Abstract
Precision agriculture requires supply of adequate amount of fertilizer application to increase crop yield and prevent environmental contamination. Objective of the study was to evaluate response of pepper under different fertigation method and amount using plant induced electrical signal (PIES) for precision agriculture. Pepper was fertigated 10 times with recommended additional nitrogen fertilizer and set as a control. Low fertilizer treatment did not receive additional urea and high fertilizer received three times higher amount of nitrogen fertilizer. Conventional treatment was fertigated as basal fertilizer and once with additional fertilizer. The PIES decreased during vegetative stage and remained constant at reproductive stage because of reduced nutrient and water uptake. The PIES showed positive relationship with soil NH4+, NO3−, stem NO3− and leaf N, which resulted in highest PIES value during reproductive stage in high fertilizer treated pepper. Plant growth parameters were also related with the PIES although yield was not affected by different fertilizer treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Climate change such as sudden temperature change and drought resulting in productivity fluctuations in open field caused environmental stress for crop growth [11]. Since nutrient and water demand of crops is also not constant due to climate change, it is important to understand how productivity and soil nutrient dynamics are affected by fertilization practices under these variable climatic conditions [38]. Therefore, it is necessary to diagnose the physiological activity of the crop at an early stress stage and manage nutrients and water based on the plant activity for stable crop production.
For stable crop productivity, it is important to manage soil nutrients by supplying an appropriate amount of fertilizer in consideration of various factors such as climate and soil conditions [26]. Excess or deficient fertilizer in the soil can cause soil nutrient imbalance. This nutrient imbalance has negative effects on both the environment and crops [22]. Runoff from overfertilized soils contains nitrogen and phosphorus, which causes eutrophication [18]. In addition, nutrient deficiency causes yellow leaves resulting in stunted growth and, in severe cases, withering, eventually leading to a decrease in crop productivity [2]. Thus, soil nutrient management contributes to the sustainability of farmland and to maintaining the yield and quality of crops.
Diagnosis of crop physiological activity for proper nutrient management is possible through monitoring crop bioinformatics. Methods for evaluating crop bioinformatics in relation to stress include analysis of chlorophyll content and chlorophyll fluorescence of leaves because environmental stress affects plant photosynthesis [5]. Analysis of chlorophyll content requires destructive sampling of the plant, while measurement of chlorophyll fluorescence is non-destructive [3, 12]. In addition, transcriptome analysis as bioinformatics tools can be used to understand regulatory and metabolic processes of plants in response to abiotic stress [37]. However, above mentioned bioinformatics analyses are expensive, take long time for analysis and have limitations in showing the plant state in real time, so it is impossible to evaluate continuous response of plant to environmental stress.
Plant induced electrical signal (PIES) measures the internal resistance through electrodes inserted on the plant stem in real time and converts it into electrical conductivity to reflect the nutrient and water absorption of the stem [29]. The PIES is used to assess effect of environmental parameters such as temperature, humidity, CO2, and light on crop growth including nutrient and water uptake and productivity [30]. Therefore, PIES can be non-destructively measured and used to diagnose crop growth and stress in various growing environments through real-time monitoring.
In our previous studies, physiological responses of broccoli (Brassica oleracea var. italica) and red pepper (Capsicum annuum L.) was evaluated in association with different climate conditions and urea application [5, 19, 20, 30]. However, the relationship between crop growth and PIES under soil conditions during varying fertigation in open filed has not been elucidated. Fertigation system is suitable for supplying balanced nutrients and water by monitoring water and nutrient levels [17]. However, it is difficult to monitor nutrient status in soil during fertigation and to determine the time and amount of fertigation. If plant activity can be measured during the fertigation and is related to plant growth, which can be applied for decision support system for efficient fertigation [15]. In order to supply adequate nutrients for crop growth, PIES monitoring as mean of plant physiological activity measurement is required and it is a priority to evaluate the relationship between nutrient supply and PIES and their association with crop productivity. Therefore, the objective of the study is to evaluate effect of fertigation on pepper growth and changes in PIES during the fertigation.
Materials and methods
Pepper growth and application of fertilizer
Pepper (Capsicum annuum L.) seedlings were planted in open field located in Jeongeup-si, Jeollabuk-do and grown for about 4 months from 22.04.30 to 22.09.16. Pepper fruits were harvested from 22.07.19 every week and yield was recorded. For characterization of the soil, soil was sampled and air-dried and sieved less than 2 mm. Soil texture was determined using a hydrometer after being dispersed with 5% sodium hexametaphosphate [10]. Soil pH and EC were measured by extracting soil in a 1:5 ratio with distilled water. Ammonium nitrogen (NH4+-N) contents were analyzed according to the indophenol-blue method [7], and nitrate nitrogen (NO3−-N) contents were measured as the VCl(III)-reduction method [8]. Available phosphate was measured by Bray No.1 method [4], and soil organic matter was estimated by Walkley–Black method [40]. Properties of the soil are presented in Table 1.
Field experiments were conducted by applying different amounts of fertilizer through fertigation system in open field and PIES of peppers under different urea application was monitored. The amounts of fertilizer for control (N1) were 22.8 g N/m2 and 23.8 g P/m2 according to the standard amount of Korean Soil Information System [21]. Fertilizer was supplied as monoammonium phosphate (MAP) and urea. The amounts of fertilizer for conventional (N2) treatment plot were same as control but treated once with MAP and once with urea. The amount of fertilizer for low fertilizer (N0) treatment plot were 4.8 g N/m2 and 23.8 g P/m2 as MAP, and urea was not applied. The amounts of fertilizer for high fertilizer (N3) treatment plot were three times as much as control. Control, low, and high fertilizer treatments were supplied 10 times as fertigation and all treatments were conducted in triplicate.
Monitoring of PIES and environmental conditions using sensors
The growth of peppers applied with different amounts of urea by fertigation was monitored by PIES. The PIES was monitored by inserting electrodes with three stainless steel needles 5 mm each on both sides of the stem (Fig. 1), and electric resistance was measured using Junsmeter II (Prumbio, Suwon, Korea), which was converted to electrical conductivity. During the experiment, the soil EC, photosynthetic photon flux density (PPFD), and air temperature and humidity were monitored by TEROS-12 (Meter group, Pullman, WA, USA), PYR (Meter group, Pullman, WA, USA) and ATMOS 14 (Meter group, Pullman, WA, USA) sensors, respectively. Precipitation data were acquired from agricultural weather of Rural Research Development [1].
Nutrient analysis of soil, and pepper stem and leaves
Soil samples near the root zone were collected on the day of harvest, and air-dried samples were sieved less than 2 mm and used for analysis. For measurement of soil pH and EC, 5 g soil was mixed with 25 mL of distilled water at 180 rpm for 30 min [33]. The pH and EC in the extracted solution were measured using a pH/conductivity meter (A215 pH/Conductivity Benchtop Multiparameter Meter, Thermo-Fisher Scientific, Waltham, MA, USA). In order to extract available nutrients in soil, 2 g soil was shaken with 20 mL of 1 N ammonium acetate in 50 mL conical tube at 180 rpm for 30 min [6]. Extracted solution was filtered through a 0.45 μm syringe filter and elemental concentrations were analyzed using ICP-OES (Avio 500, Perkin Elmer, Waltham, MA, USA). Available ammonium and nitrate contents were extracted by 2 M KCl and analyzed according to the indophenol-blue method, and VCl(III)-reduction method, respectively. Absorbance of the solution after color development was measured by UV–VIS spectrophotometer (Multiskan SkyHigh Microplate Spectrophotometer, Thermo-Fisher Scientific, Waltham, MA, USA).
To measure the EC, pH, and ion contents of pepper stems, the pepper stems were cut a length of 5 cm including the point where the PIES electrodes were inserted. After cutting, the stem was weighted and placed in 50 mL conical tube. Ions in stem were extracted with 30 mL of distilled water by shaking at 180 rpm for 2 h [41]. The pH and EC in the extracted solution were measured using a pH/conductivity meter. The ion concentrations in pepper stem extract were analyzed using ICP-OES and ammonium and nitrate concentrations were analyzed by indophenol-blue method and VCl(III)-reduction method, respectively.
After the pepper harvesting, leaves were dried at 60 ℃ and grinded. For the element content measurement 0.1 g of the leaf sample was digested with 5 mL of nitric acid at 140 ℃ until the volume was decreased to about 1 mL. The digested sample was diluted to 50 mL with deionized water and the element contents were determined using ICP-OES.
Measurement of SPAD and chlorophyll fluorescence of leaves
Before pepper harvesting, SPAD of the leaves was measured at leaves of the third joint from the top using Chlorophyll Meter (SPAD-502Plus, KONICA MINOLTA, Tokyo, Japan). Chlorophyll fluorescence was measured by FluorPen (FluorPen FP 110/D, Phyton Systems Instruments, Drásov, Czech republic), after dark adaptation of leaves using clips for 15 min.
Analysis of chlorophyll, proline, malondialdehyde (MDA) of leaves
For chlorophyll analysis, 0.2 g of frozen pepper leaves were homogenized in 5 mL of 80% acetone [12]. After centrifugation at 4000 rpm for 10 min, the absorbance was measured at 645 nm and 663 nm with 80% acetone as a blank. The contents of chlorophyll a and chlorophyll b in pepper leaves were calculated by following formula.
where Ca is the concentration of chlorophyll a (mg/g FW), Cb is the concentration of chlorophyll b (mg/g FW), D663 is the absorbance at 663 nm, D645 is the absorbance at 645 nm, V is the final volume (mL), W is the fresh weight of leaf materials (g), and d is the length of the light path in cm.
To extract proline, 10 mL of 3% sulfosalicylic acid was added to 0.5 g of frozen leaves in 50 mL of conical tube and sonicated for 30 min. The extracted solution was centrifuged at 4000 rpm for 10 min at 25 °C and filtered through a 0.45 µm syringe filter. In 2 mL of the filtrate, 2 mL of acid-ninhydrin and 2 mL of glacial acetic acid were mixed and reacted at 100 °C for 1 h. After cooling, 400 mL of toluene were added and shaken for 20 s. The absorbance of the solution was measured at 520 nm with toluene as a blank [3].
For MDA analysis, 0.2 g of frozen pepper leaves were homogenized in 2 mL of 0.1% trichloroacetic acid (TCA). After centrifugation at 13,000 rpm for 10 min, 0.5 mL of the supernatant was reacted with 1.5 mL thiobarbituric acid (TBA) at 100 °C for 15 min. After cooling and centrifugation at 13,000 rpm for 5 min, the absorbance was measured at 532 nm and 600 nm [32].
Statistical analysis
The PIES and all analytical data were calculated as average of the three replicates and presented as mean with standard deviation. Stress index of pepper was calculated based on PIES measured and modeled PIES using environmental parameters. High stress index indicates high stress on the plant. Statistical analysis of the data was performed using SPSS software (IBM, Armonk, NY, USA). Means among different treatments were compared using one-way ANOVA followed by Duncan’s multiple range test at p < 0.05. Different letters among different treatments indicate statistically significant differences. The principal component analysis (PCA) was conducted to evaluate the relationship among PIES, nutrients, plant growth and stress related parameters using Xlstat (Addinsoft, Paris, France). Variables with significant differences among treatments were selected for PCA and data were standardized. Principal components (PCs) with high eigenvalues were selected and variable loadings were examined.
Results and discussion
PIES by different urea fertilizer applications
PIES value decreased during vegetative growth and remained constant during reproductive growth. Because PIES reflects plant physiological activity, which increases when plant uptakes water and nutrients, the PIES was high when plant uptake large amount of nutrients and biomass increased at high rate. When the nutrient and water uptake of pepper is active during vegetative growth, the PIES ranged from 10 to 20 mS/m. However, after starting of harvesting pepper (22.07.19), the PIES remained relatively constant. The lowest PIES value of the pepper plant was 4 mS/m. Development stage of sweet pepper affected uptake ratio of nutrient to water during the growth. Most of nutrient uptake ratios decreased at productive stage, but demand for N and P increased during the reproductive stage [34]. As the plant growth stage progresses, more nutrients are assimilated into fruits than plant tissue synthesis [14] and leaf does not require much nutrients and amount of nutrients transported decreases [34], which might result in reduced pepper activity and low PIES.
During the vegetative stage, the PIES was high in the order of control (N1), high fertilizer (N3), low fertilizer (N0), and conventional (N2) although during reproductive period PIES of high fertilizer (N3) was higher than control (N1) and low fertilizer (N0) showed the lowest PIES (Fig. 2). For the conventional fertilizer treatment, most of fertilizer was applied as basal fertilizer, and during the reproductive stage the nutrient level in soil was similar to low fertilizer treatment because of depletion of applied nutrients. Different nitrogen application affected at the reproductive stage because N and P demand increased at reproductive stage [34].
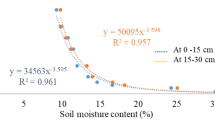
Although the growth of pepper in control (N1) was higher, there was no significant difference in the yield of pepper in all treatment (Table 2). In the case of low fertilizer, lower shoot length and fresh weight were analyzed, but no significant difference was observed in yield. The optimum temperature for growing pepper is 20–25 °C [35], but in this experiment, the air temperature and soil temperature were about 30–45 °C, and precipitation was also high. As a result, the overall growth of pepper was not good, the yield was low and significant fertigation effect was not observed because of heavy rain in August, which was not suitable for growing red pepper (Fig. 3) [13].
Nutrient concentrations of soil, and stem and leaves of pepper
Soil nutrient contents did not show significant differences in exchangeable Ca, Mg, and K according to different treatments because only different amount of nitrogen fertilizer was applied (Table 3). However, water soluble Ca and Mg was the highest in high amount of fertilizer applied plot. Liu et al. [25] also reported that urea application initially increased Ca and Mg leaching. The NH4+-N content also did not show significant difference, but the control and high fertilizer treatment showed relatively high nitrate in soil. In general, NH4+-N is rapidly converted to NO3−-N in soil and nitrogen is consumed by plants [39]. Therefore, NH4+-N content were not affected by urea application. However, the NO3−-N content in high fertilizer (N3) showed the highest although the absolute NO3−-N concentration was not high compared to applied urea (Table 3). The soil was collected on the day of pepper harvest and the applied nutrient was taken up by pepper or lost from the soil.
The nutrient content of the pepper stem extract was the highest in the high fertilizer (N3) while it was lowest in low fertilizer treatment (Table 4). The increase in water soluble Ca and Mg contents in the soil with high amount of urea might also affect the element contents in the stem. In high fertilizer treated pepper stem, NH4+-N and NO3−-N concentrations were higher than in other treatments (Table 4). Ono et al. [28] demonstrated that NH4+-N concentration in xylem sap of soybean plants increased with different type of nitrogen fertilizer application. The PIES can measure the EC of the plant stem and the EC increases with increasing sap flow as well as nutrients, so the PIES is very closely related to nutrients in the stem [29]. Comparing the nutrient concentrations of the stem extract and PIES, the same tendency was observed, which was generally high in high fertilizer and control, and low in low fertilizer and conventional treatments (Table 4) (Fig. 2).
The nutrient contents in leaves did not show a significant difference according to the fertilizer treatments indicating that assimilated amount of nutrients were not different (Table 5). In other study, there was no difference in the amount assimilated nutrients even if the nutrient contents treated in the soil was high in the high fertilizer treatment group [42]. Therefore, excessive treatment of fertilizers lowers the fertilizer use efficiency. In addition, excess nitrogen in the soil induces a phosphorus imbalance, which progressively limits plant phosphorus uptake [31]. Moreover, excessive urea application can cause inhibition of potassium absorption in plants and also accelerate chlorophyll decomposition and exacerbating K deficiency symptoms [24].
Relation of stress related parameters and PIES
The chlorophyll value was the lowest in the low fertilizer treatment although there was no significant difference among different fertilizer application. The low chlorophyll content indicates low photosynthetic activity because of low nitrogen [36]. High fertilizer showed the highest value in SPAD, which reflect high chlorophyll content by measuring the color of the leaves [43]. Because the main absorption wavelength of the SPAD instrument is close to chlorophyll a content, it is assumed that chlorophyll a is the highest in the high fertilizer [16].
The proline content, an amino acid synthesized in response to plant stress, was the highest under the high fertilizer treatment and the lowest levels in the low fertilizer treatment [23]. In contrast, MDA levels, a direct indicator of the stress state in plants, were found to be highest under low fertilization [9]. The Fv/Fm ratio, a measure of photosynthetic performance of chlorophyll, did not show significant differences in all treatment groups, with the values consistently below 0.8 (Table 6). The result suggested that the growth of plants was adversely affected by environmental stress [27]. The PIES also showed that the vitality of the plant decreased considerably before harvest at the time when Fv/Fm was measured (Fig. 2). Stress index was the highest in conventional treatment, but significant difference was not found among different treatments.
Principal component analysis (PCA) was performed to understand the relationship between the nitrogen contents in soil and plants, stress related parameters and PIES. The PC1 and PC2 explained for 39.70% and 16.39% of total variation, respectively (Table 7). The stem extract EC, leaf N, stem NH4+-N and NO3−-N, soil NO3−-N, fresh weight of pepper plant, stem height and diameter, and proline content showed positive relationship with PIES (Fig. 4). The stress index was associated with MDA content indicating that calculated stress index based on PIES reflected plant stress condition (Fig. 4). The classification of the pepper plants grown in different fertilization can be made using biplot of PC1 and PC2. The plants (N0 and N2) with high stress and relatively low growth were separated from control (N1) and high fertilizer (N3) (Fig. 4). Therefore, the PIES can be used to evaluate pepper plant growth under different conditions.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, [J.H. Park], upon reasonable request.
References
Agricultural weather, Rural Research Development. 2023. http://weather.rda.go.kr/w/weather/observation.do. Accessed 27 Mar 2023
Azimi S, Kaur T, Gandhi TK (2021) A deep learning approach to measure stress level in plants due to nitrogen deficiency. Measurement 173:108650
Bates LS, Waldren RA, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bray RH, Kurtz LT (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59(1):39–46
Cha SJ, Park HJ, Lee JK, Kwon SJ, Jee HK, Baek H, Park JH (2020) Multi-sensor monitoring for temperature stress evaluation of broccoli (Brassica oleracea var. italica). J Appl Biol Chem 63(4):347–355
Chapman HD (1965) Cation-exchange capacity. Methods Soil Anal Part 2 Chem Microbiol Prop 9:891–901
Doane TA, Horwáth WR (2003) Spectrophotometric determination of nitrate with a single reagent. Anal Lett 36(12):2713–2722
Dorich RA, Nelson DW (1983) Direct colorimetric measurement of ammonium in potassium chloride extracts of soils. Soil Sci Soc Am J 47(4):833–836
Gaweł S, Wardas M, Niedworok E, Wardas P (2004) Malondialdehyde (MDA) as a lipid peroxidation marker. Wiadomosci lekarskie (Warsaw, Poland: 1960) 57:453–455
Gee GW, Bauder JW (1979) Particle size analysis by hydrometer: a simplified method for routine textural analysis and a sensitivity test of measurement parameters. Soil Sci Soc Am J 43(5):1004–1007
Gray SB, Brady SM (2016) Plant developmental responses to climate change. Dev Biol 419(1):64–77
Guo WL, Wang SB, Chen RG, Chen BH, Du XH, Yin YX, Zhang YY (2015) Characterization and expression profile of CaNAC2 pepper gene. Front Plant Sci 6:755
Hatfield JL, Boote KJ, Kimball BA, Ziska LH, Izaurralde RC, Ort D, Wolfe D (2011) Climate impacts on agriculture: implications for crop production. Agron J 103(2):351–370
Hunt R (2012) Basic growth analysis: plant growth analysis for beginners. Springer Science & Business Media, Berlin
Incrocci L, Massa D, Pardossi A (2017) New trends in the fertigation management of irrigated vegetable crops. Horticulturae 3(2):37
Jiang C, Johkan M, Hohjo M, Tsukagoshi S, Maruo T (2017) A correlation analysis on chlorophyll content and SPAD value in tomato leaves. HortResearch 71:37–42
Kaur B, Kumar D (2013) Development of automated nutrients composition control fertigation system. Int J Comput Sci Eng Appl 3(3):67
Khan MN, Mohammad F (2014) Eutrophication: challenges and solutions. Eutrophication: causes. Consequences Control 2:1–15
Kim HN, Yi PH, Park JH (2022) Evaluation of plant-induced electrical signal and growth of Broccoli (Brassica oleracea var. italica) in response to urea application in soil. Hortic Sci Technol 40(2):210–218
Kim HN, Seok YJ, Park GM, Vyavahare G, Park JH (2023) Monitoring of plant-induced electrical signal of pepper plants (Capsicum annuum L.) according to urea fertilizer application. Sci Rep 13(1):291
Korean Soil Information System. (2023, Feb 23). http://soil.rda.go.kr/soil/sibi/cropSibiPrescript.jsp. Accessed 05 May 2022
Lee CR, Ok JH, An MS, Lee SB, Park KL, Hong SG, Park CB (2017) Soil chemical properties of long-term organic cultivation upland. Korean J Org Agric 25(1):161–170
Lehmann S, Funck D, Szabados L, Rentsch D (2010) Proline metabolism and transport in plant development. Amino Acids 39:949–962
Li J, Hu W, Lu Z, Meng F, Cong R, Li X, Lu J (2022) Imbalance between nitrogen and potassium fertilization influences potassium deficiency symptoms in winter oilseed rape (Brassica napus L.) leaves. Crop J 10(2):565–576
Liu X, Zhou J, Li W, Xu J, Brookes PC (2014) The combined effects of urea application and simulated acid rain on soil acidification and microbial community structure. Environ Sci Pollut Res 21:6623–6631
Nair KP (2019) Soil fertility and nutrient management. Intelligent soil management for sustainable agriculture: the nutrient buffer power concept. Springer, Cham, pp 165–189
Oh SJ, Goh CH, Koh SC (2007) Diurnal changes of chlorophyll fluorescence and antioxidative enzyme activity of the leaves from four subtropical plants. J Environ Sci Int 16(5):633–640
Ono Y, Fukasawa M, Sueyoshi K, Ohtake N, Sato T, Tanabata S, Toyota R, Higuchi K, Saito A, Ohyama T (2021) Application of nitrate, ammonium, or urea changes the concentrations of ureides, urea, amino acids and other metabolites in xylem sap and in the organs of soybean plants (Glycine max (L.) Merr.). Int J Mol Sci 22(9):4573
Park HJ, Park JH, Park KS, Ahn TI, Son JE (2018) Nondestructive measurement of paprika (Capsicum annuum L.) internal electrical conductivity and its relation to environmental factors. Hortic Sci Technol 36(5):691–701
Park JH, Kim HN (2021) Monitoring of plant induced electrical signal of broccoli (Brassica oleracea var. italica) under changing light and CO2 conditions. J Appl Biol Chem 64(4):351–356
Peng Y, Peng Z, Zeng X, Houx JH (2019) Effects of nitrogen-phosphorus imbalance on plant biomass production: a global perspective. Plant Soil 436:245–252
Rao KM, Sresty TVS (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157(1):113–128
Rhoades JD (1983) Soluble salts. Methods Soil Anal Part 2 Chem Microbiol Prop 9:167–179
Ropokis A, Ntatsi G, Kittas C, Katsoulas N, Savvas D (2018) Impact of cultivar and grafting on nutrient and water uptake by sweet pepper (Capsicum annuum L.) grown hydroponically under Mediterranean climatic conditions. Front Plant Sci 9:1244
Saha SR, Hossain MM, Rahman MM, Kuo CG, Abdullah S (2010) Effect of high temperature stress on the performance of twelve sweet pepper genotypes. Bangladesh J Agric Res 35(3):525–534
Sen S, Smith ME, Setter T (2016) Effects of low nitrogen on chlorophyll content and dry matter accumulation in maiz. Afr J Agric Res 11(12):1001–1007
Sreenivasulu N, Sunkar R, Wobus U, Strickert M (2010) Array platforms and bioinformatics tools for the analysis of plant transcriptome in response to abiotic stress. In: Sunkar R (ed) Plant stress tolerance: methods and protocols. Humana Press, Totowa, pp 71–93
Wang Y, Wang E, Wang D, Huang S, Ma Y, Smith CJ, Wang L (2010) Crop productivity and nutrient use efficiency as affected by long-term fertilisation in North China plain. Nutr Cycl Agroecosyst 86:105–119
Ward BB, Arp DJ, Klotz MG (eds) (2011) Nitrification. American Society for Microbiology Press, Washington, DC
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37(1):29–38
White JW, Cook ER, Lawrence JR (1985) The DH ratios of sap in trees: Implications for water sources and tree ring DH ratios. Geochim Cosmochim Acta 49(1):237–246
Xu G, Fan X, Miller AJ (2012) Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol 63:153–182
Yoo JH, Luyima D, Lee JH, Park SY, Yang JW, An JY, Yun YU, Oh TK (2021) Effects of brewer’s spent grain biochar on the growth and quality of leaf lettuce (Lactuca sativa L. var. crispa.). Appl Biol Chem 64:1–10
Acknowledgements
This work was carried out with the support of "Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ015050012023)" Rural Development Administration, Republic of Korea.
Funding
This work was carried out with the support of "Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ015050012023)" Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Contributions
JHP contributed to the study conception and design. Material preparation, data collection and analysis were performed by JYK, SKS and JHP. The first draft of the manuscript was written by JYK and JHP commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The manuscript does not have potential conflicts of interest. The research does not involve human participants or animals.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, J.Y., Sin, S.K. & Park, J.H. Electrical signal of pepper during cropping period affected by different amount of fertilizer. Appl Biol Chem 66, 62 (2023). https://doi.org/10.1186/s13765-023-00821-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-023-00821-2