemia, which is characterized by decreased serum levels of total protein, albumin, and globulin. These findings were also shown previously by other researchers [18, 24, 38, 39]. OS is one of the predisposing factors to the occurrence of these changes, as OS causes an excessive increase in the production of free radicals and/or a sharp decrease in the production of antioxidants; as a result, the oxidant/antioxidant balance is disturbed. Many studies have confirmed that these disturbances will lead to the accumulation of free radicals in vital tissues and attach macromolecules such as lipids, proteins, and nucleic acids, mainly DNA [18, 24, 38]. Besides, LA is one of the substances that accumulate chronically in vital tissues such as the kidneys, liver, brain, and reproductive organs [18]. Therefore, our study reported that in LA-induced male rabbits could be due to the high generation of ROS, which might lead to the accumulation of δ-aminolevulinic acid (ALA) [23]. The final oxidation product of ALA, 4,5-dioxovaleric acid, is an effective alkylating agent of the quinine moieties within both nucleoside and isolated DNA [23].
Importantly our findings revealed a significant increase in the serum levels of corticosteroids, MDA, and TNf-α and a substantial drop in the serum levels of CAT, SOD, GPx, total GSH, and rGSH. To ameliorate the current imbalance, GE, tocopherol TPGS-S, and TPGS-GSNP were used to balance as an alternate treatment. For that reason, the present study has targeted the impact of GE, TPGS-S, and TPGS-GSNP in inhibiting the production of free radicals or stimulating the production of antioxidants, or both together. Accordingly, the outcome of this study showed that the TPGS-GSNP is a better antioxidant agent, as it shows an effect both ways together.
Furthermore, the histopathological changes and semi-quantitative differences were consistent with the serum biochemical changes, Liver, kidneys, and adrenal glands from LA-induced toxic rabbits (T2 group) and ameliorative impacts in those treated with GE, TPGS-S, and TPGS-GSNP (T4, T6, and T8 groups).
The primary mechanism of lead toxicity is due to its ability to displace other cations such as Mg2+, Ca2+, Na+, and Fe2+, thus disrupting cellular metabolism [18]. In addition, numerous cellular functions, including protein folding, ionic transport, and enzyme regulation, may be significantly impacted by these alterations. Moreover, OS induced by lead exposure may cause damage to most cells in the body, as shown in the present histopathological sections of the liver, kidneys, and adrenal glands [18].
Similarly, the current study observes that lessening of hyperlipidemia and hypoproteinemia in response to GE, TPGS-S, and TPGS-GSNP was evidently found at various degrees, as treating toxic rabbits with these compounds significantly declined the oxidative markers and markedly elevated antioxidant markers levels. According to these results, the GE or GE-coated NPs might attenuate the production of Amadori products, which might cause protein oxidation. These results show that GE possesses strong anti-glycation capability in the tissues against OS [17, 35]. Additionally, GE has a lipid-lowering property [35, 60]. In the same way, treatment with GE alone or coated by NPs led to a significant attenuation of cholesterol-rich compounds observed in LA-induced toxic rabbits.
Building on the finding of [7], this study investigated the effect of coated NPs (TPGS-GSNP) on rabbits, and the results showed a significant increase in the antioxidant defense systems within the rabbits treated with TPGS-GSNP. This was evident through elevated levels of enzymatic antioxidants, such as GPx, CAT, and SOD and non-enzymatic antioxidants, such as total and reduced GSH, and lower. Additionally, lipid peroxidation levels were notably reduced. On the contrary, serum levels of MDA, corticosteroids, and TNFα were markedly decreased. These remarkable outcomes, surpassing the effects of GE or TPGS-S alone, strongly suggest the exceptionally high potency and/or efficacy of TPGS-GSNP as an antioxidant and a hypolipidemic agent.
Recent study established that the combination of selenium and α-tocopherol has the same profile among other antioxidants to rest lipid peroxidation [27]. For this reason, α-tocopherol neutralized lipid peroxide radicals into lipid alcohol by GPx: the selenocystine-containing enzyme [8]. Moreover, there are several organic forms of selenium, with selenocysteine (SeCyS) being the most common form [56]. These findings are consistent with our study and strongly confirm the effect of GE-coated NPs (TPGS-GSNP) on rabbits to reduce LA toxicity in vital organs. According to the biochemical functions, the selenium protein (SELENNOP) mediates immunological responses, antioxidant defense, and OS regulation [59]. SELENOP is considered the primary transporter of selenium between cells, as it is an essential element for selenium metabolism and antioxidant defense because it keeps selenium-enzyme antioxidant capacity in high activity [48, 49]. These outcomes suggest that combination of selenium with other antioxidant might be beneficial in reducing LA toxicity for various tissues.
A new study by [33] suggest that α-tocopherol effects become more activated under stress. This is likely because α-tocopherol action is associated with two selenium-containing antioxidant enzymes: thioredoxin reductase (TXNRD) and glutathione peroxidase-4 (GPX4). Additionally, α-tocopherol has high lipid solubility; it can enter cell membranes to scavenge lipid hydroperoxyl and lipid radicals, helping to prevent lipid peroxidation [25]. So, the protective role of selenium and α-tocopherol against LA toxicity in rabbits has been proved in the current study.
Furthermore, the synergism between vitamin E and selenium to fend off the OS induced by lead exposure could be through regulating inflammatory markers, lipid profile, liver functions, and antioxidant enzymes [37]. Selenium functions as an antioxidant by preventing harmful substances from being generated in large amounts during the metabolism of LA and ameliorates their potential to cause cell death. So, its presence in seleno-proteins is closely linked to several of its physiological functions [37]. Alternatively, vitamin E, as an antioxidant, is necessary for neurological processes, enzyme activity, and a number of organ functions. It performs the antioxidant role by guarding against the damaging effects of free radicals on certain lipids [37].
Besides, the current findings revealed that OS from LA exposure might be result in genotoxicity and prevent the DNA repair genes from performing their function. Based on current findings, it seems that LA-induced toxicity interferes with antioxidant activities and induces nucleic acid peroxidation, which could all be caused by this antioxidant imbalance because numerous deleterious changes were identified by ROS-mediated oxidative imbalance [18].
Data availability
Data is available within the manuscript and will be provided at the editor's request.
References
Aftab R, Ahsan S, Liaqat A, Safdar M, Chughtai MFJ, Nadeem M, Farooq MA, Mehmood T, Khaliq A (2023) Green-synthesized selenium nanoparticles using garlic extract and their application for rapid detection of salicylic acid in milk. Food Sci Technol 43:e67022
Aggarwal BB, Sundaram C, Prasad S, Kannappan R (2010) Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol 80(11):1613–1631
Ahmed Y, Mahmoud G, Farghaly A, Abo-Zeid MA, Ismail E (2012) Some studies on the toxic effects of prolonged lead exposure in male rabbits: chromosomal and testicular alterations. Glob Vet 8(4):360–366
Anu K, Singaravelu G, Murugan K, Benelli G (2017) Green-synthesis of selenium nanoparticles using garlic cloves (Allium sativum): biophysical characterization and cytotoxicity on vero cells. J Cluster Sci 28:551–563
Asdaq SMB (2015) Antioxidant and hypolipidemic potential of aged garlic extract and its constituent, s-allyl cysteine, in rats. Evid Based Complement Altern Med. https://doi.org/10.1155/2015/328545
Assi MA, Hezmee MNM, Sabri MYM, Rajion MA (2016) The detrimental effects of lead on human and animal health. Vet world 9(6):660
Balamash K, Albar O, Wang Q, Ahmed N (2012) Effect of Kyolic® aged garlic extract on glycaemia, lipidaemia and oxidative stress in patients with type 2 diabetes mellitus. J Diabetes Res Clin Metab 1(1):18
Barchielli G, Capperucci A, Tanini D (2022) The role of selenium in pathologies: an updated review. Antioxidants 11(2):251
Bashar A, El-Darawany A, Sheiha A (2022) Effect of selenium nanoparticles and/or spirulina platensis on growth, hematobiochemical, antioxidant status, hormonal profile, immunity, and apoptosis of growing rabbits exposed to thermal stress. Egypt J Rabbit Sci 32(1):77–103
Bayan L, Koulivand PH, Gorji A (2014) Garlic: a review of potential therapeutic effects. Avicenna J Phytomed 4(1):1
Block E (1985) The chemistry of garlic and onions. Sci Am 252(3):114–121
Shiefler W.Statistics for the biological sciences (2nd ed). Reading, Mass, London: Addison-Wesley Pub. Co; 1979. https://discovered.ed.ac.uk/discovery/fulldisplay?vid=44UOE_INST:44UOE_VU2&tab=Everything&docid=alma9995633502466&query=sub,exact,%20Clinical%20trials&context=L&lang=en
Carocci A, Catalano A, Lauria G, Sinicropi MS, Genchi G (2016) Lead toxicity, antioxidant defense and environment. Rev Environ Contam Toxicol 238:45–67
Chin K-Y, Ima-Nirwana S (2014) The effects of α-tocopherol on bone: a double-edged sword? Nutrients 6(4):1424–1441
D’souza HS, Dsouza SA, Menezes G, Venkatesh T (2011) Diagnosis, evaluation, and treatment of lead poisoning in general population. Indian J Clin Biochem 26:197–201
El-Ratel IT, El-Kholy KH, Mousa NA, El-Said EA (2023) Impacts of selenium nanoparticles and spirulina alga to alleviate the deleterious effects of heat stress on reproductive efficiency, oxidative capacity and immunity of doe rabbits. Anim Biotechnol 34:1–14
Elosta A, Slevin M, Rahman K, Ahmed N (2017) Aged garlic has more potent antiglycation and antioxidant properties compared to fresh garlic extract in vitro. Sci Rep 7(1):39613
Fan Y, Zhao X, Yu J, Xie J, Li C, Liu D, Tang C, Wang C (2020) Lead-induced oxidative damage in rats/mice: a meta-analysis. J Trace Elem Med Biol 58:126443
Gani BA, Asmah N, Soraya C, Syafriza D, Rezeki S, Nazar M, Jakfar S, Soedarsono N (2023) Characteristics and antibacterial properties of film membrane of chitosan-resveratrol for wound dressing. Emerg Sci J 7(3):821–842
Ghorbe F, Boujelbene M, Makni-Ayadi F, Guermazi F, Kammoun A, Murat J, Croute F, Soleilhavoup J, Feki AE (2001) Effect of chronic lead exposure on kidney function in male and female rats: determination of a lead exposure biomarker. Arch Physiol Biochem 109(5):457–463
Harborne A (1998) Phytochemical methods a guide to modern techniques of plant analysis. Springer Science & Business Media
Ahmed-Farid OA, Hassan MA. (2017) The protective effect of flaxseed oil supplemented with high source of branched chain amino acids against the rats testicular toxicity induced by lead acetate. World J Pharm Pharm Sci 6(12):30–42
Hermes-Lima M, Valle VG, Vercesi AE, Bechara EJ (1991) Damage to rat liver mitochondria promoted by δ-aminolevulinic acid-generated reactive oxygen species: connections with acute intermittent porphyria and lead-poisoning. Biochim et Biophys Acta BBA Bioenerg 1056(1):57–63
Himani, Kumar R, Ansari JA, Mahdi AA, Sharma D, Karunanand B, Datta SK (2020) Blood lead levels in occupationally exposed workers involved in battery factories of Delhi-NCR region: effect on vitamin D and calcium metabolism. Indian J Clin Biochem 35:80–87
Hondal RJ (2023) Selenium vitaminology: the connection between selenium, vitamin C, vitamin E, and ergothioneine. Curr Opin Chem Biol 75:102328
Hsu P-C, Guo YL (2002) Antioxidant nutrients and lead toxicity. Toxicology 180(1):33–44
Hu Q, Zhang Y, Lou H, Ou Z, Liu J, Duan W, Wang H, Ge Y, Min J, Wang F (2021) GPX4 and vitamin E cooperatively protect hematopoietic stem and progenitor cells from lipid peroxidation and ferroptosis. Cell Death Dis 12(7):706
Khurana A, Tekula S, Saifi MA, Venkatesh P, Godugu C (2019) Therapeutic applications of selenium nanoparticles. Biomed Pharmacother 111:802–812
Kshirsagar M, Patil J, Patil A, Ghanwat G, Sontakke A, Ayachit R (2015) Biochemical effects of lead exposure and toxicity on battery manufacturing workers of Western Maharashtra (India): with respect to liver and kidney function tests. Al Ameen J Med Sci 8(2):107–114
Kumar SR, Devi AS (2018) Lead toxicity on male reproductive system and its mechanism: a review. Res J Pharm Technol 11(3):1228–1232
Li H, Yan L, Tang EK, Zhang Z, Chen W, Liu G, Mo J (2019) Synthesis of TPGS/curcumin nanoparticles by thin-film hydration and evaluation of their anti-colon cancer efficacy in vitro and in vivo. Front Pharmacol 10:769
Liu Y, Zeng S, Liu Y, Wu W, Shen Y, Zhang L, Li C, Chen H, Liu A, Shen L (2018) Synthesis and antidiabetic activity of selenium nanoparticles in the presence of polysaccharides from Catathelasma ventricosum. Int J Biol Macromol 114:632–639
Lu X, Wang Z, Chen L, Wei X, Ma Y, Tu Y (2023) Efficacy and safety of selenium or vitamin E administration alone or in combination in ICU patients: a systematic review and meta-analysis. Clin Nutr ESPEN 57:550–560
Luna LG. Manual of histologic staining methods of the Armed Forces Institute of Pathology, 3rd edn. Blakiston Division, McGraw-Hill, New York; 1968. https://search.worldcat.org/title/manual-of-histologic-staining-methods-of-the-armed-forces-institute-of-pathology/oclc/330784
Maldonado PD, Barrera D, Medina-Campos ON, Hernández-Pando R, Ibarra-Rubio MAE, Pedraza-Chaverri J (2003) Aged garlic extract attenuates gentamicin induced renal damage and oxidative stress in rats. Life Sci 73(20):2543–2556
McCall RL, Sirianni RW (2013) PLGA nanoparticles formed by single-or double-emulsion with vitamin E-TPGS. JoVE J Vis Exp 82:e51015
Mesalam NM, Ibrahim MA, Mousa MR, Said NM (2023) Selenium and vitamin E ameliorate lead acetate-induced hepatotoxicity in rats via suppression of oxidative stress, mRNA of heat shock proteins, and NF-kB production. J Trace Elem Med Biol 79:127256
Mohameda DS, Hagagb KEA (2011) Effect of lead acetate on the histological structure of the adrenal cortex of male albino rats and the possible protective role of vitamin E. Egypt J Histol 34(3):496–504
Moreira FR, Moreira JC (2004) Os efeitos do chumbo sobre o organismo humano e seu significado para a saúde. Rev Panam Salud Publica 15(2):119–129
Mudipalli A (2007) Lead hepatotoxicity & potential health effects. Indian J Med Res 126(6):518–527
Muldarisnur M, Perdana I, Elvaswer E, Puryanti D (2023) Mapping of sensing performance of concentric and non-concentric silver nanoring. Emerg Sci J 7(4):1083–1099
Omidi F, Jafaryan H, Patimar R, Harsij M, Paknejad H (2020) Biochemical biomarkers of skin mucus in Neogobius melanostomus for assessing lead pollution in the Gulf of Gorgan (Iran). Toxicol Rep 7:109–117
Packer L, Weber SU, Rimbach G (2001) Molecular aspects of α-tocotrienol antioxidant action and cell signalling. J Nutr 131(2):369S-373S
Peh HY, Tan WD, Liao W, Wong WF (2016) Vitamin E therapy beyond cancer: tocopherol versus tocotrienol. Pharmacol Ther 162:152–169
Piaggesi A, Viacava P, Rizzo L, Naccarato G, Baccetti F, Romanelli M, Zampa V, Del Prato S (2003) Semiquantitative analysis of the histopathological features of the neuropathic foot ulcer: effects of pressure relief. Diabetes Care 26(11):3123–3128
Preston RJ, Dean BJ, Galloway S, Holden H, McFee AF, Shelby M (1987) Mammalian in vivo cytogenetic assays: analysis of chromosome aberration in bone marrow cells. Mutation Res. https://doi.org/10.1016/0165-1218(87)90021-8
Ried K (2016) Garlic lowers blood pressure in hypertensive individuals, regulates serum cholesterol, and stimulates immunity: an updated meta-analysis and review. J Nutr 146(2):389S-396S
Saito Y (2020) Selenoprotein P as an in vivo redox regulator: disorders related to its deficiency and excess. J Clin Biochem ition 66(1):1–7
Sarıkaya E, Doğan S (2020) Glutathione peroxidase in health and diseases. Glutathione Syst Oxid Stress Health Dis. https://doi.org/10.5772/intechopen.91009
Sarkar B, Bhattacharjee S, Daware A, Tribedi P, Krishnani K, Minhas P (2015) Selenium nanoparticles for stress-resilient fish and livestock. Nanoscale Res Lett 10:1–14
Satgurunathan T, Bhavan PS, Komathi S (2017) Green synthesis of selenium nanoparticles from sodium selenite using garlic extract and its enrichment on Artemia nauplii to feed the freshwater prawn Macrobrachium rosenbergii post-larvae. Res J Chem Environ 21(10):1–12
Savage J (1976) Classification and relationships of induced chromosomal structual changes. J Med Genet 13(2):103
Shahri SH, Hajinezhad MR, Jamshidian A (2021) Comparison of the histopathological effects of selenium nanoparticles and cerium oxide nanoparticles in cadmium-intoxicated rabbits. J Environ Treat Tech 9(2):528–533
Sheweita SA, El-Dafrawi YA, El-Ghalid OA, Ghoneim AA, Wahid A (2022) Antioxidants (selenium and garlic) alleviated the adverse effects of tramadol on the reproductive system and oxidative stress markers in male rabbits. Sci Rep 12(1):13958
Siddiqui A, Anwer H, Naqvi SS, Ali SA, Shah MR, Zohra RR (2022) Sensitive and rapid detection of glutamic acid in colloidal solution by surfactant mediated silver nanoparticles. J Cluster Sci 33(4):1515–1524
Steinbrenner H, Speckmann B, Klotz L-O (2016) Selenoproteins: antioxidant selenoenzymes and beyond. Arch Biochem Biophys 595:113–119
Sujatha K, Srilatha C, Anjaneyulu Y, Amaravathi P (2011) Lead acetate induced nephrotoxicity in wistar albino rats, pathological, immunohistochemical and ultra structural studies. Int J Pharm Biol Sci 2(2):B459–B469
Thomson M, Al-Qattan KK, Js D, Ali M (2015) Anti-diabetic and anti-oxidant potential of aged garlic extract (AGE) in streptozotocin-induced diabetic rats. BMC Complement Altern Med 16:1–9
Ye R, Huang J, Wang Z, Chen Y, Dong Y (2022) The role and mechanism of essential selenoproteins for homeostasis. Antioxidants 11(5):973
Yeh Y-Y, Liu L (2001) Cholesterol-lowering effect of garlic extracts and organosulfur compounds: human and animal studies. J Nutr 131(3):989S-993S
Acknowledgements
The authors acknowledge the deanery of the College of Veterinary Medicine, University of Al-Qadisiyah, and the College of Science of Sfax, University of Sfax, for their support.
Funding
No funding sources are reported.
Author information
Authors and Affiliations
Contributions
The authors have contributed to writing, designing, compiling and editing the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by Al-Qadisiyah University’s ethics and policy committee.
Competing interests
The authors declare that they have no known competing interests that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yaseen, M.A.R., Ayed, M.H. & Al-Saaidi, J.A.A. The potential modulatory impact of garlic-selenium nanoparticles coated with synthetic tocopherol polyethylene glycol-succinate against lead acetate toxicity in male rabbits. Appl Biol Chem 67, 45 (2024). https://doi.org/10.1186/s13765-024-00893-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-024-00893-8