Abstract
Intrinsic and exogenous signals in conjunction precisely regulate the initiation of flowering. Both signals influence flowering time, which is an integral part of plant reproduction. The signals converge through different pathways, and their coordinated action leads to the onset of flowering. Genetic pathways related to the regulation of flowering time are well-known from research into the molecular genetics of Arabidopsis thaliana. Specifically, crucial components of the photoperiodic pathway and floral integrators play a critically significant role in flowering. In this study, we found that GATA25 is a novel transcription factor that accelerates flowering time under long days. GATA25 encodes C-X2-C-X20-C-X2-C conserved cysteine residues of the zinc-finger domain and CCT domain which process photoperiodic flowering and regulate circadian rhythms. Flowering was accelerated by overexpression of GATA25 throughout the Arabidopsis thaliana. In contrast, GATA25 fused to SRDX (SUPERMAN repressive domain X)-motif plants showed delayed flowering. We also demonstrated that GATA25 induced the expression of floral integrator genes and photoperiodic pathway-related genes. Together, these results suggest that GATA25 might act to accelerate flowering time.
Similar content being viewed by others
Introduction
Terrestrial plants have adapted their flowering and growth to environmental changes over evolutionary time, and as a result, precisely regulate flowering patterns in different growing environments. Controlling the flowering patterns of plants can be a solution that maximizes crop yields in a limited space to overcome rapid environmental changes and reductions in crop yields caused by global warming. Molecular genetic analysis of flowering regulation in Arabidopsis thaliana to elucidate the flowering timing regulation mechanism confirms that flowering is regulated by various distinct pathways [1, 2]. As a result, regulatory proteins and genetic pathways involved in the regulation of flowering time have been identified. Typically, the control of flowering time can be attributed to both intrinsic and exogenous signals for flowering. The intrinsic signals associated with flowering include gibberellic acid (GA), autonomous signals, and aging signals whereas the exogenous signals are light intensity and day length (photoperiod). These distinct signals together converge to control activation of flowering initiation [3]. Especially for exogenous signals, it regulates the flowering pathway in a complex and sophisticated manner through circadian regulation of post-transcriptional regulation of protein stability and gene transcription by photoperiod [4, 5]. Therefore, the study of the photoperiodic pathway regulation mechanism for exogenous signals is the key to studying plant flowering patterns. The photoperiodic pathway involved in the exogenous signals regulates key components GIGANTEA (GI) and CONSTANS (CO). The CO transcription factor, which enables sensing of long days (LDs), is known to be regulated by physical interact with FLOWERING HTH1 (FHTH1) [6]. Also, The CO transcription factor is expressed in phloem companion cells and positively regulates FLOWERING LOCUS T (FT) expression under LDs photoperiods only [7, 8]. The FT protein, induced through the sequential activation of CO, interacts with FD to form the FT-FD complex [9]. The FT-FD complex activates the expression of a SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1) [10, 11]. The SOC1 gene, a type of floral integrator, is generated in the meristem during floral transition and is regulated by the phytohormone GA [12, 13]. Therefore, it can be inferred that it is an essential component for floral induction. The GI transcription factor regulates CO transcription through binding FLAVIN BINDING KELCH REPEAT F-BOX 1 (FKF1). The GI-FKF1 complex promotes the degradation of CYCLING DOF FACTOR 1 (CDF1) [14, 15]. Here, we studied the Arabidopsis gene GATA25, also known as ZIM [16, 17]. GATA25 is a member of subfamily III containing the C-X2-C-Χ20-C-Χ2-C conserved cysteine residues of the zinc-finger domain and a CONSTANS, CO-like, and TOC1 (CCT) domain [18]. The transcription factors containing the CCT domain are known to regulate flowering time and circadian rhythm [19, 20]. Therefore, we expected that the GATA25 transcription factor, including the CCT domain, would act as a regulator of flowering time. We observed that flowering initiation was accelerated when the GATA25 gene was overexpressed from the 2Χ CaMV 35S promoter. In contrast, flowering initiation was delayed when GATA25 fused to an SRDX repression motif was overexpressed from the 2Χ CaMV 35S promoter. Moreover, we demonstrated that GATA25 can control the expression of GI and CO and subsequent FT and SOC1 involved in the photoperiodic flowering response. Our results suggest that GATA25 acts as a positive regulator of flowering by regulating key floral integrators and photoperiodic pathways.
Material and methods
Plant materials and growth conditions
All of the Arabidopsis thaliana plants used in this study came from the Columbia ecotype. Plants were grown in soil in a plant growth room, at 23 ℃ with a controlled light/dark cycle (16 h light/8 h dark). Light intensity was approximately 90 μmol/ m2/ s.
Construction of vectors and generation of transgenic plants
To generate the overexpression of GATA25 (At4g24470) transgenic plants, full-length GATA25 CDS was amplified from leaf cDNA of Arabidopsis by PCR using primers linked to SpeI and XmaI sites, respectively. The PCR product was cloned into a binary pTK-BMLC vector which replaced the pCB302-3 vector with a CaMV 35S promoter to a 2Χ CaMV 35S promoter, and multiple cloning sites were XbaI, SpeI, BamHI, XmaI [21]. To generate the inducible expression of GATA25 transgenic plants, a GATA25 fragment was introduced at the SpeI site behind the GAL4 upstream activation sequence, positioned at SpeI in the pTA7002-BMLC vector, in which a CaMV 35S promoter replaced the 2Χ CaMV 35S promoter in the pTA7002 vector [22]. To generate the GATA25-SRDX chimeric repressor transgenic plant, full-length GATA25 CDS without the stop codon was amplified by PCR using primers tagged with the sequence encoding the SRDX motif (5′-LDLELRLGFA-3′) [23] and inserted into the pTK-BMLC vector. All binary vectors were verified by DNA sequencing.
Each binary vector was transformed into the Agrobacterium tumefaciens strain GV3101 used for Agrobacterium-mediated transformation of Arabidopsis thaliana (Col-0) [24, 25] and transgenic plants were screened using a 0.1% Basta solution as a selection marker.
RNA extraction and gene expression analysis
Total RNA was extracted from leaves using Trizol reagent and then was treated with RNase-free DNaseI. As previously described, the first-strand cDNA was synthesized using Superscript III reverse transcriptase [26, 27].
For semi-quantitative RT-PCR analysis, we performed PCR reactions to measure gene expression profiles using Emerald Amp GT PCR Master Mix. Amplified PCR products were separated on 1% agarose gel and stained with ethidium bromide. To compare gene transcription levels, each cDNA reaction product was normalized to PP2A (At1g13320) [28, 29].
For quantitative real-time PCR (qRT-PCR) analysis, gene expression levels were calculated using SYBR green to monitor double-strand DNA synthesis. To compare data from each cDNA reaction product, cycle threshold (CT) values for specific-target genes were normalized to the CT value of PP2A. All experiments were performed with three independent biological replicates. Details of the primers used in this study are provided in Additional file 1: Table S1.
Dexamethasone-inducible transcription activation system
The GATA25/pTA7002-BMLC transgenic plants were grown in soil for 2 weeks, and groups were divided according to their treatment with or without dexamethasone (DEX). To activate GATA25, DEX was applied by spraying at 10 μM with 0.02% silwet surfactant. The control groups were sprayed with water containing 0.01% ethanol and 0.02% silwet surfactant. After treatment, young rosette leaves were harvested at the indicated time for expression profile analysis [22].
Isolation of protoplasts and transcriptional activation analysis
Protoplasts were prepared from Arabidopsis leaves, and polyethylene glycol-mediated transformation of effector and reporter constructs was conducted as previously described [30]. For transcriptional activation analysis, protoplasts transfected with effector and reporter were lysed after incubation for 12 h, and GUS activity was calculated using the soluble extract.
To activate GATA25, which fused to the N-terminus of the glucocorticoid receptor (GR), the protoplasts were treated with 10 μM DEX. To inhibit new protein synthesis, cycloheximide was treated with 2 μM 30 min before DEX was added.
Statistics
The data are presented as mean of at least three biological replications ± standard deviation (SD). A difference was considered to be statistically significant when the P-value was less than 0.05 using a pairwise Student’s t-test.
Results
GATA25 accelerates the flowering time under LDs
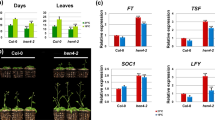
GATA25, also known as ZIM, is a transcription factor that has transcriptional activation and is localized in the nucleus [16, 17]. Transcription factors bind to a DNA promoter sequence, such as a regulatory sequence, and regulate transcription of the downstream genes [31,32,33]. To investigate the characteristics of GATA25, a transcriptional regulatory factor, we isolated twelve independent overexpression GATA25 lines and six independent chimeric repressor GATA25-SRDX lines in which there was fusion of the SRDX motif to the dominant repression of target genes. A SUPERMAN repressor domain X (SRDX), LDLELRLGFA sequence was recruited as a co-repressor to build a repressive complex on specific targeted promoters [34]. We analyzed the flowering phenotype of WT and transgenic plants under LD photoperiods, which efficiently induced flowering [10]. When strongly expressed transcription levels occurred in plants with GATA25, the transgenic plants were early flowering at the same times as WT plants. Interestingly, in contrast, GATA25-SRDX lines had significantly delayed bolting (Fig. 1A). When we counted the total number of rosette leaves to measure the flowering time [35, 36], we found that the number of rosette leaves flowering under LDs was associated with decreased overexpression of GATA25 lines as compared to WT, whereas GATA25-SRDX lines increased the number of rosette leaves at flowering (Fig. 1B). To reduce variation in the growth of plants and growth conditions within the experiment, WT and each transgenic plant were grown in the same pot (Fig. 1C, D). The results clearly showed that overexpression of GATA25 lines were early flowering and GATA25-SRDX lines were delayed in bolting compared to WT.
The early-flowering phenotype in GATA25 over-expressing plants. A Phenotypes of representative plants from wild-type (WT), GATA25-OX, and GATA25-SRDX plants. B Plants were scored for flowering time by measuring the number of rosette leaves at bolting. The mean ± SD were obtained from 6 plants. Phenotypes of GATA25-OX (C) and GATA25-SRDX (D) in a single pot with WT plants. Asterisks indicated statistically significant differences compared to WT as determined by Student’s t-test (*p < 0.05, **p < 0.01, ***p < 0.001)
Inducible expression of GATA25 affects the flowering development in Arabidopsis
Various pathways controlling flowering time regulate the floral pathway integrators in Arabidopsis [1, 2]. Floral promotive genes can serve as a molecular signal to identify the affected flowering pathways. To understand the molecular mechanism of GATA25 in the early flowering phenotype, we generated transgenic plants using transient induction of GATA25 transcription levels under the chemical control of transcription using the GVG system [37]. The DEX-inducible expression of GATA25 transgenic plants was accomplished by inserting the full-length cDNA of GATA25 into the pTA7002-BMLC vector containing a chimeric transcription factor that was driven by a 2Χ CaMV 35S promoter (Fig. 2A). It is known that key floral integrators, SOC1 and FT, converge to regulate various pathways [3, 12] and overexpression of these genes accelerate flowering [38]. To examine the expression of gene profiling, which is known to affect flowering time, transgenic plants of different lines in which GATA25 transcription was induced with DEX were collected at 0, 1, 3, and 6 h after the initial DEX treatment (Fig. 2B). When induction of GATA25 transcription with DEX led to the accumulation of floral pathway integrator transcripts, SOC1 and FT. Moreover, GI and CO involving photoperiodic pathways also accumulated with the expression of GATA25 transcription. Interestingly, the GI and CO were assigned to the photoperiodic pathway, which promoted flowering in response to long photoperiods [39,40,41] and were highly correlated with GATA25 in response to circadian rhythm [42]. Thus, our results suggest that increased transcription levels of GATA25 alter the circadian rhythm, which causes accelerated flowering.
Over-expression of GATA25 shown accelerated flowering and induced expression levels of GI, FT, CO, and SOC1. A Schematic diagram of the vector used for DEX-inducible expression of GATA25 in pTA7002-BMLC. B Expression patterns according to DEX treatment time of several flowering response-related genes of DEX-inducible GATA25 independent lines. Semi-quantitative RT-PCR was performed using the PP2A gene as control
GATA25 is a flowering time genes-regulated transcriptional activator
The transient gene expression system using Arabidopsis mesophyll protoplasts was used to analyze the functions of cellular regulatory pieces of machinery by manipulating macromolecules. Many studies extensively apply transient analysis of promoter activity using transient transcriptional activation [43,44,45]. We used a transient transcriptional activation analysis to test the hypothesis that GATA25 activates transcription of GI, CO, FT, and SOC1 (Fig. 3). As previously described, construction of an effector and reporter in the experiments was performed [46]. A 2Χ CaMV 35S promoter-driven GATA25 expression construct (effector) and 1 kb ~ 2 kb promoter-driven GUS expression construct (reporter) were co-transfected in Arabidopsis mesophyll protoplasts (Fig. 3A). As expected, when expressed effector was combined with 2Χ CaMV 35S promoter-driven GATA25, GUS activities of the reporters with the GUS gene driven by the selected promoters were significantly increased compared to control with transfection of the only reporter in protoplasts (Fig. 3B).
GATA25 activates the expression of flowering response-related genes. A Schematic diagram of the effector and reporter constructs used in the transcriptional activation analysis. B Transcriptional activation analysis showing that the promoters of GI, FT, CO, and SOC1 was activated by expressing GATA25. The activity of the GUS in the reporter construct transfected protoplasts with no effector was used as a control. Error bars represent the standard deviation (SD) of three biological replicates. Asterisks indicated statistically significant differences compared to Control as determined by Student’s t-test (*p < 0.05, **p < 0.01, ***p < 0.001)
Transcription of flowering time genes is directly activated by GATA25
To further investigate whether GATA25 directly activates the expression of GI, CO, FT, and SOC1, we used a more powerful method, the steroid receptor-based inducible activation system [47, 48]. This system uses the glucocorticoid receptor hormone-binding domain (GR) fused transcription factor that binds the heat shock protein 90 (HSP90) in the cytoplasm and allows the fusion protein to enter the nucleus by addition of DEX. Simultaneous treatment with DEX and an inhibitor of protein biosynthesis, cycloheximide (CHX), prevents the accumulation of additional protein that subsequently affects gene expression [49,50,51,52]. First, the effector was constructed such that the GATA25 gene fused to the GR (GATA25-GR) was driven by the 2Χ CaMV 35S promoter (Fig. 4A). The inducible system was tested by co-transfection of the reporters and effectors described above in Arabidopsis mesophyll protoplasts. GUS activities of the reporters in which the GUS gene was driven by the selected promoters was highly induced by the addition of DEX. However, using simultaneous treatment with CHX and DEX, GUS activities of the reporters were not induced (Fig. 4B). We found that CHX was sufficient to inhibit additional protein synthesis, and DEX could activate the inducible system. Under this condition, GATA25-GR, which was treated simultaneously with CHX and DEX, resulted in an induction of the expression of target genes over time compared to only CHX (Fig. 4C). These results suggest that GATA25 regulates transcription of downstream target genes GI, CO, FT, and SOC1 by activating each promoter [32, 53].
Direct activation of flowering response-related genes by GATA25. A Schematic diagram of the GATA25-GR (effector) and reporter constructs used in the transcriptional activation analysis. GATA25 was fused to the glucocorticoid receptor (GR) under the 2ΧCaMV 35S promoter. B GATA25 activates the promoters of GI, FT, CO, and SOC1 in the presence of DEX. Still, adding of the protein synthesis inhibitor cycloheximide at 2 μM completely abolished the DEX-induced GUS activity, indicating that the CHX treatment completely inhibited new protein synthesis. The activity of the GUS in the reporter construct transfected protoplasts with no effector was used as a control. C GATA25 directly induces the expression of flowering response-related genes by DEX activation of GATA25 occurred in the presence of CHX by performing the qRT-PCR analysis. The expression of flowering response-related genes in protoplasts, co-transfected with effector and reporter, treated with CHX was used as a control. Error bars represent the standard deviation (SD) of three biological replicates. Asterisks indicated statistically significant differences compared to Control as determined by Student’s t-test (*p < 0.05, **p < 0.01, ***p < 0.001)
Discussion
We found that Arabidopsis GATA25 is a novel regulator of the floral transition. This novel function of the GATA25 transcription factor was revealed when our study showed that transgenic plants with increased GATA25 transcription levels were early flowering, whereas the GATA25-SRDX chimeric repressor transgenic plants were late flowering (Fig. 1). This result is consistent with the fact that early flowering occurs when XTH33, known as a downstream target gene of GATA25, is overexpressed [17, 54].
Various genes have been identified that play a vital role in regulating flowering through the combination of and cooperative actions of different pathways [55,56,57,58,59]. To determine whether GATA25 affects the expression of genes involved in the flowering promotive pathway, we used the GVG system, which controls transcription of the gene of interest by DEX treatment. We confirmed that the GATA25 transcription level induced by DEX treatment increased the transcription levels of SOC1 and FT, known as key floral integrators (Fig. 2). Specifically, gibberellin (GA) signaling positively regulates the expression of floral pathway integrators [13, 60]. However, Shikata et al. (2004) showed that GATA25 leads to petiole elongation in a GA-independent pathway. This indicates that GATA25 regulates the floral pathway integrators in a GA-independent pathway. In addition, we showed that the GATA25 transcription level increased the transcription levels of GI and CO involved in the photoperiodic pathway. In the photoperiodic pathway, circadian clock control of gene transcription by light can provide control of the flowering promotive pathway [4]. We confirmed that the expression of GATA25 is regulated in response to light [42]. This suggests that GATA25 is closely related to circadian-regulated gene expression and the photoperiodic pathway.
We have demonstrated that GATA25 is a transcriptional activator of floral pathway integrators and photoperiodic pathways (Fig. 3). Also, direct target analysis using the steroid receptor-based inducible activation system reveals that GATA25 directly activates the expression of floral pathway integrators and photoperiodic pathways (Fig. 4). In addition, we confirmed that each promoter of the genes activated by GATA25 has a putative GATA binding sequence (TGATAA and AGATAA) [54] and reverse orientation putative GATA binding sequence (TTATCA and TTATCT), which has the same transcription activation as in the forward orientation [61]. Therefore, these results indicate that GATA25 binds to the promoter of the selected target genes and activates transcription levels.
In this study, we analyzed the crucial role of GATA25, clustered as members of subfamily III within the Arabidopsis GATA gene family [42]. Our results demonstrate that GATA25 upregulates the photoperiodic flowering response-related genes together with their downstream target genes. Interestingly, a loss-of-function mutation in four CDF genes (CDF1, CDF2, CDF3, and CDF5) resulted in an early flowering phenotype but still showed photoperiodic response and circadian rhythms of CO expression [62]. This result suggests that there is a novel regulator in the regulation of CO expression and photoperiodic response. Therefore, it indicates that GATA25 is a novel regulator that leads to an early flowering phenotype.
References
Henderson IR, Dean C (2004) Control of Arabidopsis flowering: the chill before the bloom. Development 131:3829–3838
Teotia S, Tang G (2015) To bloom or not to bloom: role of microRNAs in plant flowering. Mol Plant 8:359–377
Roux F, Touzet P, Cuguen J, Le Corre V (2006) How to be early flowering: an evolutionary perspective. Trends Plant Sci 11:375–381
Searle I, Coupland G (2004) Induction of flowering by seasonal changes in photoperiod. EMBO J 23:1217–1222
Song YH, Shim JS, Kinmonth-Schultz HA, Imaizumi T (2015) Photoperiodic flowering: time measurement mechanisms in leaves. Annu Rev Plant Biol 66:441–464
Sim SA, Woo SG, Hwang DY, Kim JH, Lee SS, Lim CO, Hong JC, Song YH (2019) FLOWERING HTH1 is involved in CONSTANS-mediated flowering regulation in Arabidopsis. Appl Biol Chem 62(1):1–6
Takada S, Goto K (2003) Terminal FLOWER2, an Arabidopsis homolog of heterochromatin protein1, counteracts the activation of FLOWERING LOCUS T by CONSTANS in the vascular tissues of leaves to regulate flowering time. Plant Cell 15:2856–2865
An H, Roussot C, Suárez-López P, Corbesier L, Vincent C, Piñeiro M, Hepworth S, Mouradov A, Justin S, Turnbull C, Coupland G (2004) CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131:3615–3626
Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309:1052–1056
Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D (2005) Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309:1056–1059
Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, Coupland G (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316:1030–1033
Corbesier L, Coupland G (2006) The quest for florigen: a review of recent progress. J Exp Bot 57:3395–3403
Bao S, Hua C, Shen L, Yu H (2020) New insights into gibberellin signaling in regulating flowering in Arabidopsis. J Integr Plant Biol 62:118–131
Imaizumi T, Tran HG, Swartz TE, Briggs WR, Kay SA (2003) FKF1 is essential for photoperiodic-specific light signalling in Arabidopsis. Nature 426:302–306
Sawa M, Nusinow DA, Kay SA, Imaizumi T (2007) FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 318:261–265
Shikata M, Takemura M, Yokota A, Kohchi T (2003) Arabidopsis ZIM, a plant-specific GATA factor, can function as a transcriptional activator. Biosci Biotechnol Biochem 67:2495–2497
Shikata M, Matsuda Y, Ando K, Nishii A, Takemura M, Yokota A, Kohchi T (2004) Characterization of Arabidopsis ZIM, a member of a novel plant-specific GATA factor gene family. J Exp Bot 55:631–639
Reyes JC, Muro-Pastor MI, Florencio FJ (2004) The GATA family of transcription factors in Arabidopsis and rice. Plant Physiol 134:1718–1732
Ballerini ES, Kramer EM (2011) In the light of evolution: a reevaluation of conservation in the CO–FT regulon and its role in photoperiodic regulation of flowering time. Front Plant Sci 2:81
Liu H, Zhou X, Li Q, Wang L, Xing Y (2020) CCT domain-containing genes in cereal crops: flowering time and beyond. Theor Appl Genet 133:1385–1396
Xiang C, Han P, Lutziger I, Wang K, Oliver DJ (1999) A mini binary vector series for plant transformation. Plant Mol Biol 40:711–717
Ko JH, Kim WC, Han KH (2009) Ectopic expression of MYB46 identifies transcriptional regulatory genes involved in secondary wall biosynthesis in Arabidopsis. Plant J 60:649–665
Kagale S, Rozwadowski K (2011) EAR motif-mediated transcriptional repression in plants: an underlying mechanism for epigenetic regulation of gene expression. Epigenetics 6:141–146
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Eun HD, Ali S, Jung H, Kim K, Kim WC (2019) Profiling of ACC synthase gene (ACS11) expression in Arabidopsis induced by abiotic stresses. Appl Biol Chem 62(1):1–11
Kim WC, Ko JH, Kim JY, Kim J, Bae HJ, Han KH (2013) MYB 46 directly regulates the gene expression of secondary wall-associated cellulose synthases in Arabidopsis. Plant J 73:26–36
Seo JS, Zhao P, Jung C, Chua NH (2019) PLANT U-BOX PROTEIN 10 negatively regulates abscisic acid response in Arabidopsis. Appl Biol chem 62(1):1–5
Hong SM, Bahn SC, Lyu A, Jung HS, Ahn JH (2010) Identification and testing of superior reference genes for a starting pool of transcript normalization in Arabidopsis. Plant Cell Physiol 51:1694–1706
Kim W, Park TI, Yoo SJ, Jun AR, Ahn JH (2013) Generation and analysis of a complete mutant set for the Arabidopsis FT/TFL1 family shows specific effects on thermo-sensitive flowering regulation. J Exp Bot 64:1715–1729
Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2:1565–1572
Liu L, White MJ, MacRae TH (1999) Transcription factors and their genes in higher plants functional domains, evolution and regulation. Eur J Biochem 262:247–257
Spicuglia S, Kumar S, Yeh JH, Vachez E, Chasson L, Gorbatch S, Cautres J, Ferrier P (2002) Promoter activation by enhancer-dependent and -independent loading of activator and coactivator complexes. Mol Cell 10:1479–1487
Franco-Zorrilla JM, López-Vidriero I, Carrasco JL, Godoy M, Vera P, Solano R (2014) DNA-binding specificities of plant transcription factors and their potential to define target genes. Proc Natl Acad Sci USA 111:2367–2372
Hiratsu K, Matsui K, Koyama T, Ohme-Takagi M (2003) Dominant repression of target genes by chimeric repressors that include the EAR motif, a repression domain, in Arabidopsis. Plant J 34:733–739
Mai YX, Wang L, Yang HQ (2011) A gain-of-function mutation in IAA7/AXR2 confers late flowering under short-day light in Arabidopsis. J Integr Plant Biol 53:480–492
Richter R, Bastakis E, Schwechheimer C (2013) Cross-repressive interactions between SOC1 and the GATAs GNC and GNL/CGA1 in the control of greening, cold tolerance, and flowering time in Arabidopsis. Plant Physiol 162:1992–2004
Aoyama T, Chua NH (1997) A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J 11:605–612
Yoo SK, Chung KS, Kim J, Lee JH, Hong SM, Yoo SJ, Yoo SY, Lee JS, Ahn JH (2005) Constans activates suppressor of overexpression of constans 1 through flowering locus T to promote flowering in Arabidopsis. Plant Physiol 139:770–778
Fowler S, Lee K, Onouchi H, Samach A, Richardson K, Morris B, Coupland G, Putterill J (1999) Gigantea: a circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane-spanning domains. EMBO J 18:4679–4688
Simpson GG, Gendall AR, Dean C (1999) When to switch to flowering. Annu Rev Cell Dev Biol 15:519–550
Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G (2001) CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410:1116–1120
Manfield IW, Devlin PF, Jen CH, Westhead DR, Gilmartin PM (2007) Conservation, convergence, and divergence of light-responsive, circadian-regulated, and tissue-specific expression patterns during evolution of the Arabidopsis GATA gene family. Plant Physiol 143:941–958
Skriver K, Olsen FL, Rogers JC, Mundy J (1991) cis-acting DNA elements responsive to gibberellin and its antagonist abscisic acid. Proc Natl Acad Sci USA 88:7266–7270
Sheen J (2001) Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol 127:1466–1475
Tiwari S, Wang S, Hagen G, Guilfoyle TJ (2006) Transfection assays with protoplasts containing integrated reporter genes. Methods Mol Biol 323:237–244
Im JH, Ko JH, Kim WC, Crain B, Keathley D, Han KH (2021) Mitogen-activated protein kinase 6 negatively regulates secondary wall biosynthesis by modulating MYB46 protein stability in Arabidopsis thaliana. PLoS Genet 17:e1009510
Sablowski RW, Meyerowitz EM (1998) A homolog of NO APICAL MERISTEM is an immediate target of the floral homeotic genes APETALA3/PISTILLATA. Cell 92:93–103
Yamaguchi N, Winter CM, Wellmer F, Wagner D (2015) Identification of direct targets of plant transcription factors using the GR fusion technique, in Plant Functional Genomics. Methods Mol Biol 1284:123–138
Ellis RJ, MacDonald IR (1970) Specificity of cycloheximide in higher plant systems. Plant Physiol 46:227–232
Kaufmann K, Wellmer F, Muiño JM, Ferrier T, Wuest SE, Kumar V, Serrano-Mislata A, Madueño F, Krajewski P, Meyerowitz EM, Angenent GC, Riechmann JL (2010) Orchestration of floral initiation by APETALA1. Science 328:85–89
Yamaguchi N, Wu MF, Winter CM, Berns MC, Nole-Wilson S, Yamaguchi A, Coupland G, Krizek BA, Wagner D (2013) A molecular framework for auxin-mediated initiation of flower primordia. Dev Cell 24:271–282
Yamaguchi N, Winter CM, Wu MF, Kanno Y, Yamaguchi A, Seo M, Wagner D (2014) Gibberellin acts positively then negatively to control onset of flower formation in Arabidopsis. Science 344:638–641
Fitz J, Neumann T, Steininger M, Wiedemann EM, Garcia AC, Athanasiadis A, Schoeberl UE, Pavri R (2020) Spt5-mediated enhancer transcription directly couples enhancer activation with physical promoter interaction. Nat Genet 52:505–515
Ndamukong I, Chetram A, Saleh A, Avramova Z (2009) Wall-modifying genes regulated by the Arabidopsis homolog of trithorax, ATX1: repression of the XTH33 gene as a test case. Plant J 58:541–553
Mouradov A, Cremer F, Coupland G (2002) Control of flowering time: interacting pathways as a basis for diversity. Plant Cell 14:S111–S130
Yanovsky MJ, Kay SA (2003) Living by the calendar: how plants know when to flower. Nat Rev Mol Cell Biol 4:265–275
Boss PK, Bastow RM, Mylne JS, Dean C (2004) Multiple pathways in the decision to flower: enabling, promoting, and resetting. Plant Cell 16:S18–S31
Jack T (2004) Molecular and genetic mechanisms of floral control. Plant Cell 16:S1–S17
Putterill J, Laurie R, Macknight R (2004) It’s time to flower: the genetic control of flowering time. BioEssays 26:363–373
Lee J, Lee I (2010) Regulation and function of SOC1, a flowering pathway integrator. J Exp Bot 61:2247–2254
Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6:251–264
Fornara F, Panigrahi KC, Gissot L, Sauerbrunn N, Rühl M, Jarillo JA, Coupland G (2009) Arabidopsis dof transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. Dev Cell 17:75–86
Acknowledgements
Not applicable.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIT) (No. 2020R1F1A1057487) and Korea Basic Science Institute (National research Facilites and Equipment center) grand funded by the Ministry of Education (2021R1A6C101A416).
Author information
Authors and Affiliations
Contributions
KK and W-CK conceived and designed the experiments, prepared the figures, wrote the manuscript, and analyzed the data. KK, JL, JS, BK, and T-AK performed the experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
All of the authors declare no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
List of primers used in this study
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, K., Lee, J., Kim, B. et al. GATA25, a novel regulator, accelerates the flowering time of Arabidopsis thaliana. Appl Biol Chem 65, 28 (2022). https://doi.org/10.1186/s13765-022-00698-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-022-00698-7