Abstract
Background
A long-term follow-up of close contacts to monitor their infection status is essential to formulate a promising screening strategy. The study aimed to assess the dynamics of tuberculosis (TB) infection using Interferon-γ release assay (IGRA) and determine risk factors associated with TB infection.
Methods
Definite TB patients were interviewed and their household contacts were screened for TB infection by IGRA during 12-month longitudinal investigation.
Results
We included in our analyses 184 household contacts of 92 index TB patients. 87 individuals (47.3%) in contact group progressed to TB infection, of whom 86 developed into IGRA positive within 24 weeks. Close contacts with a higher age and comorbidities are easier to exhibit TB infection. Analysis showed that risk factors for becoming IGRA-positive individuals included residence, older age, comorbidities, BCG scar and high bacterial load. Contacts with BCG scar had a lower IGRA-positive rate.
Conclusion
IGRA conversion generally occurs within 24 weeks after exposure. The TB transmission happens since subclinical TB stage and the presence of BCG scar is an independent protective factor reducing risk of TB infection among close contacts. Repeated IGRA tests are sensible to conducted among close contacts at 24 weeks after exposure to identify the IGRA-positive individuals.
Similar content being viewed by others
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB) complex, remains a serious public health threat globally [1, 2]. According to the World Health Organization (WHO) estimates, in 2022 there were 10.6 million people with TB and 1.3 million deaths [3]. This disease has been strongly associated with poverty, overcrowding, malnutrition, and smoking [4]. Moreover, approximately one-third of the world’s population is believed to be latently infected with TB, termed as latent TB (LTBI). Although an estimated 10% of LTBI individuals develop active TB during their lifetime, the huge reservoir of potential future source of active TB poses a barrier towards international targets for achieving TB control [5, 6]. The management of LTBI, therefore, is of great importance for successful achievement of the END TB Strategy.
The transmission of TB occurs mainly via inhalation of airborne droplet nuclei carrying viable bacteria [2]. The risk of acquiring infection with MTB correlates with exposure duration to an infectious source [7]. The WHO recommends systematic monitoring of population groups at high-risk for TB exposure, including household contacts of TB-afflicted individuals and healthcare workers [8]. Specially, in comparison with the intensified infection control measures for healthcare workers, the household contacts are more prone to be infected with MTB, as well as at high risk of getting TB disease [9]. Thus, screening of persons in close contact with the index case is an important strategy to decrease the incidence, especially to identify infected individuals and take action to prevent the development the presence of active TB. In China, the close contacts of infectious people with TB are asked to be screened for TB infection after obtaining personal consent. Tuberculin skin test is the most frequently used method of inferring TB infection in clinical practice. Once identified, the preventive treatment is optional for individuals with TB infection, emphasizing the urgent need for developing efficient national strategies to improve the screening and management of TB infection among close contacts [10].
Routinely, an indirect immunological test is used to directly detect LTBI by ascertaining the reactivity of host lymphocytes to mycobacterial antigens, including in vivo response with tuberculin skin test (TST) or in vitro with INF-γ release assays (IGRAs) [5, 11, 12]. Specially, numerous previous studies have demonstrated that IGRAs outperform TST in identifying LTBI [13, 14]. The high costs of equipment and consumables for IGRAs make it difficult to access in many endemic settings [12, 15]. Even in high-income, low TB burden countries, the cross-sectional IGRA-based screening is usually conducted among close contacts. However, the development of adaptive immunity requires multiple weeks in individuals infected with MTB, and the delayed adaptive immune response would undoubtedly lead to “false-negative” IGRAs. A long-term follow-up of close contacts to monitor their infection status is essential to formulate a promising screening strategy. In the present study, we aimed to assess the dynamics of TB infection using IGRAs over a follow-up period of 1 year, and determine risk factors associated with TB infection in our setting.
Materials and methods
Study populations
Between July 2022 and March 2024, a prospective cohort study of TB household contacts was conducted, 92 local TB patients with confirmed pathogenic diagnosis result were recruited as index case. Their household contacts who more than 5 years old were enrolled.
Contact investigation and contact tracing
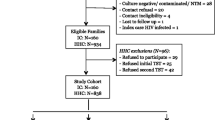
“Index case” was defined as notified bacteria-positive (Xpert, smear and/or culture-positive) TB patient. At baseline, sociodemographic and clinical characteristics information was collected included of both index patients and the household contacts (HHCs), meanwhile, the HHCs were screened by IGRA and CT scan. All contacts have a CT in the first time-point. Contacts with symptoms of TB or positive IGRA tests or CT scan underwent a clinical evaluation in Beijing Chest hospital. If participants were identified as having TB disease, they would receive medical treatment. Those HHCs with negative IGRA results were then traced for the following 12 months. At the 3rd, 6th and 12th months, HHCs with negative IGRA results were evaluated by a clinical questionnaire and IGRA test. In this cohort, all new people with TB were confirmed by IGRA combined with radiography changes (Fig. 1) and were provided timely treatment.
IFN-γ release assay (IGRA)
The commercial sandwich enzyme-linked immunosorbent assay (ELISA) kit (Leide, Guangzhou China) was used to assess interferon-gamma (IFN-γ) concentration according to the manufacturer (Leide, Guangzhou China). Three heparinized fresh blood samples in the volume of 0.6 mL each were added to three different tubes included in kit and incubated at 37 ◦C for 20 ± 2 h after gentle mixing. The first tube did not contain any stimulating agent and was used to determine the individual IFN-γ background; The second tube included MTB-specific antigen (ESAT-6 and CFP-10); the third one was the positive-control tube, which was used for controlling the stimulation ability. During the incubation, the stimulable immune cells were activated to release IFN-γ. After the incubation, the tubes were centrifuged at 4000× rpm for 5 min to obtain stimulated heparinized plasma, which could be used to determine the IFN-γ concentration. The values obtained in the three tubes were used to calculate the final concentration of IFN-γ (pg/mL) released by T lymphocytes due to their stimulation with specific antigen. The results were based on a borderline range recommended by the manufacturer, where a value ≥ 20.0 pg/mL was considered as positive result.
Statistical analyses
The data were entered using a Microsoft Excel worksheet and analyzed by R studio (v4.1.2; R Core Team 2021). Descriptive statistics were used to describe study population characteristics. SPSS version 20.0 software (IBM Corp., Armonk, NY) was used for statistical analysis of the survival curve. Logistic regression analysis (univariate and multivariate analysis) was performed using lme4 package in R studio. Variables with univariate P value less than 0.02 in the univariate analysis were considered in the multivariable models. After using backward elimination, we kept variables if they had a 2-sided significance level less than 0.05. Statistically significant differences were defined using the chi-square or Fisher’s exact test.
Ethics statement
Written informed consent was obtained from all index cases and HHCs. The study was approved by the Ethics Committee of the Beijing Chest Hospital, Capital Medical University (approval No.: YJS-2022-03). The information of all individuals involved in the study were anonymized.
Result
Demographic characteristics of close contacts and index patients
Characteristics of the 92 index patients and 184 close contacts enrolled in the study are shown in Tables 1 and 2. All close contacts completed a 12-month follow-up by the end of March 2024. At enrollment of the index patients, the section of median age was 48 years and 38.0% (n = 35) were older than 60 years of age. About 71.7% (n = 66) of the index patients were male. In addition, 38.0% (n = 35) patients had positive sputum culture and 28.3% (n = 26) had cavitation on chest radiography, only 14 (15.2%) index patients progressed to the calcification stage of TB (Table 1).
Among the contacts, approximately 63.6% (n = 117) of the close contacts were female, and 54.9% (n = 101) were categorized as overweight or obese. Nearly none of the follow-up contacts had a history of tuberculosis, while three-quarters of the close contacts (n = 138) exhibited a scar from Mycobacterium bovis BCG vaccination. 20% of the contact study population (n = 39) had additional comorbidities, such as diabetes mellitus, cardiopathy, hypertension, and other diseases (Table 2).
Progression of household contacts
Over the course of more than one year of follow-up, 62 individuals (33.7%) within the contact group had positive IGRA results at baseline and 25 individuals (13.6%) within the contact group converted to a positive IGRA result. Thus 81 (44.0%) of the contacts had a positive IGRA result within 12 weeks (Fig. 2). IGRA conversion predominantly took place within 24 weeks (86/87, 98.9%) from the commencement of this follow-up study. Only one close contact converted to IGRA-positive result after 24 weeks (48 weeks).
Risk and protective effects associated with positive Interferon-γ release assay
In univariate analyses of positive IGRA result among close contacts, notable distinctions emerged between negative IGRA contacts and positive IGRA contacts across several factors. These factors encompassed residence (P = 0.016), advanced age (P = 0.013), comorbidities (P = 0.006), presence of BCG scar (P = 0.005), and extent of Xpert (P<0.001). In univariate analyses of IGRA conversion among close contacts, there was no significant difference between HHCs who were negative at baseline and subsequently had IGRA conversion and HHCs where IGRA remained negative by 48 weeks (Table S1).
In a multivariable model that encompassed HHCs with positive and negative IGRA results, several risk factors and protective factors for positive results emerged. These included migrant (defined as domicile was not in Beijing) (OR [95%CI]: 2.621[1.363–5.149], P = 0.004), advanced age (OR [95%CI]: 3.449[1.063–12.479], P = 0.045), comorbidities (OR [95%CI]: 2.726[1.199–6.412], P = 0.018), presence of BCG scar (OR [95%CI]: 0.424[0.199–0.877], P = 0.022) (Table 3).
Moreover, individuals with BCG scars among the contacts exhibited a reduced positive IGRA rate (57/138, 41.3%) in contrast to those without BCG scars (30/46, 65.2%). This disparity suggests that contacts possessing a BCG scar experienced a diminished risk of tuberculosis (Table 3). The rate of positive IGRA in close contacts of patients with subclinical symptoms (defined as bacteriologically confirmed but negative on symptom screening) closely mirrored the rate observed in the close contacts of patients with active tuberculosis (47.1% for the symptomatic patient group versus 47.3% for the asymptomatic patient group, P = 1.000) (Table 2).
Discussion
Early identification and intervention for TB infections among close contacts decrease the likelihood of TB transmission within the community [16]. Conventional screening of contacts of people with infectious TB at baseline inevitably results in underestimation of TB infections due to time lags in developing adaptive immune responses against MTB. In this study, our data demonstrated that IGRA conversion majorly occurred within 24 weeks since the investigation began, and only one close contact showed a positive IGRA response at 48 weeks. In a previous cohort study in Korea, Lee and colleagues found that IGRA conversion generally occurred within 22 weeks after exposure, and no conversion was noted 30 weeks after the outbreak investigation [17]. The different intervals of IGRA conversion could be explained by individual diversity in the generation of a lymphocyte memory immune response. Although the memory T cell formation generally takes weeks, this duration varies across individuals, which depends on the immune status of the host organism, as well as pathogenicity of tubercle bacilli. The inclusion of close contact in one TB outbreak may not reflect the diversity of MTB isolates, thereby resulting in biased estimation of IGRA conversion. Previous guidelines endorse repeating the IGRA test at 6–10 weeks after TB exposure among close contacts with a negative IGRA result at initial [18, 19]. Based on the results of our study, repeated IGRA tests should be conducted among close contacts at 24 weeks after exposure to identify the maximum individuals infected with MTB.
Subclinical TB poses a substantial challenge to TB control. Individuals with subclinical TB would be missed if the screening approach had included patients with clinical symptoms suggestive of active TB. In this study, 17 subclinical index cases were identified. Several modeling studies have demonstrated that the evaluation and diagnosis of subclinical index cases of TB disease could contribute to retarding MTB transmission [20]; however, this hypothesis has not emerged from observed data, but from modeling analysis. The results of the present study revealed that the TB transmission occurred from exposure to individuals with subclinical TB. Theoretically, the cycle of TB transmission requires inhalation of an infectious droplet nucleus into the alveolus of a TB-naïve person. For most active TB patients, coughing is assumed to be the main way to produce infectious airborne droplet nuclei. Interestingly, a recent report of face-mask sampling indicated that cough was not necessary for MTB transmission, and the exhaled MTB output was not associated with cough frequency and disease severity [21]. Similarly, Wang and coresearchers identified potential TB transmission among detainee at subclinical TB stage [22]. We consider that subclinical index patients still have atypical clinical symptoms. Breathing, talking and other respiratory maneuvers may play an important role in TB transmission on a population scale. Our findings, combined with previous evidence, highlights an urgent need to address subclinical presentations of the disease.
Although BCG vaccination protects against miliary and meningeal TB in infants and children, its protective effect against adult TB is controversial. In multiple randomized controlled trials and observational studies, the protective effect of BCG vaccination ranges from negative to 100% [23]. In the present study, our data demonstrated that the presence of BCG scar, a useful indicator of an individual’s immune response to BCG vaccination, was an independent protective factor reducing risk of TB infection among close contacts [23], suggesting that BCG vaccination may provide consistent protection in adults. In addition, we found significantly increased risk of TB infection among elderly close contacts. This phenomenon may reflect poor innate immune responses against MTB in these individuals and a higher prevalence of remote infection (infection from the past). Previous experimental studies support our findings at the cellular level, the alveolar macrophages from elderly mice were more permissive to MTB growth and survival [24, 25]. The failure of early clearance of invasive MTB would undoubtedly facilitate antigen presentation and development of adaptive immunity against MTB. In order to achieve the EndTB Strategy target to eliminate the TB epidemic by 2035, more effective vaccines are also urgently needed to protect these vulnerable populations from new MTB infections.
Our study had several obvious limitations. First, our data should be interpreted with caution, as the results came from HIV-naïve populations; bias in the analysis may occur when our conclusion is extended to HIV-positive populations. Second, because the majority of MTB-infected individuals would not ultimately progress to TB disease, we did not perform genotyping to distinguish transmission sources. Additionally, the TST could boost the response of subsequent IGRA test [26, 27], thereby resulting in false-positive IGRA results. Taken together, the infection rate among close contacts may be overestimated in our analysis. Third, adaptive immunity always takes approximately 3–8 weeks after MTB infection [28]. In our cohort, 95.8% (23/24) of close contact showed IGRA conversion by 24 weeks after exposure. Besides the delayed immunological response to TB infection, one plausible explanation for this observation may be associated with the subsequent exposure to a unrecorded index case. Fourth, the time lag between onset of TB symptoms and enrolment on treatment is an important determinant of spread of TB infection within household contacts. However, considering that the onset self-reported symptoms of the index patient is a subjective measure and many factors influence a patients answer, this time log was not recorded in our analysis, which hampered our ability to provide an accurate estimate of window period of conversion after exposure to MTB. Fifth, although the benefits of TB preventive therapy have been addressed by several TB contact cohort studies [29,30,31], the majority of individuals refuse preventive therapy in China, which limits our ability to evaluate the efficacy of this intervention for preventing development of active disease. Finally, our data came from a single pilot, potentially limiting the generalizability of our results to other settings.
In conclusion, our data demonstrates that IGRA conversion generally occurs within 24 weeks after exposure. In addition, the results of the present study reveal that the TB transmission can happen during a subclinical TB stage. The presence of BCG scar is an independent protective factor reducing risk of TB infection among close contacts; whereas we identified significantly increased risk of TB infection among the elderly individuals. Based on the results of the represent study, repeated IGRA tests should be conducted among close contacts at 24 weeks after exposure to identify the maximum individuals infected with TB.
Data availability
No datasets were generated or analysed during the current study.
References
Freschi L, Vargas R Jr., Husain A, Kamal SMM, Skrahina A, Tahseen S, Ismail N, Barbova A, Niemann S, Cirillo DM, et al. Population structure, biogeography and transmissibility of Mycobacterium tuberculosis. Nat Commun. 2021;12(1):6099.
Churchyard G, Kim P, Shah NS, Rustomjee R, Gandhi N, Mathema B, Dowdy D, Kasmar A, Cardenas V. What we know about Tuberculosis Transmission: an overview. J Infect Dis. 2017;216(suppl6):S629–35.
World Health Organization. Global tuberculosis report 2023. Geneva: World Health Organization; 2023.
Millet JP, Moreno A, Fina L, del Baño L, Orcau A, de Olalla PG, Caylà JA. Factors that influence current tuberculosis epidemiology. Eur Spine J. 2013;22(Suppl 4):539–48.
Carranza C, Pedraza-Sanchez S, de Oyarzabal-Mendez E, Torres M. Diagnosis for Latent Tuberculosis Infection: New Alternatives. Front Immunol 2020, 11:2006.
Rao M, Ippolito G, Mfinanga S, Ntoumi F, Yeboah-Manu D, Vilaplana C, Zumla A, Maeurer M, Latent TB. Infection (LTBI) - Mycobacterium tuberculosis pathogenesis and the dynamics of the granuloma battleground. Int J Infect Dis. 2019;80s:S58–61.
Rieder HL. Contacts of tuberculosis patients in high-incidence countries. Int J Tuberc Lung Dis. 2003;7(12 Suppl 3):S333–336.
Paleckyte A, Dissanayake O, Mpagama S, Lipman MC, McHugh TD. Reducing the risk of tuberculosis transmission for HCWs in high incidence settings. Antimicrob Resist Infect Control. 2021;10(1):106.
Martinez L, Shen Y, Mupere E, Kizza A, Hill PC, Whalen CC. Transmission of Mycobacterium Tuberculosis in households and the community: a systematic review and Meta-analysis. Am J Epidemiol. 2017;185(12):1327–39.
Jiang Q, Lu L, Wu J, Yang C, Prakash R, Zuo T, Liu Q, Hong J, Guo X, Gao Q. Assessment of Tuberculosis contact investigation in Shanghai, China: an 8-year cohort study. Tuberculosis (Edinb). 2018;108:10–5.
Hamada Y, Cirillo DM, Matteelli A, Penn-Nicholson A, Rangaka MX, Ruhwald M. Tests for tuberculosis infection: landscape analysis. Eur Respir J 2021, 58(5).
Goletti D, Delogu G, Matteelli A, Migliori GB. The role of IGRA in the diagnosis of tuberculosis infection, differentiating from active tuberculosis, and decision making for initiating treatment or preventive therapy of tuberculosis infection. Int J Infect Dis. 2022;124(Suppl 1):S12–9.
Sousa S, Rocha D, Silva JC, Ribeiro AI, Gonçalves G, Almeida Á, Correia AM, Duarte R, Carvalho C. Comparing the cost-effectiveness of two screening strategies for latent tuberculosis infection in Portugal. Pulmonology. 2021;27(6):493–9.
Gualano G, Mencarini P, Lauria FN, Palmieri F, Mfinanga S, Mwaba P, Chakaya J, Zumla A, Ippolito G. Tuberculin skin test - outdated or still useful for latent TB infection screening? Int J Infect Dis. 2019;80s:S20–2.
Kowada A, Takasaki J, Kobayashi N. Cost-effectiveness of interferon-gamma release assay for systematic tuberculosis screening of healthcare workers in low-incidence countries. J Hosp Infect. 2015;89(2):99–108.
Fox GJ, Johnston JC, Nguyen TA, Majumdar SS, Denholm JT, Asldurf H, Nguyen CB, Marks GB, Velen K. Active case-finding in contacts of people with TB. Int J Tuberc Lung Dis. 2021;25(2):95–105.
Lee SW, Oh DK, Lee SH, Kang HY, Lee CT, Yim JJ. Time interval to conversion of interferon-gamma release assay after exposure to tuberculosis. Eur Respir J. 2011;37(6):1447–52.
National Collaborating Centre for, Chronic C. Centre for Clinical Practice at N: National Institute for Health and Clinical Excellence: Guidance. In: Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for Its Prevention and Control edn. London: National Institute for Health and Clinical Excellence (UK) © 2006, Royal College of Physicians of London. Updated text, Copyright © 2011, National Institute for Health and Clinical Excellence.; 2011.
Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using Interferon Gamma release assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59(Rr–5):1–25.
Nguyen HV, Tiemersma E, Nguyen NV, Nguyen HB, Cobelens F. Disease transmission by patients with subclinical tuberculosis. Clin Infect Dis. 2023;76(11):2000–6.
Turner RD, Bothamley GH. Cough and the transmission of tuberculosis. J Infect Dis. 2015;211(9):1367–72.
Wang Z, Li H, Song S, Sun H, Dai X, Chen M, Xu H, Zhang H, Pang Y. Transmission of tuberculosis in an incarcerated population during the subclinical period: a cross-sectional study in Qingdao, China. Front Public Health. 2023;11:1098519.
Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, Rodrigues LC, Smith PG, Lipman M, Whiting PF, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58(4):470–80.
Canan CH, Gokhale NS, Carruthers B, Lafuse WP, Schlesinger LS, Torrelles JB, Turner J. Characterization of lung inflammation and its impact on macrophage function in aging. J Leukoc Biol. 2014;96(3):473–80.
Guerra-Laso JM, González-García S, González-Cortés C, Diez-Tascón C, López-Medrano R, Rivero-Lezcano OM. Macrophages from elders are more permissive to intracellular multiplication of Mycobacterium tuberculosis. Age (Dordr). 2013;35(4):1235–50.
Naseer A, Naqvi S, Kampmann B. Evidence for boosting Mycobacterium tuberculosis-specific IFN-gamma responses at 6 weeks following tuberculin skin testing. Eur Respir J. 2007;29(6):1282–3.
Igari H, Watanabe A, Sato T. Booster phenomenon of QuantiFERON-TB gold after prior intradermal PPD injection. Int J Tuberc Lung Dis. 2007;11(7):788–91.
Jasenosky LD, Scriba TJ, Hanekom WA, Goldfeld AE. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol Rev. 2015;264(1):74–87.
Liu E, Cheng S, Wang X, Hu D, Zhang T, Chu C. A systematic review of the investigation and management of close contacts of tuberculosis in China. J Public Health (Oxf). 2010;32(4):461–6.
Reichler MR, Khan A, Sterling TR, Zhao H, Chen B, Yuan Y, Moran J, McAuley J, Mangura B. Risk factors for tuberculosis and effect of preventive therapy among close contacts of persons with infectious tuberculosis. Clin Infect Dis. 2020;70(8):1562–72.
Sayedi SM, Seddiq MK, Rashidi MK, Qader G, Ikram N, Melese M, Suarez PG. Active household contact screening for tuberculosis and provision of isoniazid preventive therapy to under-five children in Afghanistan. PLoS ONE. 2020;15(10):e0240031.
Acknowledgements
The authors would like to thank anonymous reviewers for their valuable comments, and the editors for their carefully check about this paper.
Funding
This study was supported by the National Key Research and Development Program of China (Grant No. 2022YFC2302900) and Beijing Tong Zhou District Science and Technology Program (Grant No. KJ2023CX047).
Author information
Authors and Affiliations
Contributions
SJD and YP contributed to the conception and design of the study, WW and ZCM contributed to acquisition of data, or analysis and interpretation of data, XXZ, SSL, YYS and RML contributed to drafting the article. MQG and JFY contributed to the editing. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
This study was approved by the Ethics Committee of Beijing Chest Hospital, Capital Medical University (YJS-2022-03). Informed consent from all participating subjects was obtained.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ma, Z., Duan, S., Wang, W. et al. Surveillance of close contacts of patients with infectious tuberculosis: a prospective cohort study. Antimicrob Resist Infect Control 13, 59 (2024). https://doi.org/10.1186/s13756-024-01419-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-024-01419-z