Abstract
In current study, we addressed the anti-cancer effect of oleifolioside A and its mechanism on the regulation of cell death in HCT-116 human colorectal cancer cells. Oleifolioside A inhibited HCT-116 cell proliferation and caused apoptosis associated with sequential activation of caspases 8 and 3, followed by PARP cleavage. Moreover, anti-LC3-positive granules and the increased LC3-II level were observed in HCT-116 cells treated with oleifolioside A, which is the specific characteristics of autophagy. Treatment of autophagy inhibiors, 3-MA and Wort, markedly accelerated the cell death by oleifolioside A and, furthermore, knockdown of Beclin-1 and Atg7 using shRNA increased oleifolioside A-induced apoptosis, suggesting a cytoprotective function of autophagy against oleifolioside A-triggered apoptosis. Treatment of HCT-116 cells with oleifolioside A time-dependently activated extracellular signal-regulated kinase (ERK). Oleifolioside A-induced autophagy was dramatically inhibited by pretreatment with an ERK inhibitor, U0126, which resulted in a marked reduction in cell viability. These findings indicate that oleifolioside A induce autophagy through ERK activation in HCT-116 cells and that autophagy suppression enhances apoptosis induced by oleifolioside A.
Similar content being viewed by others
Introduction
Dendropanax morbifera Leveille (D. morbifera) has long been used in South Korea as a traditional medical material to treat various diseases such as infectious diseases, migraine headache, dysmenorrhea, and skin diseases [1]. Many studies have proved that D. morbifera extracts possess various pharmacological activities, such as anti-inflammatory [2,3,4] and anti-cancer effects [5] as well as protective effects on kidney function [6] and hippocampus [7,8,9]. Although some compounds isolated from D. morbifera extracts exhibited anti-diabetic [10], anti-obesity [11], anti-thrombotic [12], anti-inflammatory [13], and anti-complementary activities [14], systematic studies of the pharmacological efficacy of the active ingredients of D. morbifera are still not sufficient.
It has been previously reported that oleifolioside A, a bioactive compound isolated from D. morbifera, triggered caspase-independent apoptosis through nuclear translocation of AIF and EndoG in human cervical carcinoma HeLa cells [15]. Although this study has investigated apoptosis-inducing effects of oleifolioside A, its function in autophagy has not been addressed.
The currrent study aimed to explore whether oleifolioside A was able to generate autophagy in HCT-116 human colorectal cancer cells. In addition, mechanisms underlying interactions between autophagy and apoptosis induced by oleifolioside A was investigated.
Results
Oleifolioside A hinders cell proliferation and triggers apoptosis in HCT-116 cells
First, we investigated the inhibitory effect of cell viability by oleifolioside A in HCT-116 cells. Cell viability was assessed by MTT assay after different concentrations of oleifolioside A were treated for 12 h or 24 h. As shown in Fig. 1A, cell viability was diminished in a time- and concentration-dependent way after treatment with oleifolioside A.
Oleifolioside A induces caspase-dependent apoptosis in HCT-116 cells. A Cells were treated with various concentrations of oleifolioside A for 12 and 24 h and then cell viability were measured by MTT assay. B After treatment with different concentrations of oleifolioside A for 24 h and then apoptotic cells were stained with Annexin V and PI and detected by flow cytometry. C Bar graph indicates the percentage of apoptotic cells by quantitative analysis of Av + /PI- (early apoptosis) and Av + /PI + (late apoptosis) cells. D Cells were treated with different concentrations of oleifolioside A for 24 h and then analyzed by immunoblotting with an antibody against caspase-3, caspase-8, caspase-9 and PARP. GAPDH was used as loading control of immunoblotting analysis. E Cells were pre-treated with or without each caspase inhibitor, Z-VAD-FMK (pan-caspase inhibitor), Z-IETD-FMK (caspase-8 inhibitor) and Z-LEHD-FMK (caspase-9 inhibitor) for 1 h and then treated with oleifolioside A for 24 h. Cell viability was measured by MTT assay. Data were expressed as the mean ± SEM of three independent experiments. *p < 0.05 and **p < 0.01 compared with control. PF, precursor form; CF, cleaved form
To evaluate whether the decrease in cell viability was due to apoptosis, after treatment with different concentrations of oleifolioside A for 24 h, cells were stained with Annexin V-FITC and PI. Flow cytometry analysis of Annexin V/PI-stained cells were conducted. As shown in Fig. 1B, C, oleifolioside A significantly increased apoptotic cell populations in a concentration-dependent way, suggesting induction of apoptosis by oleifolioside A in HCT-116 cells. To further confirm oleifolioside A-induced apoptosis, we examined the activation of caspase-3, a core protease in the apoptotic process and the cleavage of PARP, an endogenous caspase-3 substrate, by Western blot analysis. As shown in Fig. 1D, oleifolioside A treatment elevated the cleaved forms of caspase-3 and PARP in a concentration-dependent way. It is well documented that after synthesis as procaspase-3, caspase-3 activation is occurred by caspase-8 and caspase-9 mediating extrinsic and intrinsic apoptosis pathways, respectively [19, 20]. To investigate which of these two caspases triggers the activation of caspase-3 in oleifolioside A-induced apoptosis, therefore, caspase-8 and -9 activations in HCT-116 cells treated with oleifolioside A were monitored by immunoblotting. Oleifolioside A treatment elevated the cleaved forms (18 and 43 kDa) of caspase-8 in a dose-dependent way, but did not those of caspase-9 (Fig. 1D). To further validate caspase-dependent apoptosis by oleifolioside A, cell viability after treatment with caspase inhibitors was monitored. As shown in Fig. 1E, compared with oleifolioside A treatment alone, a marked increase of the cell viability was observed in cells pretreated with the Z-VAD-FMK. In addition, pretreatment with z-IETD-FMK also notably increased the cell viability, whereas z-LEHD-FMK did not reveal a noticeable increment compared with the oleifolioside A alone-treated group. Taken together, these results indicate that oleifolioside A generates apoptosis through caspase-8 activation followed by subsequent caspase-3 activation in HCT-116 cells.
Oleifolioside A causes autophagy in HCT-116 cells
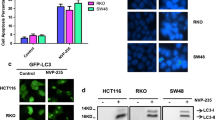
Several natural product compounds have been reported to trigger both apoptosis and autophagy in diverse human cancer cells, including colon cancer cells [21, 22]. To address whether oleifolioside A triggers autophagy in HCT-116 cells, we analyzed the change in a key autophagy marker protein, LC3-II, in HCT-116 cells treated with oleifolioside A by immunoblotting. As shown in Fig. 2A, B, the conversion of LC3-I to LC3-II, which is a core step in autophagy induction [22], was markedly increased in a time-dependent way after oleifolioside A treatment. Caspase-8, caspase-3 and PARP cleavages were simultaneously elevated in a time-dependent manner. Moreover, by LC3 immunofluorescence through confocal microscope anti-LC3-positive granules were detected in oleifolioside A-treated cells compared to control, untreated cells (Fig. 2C). These results indicate that oleifolioside A causes autophagy as well as apoptosis in HCT-116 cells. Induction of autophagy by oleifolioside A was also observed in A549 human lung cancer cells and MCF-7 human breast cancer cells (Additional file 1: Figure S1).
Oleifolioside A induces autophagy in HCT-116 cells. A Cells were treated with oleifolioside A (50 µM) for different times and then analyzed by immunoblotting with an antibody against LC3, caspase-3, caspase-8 and PARP. GAPDH was used as loading control of immunoblotting analysis. B Bar graph shows densitometry analysis of LC3-II/GAPDH ratio. C Cells were treated with oleifolioside A (50 µM) for 24 h and then, after fixation, cells were immunostained with anti-LC3 antibodies (FITC; green). Nuclei were stained with DAPI (blue). Nuclei were stained with DAPI (blue). Images were taken under confocal microscope (scale bars: 10 µm). Data were expressed as the mean ± SEM of three independent experiments. *p < 0.05 and **p < 0.01 compared with control treated for 0 h. PF, precursor form; CF, cleaved form
Inhibition of autophagy decreases cell viability in HCT-116 cells
Many studies have recently reported that autophagy inhibitor, such as Wort and 3-MA, could strengthen apoptosis in human cancer cells treated with natural product compounds [23,24,25,26,27,28]. We treated 3-MA and Wort to repress the autophagy produced by oleifolioside A treatment and measured cell viability. As shown in Fig. 3A, B, cell viability was dose-dependently decreased when oleifolioside A-induced autophagy was inhibited by 3-MA and Wort (Additional file 2: Figure S2). Next, to verify whether autophagy can control apoptosis, Atg7 and Beclin-1, crucial regulators of autophagy, were knocked down using shRNA and then the changes in LC3, PARP and caspase-3 were checked by immunostaining. As shown in Fig. 3C, knockdowns of Atg7 and Beclin-1 gene remarkably repressed Atg7 and Beclin-1 protein expression. Simultaneously, caspase-3 and PARP cleavages were significantly enhanced compared with oleifolioside A-treated shControl cells. Collectively, these results indicate that autophagy inhibiton by 3-MA and Wort or by Atg7 and Beclin-1 knockdowns promotes oleifolioside A-induced apoptosis and cell death in HCT-116 cells.
Inhibition of oleifolioside A-induced autophagy increases cell death in HCT-116 cells. Cells were pre-treated with or without different concentrations of 3-MA A or wortmannin B for 1 h and then treated with 50 µM oleifolioside A for 24 h. Cell viability was measured by MTT assay. Data were expressed as the mean ± SEM of three independent experiments. *p < 0.05 and **p < 0.01 compared with control. C Knockdown cells (shControl, shAtg7 and shBeclin-1) were treated with oleifolioside A (50 µM) for 24 h. Immunoblot analysis was carried out with antibodies against Atg7, Beclin-1, LC3, PARP-1/2 and caspase-3, respectively. GAPDH was used as loading control. PF, precursor form; CF, cleaved form
Oleifolioside A generates autophagy through activation of ERK pathway
Induction of autophagy by ERK/JNK/p38 MAPK signaling pathway has been recently reported [17, 29,30,31,32,33]. To explore the pathway which contributes to autophagy induction by oleifolioside A in HCT-116 cells, we investigated MAPK signaling pathway by immunoblotting. As shown in Fig. 4A, the active phosphorylated forms of ERK and JNK were time-dependently elevated. To investigate the effects of ERK and JNK MAPK signal pathways on cell death, we measured cell viability after oleifolioside A treatment in HCT-116 cells pre-treated with MAPK inhibitors. As shown in Fig. 4B, cell viability was significantly decreased by U0126, but not by SB203580 and SP600125. In addition, the protein level of LC3-II increased by oleifolioside A treatment was also notably decreased after the combined treatment with U0126 and oleifolioside A (Fig. 4C, D). Taken together, these results indicate that oleifolioside A-triggered autophagy is mediated by ERK signaling pathway, and autophagy inhibition by U0126 augments cell death induced by oleifolioside A in HCT-116 cells.
Effects of oleifolioside A on MAPK signaling pathway in HCT-116 cells. A Cells were treated with oleifolioside A (50 µM) for different times and then analyzed by immunoblotting with antibodies against p-ERK1/2, ERK1/2, p-p38, p38, p-JNK and JNK. GAPDH was used as loading control of immunoblotting analysis. B Cells were pre-treated with or without different concentrations (20 µM and 40 µM) of U0126, SB203580 and SP600125 for 1 h and then treated with 50 µM oleifolioside A for 24 h. Cell viability was measured by MTT assay. C Cells were pre-treated with or without 40 µM U0126 for 1 h and then treated with 50 µM oleifolioside A for 24 h. Immunoblot analysis was performed with antibody against LC3. D Bar graphs show densitometry analysis of LC3-II/GAPDH ratio. Data were expressed as the mean ± SEM of three independent experiments. **p < 0.01 compared with control; # p < 0.05 compared with oleifolioside A-treated group
Discussion
Although a previous study reported that oleifolioside A, a new component extracted from Dendropanax morbifera Leveille, triggers caspase-independent apoptosis in human cervical carcinoma HeLa cells [15], its role in relation to autophagy has yet to be identified. Thus, in this study, we addressed the role of oleifolioside A on autophagy in HCT-116 human colorectal cancer cells. Here we have demonstrated for the first time that oleifolioside A can trigger autophagy and apoptosis in HCT-116 cells. Unlike a previous report [15] showing the caspase-independent apoptosis by oleifolioside A in HeLa cells, our current results clearly indicated that oleifolioside A triggered caspase-dependent apoptosis, as proven by the increase of caspase-8, -3 and PARP cleavages, as well as the rescue from cell death by caspase inhibitors, z-VAD and z-IETD. These findings suggest that oleifolioside A-induced apoptosis is possibly cell type-dependent.
The level of LC3-II expression is the most extensively used as a typical marker of the autophagosome formation, because LC3-I to LC3-II conversion is a hallmark showing the extent of autophagosome [34, 35]. In addition to apoptosis induction by oleifolioside A in HCT-116 cells, in current study, we demonstrated that oleifolioside A triggers autophagy in HCT-116 cells, as evidenced by LC3 immunostaining and immunoblot analysis showing the marked elevation of LC3-II expression levels. Numerous studies have shown that the suppression of autophagy by its specific inhibitor could enhance apoptosis and consequently result in the increase of cell death in human colon cancer cells treated with natural compounds [23,24,25,26,27,28]. Consistent with these observations, our present results showed that autophagy inhibition by its specific inhibitor, 3-MA or wortmannin, led to the increase in cell death in HCT-116 cells, as characterized by the significant reduction in cell viability. Moreover, knockdowns of Atg7 and Beclin-1 genes markedly enhanced oleifolioside A-triggered apoptosis in HCT-116 cells, as proven by increases of caspase-3 and PARP cleavages. These results suggest that autophagy might have cytoprotective role against apoptotic cell death in oleifolioside A-treated HCT-116 cells.
It had been previously reported that ERK, one of MAPK family members, plays a key role in regulation of autophagy [17, 36,37,38]. For instance, Sivaprasad and Basu reported that TNF induced autophagy in MCF-7 cells via the ERK signaling pathway and inhibition of ERK signaling enhanced TNF-triggered cell death [36]. Wen et al. showed that orexin A, a class of peptides, triggered autophagy through the ERK1/2 activation in HCT-116 cells and ERK inhibition reduced the LC3 II level increased by orexin A treatment [27]. In addition, we have also previously reported that dendropanoxide (DP), another natural compound from Dendropanax morbifera Leveille, induces autophagy through the activation of ERK1/2 in MG-63 human osteosarcoma cells and autophagy inhibition elevates DP-triggered apoptosis [17]. In accordance with these findings, in the present study, we revealed that oleifolioside A activated ERK1/2 and ERK1/2 inhibition by U0126 notably decreased the LC3-II level enhanced by oleifolioside A, which leads to augment the cell death by oleifolioside A.
Material and methods
Materials
The ERK1/2 inhibitor U0126, was obtained from Promega (Madison, WI, USA). Z-IETD-FMK (caspase-8 inhibitor), Z-LEHD-FMK (caspase-9 inhibitor), Z-VAD-FMK (pan-caspase inhibitor), SB203580, SP600125 and wortmannin (Wort) were purchased from Calbiochem (Darmstadt, Germany). Antibodies for PARP-1/2, p-ERK and ERK were obtained from Santa Cruz Biotechnology (CA, USA); antibodies for Atg7, Beclin-1, caspase-3, caspase-8, caspase-9, LC3, p-p38, p38, p-JNK and JNK were purchased from Cell Signaling Technology (Dancers, Mass, USA); antibody for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and polyvinylidene difluoride membrane (PVDF) were obtained from Millipore (Milford, MA, USA); fluorescein isothiocyanate (FITC)-conjugated and horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Vector Laboratories (CA, USA) and Enzo Life Science (Farmingdale, NY, USA), respectively. 3-Methyladenine (3-MA) and 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI) were purchased from Sigma-Aldrich. (St. Louis, MO, USA). Annexin V-FITC apoptosis detection kit and BCA protein assay kit were purchased from BD Biosciences (San Jose, CA, USA) and Thermo (Rockford, IL, USA), respectively. Oleifolioside A isolated from D. morbifera was prepared as described previously [16], dissolved in dimethyl sulfoxide (DMSO) as a stock solution at 30 mM concentration, and stored in aliquots at − 20 °C.
Cell cultures
Human colon cancer cell line HCT-116 was obtained from American Type Culture Collection (Rockville, MD, USA). Cells were cultured in plastic dishes containing Dulbecco’s modified Eagle’s medium (DMEM; WelGENE Co., Korea) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS), 100 μg/ml streptomycin and 100 U/ml penicillin at 37 °C under 5% CO2.
Cell viability
The cytotoxic effect of oleifolioside A on HCT-116 cells was measured by MTT assay as previously described [17]. Briefly, when the cells reached about 70% confluence, they were treated with various concentrations of oleifolioside A for 12 and 24 h. Cell viability was determined by measuring the optical density (OD) at 590 nm and quantified as a percentage compared to the control.
Westernblot analysis
Westernblot analysis was conducted as described previously [17]. Antibodies for caspase-3, caspase-8, caspase-9, LC3, PARP-1/2, p-ERK, ERK p-p38, p38, p-JNK, JNK and GAPDH were utilized.
Immunofluorescence staining
Immunofluorescence staining analysis was performed with anti-LC3 antibody and FITC-conjugated secondary antibody according to a procedure as previously described [17]. Fluorescent signals in cells were observed with LSM 700 confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany).
Annexin V and PI staining
After treatment with the indicated concentrations of oleifolioside A for 24 h, the cells were harvested, washed with cold PBS, and then stained with Annexin V-FITC and propidium iodide (PI) in accordance with the manufacturers’ instructions and finally analyzed with Beckman-Coulter Cytomics FC500 flow cytometer (Beckman-Coulter, Miami, FL, USA).
RNA interference
Plasmids pLKO.1 lentiviral plasmid containing shRNA against Beclin-1 (TRCN0000033552) and Atg7 (TRCN0000007587) were purchased from Sigma-Aldrich (Mission shRNA). Lentivirus productions and lentiviral infections were carried out according to the supplier's protocol. After puromycin selection, cells were treated with oleifolioside A and the silencing efficacy of shRNAs was proved using by immunoblot analysis, as described previously [18].
Statistical analysis
All experiments data are expressed as means ± SEM of at least three replicates in each group. Comparisons between the two groups were determined by Student’s t-test and *P < 0.05 was considered statistically significant.
Availability of data and materials
The datasets used in this study are available from the corresponding authors upon request.
References
Bae K (2000) The Medicinal Plants of Korea. Kyo-Hak Publishing Co Ltd, Seoul
Akram M, Kim KA, Kim ES, Syed AS, Cy K, Lee JS et al (2016) Potent anti-inflammatory and analgesic actions of the chloroform extract of dendropanax morbifera mediated by the Nrf2/HO-1 pathway. Biol Pharm Bull 39:728–736
Choo GS, Lim DP, Kim SM, Yoo ES, Kim SH, Kim CH et al (2019) Anti-inflammatory effects of Dendropanax morbifera in lipopolysaccharide-stimulated RAW264.7 macrophages and in an animal model of atopic dermatitis. Mol Med Rep 19:2087–2096
Shim HJ, Park S, Lee JW, Park HJ, Baek SH, Kim EK et al (2016) Extracts from Dendropanax morbifera leaves have modulatory effects on neuroinflammation in microglia. Am J Chin Med 44:119–132
Lee JW, Park C, Han MH, Hong SH, Lee TK, Lee SH et al (2013) Induction of human leukemia U937 cell apoptosis by an ethanol extract of Dendropanax morbifera Lev. through the caspase-dependent pathway. Oncol Rep 30:1231–1238
Kim ES, Lee JS, Akram M, Kim KA, Shin YJ, Yu JH et al (2015) Protective activity of Dendropanax morbifera against cisplatin-induced acute kidney injury. Kidney Blood Press Res 40:1–12
Kim W, Kim DW, Yoo DY, Jung HY, Nam SM, Kim JW et al (2014) Dendropanax morbifera Leveille extract facilitates cadmium excretion and prevents oxidative damage in the hippocampus by increasing antioxidant levels in cadmium-exposed rats. BMC Complement Altern Med 14:e428
Kim W, Kim DW, Yoo DY, Jung HY, Kim JW, Kim DW et al (2015) Antioxidant effects of Dendropanax morbifera Leveille extract in the hippocampus of mercury-exposed rats. BMC Complement Altern Med 15:e247
Jung HY, Kwon HJ, Hahn KR, Yoo DY, Kim W, Kim JW et al (2018) Dendropanax morbifera Léveille extract ameliorates cesium-induced inflammation in the kidney and decreases antioxidant enzyme levels in the hippocampus. Mol Cell Toxicol 14:193–199
Moon HI (2011) Antidiabetic effects of dendropanoxide from leaves of Dendropanax morbifera Leveille in normal and streptozotocin-induced diabetic rats. Human Exp Toxicol 30:870–875
Kang MJ, Kwon EB, Ryu HW, Lee S, Lee JW, Kim DY et al (2018) Polyacetylene from Dendropanax morbifera alleviates diet-induced obesity and hepatic steatosis by activating AMPK signaling pathway. Front Pharmacol 9:e537
Choi JH, Kim DW, Park SE, Lee HJ, Kim KM, Kim KJ et al (2015) Anti-thrombotic effect of rutin isolated from Dendropanax morbifera Leveille. J Biosci Bioeng 120:181–186
Hyun TK, Ko YJ, Kim EH, Chung IM, Kim JS (2015) Anti-inflammatory activity and phenolic composition of Dendropanax morbifera leaf extracts. Ind Crops Prod 74:263–270
Chung IM, Song HK, Kim SJ, Moon HI (2011) Anticomplement activity of polyacetylenes from leaves of Dendropanax morbifera Leveille. Phytother Res 25:784–786
Yu HY, Jin CY, Kim KS, Lee YC, Park SH, Kim GY (2012) Oleifolioside A mediates caspase-independent human cervical carcinoma HeLa cell apoptosis involving nuclear relocation of mitochondrial apoptogenic factors AIF and EndoG. J Agric Food Chem 60:5400–5406
Yu HY, Kim KS, Lee YC, Moon HI, Lee JH (2012) Oleifolioside A, a new active compound, attenuates LPS-stimulated iNOS and COX-2 expression through the downregulation of NF-κB and MAPK activities in RAW 2647 macrophages. Evid Based Complemen Alternat Med 2012:e637512
Lee JW, Kim KS, An HK, Kim CH, Moon HI, Lee YC (2013) Dendropanoxide induces autophagy through ERK1/2 activation in MG-63 human osteosarcoma cells and autophagy inhibition enhances dendropanoxide-induced apoptosis. PLoS ONE 8:e83611
An HK, Kim KS, Lee JW, Park MH, Moon HI, Park SJ et al (2014) Mimulone-induced autophagy through p53-mediated AMPK/mTOR pathway increases caspase-mediated apoptotic cell death in A549 human lung cancer cells. PLoS ONE 9:e114607
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Boatright KM, Salvesen GS (2003) Mechanisms of caspase activation. Curr Opin Cell Biol 15:725–731
Zhang X, Chen LX, Ouyang L, Cheng Y, Liu B (2012) Plant natural compounds: targeting pathways of autophagy as anti-cancer therapeutic agents. Cell Prolif 45:466–476
Rahman A, Rahman H, Hossain S, Biswas P, Islam R, Uddin J et al (2020) Molecular insights into the multifunctional role of natural compounds: autophagy modulation and cancer prevention. Biomedicines 8:517
Jiang F, Zhou JY, Zhang D, Liu MH, Chen YG (2018) Artesunate induces apoptosis and autophagy in HCT116 colon cancer cells, and autophagy inhibition enhances the artesunate-induced apoptosis. Int J Mol Med 42:1295–1304
Fan XJ, Wang Y, Wang L, Zhu M (2016) Salidroside induces apoptosis and autophagy in human colorectal cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol Rep 36:3559–3567
Zhu M, Zhang P, Jiang M, Yu S, Wang L (2020) Myricetin induces apoptosis and autophagy by inhibiting PI3K/Akt/mTOR signalling in human colon cancer cells. BMC Complement Med Ther 20:209
Nishikawa T, Tsuno NH, Okaji Y, Shuno Y, Sasaki K, Hongo K et al (2010) Inhibition of autophagy potentiates sulforaphane-induced apoptosis in human colon cancer cells. Ann Surg Oncol 17:592–602
Wen J, Zhao Y, Guo L (2016) Orexin A induces autophagy in HCT-116 human colon cancer cells through the ERK signaling pathway. Int J Mol Med 37:126–132
Lee Y, Sung B, Kang YJ, Kim DH, Jnag JY, Hwang SY et al (2014) Apigenin-induced apoptosis is enhanced by inhibition of autophagy formation in HCT116 human colon cancer cells. Int J Oncol 44:1599–1606
Corcelle E, Djerbi N, Mari M, Nebout M, Fiorini C, Fenichel P et al (2007) Hofman P, Poujeol P & Mograbi B, Control of the autophagy maturation step by the MAPK ERK and p38: lessons from environmental carcinogens. Autophagy 3:57–59
Zhou YY, Li Y, Jiang WQ, Zhou LF (2015) MAPK/JNK signalling: a potential autophagy regulation pathway. Biosci Rep 35:e00199
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang Q et al (2014) p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett 344:174–179
Cagnol S, Chambard JC (2010) ERK and cell death: Mechanisms of ERK-induced cell death apoptosis, autophagy and senescence. FEBS J 277:2–21
Aredia F, Guamán Ortiz LM, Giansanti V, Scovassi AI (2012) Autophagy and cancer. Cell 1:520–534
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T et al (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728
Mizushima N, Yoshimori T (2007) How to interpret LC3 immunoblotting. Autophagy 3:542–545
Sivaprasad U, Basu A (2008) Inhibition of ERK attenuates autophagy and potentiates tumour necrosis factor-α-induced cell death in MCF-7 cells. J Cell Mol Med 12:1265–1271
Zeng Y, Yang X, Wang J, Fan J, Kong Q, Yu X (2012) Aristolochic acid I induced autophagy extenuates cell apoptosis via ERK 1/2 pathway in renal tubular epithelial cells. PLoS ONE 7:e30312
Jo C, Kim S, Cho SJ, Choi KJ, Sm Y, Koh YH et al (2014) Sulforaphane induces autophagy through ERK activation in neuronal cells. FEBS Lett 588:3081–3088
Acknowledgements
We thank Dr. Hyung-In Moon (SKEDERM Cosmetic R&D Center, Seoul, Republic of Korea) for providing oleifolioside A.
Funding
This work was supported by the Dong-A University research fund.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: S-YA, H-KA, Y-CL, S-HK. Performed the experiments: S-YA, H-KA. Analyzed the data: S-YA, H-KA, K-SK, Y-CL, S-HK. Contributed reagents/materials/analysis tools: K-SK, S-HK. Wrote the paper: S-YA, Y-CL, S-HK. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declares that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Induction of authophagy by oleifolioside A in A549 human lung cancer cells and MCF-7 human breast cancer cells. A Cells were treated with different concentration of oleifolioside Afor 24 h and then analyzed by immunoblotting with an antibody against LC3. GAPDH was used as loading control of immunoblotting analysis. B Cells were treated with oleifolioside Afor 24 h and then, after fixation, cells were immunostained with anti-LC3 antibodies. Nuclei were stained with DAPI. Images were taken under confocal microscope.
Additional file 2: Figure S2.
Effects of oleifolioside A on MAPK signalling pathway in HCT-116 cells. Cells were pre-treated with or without 10 mM Methyladenineor 10 μM Wortmanninfor 1 h and then treated with 50 μM oleifolioside A for 24 h. Immunoblot analysis was performed with antibody against LC3. Bar graphs show densitometry analysis of LC-3II/GAPDH ratio. Data were expressed as the mean ± SEM of three independent experiments. **p<0.01 compared with control; # p<0.05 compared with oleifolioside A-treated group.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
An, SY., An, HK., Kim, KS. et al. Induction of autophagy by oleifolioside A in HCT-116 human colorectal cancer cells. Appl Biol Chem 66, 32 (2023). https://doi.org/10.1186/s13765-023-00791-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-023-00791-5