Abstract
In this study, lemon peel flavonoids (LPF) were administered to investigate its effect on the anti-fatigue and antioxidant capacity of mice that undergo exercise until exhaustion. LPF (88.36 min in LPFH group mice) significantly increased the exhaustion swimming time compare to the untreated mice (40.36 min), increased the liver glycogen and free fatty acid content in mice and reduce lactic acid and BUN content in a dose-dependent manner. As the concentration of lemon peel flavonoids increased, the serum creatine kinase, aspartate aminotransferase, and alanine aminotransferase levels of mice gradually decreased. LPF increases superoxide dismutase (SOD) and catalase (CAT) levels in mice and reduces malondialdehyde levels in a dose-dependent manner. And LPF raises hepatic tissue SOD, CAT activities and reduces skeletal muscle tissue iNOS, TNF-α levels of mice compared to the control group. LPF also enhanced the expression of copper/zinc-superoxide dismutase (Cu/Zn-SOD), manganese-superoxide dismutase (Mn-SOD), and CAT mRNA in mouse liver tissue. LPF also enhanced the expression of alanine/serine/cysteine/threonine transporter 1 (ASCT1) mRNA and attenuate the expression of syncytin-1, inducible nitric oxide synthase (iNOS), and tumor necrosis factor (TNF)-α in mouse skeletal muscle. According to high-performance liquid chromatography (HPLC) analysis, it was found that LPF contains flavonoids such as rutin, astragalin, isomangiferin, naringin, and quercetin. Our experimental data show that LPF has good anti-fatigue effects and anti-oxidation ability. In summary, LPF has high prospects to be developed and added to nutritional supplements.
Similar content being viewed by others
Introduction
Lemon (Citrus limon) is a common fruit that is rich in beneficial ingredients such as vitamin C, sugar, calcium, phosphorus, iron, vitamin B1, vitamin B2, niacin, citric acid, quinic acid, malic acid, hesperidin, naringin, and coumarin. It contains high potassium and low sodium and is very beneficial for the human body [1]. The beneficial ingredients contained in lemon can assist with maintaining the production of various tissues and intercellular substances in the human body, and also maintaining their normal physiological functions [2]. Lemon promotes the production of body fluid, prevents cardiovascular diseases, clears away heat and disperses phlegm, and possesses antibacterial and anti-inflammatory effects as well as anti-aging effects [3,4,5,6]. The pulp and juice of lemon are commonly used, and lemon leaves can be used as a seasoning. The skin of fresh lemon fruit can be used to produce lemon essential oil, and edible oil can be obtained from squeezing lemon seeds [7, 8]. However, lemon peels are not commonly used in the processing and application of lemons, and most of the lemon peels created during the industrial process of lemons are discarded as waste. Exploring the application value of lemon peels, including functional applications, can effectively increase the value of lemons, which is beneficial for the full development and utilization of agricultural products.
Fatigue caused by sports has become a common phenomenon among athletes, students, office workers, and urban fitness groups. Fatigue is mainly an uncomfortable physiological phenomenon caused by the body’s inability to maintain exercise intensity. Its causes are fairly complicated, such as exercise overload, irregular work and rest, mental factors, and disease factors [9]. The occurrence of exercise fatigue is mainly related to the excessive consumption of energy biochemicals in the body caused by continuous high-intensive exercise, which promotes the transformation of energy metabolism and leads to the excessive accumulation of a series of fatigue-related metabolites [10]. Long-term or strenuous exercise by humans or animals can cause the source of cellular energy to change from aerobic respiration to anaerobic glycolysis. The body then accumulates a large amount of lactic acid (LA), the pH and osmotic pressure of the internal environment become unbalanced, and the level of reactive oxygen species (ROS) increases. These physiological changes are all important factors that contribute to the generation of fatigue [11]. Studies have shown that ROS can damage the integrity of cell membranes through lipid peroxidation and cause oxidative fatigue of skeletal muscles. Effectively scavenging free radicals is the central link in relieving fatigue [12].
A number of studies have shown that food and some medicinal plants eaten by the general public not only have high edible value, but the active compounds they contain also have high medicinal and health value. The flavonoids in food exert certain anti-oxidation, anti-fatigue, and anti-aging effects, which can also enhance exercise capacity and prevent cardiovascular and cerebrovascular diseases [13,14,15]. Because flavonoids extracted from plants are generally safer than synthetic drugs, they have better application prospects for specific populations, such as the elderly and athletes. However, the effect of most natural plant flavonoids on exercise-induced fatigue is still unclear, including the effect and mechanism of lemon peel flavonoids. Therefore, this study investigated the effects of lemon peel flavonoids on the fatigue and antioxidant capacity of mice exhausted from swimming in order to provide a theoretical basis for the development of sports nutritional supplements.
Materials and methods
Extraction of lemon peel flavonoids (LPF)
Lemon peels (Chongqing Huida lemon Technology Group Co., Ltd, Chongqing, China) were freeze-dried and crushed into powder, and then added to 95% ethanol for ultrasonic extraction at 1:10 (w/v). The crude flavonoids from the peel were sonicated for 60 min (at 50 °C and 200 W), and finally, the crude extract of the sample was obtained by suction filtration. Then, the crude extract was passed through macroporous resin AB-8 (Solarbio Life Sciences, Beijing, China) to separate and purify the flavonoids, and finally, purified LPF was obtained by rotary evaporation.
Determination of cell survival rate (MTT assay)
Human kidney 293 T cell suspension (1 × 104 cells/mL, National Collection of Authenticated Cell Cultures, Shanghai, China) was inoculated into 96 well cell culture plate (60 μL cell solution + 100 μL DEME medium, Solarbio Life Sciences), and cultured at 37 °C for 24 h. After adhering to the wall, 20 μL hydrogen peroxide (0.3 mmol/L) was added for 4 h to prepare the oxidative damage model. Continue to add 20 μL LPF aqueous solution (0–20 mg/mL) for 24 h. After that, 20 μL MTT (Solarbio Life Sciences) was added and cultured for 4 h. The upper medium was removed, and 150 μL DMSO was added. After shaking for 30 min at 37 °C, the OD value was measured at 570 nm.
Animal grouping and handling
Seventy-five inverted cytokine receptor (ICR) mice (Kaixue Biotechnology (Shanghai) Co., Ltd, Shanghai, China) were randomly divided into 5 groups: control group (Control), swimming group (Swimming), vitamin C group (Vitamin C), low-dose lemon peel flavonoid group (LPFL), and high-dose lemon peel flavone group (LPFH), with 15 mice per group. Mice in the vitamin C group received intragastric administration of vitamin C solution at a dose of 100 mg/kg per day, and mice in the LPFL and LPFH groups received intragastric administration of LPF at a dose of 50 and 100 mg/kg, respectively. The control group and swimming group received intragastric administration of 0.2 mL of saline per day. The five groups of mice continuously received intragastric administration for 4 weeks.
Swimming exhaustion experiments
Starting with intragastric administration of vitamin C and LPF, mice in the swimming group, vitamin C group, LPFL group, and LPFH group performed three 30-min swimming trainings during the first week. After that, the swimming time was increased by 10 min per week, and swimming was still performed 3 times a week (KW-QP forced swimming system in mice, Nanjing Calvin Biotechnology Co., Ltd, Nanjing, Jiangsu, China). After the last LPFH gavage, which occurred 4 weeks later, the mice were subjected to swimming exhaustion experiments. Before the experiment, each mouse was weighed, and then a lead wire weighing 5% of the mouse's body weight was tied to the tail of the mouse. The mice were then placed in a water tank with a water depth of 30 cm and a water temperature of 30 °C for swimming exhaustion experiments. When the mice were submerged in the water and did not rise to the surface within 10 s, they were judged to be exhausted, and the time of exhaustion was recorded [16]. The protocol for these experiments was approved by the Ethics Committee of Chongqing Collaborative Innovation Center for Functional Food (202005022B), Chongqing, China.
Energy metabolism index measurement
Liver glycogen was determined according to a previously published method [17]. Mouse livers was homogenized in 0.5 mL of perchloric acid and centrifuged at 15 °C and 15,000 r/min for 15 min. The supernatant was maintained on ice, and 30 μL of the supernatant or glycogen standard was added to a 96-well microplate. Then, 200 μL of potassium iodide reagent was added to each well, and after standing for 10 min, the absorbance at 460 nm was recorded. The serum LA content was determined by the lactate oxidase method [18]; the blood urea nitrogen (BUN) content was determined by the diacetyl monoxime color method [19], and the serum nonesterified fatty acid (NEFA) content was determined by the copper ion color method [20].
Sports injury index determination
After the swimming exhaustion experiments were completed, the mice were euthanized by cervical dislocation, and blood was collected from their hearts. The blood was centrifuged at 4000 r/min for 20 min, and the supernatant serum was reserved for testing. Mouse serum creatine kinase (CK), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels were detected using kits according to the manufacturer’s instructions (Solarbio Life Sciences, Beijing, China).
Antioxidant index determination
The superoxide dismutase (SOD) activity of mouse serum was detected by the pyrogallol colorimetric method, the catalase (CAT) activity was measured by the visible light method, and the activity of malondialdehyde (MDA) was determined by the thiobarbituric acid colorimetric method (Solarbio Life Sciences, Beijing, China) [21].
Determination of SOD, CAT activities in hepatic tissue, and iNOS, TNF-α levels in skeletal muscle tissue
Mice liver and skeletal muscle tissues were prepared into 10% homogenate with normal saline and centrifuged at 4000 rpm for 10 min. The supernatant was removed and the liver tissue SOD, CAT activities and skeletal muscle tissue iNOS, TNF-α levels were measured using the kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
Hematoxylin and eosin (H&E) staining
Five mice in each group did not perform swimming exhaustion experiments, and the remaining 10 mice were subjected to swimming exhaustion experiments. After the mice were euthanized before and after swimming exhaustion experiments, the mouse livers were washed with normal saline, and 0.5 × 0.5 cm2 tissue was fixed in 10% formalin, embedded in paraffin, and 4-μm thick sections were cut. The sections were stained with H&E to evaluate the pathological changes in the mouse liver, and the staining was observed under an optical microscope (BX43, Olympus, Tokyo, Japan).
Quantitative real-time polymerase chain reaction (qPCR) experiment
The mouse liver tissue and skeletal muscle were collected and homogenized. RNA was extracted from the tissue with TRIzol™ reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and diluted to 1 μg/μL. Then, reverse transcription was performed on 1 μL of the diluted RNA solution according to the reverse transcription kit method (Solarbio Life Sciences, Beijing, China) to obtain a cDNA template. For PCR, 1 μL of cDNA template was mixed with 10 μL of SYBR Green PCR Master Mix, 1 μL of upstream and downstream primers (Thermo Fisher Scientific, Inc., Table 1), and 7 μL of sterile distilled water. The PCR reaction was performed at 95 °C for 60 s and then at 95 °C for 40 cycles, and each cycle was 15 s. Then, the mixture was reacted at 55 °C for 30 s, 95 °C for 30 s and 55 °C for 35 s before the reaction ended. The relative gene expression was calculated by the 2−ΔΔCt method (Stepone Plus qPCR instrument, Thermo Fisher Scientific, Inc.), with GAPDH being the internal reference for expression [22].
HPLC
For HPLC preparations, 2 mg of dry standard (Beijing Putian Tongchuang Biotechnology Co., Ltd., Beijing, China) was mixed with sufficient methanol to bring the volume to 2 mL, and a 1 mg/mL solution was prepared. Then, the sample solution was aspirated into the injection bottle with a disposable needle and filter membrane, with the volume of 0.5–1.0 mL. The chromatographic conditions were as follows: Accucore-C18 (2.6 μm, 4.6 × 150 mm); column temperature: 30 °C; mobile phase: (A) 0.5% acetic acid aqueous solution, (B) acetonitrile; flow rate: 0.5 mL/min; detection wavelength: 285 nm; injection volume: 10 μL (UltiMate3000 HPLC system, Thermo Fisher Scientific, Inc.). A standard curve area was drawn according to the standard concentration of R2. Then, the content of flavonoids in the sample was calculated according to the peak area of the sample, the peak area of the standard in the mixed standard, the concentration of the standard in the mixed standard, and the injection volume of the standard in the mixing ratio.
Statistical analysis
Excel 2010 software was applied to organize the data and draw the graphs. The data is expressed as the mean ± standard deviation. SPSS 18.0 software was applied for data analysis. The test methods used were one-way analysis of variance (ANOVA) and the least significant difference (LSD) test, and the significance level was p < 0.05.
Results
Swimming exhaustion time of mice
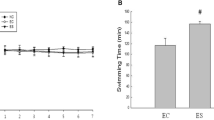
As shown in Fig. 1, LPF can reduce the oxidative damage induced by hydrogen peroxide. With the increase of LPF concentration, the survival rate of oxidative damage cells increased. When the concentration of LPF reached 12.5 mg/mL, the survival rate of oxidative damage cells did not significantly improve. Therefore, 12.5 mg/mL (equivalent to 100 mg/kg b.w.) was selected as the high concentration for further animal experiments.
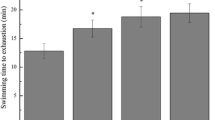
Swimming exhaustion time of mice
After analyzing the swimming exhaustion time of the mice in each group (Fig. 2), it was found that 4 weeks of intragastric administration of vitamin C and different doses of LPF solutions significantly extended the swimming exhaustion time of mice, which was significantly longer than that of the control group and swimming group. In addition, the exhaustion time of the LPFH group was significantly longer than that of vitamin C group. As the concentration of LPF solution increased, the time until exhaustion for the mice also increased.
Energy metabolism in mice
The main energy metabolism indexes of all mice from each group were detected after swimming exhaustion experiments. The current study found (Table 2) that the liver glycogen and free fatty acid content of mice in the vitamin C group and various doses in the LPF groups were significantly higher than those in the control group and swimming group. The amounts of liver glycogen and free fatty acids in the LPFH group were significantly higher than those in the vitamin C group. Supplementation with LPF solution increased the liver glycogen and free fatty acid content of mice in a dose-dependent manner. However, the amounts of LA and BUN in the vitamin C group and various doses in the LPF groups were significantly lower than those in the swimming group. The amounts of LA and BUN in the LPFH group were significantly lower than those in the vitamin C group. With the increase in LPF concentration, there was a gradual decrease in LA and BUN.
Sports injuries in mice
In the current study, the serum creatine kinase, AST, and ALT levels in mice in the vitamin C group and various doses of the LPF groups were significantly higher than those in the swimming group (Table 3). The above 3 indicators from the LPFH group were significantly lower than those from the vitamin C group. As the concentration of LPF increased, the levels of the 3 indicators gradually decreased.
Serum oxidation level of mice
Table 4 shows that the serum SOD and CAT levels of the vitamin C group and various doses of the LPF groups were significantly higher than those of the swimming group, and supplementation with LPF increased the levels of SOD and CAT in a dose-dependent manner. The MDA levels of the vitamin C group and various doses of the LPF groups were significantly lower than those of the swimming group, and supplementation with LPF reduced MDA levels in a dose-dependent manner.
Hepatic tissue SOD, CAT activities and skeletal muscle tissue iNOS, TNF-α levels of mice
The activities of SOD and CAT in the control group were the weakest, and swimming could improve the activities of SOD and CAT in liver tissue of mice. LPF can further enhance the activities of SOD and CAT in swimming mice, and the enzyme activities also increase with the increase of LPF concentration (Table 5). The skeletal muscle tissue iNOS, TNF-α levels showed the opposite trends, these levels were highest in control group mice, LPF can reduce these levels, the effects were better than vitamin C, and the effect of high concentration (LPFH) was better.
Pathological observation of liver
Before the swimming exhaustion experiments, this study applied H&E staining to evaluate the pathological changes in mouse livers. The results (Fig. 3a) showed that the cell nuclei from liver cells of each group of mice were basically uniformly stained, the structure of the mouse liver tissue cells was normal, and the liver cells were radially distributed around the central vein, indicating that vitamin C and LPF had no obvious toxic or side effect on the liver of the mice. After the swimming exhaustion experiments, the mouse liver cells from each group (Fig. 3b) appeared unevenly arranged, the central veins also became irregular, and some cell structures were destroyed and necrotic. Both vitamin C and LPF alleviated the liver damage caused by exhausted swimming, although it was observed that a stronger effect was caused by LPFH.
Expression of Cu/Zn-SOD, Mn-SOD, and CAT mRNA in mouse liver
Figure 4 shows that the expression of Cu/Zn-SOD, Mn-SOD, and CAT mRNA in the control group was the weakest, and the expression of Cu/Zn-SOD, Mn-SOD, and CAT mRNA in the swimming group was stronger than that in the control group. The above expression in the Vitamin C, LPFL, and LPFH group mice was significantly stronger than that of the control group and swimming group, and the LPFH group showed the strongest expression.
The mRNA expression of syncytin-1, ASCT1, iNOS, and TNF-α in mouse skeletal muscle
Figure 5 shows that the mRNA expression intensities, in descending order, of syncytin-1, inducible nitric oxide synthase (iNOS), and tumor necrosis factor (TNF)-α in mouse skeletal muscle were the LPFH group, Vitamin C group, LPFL group, swimming group, and control group. ASCT1 exhibited the opposite trend, with the expression intensity from high to low as follows: the control group, swimming group, LPFL group, Vitamin C group, and LPFH group.
Composition of LPF
By HPLC analysis, the experimental results showed that LPF contained isomangiferin, rutin, astragalin, naringin, and quercetin (Fig. 6). The content of isomangiferin was highest, followed by rutin (Table 6).
Discussion
The mechanism of sports fatigue is complicated, and primary mechanisms include metabolic energy failure, accumulation of metabolites, and free radical attack [23]. Energy failure refers to the excessive consumption of energy materials in the body due to strenuous or excessive exercise, causing body fatigue due to insufficient capacity supply. Accumulation of metabolites refers to the fact that strenuous or excessive exercise affects the body’s energy supply, resulting in the conversion of aerobic energy to anaerobic glycolysis. A large amount of accumulation occurs of metabolites of anaerobic glycolysis, such as LA and ammonia, which disrupts the acid–base balance and causes disorder of the intracellular environment, then which induces fatigue. The theory of free radical attack refers to the fact that anaerobic glycolysis can increase the level of oxygen free radicals in the body, destroy the fluidity and permeability of cell membranes, cause damage to tissues, and induce fatigue.
Most pathological fatigue requires treatment with drugs. However, in healthy people, drug intervention for sports fatigue is usually accompanied by undesirable side effects. Therefore, for healthy people, the main methods for relieving fatigue are physiotherapy and nutritional supplements [24]. Physiotherapy mainly includes tuina, massage, and rehabilitation exercises. However, physiotherapy only temporarily relieves fatigue and cannot increase the ability of the body to manage fatigue. Nutritional supplements are currently the main anti-fatigue research area. Existing studies have shown that some foods can increase the body’s immunity, with anti-aging and anti-oxidation effects, and prevent cardiovascular diseases, and thus, the foods also confer certain anti-fatigue effects [25]. However, these studies have not yet reported the mechanism of action of these foods against sports fatigue.
It is a common in vivo animal experiment to observe mice develop increased ability to exercise and increase their resistance to fatigue through weight-bearing swimming exhaustion experiments [26]. In the current study, the anti-fatigue effect and anti-oxidation ability of LPF were investigated through swimming exhaustion experiments with mice, and vitamin C with satisfactory anti-fatigue and anti-oxidation effects was used as a positive control. After 4 weeks of intragastric administration of vitamin C and different doses of LPF, the mice significantly increased their swimming exhaustion time. The exhaustion time for the LPF group mice was significantly longer than that of the vitamin C group, when both groups received identical doses. In addition, when the LPF concentration was increased, the exhaustion time of mice was also prolonged, indicating that LPF had a stronger anti-fatigue effect.
Hepatic glycogen, LA, BUN, and FFAs are all related to the body’s energy supply [27]. Liver glycogen is mainly formed by the polymerization of glucose molecules and is stored in the liver. Liver glycogen is broken down into glucose to supply the body with energy in response to an energy deficit. Therefore, the level of liver glycogen is an important indicator to evaluate the degree of fatigue. FFAs are the breakdown products of fats. When vigorous or prolonged exercise is performed, fat mobilization is enhanced, and the FFA level increases so as to provide energy for the body and relieve fatigue [28].
The current study found that after exhaustive exercise, the liver glycogen and FFAs of the mice in the Vitamin C group and the LPF group were significantly higher than those in the control group and swimming group. Additionally, the liver glycogen and FFAs of the mice in the LPFH group were significantly higher than those of the Vitamin C group. LPF supplementation increased liver glycogen and FFAs in mice in a dose-dependent manner, and thus increased the liver glycogen storage capacity of mice, promoted fat mobilization, and relieved fatigue.
Strenuous and prolonged exercise induces anaerobic glycolysis to supply energy. Glycolytic metabolism produces a large amount of LA, which lowers the pH value in the body’s internal environment, affects the body’s muscle contraction function, and induces fatigue [29]. In addition, strenuous and prolonged exercise also causes degradation of body protein to produce BUN, which is one of the main evaluation indexes of body protein consumption [30]. The current study found that after exhaustive exercise, the LA and BUN levels of the mice in the vitamin C group and the LPF group were significantly lower than those of the mice in the swimming group. The LA and BUN levels of the mice in the LPFH group were also significantly lower than those of the vitamin C group. With the increase in the LPF concentration, the amounts of LA and BUN gradually decreased. This showed that supplementing with LPF reduces the accumulation of LA, inhibits protein degradation, and relieves fatigue by positively regulating the body's energy metabolism.
Muscles and the liver are more prone to damage during exercise. When the body is damaged due to excessive exercise, it is usually accompanied by an increase in the levels of CK, AST, and ALT in the body [31]. The current study found that the serum CK, AST, and ALT levels of mice in the vitamin C group and LPF group were significantly higher than those in the swimming group. The above 3 indicators were significantly lower in the LPFH mouse group as compared to those in the vitamin C mouse group. As the concentration of LPF increased, the levels of the 3 indicators gradually decreased, suggesting that LPF can adequately function to prevent sports injuries.
Excessive exercise can promote the massive generation and accumulation of superoxide anion free radicals, which have great destructive effects on the cell membrane system and cell metabolism, and cause oxidative stress damage to the body. SOD and CAT are important antioxidant enzymes in the body. In addition to antioxidant effects, SOD can also repair damaged cells. In vertebrates, SOD is mainly expressed in Cu/Zn-SOD and Mn-SOD [32]. In the body, SOD converts free radicals into less toxic H2O2, and then H2O2 is catalyzed by CAT to generate H2O, thereby scavenging free radicals [33]. The current study found that the levels of SOD and CAT in the Vitamin C group and the LPF group were significantly higher than those in the swimming group, and LPF supplementation increased the levels of SOD and CAT in a dose-dependent manner. This showed that LPF has satisfactory antioxidant capacity. MDA is the oxidation product of cell membrane lipids and is a sensitive indicator of lipid peroxidation. When the human body is fatigued, the level of MDA increases [34]. In the current study, the MDA levels in the vitamin C group and the LPF group were significantly lower than those in the swimming group, and supplementation with LPF reduced MDA levels in a dose-dependent manner, indicating that LPF can protect cells and act as an antioxidant by reducing lipid peroxidation.
Studies have shown that there is high expression of syncytin-1 in response to damage, inflammation, or atrophy of muscle tissues, which affects exercise ability [35]. The increase in syncytin-1 expression level inhibits the expression of its receptor, ASCT1, and the decrease in ASCT1 expression is regulated by nitric oxide (NO) [36]. When there is inflammation in the body, the level of iNOS increases, which produces a large amount of NO and inhibits the expression of ASCT1 [36, 37]. TNF-α induces and enhances the expression of syncytin-1 in muscles, causing the production of free radicals and cytoinflammatory factors, which in turn inhibits the expression of ASCT1 [38]. Our study also showed that vitamin C and LPF upregulate the expression of ASCT1 and downregulate the expression of syncytin-1, iNOS, and TNF-α in the skeletal muscles of mice after exhaustive exercise.
Most anti-fatigue chemicals are cortical stimulants. When the cerebral cortex is in a depressed state, the effect of these drugs is more significant. Anti-fatigue drugs excite the brain and eliminate drowsiness, which allows users to continue to function for a prolonged amount of time. Representative drugs in this category include caffeine, amphetamine, ritalin, meclofenac axetil, piracetam, and modafinil, but most of these anti-fatigue chemicals are addictive and have significant side effects [39]. Therefore, there has been an urgent need to search for anti-fatigue factors from medicinal and edible plants.
Research reveals that active flavonoids from plants have anti-fatigue effects, which provides the material basis for the research and development of anti-fatigue functional food. Isomangiferin, rutin, astragalin, naringin, and quercetin have strong antioxidant effects, they can reduce the damage of oxidative stress caused by high intensity exercise [13, 40,41,42,43]. At the same time, these plant flavonoids can remove LA, pyruvic acid, ammonia, and other metabolic wastes produced after exercise, and assist with maintaining the normal metabolism of skeletal muscle cells [44]. In addition, these plant flavonoids can improve energy metabolism and promote energy recovery after exercise [45]. Our study also confirmed that these plant flavonoids are multi-functional, as LPF acts as both an antioxidant and an anti-fatigue agent.
Availability of data and materials
All data and materials used in this study are included in this published article.
Change history
06 August 2023
Missing Funding section has been added.
References
Gao SJ, Mao J (2014) Study on the protective effect of erythritol on vitamin C in lemon juice. Sci Technol Food Ind 35:49–51
Miyake Y, Yamamoto K, Tsujihara N, Osawa T (1998) Protective effects of lemon flavonoids on oxidative stress in diabetic rats. Lipids 33:689–695
Zhou XY, Zhu CH, Shen ZS, Yang X, Gong Q, Yue JQ (2014) Research progress in anticancer active constituents of lemon. Acta Agri Jiangxi 2014:49–53
Zeng XF, Li LB, Yu LM, Feng WH, Chen HG, Bai WD (2018) Research progress on efficacy of trace elements in lemon. Mod Agri Sci Technol 2018:243–244
Zhu C, Zhou X, Li J, Gao J, Du Y, Duo J, Yue J (2019) Research progress of nutrient components and health benefits of grape-fruit. China Med Herald 16:29–32
Zhu CH, Zhou XY, Li JX, Du YX, Liu HX, Pan SY, Yue JQ (2018) Advances in R&D of main nutritional functions of lemon and products in China. Packag Food Mach 36:48–53
Wang X, Li Z, Liu H, Li H (2019) Research progress of lemon essential oil extraction technology. Shandong Chem Ind 48:82–84
Li G, Tan F, Zhang Q, Tan A, Cheng Y, Zhou Q, Liu M, Tan X, Huang L, Rouseff R, Wu H, Zhao X, Liang G, Zhao X (2020) Protective effects of polymethoxyflavone-rich cold-pressed orange peel oil against ultraviolet B-induced photoaging on mouse skin. J Funct Foods 67:103834
Guenette JA, Romer LM, Querido JS, Chua R, Eves ND, Road JD, McKenzie DC, Sheel AW (2010) Sex differences in exercise-induced diaphragmatic fatigue in endurance-trained athletes. J Appl Physiol 109:35–46
Vashistha V, Singh B, Kaur S, Prokop LJ, Kaushik D (2016) The effects of exercise on fatigue, quality of life, and psychological function for men with prostate cancer: systematic review and meta-analyses. Eur Urol Focus 2:284–295
Powers SK, Nelson WB, Hudson MB (2010) Exercise-induced oxidative stress in humans: cause and consequences. Free Rad Biol Med 51:942–950
Fittipaldi S, Dimauro I, Mercatelli N, Caporossi D (2013) Role of exercise-induced reactive oxygen species in the modulation of heat shock protein response. Free Rad Res 48:52–70
Su KY, Yu CY, Chen YW, Huang YT, Chen CT, Wu HF, Chen YL (2014) Rutin, a flavonoid and principal component of saussurea involucrata, attenuates physical fatigue in a forced swimming mouse model. Int J Med Sci 11:528–537
Lin Y, Liu HL, Fang J, Yu CH, Xiong YK, Yuan K (2014) Anti-fatigue and vasoprotective effects of quercetin-3-O-gentiobiose on oxidative stress and vascular endothelial dysfunction induced by endurance swimming in rats. Food Chem Toxicol 68:290–296
Duan FF, Guo Y, Li JW, Yuan K (2017) Antifatigue effect of luteolin-6-c-neohesperidoside on oxidative stress injury induced by forced swimming of rats through modulation of Nrf2/ARE signaling pathways. Oxid Med Cellular Longe 2017:3159358
Jia J (2020) Effect of Plesurotus nebrodensis polysaccharide on oxidative stress in exercised mice. Gen Appl Biol 39:895–901
Mojibi N, Rasouli M (2017) Comparison of methods to assay liver glycogen fractions: the effects of starvation. J Clin Diagn Res JCDR 11:17–20
Xie Q, Chen Z, Zhu S (2020) Xia L (2020) Levels and clinical significance of serum D-lactic acid, endotoxin and DAO at early stage in patients with acute pancreatitis. Chongqing Med 49:1421–1424
Jainu M, Vijaimohan K, Kannan K (2010) Cissus quadrangularis L. extract attenuates chronic ulcer by possible involvement of polyamines and proliferating cell nuclear antigen. Pharm Mag 6:225–233
Chromý V, Gergel J, Voznícek J, Krombholzová L, Musil J (1977) Assay of serum free fatty acids by extraction-photometric procedure. Clin Chim Acta 80:327–332
Pan Y, Wang H, Tan F, Yi R, Li W, Long X, Mu J, Zhao X (2020) Lactobacillus plantarum KFY02 enhances the prevention of CCl4-induced liver injury by transforming geniposide into genipin to increase the antioxidant capacity of mice. J Funct Foods 73:104128
Long X, Zeng X, Tan F, Yi R, Pan Y, Zhou X, Mu J, Zhao X (2020) Lactobacillus plantarum KFY04 prevents mice obesity through the PPARs pathway and alleviate oxidative damage and inflammation caused by obesity in mice. Food Funct 11:5460–5472
Gao ZY, Zhou HT, Lin Q (2011) Effects of herba cistanches on the ability of resistance exercise-induced fatigue in rats and free radical in brain tissue. J Anhui Agri Sci 39:9592–9593
Lambert EV, Hawley JA, Goedecke J, Noakes TD, Dennis SC (1997) Nutritional strategies for promoting fat utilization and delaying the onset of fatigue during prolonged exercise. J Sports Sci 15:315–324
Williams C (1985) Nutritional aspects of exercise-induced fatigue. Proc Nutr Soc 44:245–256
Wu J, Gao W, Wei J, Yang J, Pu L, Guo C (2012) Effect of micronutrient compound on swimming endurance in mice with nutritional deficiency and its mechanism. Med J Chin People’s Liberat Army 37:695–698
Shang Y, Li J (2018) Research on the mechanism of resistance to exercise-induced fatigue of codonopsis extract. J Southwest Univ 40:9–14
Liu Y, Zhou Y, Nirasawa S, Tatsumi E, Cheng Y, Li L (2014) In vivo anti-fatigue activity of sufu with fortification of isoflavones. Pharmacog Mag 10:367–373
Wilkie MP, Bradshaw PG, Joanis V, Claude JF, Swindell SL (2001) Rapid metabolic recovery following vigorous exercise in burrow-dwelling larval sea lampreys (Petromyzon marinus). Physiolog Biochem Zool PBZ 74:261–272
Zhou L, Zhou X, Xu X, Liang Y, Gao F, Zhang C, Sun L, Ma X (2017) Experimental study on the effect of moxibustion at Shenque (CV 8) for long-term exercise-induced fatigue. J Acupunct Tuina Sci 15:387–391
Pal S, Chaki B, Chattopadhyay S, Bandyopadhyay A (2018) High-intensity exercise induced oxidative stress and skeletal muscle damage in postpubertal boys and girls: a comparative study. J Strength Cond Res 32:1045–1052
Wang X, Wang Y, Xiong Z (2013) Protection of Eucommia ulmoides Oliver extract on the oxidative damage of rat’s liver tissue for taking exercise training. J Northwest A&F Univ 41:41–45
Kiruthiga PV, Shafreen RB, Pandian SK, Arun S, Govindu S, Devi KP (2007) Protective effect of silymarin on erythrocyte haemolysate against benzo(a)pyrene and exogenous reactive oxygen species (H2O2) induced oxidative stress. Chemosphere 68:1511–1518
Hou Y, Tang Y, Wang X, Ai X, Wang H, Li X, Chen X, Zhang Y, Hu Y, Meng X, Zhang J (2020) Rhodiola Crenulata ameliorates exhaustive exercise-induced fatigue in mice by suppressing mitophagy in skeletal muscle. Exp Ther Med 20:3161–3173
Frese S, Ruebner M, Suhr F, Konou TM, Tappe KA, Toigo M, Jung HH, Henke C, Steigleder R, Strissel PL, Huebner H, Beckmann MW, van der Keylen P, Schoser B, Schiffer T, Frese L, Bloch W, Strick R (2015) Long-term endurance exercise in humans stimulates cell fusion of myoblasts along with fusogenic endogenous retroviral genes in vivo. PLoS ONE 10:e0132099
Arthur S, Kekuda R, Sundaram U (2012) Mo1817 PKC mediated phosphorylation of RKIP regulates the LTD4 mediated inhibition of ASCT1 activity in IEC-18 cells. Gastroenterology 5:S692
Rodríguez-Lobato LG, Ganzetti M, Fernández de Larrea C, Hudecek M, Einsele H, Danhof S (2020) CAR T-cells in multiple myeloma: state of the art and future directions. Front Oncol 10:1243
Perdigón G, Maldonado Galdeano C, Valdez JC, Medici M (2002) Interaction of lactic acid bacteria with the gut immune system. Eur J Clin Nutr 56:21–26
Lorist MM, Tops M (2003) Caffeine, fatigue, and cognition. Brain Cognit 53:82–94
Yuan X, Han LH, Zhang N, Zhao QF, Yang ZE, Liu JP (2019) Isomangiferin attenuates high fat diet induced liver injury in rats. J Nanjing Univ Tradit Chin Med 35:453–457
Ala M, Li S, Li YW, Wang L (2009) Influence of astragaloside on gastrin gene expression and the free radical content of gastric mucosa with exercise fatigue rat. J Xi’an Inst Phy Educ 26:461–463
Zamanian M, Hajizadeh M, Shamsizadeh A, Moemenzadeh M, Amirteimouri M, Elshiekh M, Allahtavakoli M (2017) Effects of naringin on physical fatigue and serum MMP-9 concentration in female rats. Pharm Biol 55:423–427
Bigelman KA, Chapman DP, Freese EC, Trilk JL, Cureton KJ (2011) Effects of 6 weeks of quercetin supplementation on energy, fatigue, and sleep in ROTC cadets. Mil Med 176:565–572
Sosa T, Chaves N, Alias JC, Escudero JC, Henao F, Gutiérrez-Merino C (2004) Inhibition of mouth skeletal muscle relaxation by flavonoids of Cistus ladanifer L.: a plant defense mechanism against herbivores. J Chem Ecol 30:1087–1101
Theriault A, Wang Q, Van Iderstine SC, Chen B, Franke AA, Adeli K (2000) Modulation of hepatic lipoprotein synthesis and secretion by taxifolin, a plant flavonoid. J Lipid Res 41:1969–1979
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
FL performed the majority of the experiments and wrote the manuscript; HL, GR, XL and RY contributed to the data analysis; FT and XZ designed and supervised the study, and checked the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ethics Committee of Xi'an University of Science and Technology, Xi’an, Shaanxi, China.
Competing interests
The authors of this manuscript state that they do not have conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of the article was revised: Funding note has been updated.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bao, G., Zhang, Y. & Yang, X. Effect of lemon peel flavonoids on anti-fatigue and anti-oxidation capacities of exhaustive exercise mice. Appl Biol Chem 63, 85 (2020). https://doi.org/10.1186/s13765-020-00573-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-020-00573-3