Abstract
The prevalence of gastritis in South Korea is rapidly increasing owing to the prevalence of Helicobacter pylori infection and fast eating habit. The usual treatment for acute gastritis following a long intake of non-steroidal anti-inflammatory drugs (NSAIDs) or alcohol is to stop the causal factors. Metronidazole and lansoprazole are recommended for the treatment of H. pylori infection gastritis. Omeprazole a proton pump inhibitor, is used to decrease gastric acid production. However, owing to the side effects and refractoriness of the drug, a safe and efficient treatment is required. Plant-derived phytochemicals have emerged as novel agents against chronic disorders. In this study, firstly, to explore the potential of pharmacological activities, including efficacy and mechanisms of Cinnamomum cassia against gastritis, a literature review was performed based on 20 studies out of a total of 749 records obtained using a search strategy. From the literature review, the therapeutic targets of C. cassia extract and cinnamaldehyde, a compound of C. cassia, were found to be related with NFκB activity, and their signaling pathway were verified by experiments. C. cassia extract plays a role in protection of gastric ulcers induced in four ways (immersion stress-induced, ethanol-induced, hydrochloric acid-induced, or NSAIDs-induced ulcer). None of the clinical studies on C. cassia extracts or compounds met our criteria. When the standardized extract of C. cassia (ECC) was orally administered repeatedly to Beagle Dog for 4 weeks, no toxicologically harmful changes were observed. Therefore, under the test condition, the no observed adverse effect level (NOAEL) of ECC was judged to be 1000 mg/kg/day for both sexes, and no toxic target organ was observed. Administration of ECC in the Sprague–Dawley rat model of acute gastric injury caused by indomethacin administration significantly increased gastric mucus volume. Administration of ECC in the acute gastric injury model caused by indomethacin administration is considered effective in improving gastric injury. However, research and efforts to develop a reliable ‘standardization of natural drugs’ by establishing the best quality evaluation system are limited. Despite the pharmacological potential of ECC, further well-designed experimental studies such as in vitro, in vivo, and clinical trials are required to validate these findings and the underlying mechanisms of ECC.
Similar content being viewed by others
Introduction
Plant-derived phytochemicals have emerged as novel agents for protection against chronic disorders, including gastritis [1]. In particular, the stomach is an organ that does not metabolize and absorb other than protein denaturation, and is a place where natural products can exert their effects. Owing to the diversity of phytochemicals, they cover a wide spectrum of therapeutic indications against gastrointestinal diseases such as gastritis, hyperacidity, and reflux esophagitis, and have been a productive source of major compounds for the development of novel medications [2].
Gastric ulcer is a common disease with multiple cause and is defined as a morphological defect in the gastric mucosa penetration through the muscularis mucosa [3]. The pathophysiology of gastric ulcers is unclear, but it is commonly known to be associated with an imbalance between aggressive factors (physical, chemical, or psychological) and cytoprotective factors of the gastric mucous membrane (mucus and bicarbonate secretion), and is involved in the production of gastric protective endogenous factors such as prostaglandins, plyamies, nitric oxide (NO) and dopamine [4,5,6]. Gastric ulcer is also caused by environmental factors such as the use of alcoholic beverage and non-steroidal anti-inflammatory drug (NSAID), and Helicobacter pylori [7,8,9].
The usual treatment for acute gastritis following a long intake of NSAIDs or alcohol is to stop their consumption. Metronidazole and lansoprazole are recommended for H. pylori infection gastritis treatment, with antibiotics such as clarithromycin and amoxicillin for 10–14 days [10, 11]. Omeprazole is a proton pump inhibitor used to decrease gastric acid production [12]. In addition, medicinal plants with tannins and flavonoids contribute not only to antioxidant and anti-inflammatory effects, but also to anti-ulcerogenic effects and wound healing [13]. Curcumin extract and its flavonoid content have significant effects on mucosal lesions, such as gastric ulcers [14, 15].
Cinnamomum cassia is a type of deciduous tree that is distributed in China, India, Vietnam, Japan, and Korea and is widely used worldwide for its fragrance and spicy flavor. Cinnamomi cortex is the bark of C. cassia, which is used as a food supplement in America, and as a traditional drug in Asia [16]. C. cassia has been used as a medicine to treat various inflammatory diseases [17, 18], atopic dermatitis [19], and anti-diabetic activities [20]. In addition, almost every part of the cinnamon tree, including fruits, roots, flowers, leaves, and bark, has some medicinal activity. The constituents of C. cassia have been separated and identified in many studies [21, 22].
Recently, the pharmaceutical industry has faced challenges such as increased drug development costs, high failure rates, increased competition for proven targets, demand for new targets and pharmacological mechanism-based drug development [23]. Moreover, there are several difficulties in quality control, such as activity, purity, stability, and equivalence management when developing natural medicines. First, natural medicinal products are composed of a mixture of various compounds, and it is often not possible to identify all the structures and characteristics of the compounds that they contain. Second, the correlation between each component and the activity contained in natural medicinal products is often unclear. Therefore, in the case of natural medicinal products, there is a tendency to recognize the composition and content of components as integrated active ingredients. Third, the composition and content of ingredients that make up natural medicinal products are highly volatile. This is inevitable because of the natural deviation of crude drugs, which is the starting point for natural medicinal products. In addition, there may be differences in the composition and content of the components in the extract depending on the processing conditions during the manufacturing process of the drug.
Therefore, it is important to research and develop new natural products at an international level through reliable “standardization of natural drugs” by establishing the best quality evaluation system within the given scientific limits. In this study, the possibility of developing natural medicines from C. cassia was investigated through literature review on its effects on gastritis, preparation of standardized extracts, and evaluation of toxicity and activity.
Part 1: Literature findings on the beneficial effects of C. cassia on gastritis
This review introduces the current status of cell-, and animal-based studies and clinical studies on the effects of C. cassia on gastritis. We collected and identified literature that studied the effectiveness of extracts or compounds from C. cassia on gastritis using the search strategy. Considering that C. cassia is being used by various scientific names, the following search terms were set through related reviews and databases [National Herbal Medicine Information (NHMI, www.nifds.go.kr) and Korean Traditional Knowledge Portal (www.koreantk.com)]: “Cinnamomum cassia J. Presl”; “Cinnamomum cassia,” “Chinese cinnamon,” “Chinese cassia,” “Cinnamomum cassia Presl,” “Cinnamomi Cortex,” “Cinnamon Bark,” “Cinnamomi Cortex Spissus,” “Cassia bark,” or “Cinnamomum cassia Blume.” The literature search was conducted on PubMed (Pubmed. ncbi. nlm. nih. gov) on September 16, 2021, and there were no restrictions on publication date or language during the search process. The literature on clinical and basic experimental studies evaluating the effectiveness of extracts or compounds from C. cassia for gastritis (including gastric ulcer in a broader view), were included in our review. The following studies were excluded: 1) studies not related to C. cassia; 2) studies that contained a combination of substances other than extracts or compounds from C. cassia (e.g., prescriptions); 3) studies in which efficacy results are difficult to estimate or secondary research that is not an experimental study (e.g., letter, review, editorial, review, conference abstract); 4) studies that did not target the gastritis animal model or cells that could reveal effects similar to gastritis; or 5) studies not written in English. Two researchers independently screened and assessed the eligibility of all titles, abstracts, or full texts of publications. From the included literature, two researchers extracted data on items determined in advance (e.g., animal or cell type; disease-induced methods; details of intervention, including types of herb, extraction methods, doses; and outcome measures) and reviewed them with each other. Where necessary, in the process of literature selection and data extraction, disagreements were discussed to reach a consensus among the researchers. The extracted data are presented through tables and narrative descriptions.
A total of 749 records were searched for using the search strategy. Studies corresponding to the exclusion criteria were primarily excluded, and others were classified into cell- or animal-based experiments, clinical studies, or reviews by checking the abstract and full text in the articles of the remaining literature. Articles classified as reviews went through the another checking process to determine whether additional studies met the inclusion criteria. Through this process, a total of 20 articles were included in our review (Fig. 1). Of the 20 studies, 17 the studies evaluated in vitro effects of C. cassia, four studies were conducted on animals, in which one of the studies was in vitro and in vivo studies on gastritis, and none of the clinical trials were reported.
In vitro studies of C. cassia extracts or its compounds on gastritis
The in vitro tests for the gastritis model induced by H. pylori infection were performed in two studies; they used 70% EtOH extraction for C. cassia and cinnamaldehyde, a compound of C. cassia, as therapeutic interventions (Table 1). Zaidi et al. (2012) [24] evaluated the inhibitory effect of 70% EtOH extracts from 24 species of medicinal plants, including C. cassia, on IL-8 and ROS production in H. pylori infection induced AGS gastric epithelial cells. Among them, C. cassia extract was reported to have a greater inhibitory effect than other medicinal plant extracts. In the study by Muhammad et al. (2015) [25], cinnamaldehyde, a compound of C. cassia, dose-dependently decreased IL-8 secretion and NFκB activity in H. pylori-infected human epithelial cell lines (AGS and MKN-45). The gastritis model, there were studies that confirmed the effects of inflammation induced by LPS using C. cassia extracts (n = 2, EtOH or MeOH solvent extracts, respectively), or compounds derived from C. cassia (n = 9); however, they were not in vitro tests of the gastritis model. In addition, studies of C. cassia extracts and its compounds confirmed their anti-inflammatory activity and identified active constituents (n = 4), such as cinncassin D, cinncassin E, (+)-threo-(7S,8S)-guaiacylglycerol-b-coniferyl aldehyde ether, (+)-erythro-(7S,8R)-guaiacylglycerol-β-coniferyl aldehyde ether, (−)-erythro-(7S,8R)-syringylglycerol-8-O-4′-(sinapoyl alcohol) ether, (7S,8R)-lawsonicin and (+)-(7′R,8R,8′R)-5,5′-dimethoxylariciresinol, trans -cinnamaldehyde, (−)-aromadendrene, caryophyllene oxide, t-cadinol, and a-cadinol, trans-cinnamaldehyde, p-cymene, eugenol, and cinnamic acid, and a study on the anti-inflammatory effect of cinnamate-zinc layered hydroxide processed with compounds derived from C. cassia (n = 1) were reported. In addition, 14 articles reported the inhibitory effect of the LPS-induced inflammatory response (Table 1). Two of the articles confirmed the inhibitory ability of C. cassia EtOH and MeOH extracts, and 9 articles reported the anti-inflammatory effect of compounds derived from C. cassia. Four articles confirmed the anti-inflammatory effect of C. cassia extracts and identified the anti-inflammatory active compound of the extract. In addition, one study confirmed the inhibitory response and activity of cinnamate-zinc layered hydroxide with a compound derived from C. cassia on LPS-induced inflammation. To evaluate the anti-inflammatory efficacy in each study, the inhibitory efficacy on LPS-induced NO production, COX-2 activity, PGE2 production, pro-inflammatory cytokine production, and inflammatory signaling pathways (NFκB and MAPK pathways) using most macrophages (some myocytes or microglia cells) was confirmed.
In vivo studies of the effects of C. cassia extracts or compounds on gastritis
All four studies on the efficacy evaluation of C. cassia extracts or compounds in animal models of gastritis and gastric ulcer were conducted in Japan, two in the 1980s and one in 2010. Among them, two studies conducted in the 1980s induced gastric ulcer after pretreatment with oral administration for one day in rats, and the pretreatment drugs were Chinese cinnamon (hot water extracts) and Cinnamomum cassia Blume, Lauraceae (EtOH, MeOH extract) (Table 2). In the study by Akira et al. [41], cimetidine was used as a control group, and for each of the three gastric ulcer-inducing methods (cold-stress, water immersion stress, and serotonin-induced method), the results were compared between groups. Chinese cinnamon inhibited serotonin-induced ulcers that could not be controlled by cimetidine, although cinnamon extract was less effective (28.6%) than cimetidine (78.8%) at a dose of 50 mg/kg.
In the study by Tanaka et al. [42], active compounds isolated from Cinnamomum cassia Blume, Lauraceae reduced and inhibited serotonin-induced ulcers by up to 54%, while mianserin, a serotonin receptor (5HT2) antagonist as a control, showed 52% inhibition. In a study by Manjuh et al. [43], gastric ulcers were induced in four ways (immersion stress-induced, ethanol-induced, hydrochloric acid-induced, or NSAID-induced ulcer) in ddY mice; pretreatment of the mice with Cinnamomum cassia Blume (Lauraceae) powder mixed with feed showed a protective effect on gastric ulcer. The treatment group showed a significantly lower ulcer index value of 4.2 mm than the control group (sucralfate). In addition, Jung et al. (2011) [26] performed experiments on the protective effect of gastric ulcer by evaluating gastric lesions and gastric secretions in HCl/EtOH-induced rat gastric ulcer in vivo. The eugenol and cinnamic acid, compounds derived from C. Ramulus, played major role in protective effects of gastritis (Table 2).
As described above, the efficacy and clinical efficacy for gastritis have been revealed in the literature; however, it is necessary to analyze the active ingredients, test for safety, and verify the efficacy of standardized extracts. This process will be introduced in the rest of this article as excerpts from reports conducted by specialized research companies and toxic and efficacy testing institutions.
Part 2: Safety of ECC
ECC toxicity study on animals was conducted by ChemOn Co., Ltd. and was approved by the non-clinical animal laboratory steering committee (Review No.: 17-D290). HPLC analysis was performed to confirm the components on the ECC (Fig. 2). Two major compounds were identified as coumarin and cinnamic acid in ECC. The test substance was weighed according to the body weight of the animals and administered orally. Beagle dogs (no specific pathogen) were purchased from Beijing Marshall Miotechnology Co., Ltd. (Wayao Village, Ryuchunjin, Changping District, Beijing, China) from May to June and used after 1 month of adaptation.
HPLC profiling of ECC. ECC was manufactured by Chong Kun Dang (Yongin, Korea). C. cassia was extracted with distilled water using a heat-reflux extractor for 5 h. The extraction was concentrated by using reduced pressure in evaporator and dry-powdered. The standardized extract of C. cassia (ECC) was analyzed by Alliance HPLC (Waters e2695, MA, USA). The result was detected by UV (305 nm) with Phenomenex Gemini column (4.6 × 250 nm, 5 μm) and sample was injected with 10 μL and the flow rate was 1.0 mL/min. Standard reagents were coumarin and cinnamic acid (Sigma-Aldrich, MO, USA)
Mortality, clinical signs, body weight, and food intake were monitored for 28 days. The start date of administration was set as day 1. All animals underwent extraocular examinations prior to dosing, 2 weeks after the initiation of dosing, and within 1 week of scheduled necropsy. Urinalysis was performed on samples collected using a urinalysis strip (Roche Diagnostics, Mannheim, Germany) and an automated urine analyzer (Clinitek Advantus, Siemens, Erlangen, Germany).
The animals were fasted overnight prior to blood collection or necropsy. Blood was drawn from the posterior vena cava under isoflurane anesthesia. Samples were collected in CBC bottles containing EDTA-2K (Suwon Medical, Suwon, Korea) and analyzed to measure the blood components.
For all animals, fixed organs were cut and embedded in paraffin, and 2–5 μm pieces were prepared through H&E staining, and histopathological examination was performed. Histopathological findings were entered into the Pristina® (Xybion, Connecticut, Stamford, USA) program and diagnostic terms were displayed on the Prestima® Lexicon (Version 6.1.0 Build 31., Xybion Medical Systems). For standardized nomenclature and diagnostic criteria, the guide to toxic pathology of the American Society of Toxicological Society and Covance was referred to [44].
Results of toxicity study
No clinically abnormal signs were observed in Beagle dogs treated with ECC. In contrast, salivation was observed in all animals in the 1000 mg/kg group and in one animal in male Beagle dogs in the 500 mg/kg and 1000 mg/kg groups. Diarrhea was observed in all animals in the 250 mg/kg group and those in the 500 mg/kg male group. The above symptoms are natural reactions in experiments with Beagle dogs. No death occurred in the sex or vehicle control groups at any dose.
There was no significant difference in body weight between the vehicle-only control group and the treatment group. However, in the female group, body weight was significantly reduced at 250 and 500 mg/kg, but there was no change on the basis of dose, and it was judged that there was no effect of the test substance. Food did not show significant difference. No effect of the test substance on electrocardiogram was observed. Ophthalmic examination revealed no treatment-related ocular lesions in the animals.
There were no treatment-related gross pathological changes at necropsy except for a decrease in epididymal size (n = 1) in the 1000 mg/kg male group. In females, no serious pathological finding was observed in any group. For absolute organ weights, the lungs decreased in the 250 mg/kg group. The 250 mg/kg male group showed an increase in the liver. There was no significant difference in absolute organ weight between the vehicle-only control group and the treatment group. Finally, there was no significant difference in the relative organ weights between the vehicle-only control and treatment groups for either sex.
There was no significant difference in hematology tests in any group. In contrast, the 500 mg/kg male group had significantly prolonged PT. There was no significant difference in serum biochemistry and urinalysis values between the vehicle-only control group and the treatment group (Table 3).
Administration of ECC did not induce histopathological changes in the liver or kidneys at any dose level. ECC administration also did not induce electrocardiographic changes at any dose level (Table 4).
Part 3: Protective effect of ECC on gastritis induced by indomethacin in rats
C. cassia was purchased from YingBai, Vietnam and voucher specimens were obtained from the Chong Kun Dang Research institute, Korea. The standardized extract of C. cassia (ECC) was analyzed using Alliance HPLC (Waters e2695, MA, USA) and was used for animal test.
Sixty male SD rats were purchased from Orient Bio (Gyeonggi-do, Korea). All animals were divided into six groups (each group was 10): control, indomethacin-induced acute stomach injury group, positive control (Artemisia extract (AE) and rebamipide), and experimental group (ECC). All animals were cared for at 22 ± 2 °C with 55 ± 5% humidity and light and dark cycle of 12/12 h. Mice were fasted for 48 h and then AE and ECC (300 mg/kg) were orally injected. After 30 min, indomethacin (80 mg/kg) was administered orally to induce acute stomach injury. Seven hours later, all animals were sacrificed, and their stomach and serum were extracted for further experiments. All diets and water were provided ad libitum. This study was approved by the Institutional Animal Care and Use Committee of the Korea Animal Medical Science Institute (KAMSI IACUC 14-KE-134).
Alcian blue (Sigma-Aldrich) binding assay was performed according to a previously published method [45]. Blood was analyzed after serum separation and sampling according to the protocol provided by the manufacturer of the ELISA kit for measurement of prostaglandin E2 (PGE2), glutathione (GSH), and myeloperoxidase (MPO).
Potential beneficial effect of ECC on gastritis
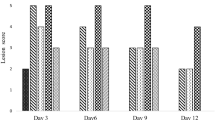
This study was conducted to evaluate the effect of administration of test substances (ECC) on gastric mucus volume in a Sprague–Dawley rat model of acute gastric injury caused by indomethacin administration. It was found that the amount of gastric mucus in the negative control group was significantly lower than that in the normal control group (Fig. 3). The amount of gastric mucus in the substance-administered group was significantly higher than that on the negative control group (Fig. 3).
Indomethacin, a non-selective cyclooxygenase (COX) inhibitor, causes relatively strong gastric mucosal damage among various non-steroidal anti-inflammatory drugs (NSAIDs) [46]. As a defense mechanism, mucus is secreted as a defense factor to protect the mucosal layer, and gastric mucus can protect the stomach; therefore, increasing the amount of gastric mucus can improve gastric damage [47].
Considering this aspect, the amount of gastric mucus in the AE- and ECC-administered groups was significantly higher than that in the negative control group, and there was a significant difference compared with that in the normal control group. Since the reduced amount of gastric mucus was recovered, the administration of the test substance was considered to be effective in the improvement of acute gastric injury.
Stomach damage is caused by various factors, and it has been recently reported that gastric damage and antioxidant defense system disturbance are closely related [4, 48]. Gastric mucosal damage is caused by the influence of various defense factors constituting the gastric mucosa, and disorders of the antioxidant defense system, such as lipid peroxidation by reactive oxygen species (ROS), have recently emerged as a strong cause [49]. In fact, the protective effects of various antioxidants against gastric mucosal damage have been reported. Glutathione is a representative endogenous antioxidant known to counteract gastric mucosal damage caused by reactive oxygen species, and a significant decrease in glutathione levels has been reported during gastric mucosal damage caused by nonsteroidal anti-inflammatory drugs [50]. Result of glutathione measurement in the serum showed that the glutathione levels of all test substance administration groups did not show a significant difference compared to the negative control group; however, it showed a relatively higher tendency than the negative control group, and was significantly higher than that of the normal control group (Table 5).
In contrast, myeloperoxidase is an enzyme secreted by neutrophils, and the increase in the activity of myeloperoxidase in the gastric mucosa is used as an indicator of the increase in neutrophils in the gastric mucosa when the gastric mucosa is damaged [51, 52]. The increase in myeloperoxidase activity owing to neutrophil infiltration into the induced lesion is well known. Result of myeloperoxidase measurement in the serum showed that, the myeloperoxidase level of the test substances in the ECC and AE administered group was significantly lower than that of the negative control group and positive control group (Table 6). There was no significant difference compared to that in the control group.
Prostaglandin, which is abundantly contained in gastric mucosa, promotes secretion of mucus and HCO3- and suppresses gastric acid secretion. It is important for maintaining gastric mucosa blood flow and epithelial cell repair mechanisms, and is also important for cellular integrity of gastric mucosa [53]. Result of prostaglandin E2 measurement in serum showed that there was no significant difference in the level of prostaglandin E2 in the group administered ECC and AE compared with the normal control group (Table 7). Combining the results of the analysis of serum glutathione, prostaglandin E2, and myeloperoxidase levels in this study, administration of the test substance to the acute gastric injury model induced a significant decrease in myeloperoxidase levels, although there was no significant change in glutathione and prostaglandin. levels. The increasing trend in E2 levels indicates that the test substance probably has a gastric protective effect against acute gastric injury.
In conclusion, administration of the test substances ECC and AE in a Sprague–Dawley rat model of acute gastric injury caused by indomethacin administration significantly increased gastric mucus volume. Analysis of serum glutathione, prostaglandin E2, and myeloperoxidase levels revealed that there was a significant decrease in myeloperoxidase levels and an increase in glutathione and prostaglandin E2 levels. Therefore, administration of ECC to the acute gastric injury model caused by the administration of indomethacin is considered to be effective in improving gastric injury.
This study revealed the therapeutic potential of ECC against gastritis and its future prospect: (1) ECC shows the pharmacological potential for gastritis treatment in both the results of the literature review and experimental tests. (2) According to the literature review, C. cassia extract and cinnamaldehyde play a strong anti-inflammatory and antioxidant effect roles and an inhibitory effect role against the secretion of IL-8 and ROS production by H. pylori. Furthermore, C. cassia extract plays an anti-inflammatory effect role in the protection of gastric ulcers induced in four ways (immersion stress-induced, ethanol-induced, hydrochloric acid-induced, and NSAID-induced ulcer) and prevention of gastric mucosal lesions. Lastly, the therapeutic mechanism of C. cassia is related to NFκB activity and its signaling pathway. Unfortunately, no clinical studies of C. cassia extracts or compounds on gastritis have been conducted to date. (3) In the safety tests, the no observed adverse effect level of ECC was judged to be 1000 mg/kg/day for both sexes, and no toxic target organ was observed. Finally, (4) it was confirmed that the standard extract (ECC) exerts an effect on indomethacin-induced gastritis, which is consistent with the results of the literature.
Although there have been several positive effects and studies on gastritis, this study has several limitations. Thus, well-planned clinical studies need to be conducted. There are many natural medicines that are not effective in clinical practice, regardless of how good they appeared in cellular and animal experiments. The next problem to be solved is the research on ingredient standardization that meets international standards. Various ingredients have complex effects because of the nature of natural products; however, some are standardized with minimal ingredients, for example, cinnamaldehyde. Therefore, efforts should be made to study the mechanism of efficacy at the level of compound drugs owing to the difference in efficacy, and various studies have reported inconsistent effects on cytokines or inflammation-related biomarkers. In addition, it is necessary to elucidate the mechanism of action of each complex component that exerts its effects on gastritis. There are also many factors to consider in the study of natural ingredients, for example, how much of these ingredients are absorbed by the body and the metabolites of these ingredients. This study has limitations; thus, further well-designed experimental studies such as in silico, in vitro, in vivo, and clinical trials are required to validate these findings and the underlying mechanisms of ECC.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- EtOH:
-
Ethanol
- MeOH:
-
Methanol
- NA:
-
Not available
- H. pylori :
-
Helicobacter pylori
- LPS:
-
Lipopolysaccharide
- NO:
-
Nitric oxide
- iNOS:
-
Inducible NO synthase
- COX-2:
-
Cyclooxygenase-2
- PGE2:
-
Prostaglandin E2
- ILs:
-
Interleukin
- TNF-α:
-
Tumor necrosis factor-alpha
- NFκB:
-
Nuclear factor kappa-light-chin-enhancer of activated B cells
- TLRs:
-
Toll-like receptors
- MAPK:
-
Mitogen activated protein kinase
- ERK:
-
Extracellular signal-related protein
- JNK:
-
C-jun N-terminal kinase
- ROS:
-
Reactive oxygen species
- SD:
-
Sprague Dawley
- RBC:
-
Red blood cell
- HGB:
-
Hemoglobin
- HCT:
-
Hematocrit
- MCV:
-
Mean corpuscular volume
- MCH:
-
Mean corpuscular hemoglobin
- MCHC:
-
Mean corpuscular hemoglobin con-centratio
- RDW:
-
Red cell distribution width
- HDW:
-
Hemoglobin distribution width
- RET:
-
Reticulocyte
- PLT:
-
Platelet
- MPV:
-
Mean platelet volume
- WBC:
-
White blood cell
- NEU:
-
Neutrophil
- LYM:
-
Lymphocyte
- MONO:
-
Monocyte
- EOS:
-
Eosinophil
- BASO:
-
Basophil
- QRS:
-
Ventricular depolarization
- QT:
-
Repolarization of the ventricles
References
Merecz-Sadowska A, Sitarek P, Śliwiński T, Zajdel R (2020) Anti-inflammatory activity of extracts and pure compounds derived from plants via modulation of signaling pathways, especially PI3K/AKT in macrophages. Int J Mol Sci 21:9605
Kang KS (2021) Phytochemical constituents of medicinal plants for the treatment of chronic inflammation. Multidisciplinary Digital Publishing Institute
Yeomans ND, Naesdal J (2008) Systematic review: ulcer definition in NSAID ulcer prevention trials. Aliment Pharmacol Ther 27:465–472
Ramakrishnan K, Salinas RC (2007) Peptic ulcer disease. Am Fam Physician 76:1005–1012
Tsukimi Y, Nakai H, Itoh S, Amagase K, Okabe S (2001) Involvement of heat shock proteins in the healing of acetic acid-induced gastric ulcers in rats. J Physiol Pharmacol 52
Li W-F, Hao D-J, Fan T, Huang H-M, Yao H, Niu X-F (2014) Protective effect of chelerythrine against ethanol-induced gastric ulcer in mice. Chem Biol Interact 208:18–27
Konturek S, Konturek P, Brzozowski T, Konturek J, Pawlik W (2005) From nerves and hormones to bacteria in the stomach; Nobel prize for achievements in gastrology during last century. J Physiol Pharmacol 56:507–530
Graham DY, Lew GM, Klein PD, Evans DG, Evans DJ Jr, Saeed ZA, Malaty HM (1992) Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer: a randomized, controlled study. Ann Intern Med 116:705–708
Hollander D, Tarnawski A, Krause WJ, Gergely H (1985) Protective effect of sucralfate against alcohol-induced gastric mucosal injury in the rat: macroscopic, histologic, ultrastructural, and functional time sequence analysis. Gastroenterology 88:366–374
Scherübl H, Fischbach W, Glocker E, Malfertheiner P (2015) Was ist neu bei der Behandlung der Helicobacter-pylori-Infektion? DMW-Deutsche Medizinische Wochenschrift 140:277–280
Wang T, Zhang Y, Zhang X, Cheng H, Hu F, Han H, Chen X, Li J, Lai Y, Liu Y (2013) Jinghuaweikang gelatin pearls plus proton pump inhibitor-based triple regimen in the treatment of chronic atrophic gastritis with Helicobacter pylori infection: a multicenter, randomized, controlled clinical study. Zhonghua Yi Xue Za Zhi 93:3491–3495
Sakamoto Y, Shimoyama T, Nakagawa S, Mikami T, Fukuda S (2014) Proton pump inhibitor treatment decreases the incidence of upper gastrointestinal disorders in elderly Japanese patients treated with NSAIDs. Intern Med 53:1107–1111
Tonino P (2011) Gastritis and Gastric Cancer: New Insights in Gastroprotection, Diagnosis and Treatments, BoD–Books on Demand
Tuorkey M, Karolin K (2009) Anti-ulcer activity of curcumin on experimental gastric ulcer in rats and its effect on oxidative stress/antioxidant, IL-6 and enzyme activities. Biomed Environ Sci 22:488–495
Elseweidy MM, Younis NN, Amin RS, Abdallah FR, Fathy AM, Yousif ZA (2008) Effect of some natural products either alone or in combination on gastritis induced in experimental rats. Dig Dis Sci 53:1774–1784
Wang Y-H, Avula B, Nanayakkara ND, Zhao J, Khan IA (2013) Cassia cinnamon as a source of coumarin in cinnamon-flavored food and food supplements in the United States. J Agric Food Chem 61:4470–4476
Seo U-K, Lee Y-J, Kim J-K, Cha B-Y, Kim D-W, Nam K-S, Kim C-H (2005) Large-scale and effective screening of Korean medicinal plants for inhibitory activity on matrix metalloproteinase-9. J Ethnopharmacol 97:101–106
Lim C-S, Kim E-Y, Lee H-S, Soh Y, Sohn Y, Kim SY, Sohn N-W, Jung H-S, Kim Y-B (2010) Protective effects of Cinnamomum cassia Blume in the fibrogenesis of activated HSC-T6 cells and dimethylnitrosamine-induced acute liver injury in SD rats. Biosci, Biotechnol, Biochem 1001281834–1001281834
Sung Y-Y, Yoon T, Jang JY, Park S-J, Jeong G-H, Kim HK (2011) Inhibitory effects of Cinnamomum cassia extract on atopic dermatitis-like skin lesions induced by mite antigen in NC/Nga mice. J Ethnopharmacol 133:621–628
Verspohl EJ, Bauer K, Neddermann E (2005) Antidiabetic effect of Cinnamomum cassia and Cinnamomum zeylanicum in vivo and in vitro. Phytother Res 19:203–206
Shen Q, Chen F, Luo J (2002) Comparison studies on chemical constituents of essential oil from ramulus cinnamomi and cortex cinnamomi by GC-MS. Zhong yao cai Zhongyaocai J Chin Med Materials 25:257–258
Gruenwald J, Freder J, Armbruester N (2010) Cinnamon and health. Crit Rev Food Sci Nutr 50:822–834
Arden NS, Fisher AC, Tyner K, Yu LX, Lee SL, Kopcha M (2021) Industry 40 for pharmaceutical manufacturing: preparing for the smart factories of the future. Int J Pharm 602:120554
Zaidi SF, Muhammad JS, Shahryar S, Usmanghani K, Gilani A-H, Jafri W, Sugiyama T (2012) Anti-inflammatory and cytoprotective effects of selected Pakistani medicinal plants in Helicobacter pylori-infected gastric epithelial cells. J Ethnopharmacol 141:403–410
Muhammad JS, Zaidi SF, Shaharyar S, Refaat A, Usmanghani K, Saiki I, Sugiyama T (2015) Anti-inflammatory effect of cinnamaldehyde in Helicobacter pylori induced gastric inflammation. Biol Pharm Bull 38:109–115
Jung J, Lee J-H, Bae KH, Jeong C-S (2011) Anti-gastric actions of eugenol and cinnamic acid isolated from Cinnamomi Ramulus. Yakugaku Zasshi 131:1103–1110
Hong CH, Hur SK, Oh O-J, Kim SS, Nam KA, Lee SK (2002) Evaluation of natural products on inhibition of inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells. J Ethnopharmacol 83:153–159
Yu T, Lee S, Yang WS, Jang H-J, Lee YJ, Kim TW, Kim SY, Lee J, Cho JY (2012) The ability of an ethanol extract of Cinnamomum cassia to inhibit Src and spleen tyrosine kinase activity contributes to its anti-inflammatory action. J Ethnopharmacol 139:566–573
Reddy AM, Seo JH, Ryu SY, Kim YS, Kim YS, Min KR, Kim Y (2004) Cinnamaldehyde and 2-methoxycinnamaldehyde as NF-κB inhibitors from Cinnamomum cassia. Planta Med 70:823–827
Lee SH, Lee SY, Son DJ, Lee H, Yoo HS, Song S, Oh KW, Han DC, Kwon BM, Hong JT (2005) Inhibitory effect of 2′-hydroxycinnamaldehyde on nitric oxide production through inhibition of NF-κB activation in RAW 264.7 cells. Biochem Pharmacol 69:791–799
Hwang H, Jeon H, Ock J, Hong SH, Han Y-M, Kwon B-M, Lee W-H, Lee M-S, Suk K (2011) 2′-Hydroxycinnamaldehyde targets low-density lipoprotein receptor-related protein-1 to inhibit lipopolysaccharide-induced microglial activation. J Neuroimmunol 230:52–64
He S, Jiang Y, Tu P-F (2016) Three new compounds from Cinnamomum cassia. J Asian Nat Prod Res 18:134–140
He S, Zeng K-W, Jiang Y, Tu P-F (2016) Nitric oxide inhibitory constituents from the barks of Cinnamomum cassia. Fitoterapia 112:153–160
Fu Y, Yang P, Zhao Y, Zhang L, Zhang Z, Dong X, Wu Z, Xu Y, Chen Y (2017) trans-Cinnamaldehyde inhibits microglial activation and improves neuronal survival against neuroinflammation in BV2 microglial cells with lipopolysaccharide stimulation. Evid-Based Complement Altern Med 2017
Kim ME, Na JY, Lee JS (2018) Anti-inflammatory effects of trans-cinnamaldehyde on lipopolysaccharide-stimulated macrophage activation via MAPKs pathway regulation. Immunopharmacol Immunotoxicol 40:219–224
Park C, Lee H, Hong S, Molagoda IMN, Jeong J-W, Jin C-Y, Kim G-Y, Choi SH, Hong SH, Choi YH (2021) Inhibition of lipopolysaccharide-induced inflammatory and oxidative responses by trans-cinnamaldehyde in C2C12 myoblasts. Int J Med Sci 18:2480
Lee H-S, Kim B-S, Kim M-K (2002) Suppression effect of Cinnamomum cassia bark-derived component on nitric oxide synthase. J Agric Food Chem 50:7700–7703
Tung Y-T, Yen P-L, Lin C-Y, Chang S-T (2010) Anti-inflammatory activities of essential oils and their constituents from different provenances of indigenous cinnamon (Cinnamomum osmophloeum) leaves. Pharm Biol 48:1130–1136
Schink A, Naumoska K, Kitanovski Z, Kampf CJ, Fröhlich-Nowoisky J, Thines E, Pöschl U, Schuppan D, Lucas K (2018) Anti-inflammatory effects of cinnamon extract and identification of active compounds influencing the TLR2 and TLR4 signaling pathways. Food Funct 9:5950–5964
Adewoyin M, Mohsin SMN, Arulselvan P, Hussein MZ, Fakurazi S (2015) Enhanced anti-inflammatory potential of cinnamate-zinc layered hydroxide in lipopolysaccharide-stimulated RAW 264.7 macrophages. Drug Design, Dev Therapy 9:2475
Akira T, Tanaka S, Tabata M (1986) Pharmacological studies on the antiulcerogenic activity of Chinese cinnamon. Planta Med 52:440–443
Tanaka S, Yoon YH, Fukui H, Tabata M, Akira T, Okano K, Iwai M, Iga Y, Yokoyama K (1989) Antiulcerogenic compounds isolated from Chinese cinnamon. Planta Med 55:245–248
Tankam JM, Sawada Y, Ito M (2013) Regular ingestion of cinnamomi cortex pulveratus offers gastroprotective activity in mice. J Nat Med 67:289–295
Keenan C, Hughes-Earle A, Case M, Stuart B, Lake S, Mahrt C, Halliwell W, Westhouse R, Elwell M, Morton D (2002) The north American control animal database: a resource based on standardized nomenclature and diagnostic criteria. Toxicol Pathol 30:75–79
Ribeiro ARS, do Nascimento Valença JD, da Silva SJ, Boeing T, da Silva LM, de Andrade SF, Albuquerque-Júnior RL, Thomazzi SM (2016) The effects of baicalein on gastric mucosal ulcerations in mice: protective pathways and anti-secretory mechanisms. Chemico-Biol Interact 260:33–41
Slomiany B, Piotrowski J, Slomiany A (1997) Induction of tumor necrosis factor-α and apoptosis in gastric mucosal injury by indomethacin: effect of omeprazole and ebrotidine. Scand J Gastroenterol 32:638–642
Phillipson M, Johansson ME, Henriksnas J, Petersson J, Gendler SJ, Sandler S, Persson AEG, Hansson GC, Holm L (2008) The gastric mucus layers: constituents and regulation of accumulation. Am J Physiol-Gastrointest Liver Physiol 295:G806–G812
Jeon W-Y, Lee M-Y, Shin I-S, Jin SE, Ha H (2015) Curcuma aromatica water extract attenuates ethanol-induced gastritis via enhancement of antioxidant status. Evid-Based Complement Altern Med 2015
Kwiecien S, Jasnos K, Magierowski M, Sliwowski Z, Pajdo R, Brzozowski B, Mach T, Wojcik D, Brzozowski T (2014) Lipid peroxidation, reactive oxygen species and antioxidative factors in the pathogenesis of gastric mucosal lesions and mechanism of protection against oxidative stress-induced gastric injury. J Physiol Pharmacol 65:613–622
Robert A, Eberle D, Kaplowitz N (1984) Role of glutathione in gastric mucosal cytoprotection. Am J Physiol-Gastrointest Liver Physiol 247:G296–G304
Tepperman B, Besco J, Kiernan J, Soper B (1991) Relationship between myeloperoxidase activity and the ontogenic response of rat gastric mucosa to ethanol. Can J Physiol Pharmacol 69:1882–1888
Zhao W, Zhu F, Shen W, Fu A, Zheng L, Yan Z, Zhao L, Fu G (2009) Protective effects of DIDS against ethanol-induced gastric mucosal injury in rats. Acta Biochim Biophys Sin 41:301–308
Wallace JL (2008) Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev 88:1547–1565
Acknowledgements
Not applicable.
Funding
This research was funded by Chong Kun Dang (CKD) Pharm Research Institute.
Author information
Authors and Affiliations
Contributions
Conceptualization: S-YK, SJP and KSK; methodology, JWL, DHP, HJS and SHL; investigation, JHL and DHP; writing—original draft preparation, JHL and DHP; writing—review and editing, KSK; supervision, S-YK, SJP and KSK; project administration, KSK. All authors have read and agreed to the published version of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, J.H., Park, D.H., Lee, S. et al. Potential and beneficial effects of Cinnamomum cassia on gastritis and safety: Literature review and analysis of standard extract. Appl Biol Chem 64, 95 (2021). https://doi.org/10.1186/s13765-021-00661-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-021-00661-y