Abstract
A new solid phase microextraction (SPME)-Arrow method was evaluated for the analysis of volatile compounds in kanari-aekjeot, a Korean traditional salt–fermented sand lance sauce, and compared it to the standard headspace–SPME method. Factors observed to affect the extraction, including the fiber used, extraction temperature, extraction time, and NaCl concentration were carefully optimized. The Carboxen/Polydimethylsiloxane fiber exhibited the highest extraction efficiency for both analytical methods and was selected for further optimization of the extraction. The major volatile compounds extracted using both methods were 3-methyl butanoic acid, butanoic acid, acetic acid, 2,6-dimethylpyrazine, and benzaldehyde. The relative concentration (mg/L) of 3-methyl butanoic acid was 1.4-fold higher when using SPME. However, the SPME-Arrow method was more effective at extracting aromatic compounds including alcohol, aldehydes, and pyrazine. In particular, 3-methyl-1-butanol, 2-furanmethanol, and phenylethyl alcohol could only be detected using SPME-Arrow due to its larger sorbent volume. Thus, SPME-Arrow was evaluated as being more suitable for the extraction of pyrazines in sand lance fish sauce and might be useful for determining a broader range of volatile compounds in complex fermented foods.
Similar content being viewed by others
Introduction
Fish sauce is widely consumed in Southeast Asian nations and is also a traditional Korean fermented product. It is manufactured using various salt–fermented fish species including anchovy, sand lance, sardine, hairtail, tuna, and shrimp [1]. The fish sauce is produced from a mixture of fish and salt (4:1) and is fermented for an extended period of 6 months to one year at 30–35 °C. The resulting product has a distinctive odor and flavor, which develops progressively as the fermentation progresses.
Sand lance (Ammodytes personatus) sauce, called kanari-aekjeot has seen increasing demand in Korea recently, and is one of the most popular fish sauces used to ferment other foods such as kimchi due to its preferable taste and flavor compared to anchovy sauce [2]. The unique taste and aroma of sand lance sauce is largely due to the abundant amino acids present in the fish protein during fermentation [3]. The odor has been described as a blend of ammoniacal, cheesy, and meaty notes [4, 5]. Several studies have been conducted on the flavors and volatile compounds present in fish sauces [6,7,8,9]. However, little information exists in relation to the aromatic volatile compounds in sand lance sauce.
Over the past year, various headspace techniques have been used for the determination of volatile compounds in food products, such as dynamic headspace (DHS), solid phase microextraction (SPME), stir-bar sorptive extraction (SBSE) and gas chromatography (GC) coupled with various detectors [7, 9]. Such headspace extraction methods have many advantages, including solvent-free sample preparation, a short extraction time, the small amount of sample required, and lower labor requirements compared to liquid–liquid extraction. SPME is the most widely used headspace analysis for the extraction of volatiles because it offers a fully automated approach at a lower cost than other headspace techniques such as DHS and SBSE. In recent years, a new prototype SPME-Arrow approach combining the advantages of SPME and SBSE approaches has been developed. This new method uses a larger amount of sorbent material to improve detection limits in SPME, which is less fragile than the traditional SPME fibers [10]. The SPME-Arrow technique has been applied to waste and drinking water [11] and atmospheric air samples since it was introduced [12]. To our knowledge, SPME-Arrow has not previously been used for aromatic characterization of food materials.
The identification of volatile compounds is still incomplete and the possible factors responsible for the characteristic odor of fish sauce have not been fully established. The aim of this study was to optimize the analytical conditions for both SPME and SPME-Arrow and compare their suitability in identifying volatile compounds in sand lance fish sauce.
Materials and methods
Materials and reagents
Four SPME fibers, Polydimethylsiloxane (PDMS)/divinylbenzene (DVB) (65 µm × 10 mm, 0.6 µL), PDMS (100 µm × 10 mm, 0.6 µL), DVB/CAR/PDMS (50/30 µm × 10 mm, 0.6 µL), and Carboxen/Polydimethylsiloxane (CAR/PDMS) (75 µm × 10 mm, 0.6 µL) were purchased from Supelco (Bellefonte, PA, USA). Five SPME-Arrow fibers PDMS (100 µm × 20 mm) and PA (100 µm × 20 mm), DVB/PDMS (120 µm × 20 mm) and CAR/PDMS (120 µm × 20 mm), and PDMS (250 µm × 20 mm) for SPME-Arrow analysis were all purchased from CTC Analytics AG (Zwingen, Switzerland). The 20 mL screw cap vial for SPME-Arrow and screw cap including silicone septa for SPME–GC–MS were purchased from Supelco. The internal standard consisting of 4-methyl-2-pentanol and sodium chloride were supplied by Sigma-Aldrich (St. Louis, MO, USA). All of the reagents used were analytical grade.
Sample preparation
Sand lance fish sauce (CJ Cheiljedang Co. Ltd., Korea, Anseong-si) was purchased from a general market and stored at 4 °C before analysis. First, 1 mL of sample was transferred into 20 mL screw cap vial. Before extracting the sample, 20 µL of the internal standard stock solution 4-methyl-2-pentanol (100 ppm (v/v) in water) was added. The screw cap was then sealed and the vials were placed in a Triplus RSH autosampler tray for GC–MS analysis.
Experimental design for the establishment of optimal conditions
Different sorbents for SPME (100 µm PDMS, 65 µm DVB/PDMS, 75 µm CAR/PDMS, 50/30 µm DVB/CAR/PDMS) and SPME-Arrow (100 µm PDMS, 250 µm PDMS, 100 µm PA, 120 µm CAR/PDMS, 120 µm DVB/PDMS) were used to identify the most effective fiber for further analysis. Based on the chromatographic peak areas, extraction time (10, 30, and 60 min), temperature (40, 50, and 60 °C) and salt addition (0, 4, and 8%, w/v) were optimized to optimize the extraction conditions.
Gas chromatography-mass spectrometry
The SPME–GC–MS and SPME-Arrow–GC–MS analyses were performed using a Triplus RSH Autosampler coupled with a TRACE 1310 GC system, and ISQ LT single quadrupole mass detector instrument from Thermo Scientific (West Palm Beach, FL, USA). The analytes were incubated for 5 min. The injector temperature was set at 250 °C and desorption was processed in split mode (5:1) for 2 min. Separation was performed on a DB-WAX column (60 m × 0.25 mm i.d. × 0.25 µm) with helium as a carrier gas at a flow rate of 1.0 mL/min. The initial oven temperature of 50 °C was held for 2 min, and raised to 210 °C at a rate of 2.5 °C/min and held for 2 min. The MS transfer line, source, and quadrupole temperatures were 280, 230, and 150 °C, respectively. The analysis was performed in full scan mode (mass range: 50–450 m/z) and the electron ionization mass spectrometer was operated at 70 eV in the range of 40–400 amu. All data were analyzed with Xcalibur. The volatile compounds were identified on the basis of a mass spectra library (Wiley/NIST 2008) using Kovats retention index (KI) based on the retention time of standard n-alkanes. KI for the unknown compounds was calculated using the following equation [13]:
where: I = Kováts index of compound A, n = number of carbon atoms in the smaller n-alkane eluting before compound A, N = number of carbon atoms in the larger n-alkane eluting after compound A, Tr(A) = retention time of compound A, Tr(n)= retention time of the smaller n-alkane eluting before compound A, Tr(N) = retention time of the larger n-alkane eluting after compound A.
Quantitative analysis was expressed as a relative ratio of the area of the volatile compound in the sample with the highest volatile compounds area of the sample.
Blank samples with an empty screw vial were run before and after each sample to remove any possible contaminants.
Statistical analysis
All experiments were performed at least in triplicate and the results of the analyses are expressed as mean values ± standard deviation (SD). Statistical analyses were processed with SPSS Statistics 23 (IBM, Armonk, N.Y., USA) and calculations were carried out using Sigmaplot 12.0 (Systat Software, San Jose, CA, USA). Significant differences between groups were assessed using ANOVA and Duncan’s multiple range test.
Results and discussion
Optimization of extraction parameters: effect of different fibers
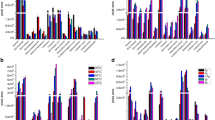
To establish optimal conditions for the analysis of volatile compounds in the fish sauce, factors affecting extraction efficiency including the fiber used, extraction temperature, extraction time, and salt concentration were analyzed. The extraction efficiency and identification of major compounds depending on the different sorbents used for SPME and SPME-Arrow analysis were compared based on percentages of the normalized peak area. The SPME and SPME-Arrow methods feature a difference in fiber thickness, and this has an influence on extraction efficiency for the flavor compounds. To select the most appropriate coating for HS–SPME, four commercial SPME fibers were evaluated, including 100 μm PDMS, 65 μm PDMS/DVB, 75 μm CAR/PDMS fibers and 50/30 μm DVB/CAR/PDMS. As shown in Fig. 1a, significantly higher concentrations of the major volatile compounds including 3-methyl butanoic acid, butanoic acid, 2-methyl-propanoic acid, propanoic acid, acetic acid and 1-penten-3-ol were extracted using the 75 μm CAR/PDMS fiber (p < 0.05). Generally, DVB/CAR/PDMS fiber enables the detection of a wide range of volatile and semi-volatile compounds [14]. However, in this study, 50/30 µm DVB/CAR/PDMS extracted a much lower normalized peak area (%) than that extracted with 75 µm CAR/PDMS. CAR/PDMS is a porous coating material that allows for absorption with high selectivity and sensitivity for semi-polar compounds and is thus well suited to the extraction of relatively small molecules [15, 16]. Based on the results, 75 µm CAR/PDMS was selected as the most suitable fiber for HS–SPME flavor compound analysis and was used for all further experiments.
Different sorbents for SPME-Arrow were compared during the extraction of seven major volatile compounds (Fig. 1b). The normalized peak areas (%) differed depending on each of the four sorbents used: PDMS, PA, CAR/PDMS and DVB/PDMS. The 120 μm CAR/PDMS fibers extracted the highest concentrations of compounds, followed by the 250 μm PDMS and 100 μm PA fiber. The CAR/PDMS-coated fiber has micropores that are more effective at trapping low-molecular weight compounds [11]. On the other hand, PDMS and PA have limited affinity for highly volatile and highly polar compounds [17]. Compared to the 100 μm and 250 μm PDMS fibers, the larger sorbent volume in SPME-Arrow resulted in significantly higher extraction efficiency for the seven volatile compounds (4-methyl-pentanoic acid, 3-methyl butanoic acid, 2-furanmethanol, butanoic acid, 2-methyl propanoic acid, acetic acid and propanoic acid), with the exception of ethanol and 2-butanone. Among the extracted volatile compounds, ethanol and propanoic acid were most effectively extracted using PA fiber, while butanoic acid and 2-furamethanol were more effectively extracted with the CAR/PDMS fiber. Various volatile compound patterns were observed due to the different polarities of the coatings [18]. The CAR/PDMS fiber was more effective in extracting volatile compounds using the HS–SPME method. Both CAR/PDMS and PDMS fiber were effective at extracting highly volatile compounds with the SPME-Arrow method. Thus, the CAR/PDMS fiber was selected for further optimization of the extraction process.
Extraction time and temperature
The duration of extraction time and temperature is known to affect extraction efficiency, and extended durations may impact the sensitivity depending on the compound’s absorptive affinity with the fiber. In this study, the extraction was carried out from at least 10 min to 2 h 30 min using the CAR/PDMS fiber. When volatile compounds were extracted using HS–SPME–GC/MS (Fig. 2a), the maximum normalized peak area (%) of 3-methyl butanoic acid and 2-methyl propanoic acid were observed at 1 h 30 min. The extraction of 1-penten-3-ol was not significantly different when durations of 30 and 90 min were compared. In particular, low-molecular mass 2-propanone and propanoic acid reached maximum extraction efficiency at 30 min and 120 min, respectively. The optimal extraction time for each major compound varied depending on the compound’s competitive absorption on the fiber. Considering the higher extraction time of most compounds, 1 h 30 min was selected as a reasonable duration for the HS–SPME–GC/MS method. On the other hand, all major compounds reached equilibrium at 30 min and began to decrease at 60 min in the SPME-Arrow method (Fig. 2b), suggesting that 30 min of extraction time was selected for the SPME-Arrow method. The SPME-Arrow method was more rapid and effective at extracting the major volatile compounds than the SPME method.
The effect of extraction temperature was investigated at 40, 50, 60, and 70 °C. Higher temperatures promote extraction efficiency but also induce desorption of the components from the fibers [17]. For this reason, most compounds were extracted at 50 °C with the SPME–GC/MS method (Fig. 3a). On the other hand, an increase in temperature (60 °C) was effective with the SPME-Arrow method for ethanol and volatiles with lower boiling points such as 2,6-dimethylpyrazine, but did not show a significant difference at 40–50 °C for other compounds (Fig. 3b). The duration and temperature for successful absorption of all major compounds from the selected fiber were set at 1 h 30 min at 50 °C for SPME–GC/MS and 30 min at 60 °C for the SPME-Arrow–GC/MS method.
Salt effect
The addition of salt improves sample extraction efficiency due to its salting-out effect which reduces the solubility of hydrophobic compounds and retains ionic strength [17]. As expected, the alcohols and acids extracted using the SPME-Arrow method increased with increasing salt concentration until a concentration of 8% (Fig. 4b). Of particular note, ethanol was extracted considerably well. However, the results revealed that the extraction of volatile compounds using the SPME method was significantly reduced by adding NaCl (Fig. 4a). The 4–8% NaCl concentration range reduces the sensitivity of all volatile compounds, although no significant changes in absorption were observed for 2,6-dimethylpyrazine by altering salt concentration.
Comparison of volatile compounds in fish sauce analyzed by SPME and SPME-Arrow
The detection of volatile compounds using different extraction methods were directly compared (Table 1). The SPME method primarily extracted acetic acid and ethanol, whereas the SPME-Arrow–GC/MS method was effective for the detection of broader types of alcohol and acid compounds using the CAR/PDMS coated fiber (Fig. 5). A total of 22 volatiles were identified in sand lance fish sauce including 5 alcohols, 1 ketone, 3 aldehydes, 7 acids, and 6 pyrazines. Of these, 3-methyl butanoic acid, butanoic acid, acetic acid, and benzaldehyde were the most abundant acids and aldehydes extracted with both SPME and SPME-Arrow methods, whereas the relative concentrations varied between the extraction methods. 3-methyl butanoic acid, butanoic acid, and acetic acid (which are associated with cheesy, sweaty and sour odors) were the most abundant volatile compounds contributing to more than 56% and 70% of the total volatile content extracted by SPME-Arrow and SPME, respectively, in agreement with the previous results [19]. There have been very few studies conducted to date focusing on volatile compounds in sand lance fish sauce. According to previous studies, trimethylamine, benzaldehyde, and C4–C7 carbon chain acids are the most important contributors to the aroma of fish sauce. Yimdee and Wang [20] reported that isobutyronitrile, tetrahydro-3-methylfuran, 2-methylbutanenitrile, 3-(methylthio)-propanenitrile, and benzylnitrile were major aromatic active compounds present in sand lance fish sauce. Propanoic acid, butanoic acid, 3-methylbutanal, and 2-methylbutanal are also common aromatic compounds present in Chinese, Thailand, and Malaysian fish sauces [21,22,23]. The relative concentrations of benzaldehyde and acetic acid extracted from the fish sauce was twofold higher with SPME-Arrow, but the other aldehydes and acids were at similar or lower concentrations when the samples were extracted with SPME fiber. Benzaldehyde is considered to enhance flavor quality and produces sweet, fruity, nutty, and caramel-like odors [24].
All components, including the 3 aldehydes, 4 alcohols and 6 pyrazines, with the exception of 1-pentenol, were more effectively detected using the SPME-Arrow method. Alcohols and higher alcohols are important immediate precursors of the flavor-active esters and were the second largest chemical group represented. In particular, 3-methyl-1-butanol, 2-furanmethanol and phenylethyl alcohol were only detected when SPME-Arrow was used for extraction, at 0.122 mg/L, 0.884 mg/L and 1.288 mg/L, respectively. Unlike the competitive adsorption feature of SPME fiber, SPME-Arrow detected a broader range of compounds, probably due to the larger sorbent capacity. On the other hand, the concentration of ketones and acid compounds were relatively higher for the SPME method. Pyrazines, which produce cocoa and roasted flavors [25], were detected with the SPME-Arrow method. Free amino acids and monosaccharides produce pyrazines via Malliard reactions [26]. Although the SPME extraction was more effective for detecting acid compounds, there were lower levels of pyrazines present. Our results demonstrate that SPME-Arrow is more effective for the detection of nitrogen-containing heterocyclic compounds (pyrazines) than the classical SPME fiber extraction. Due to this selective sensitivity, SPME-Arrow can be used for the detection of volatile alcoholic compounds at trace levels in food.
In this study, the SPME and SPME-Arrow methods were optimized and compared for the analysis of volatiles present in salt–fermented sand lance fish sauce. The extraction methods were optimized by focusing on different aspects of the extraction conditions such as sorbent type, temperature, addition of salt and extraction time. The primary flavoring agents, pyrazine compounds, were not detected by SPME, but could be detected sensitively with SPME-Arrow. SPME-Arrow was effective for the extraction of major flavoring factors such as ethanol, higher alcohols and acetic acid. In conclusion, SPME-Arrow represents a promising new system for future analytical studies with a broad capacity for the identification of volatile compounds.
References
Um IS, Park KS (2015) Biogenic amine contents of commercial salted and fermented sand lance Ammodytes personatus sauces. Korean J Fish Aquat Sci 48:883–887
Moon KW, Kim SY, Kim JM, Chang UJ, Bae SH, Suh HJ (2008) Prevention of precipitation in sand lance fish sauce by chelating agents. Food Sci Biotechnol 17:114–117
Oh KS (1999) Quality characteristics of salt–fermented anchovy sauce and sandlance sauce. Korean J Fish Aquat Sci 32:252–255
Dougan J, Howard GE (1975) Some flavoring constituents of fermented fish sauces. J Sci Food Agric 26:887
Beddows CG, Ismail M, Steinkraus KH (1976) The use of bromelain in the hydrolysis of mackerel and the investigation of fermented fish aroma. J Food Technol 11:379
Peralta RR, Shimoda M, Osajima Y (1996) Further identification of volatile compounds in fish sauce. J Agric Food Chem 44:3606–3610
Shimoda M, Peralta RR, Osajima Y (1996) Headspace gas analysis of fish sauce. J Agric Food Chem 44:3601–3605
Fukami K, Ishiyama S, Yaguramaki H, Masuzawa T, Nabeta Y, Endo K, Shimoda M (2002) Identification of distinctive volatile compounds in fish sauce. J Agric Food Chem 50:5412–5416
Ochiai N, Sasamoto K, Takino M, Yamashita S, Daishima S, Heiden AC, Hoffmann A (2002) Simultaneous determination of preservatives in beverages, vinegar, aqueous sauces, and quasi-drug drinks by stir-bar sorptive extraction (SBSE) and thermal desorption GC–MS. Anal Bioanal Chem 373:56–63
Bruheim I, Liu X, Pawliszyn J (2003) Thin-film microextraction. Anal Chim 75:1002–1010
Helin A, Rönkkö T, Parshintsev J, Hartonen K, Schilling B, Läubli T, Riekkola ML (2015) Solid phase microextraction Arrow for the sampling of volatile amines in wastewater and atmosphere. J Chromatogr A 1426:56–63
Feijó Barreira LM, Duporté G, Rönkkö T, Parshintsev J, Hartonen K, Hyrsky L, Heikkinen E, Jussila M, Kulmala M, Riekkola ML (2018) Field measurements of biogenic volatile organic compounds in the atmosphere using solid-phase microextraction Arrow. Atmos Meas Tech 11:881–893
Lubeck A, Sutton D (1983) Kovats retention indices of selected hydrocarbons through C10 on bonded phase fused silica capillaries. J High Resolut Chromatogr 6:328–332
Mestres M, Busto O, Guasch J (2002) Application of headspace solid-phase microextraction to the determination of sulphur compounds with low volatility in wines. J Chromatogr A 945:211–219
Kataoka H, Lord HL, Pawliszyn J (2000) Applications of solid-phase microextraction in food analysis. J Chromatogr A 880:35–62
Wardencki W, Michulec M, Curyło J (2004) A review of theoretical and practical aspects of solid-phase microextraction in food analysis. Int J Food Sci Tech 39:703–717
Pawlizsyn J (2009) Handbook of solid phase microextraction. Chemical IndustryPress, Beijing
Huang LF, Zhou SY (2007) Headspace solid-phase microextraction and gas chromatography-mass spectrometry for Rhizoma Atractylodis Macrocephalae volatile components analysis. Chromatogr 25:43–47
Lapsongphon N, Yongsawatdigul J, Cadwallader KR (2015) Identification and characterization of the aroma-impact components of Thai fish sauce. J Agric Food Chem 63:2628–2638
Yimdee T, Wang XC (2016) Comparison of odor and taste of commercial brand fish sauces from east and south east Asian countries. Int J Food Prop 19:873–896
Jiang JJ, Zeng QX, Zhu ZW (2011) Analysis of volatile compounds in traditional Chinese fish sauce (Yu Lu). Food Bioproc Tech 4:266–271
Wichaphon J, Thongthai C, Assavanig A, Lertsiri S (2012) Volatile aroma components of Thai fish sauce in relation to product categorization. Flavour Fragr J 27:149–156
Mohamed HN, Man YC, Mustafa S, Manap YA (2012) Tentative identification of volatile flavor compounds in commercial budu, a Malaysian fish sauce, using GC-MS. Molecules 17:5062–5080
Zhao Q, Shen Q, Guo R, Wu J, Dai ZY (2016) Characterization of flavor properties from fish (Collichthys niveatus) through enzymatic hydrolysis and the maillard reaction. J Aquat Food Prod T 25:482–495
Xiao L, Lee J, Zhang G, Ebeler SE, Wickramasinghe N, Seiber J, Mitchell AE (2014) HS–SPME GC/MS characterization of volatiles in raw and dry-roasted almonds (Prunus dulcis). Food Chem 151:31–39
Coghe S, Gheeraert B, Michiels A, Delvaux FR (2006) Development of Maillard reaction related characteristics during malt roasting. J Inst Brew 112:148–156
Authors’ contributions
HWJ conceived and designed the experiments. N-ES and J-YL performed most of experiments and wrote the manuscript. Y-YL helped with the preparation of the experiments and conducted data analysis. JDP assisted the revision of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This research was supported by the Food Analysis Center of Korea Food Research Institute (Project No. E0193115-01) and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food, and Rural Affairs (MAFRA) (Project number 171000-02).
Competing interests
The authors declare that they have no competing interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Song, NE., Lee, JY., Lee, YY. et al. Comparison of headspace–SPME and SPME-Arrow–GC–MS methods for the determination of volatile compounds in Korean salt–fermented fish sauce. Appl Biol Chem 62, 16 (2019). https://doi.org/10.1186/s13765-019-0424-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-019-0424-6