Abstract
The rising prevalence of vancomycin-resistant enterococci (VRE) is a matter of concern in hospital settings across Europe without a distinct geographical pattern. In this scoping review, we compared the epidemiology of vancomycin-resistant Enterococcus spp. in hospitals in the Netherlands and Germany, between 1991 and 2022. We searched PubMed and summarized the national antibiotic resistance surveillance data of the two countries. We included 46 studies and summarized national surveillance data from the NethMap in the Netherlands, the National Antimicrobial Resistance Surveillance database in Germany, and the EARS-Net data. In total, 12 studies were conducted in hospitals in the Netherlands, 32 were conducted in German hospitals, and an additional two studies were conducted in a cross-border setting. The most significant difference between the two countries was that studies in Germany showed an increasing trend in the prevalence of VRE in hospitals, and no such trend was observed in studies in the Netherlands. Furthermore, in both Dutch and German hospitals, it has been revealed that the molecular epidemiology of VREfm has shifted from a predominance of vanA towards vanB over the years. According to national surveillance reports, vancomycin resistance in Enterococcus faecium clinical isolates fluctuates below 1% in Dutch hospitals, whereas it follows an increasing trend in German hospitals (above 20%), as supported by individual studies. This review demonstrates that VRE is more frequently encountered in German than in Dutch hospitals and discusses the underlying factors for the difference in VRE occurrence in these two neighboring countries by comparing differences in healthcare systems, infection prevention control (IPC) guidelines, and antibiotic use in the Netherlands and Germany.
Similar content being viewed by others
Background
Enterococci are among the most common nosocomial pathogens in the world [1]. The spread of multidrug-resistant enterococci in healthcare, the majority attributed to Enterococcus faecium, and their adaptation to the hospital environment have been of concern since the 1970s [1, 2].
Enterococci can acquire antibiotic resistance by sporadic chromosomal mutations or exogenous gene exchange, besides being intrinsically resistant to many antibiotics such as cephalosporins, trimethoprim-sulfamethoxazole, and lincosamides [3]. High-level resistance to aminoglycosides and resistance to ampicillin and glycopeptides are well-known examples of acquired antibiotic resistance in enterococci [3, 4]. The first case of vancomycin-resistant enterococci (VRE) was reported in France in 1986; since then, it has emerged as a major cause of nosocomial infections worldwide [5,6,7]. Vancomycin resistance has been attributed to the acquisition of gene clusters that alter the nature of peptidoglycan precursors; and to date, nine different gene clusters have been identified [8]: vanA, vanB, vanC, vanD, vanE, vanG, vanL, vanM, vanN. However, vanA and vanB are the major circulating gene clusters in human VRE colonization and infections, both in Europe and worldwide [5, 9].
Given the fact that VRE are resistant to first-line antibiotics in hospital settings, there are a limited number of therapeutic options, such as linezolid, tigecycline, and daptomycin [10]. However, increasing resistance to these last-resort antibiotics has been reported [10,11,12,13,14]. Therefore, prevention of VRE infections is crucial to avoid treatment challenges [15].
Over the past two decades, studies have provided information on the burden of VRE infections in hospitals [5, 16,17,18,19,20]. Compared to vancomycin-susceptible enterococci (VSE) infections, VRE infections are associated with higher morbidity, cost of care, longer length of hospital stay, and mortality [19, 21, 22]. Unsurprisingly, the World Health Organization (WHO) included VRE as a high-priority pathogen in its global list of important antibiotic-resistant bacteria in 2017 [23]. Data from the European Antimicrobial Resistance Surveillance Network (EARS-Net) justified the WHO’s decision by showing that the prevalence of VRE across Europe doubled from 2015 to 2019 [24]. According to this report, an increase in vancomycin resistance was reported across Europe due to the increasing prevalence of vancomycin-resistant E. faecium (VREfm) [24]. Interestingly, two neighboring countries, Germany and the Netherlands, are at both ends of the scale of the proportion of VREfm in all invasive E. faecium isolates according to EARS-Net (< 1% in the Netherlands and 22.3% in Germany). The underlying reasons for this difference are not yet fully understood [24]. Although Germany and the Netherlands have many common historical, cultural, and social values, they differ in many aspects regarding healthcare. These differences include amongst others the healthcare structure, antibiotic prescription habits, and local and national infection prevention and control (IPC) guidelines for multidrug-resistant microorganisms (MDRO) [25,26,27]. All these aspects taken together may be the cause for the differences in VRE rates encountered in these two neighboring countries [25, 27,28,29].
Despite the available evidence for the difference in the prevalence of VRE in the Netherlands and Germany, there are no nationwide comparative studies detailing this situation to date. Therefore, this review aims to describe the epidemiology of vancomycin-resistant Enterococcus spp. by presenting the outbreaks, VRE colonization prevalence, and VRE proportions in clinical isolates in hospitals in Germany and the Netherlands based on the literature and national and European surveillance data.
Methods
We performed a scoping review using PubMed to search for publications in English, Dutch, and German providing data on VRE colonization and infection prevalence, incidence, surveillance, and outbreaks in hospital settings in the Netherlands and Germany. The review was performed following the recommendations of PRISMA-ScR [30]. We performed a peer-reviewed search strategy, executed on December 30, 2022. The search term (Additional file 1) was externally reviewed by a research librarian from the University of Groningen. The authors (CC and MSB) independently searched and extracted data using a peer-reviewed search strategy to avoid missing any relevant studies. No inconsistencies were encountered with this strategy. The dataset is available in Additional file 2, and those who are interested can reach out to the corresponding author for any further inquiries.
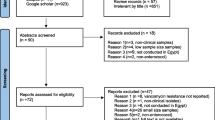
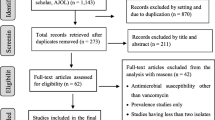
The relevance of the publications was assessed and included following a defined flowchart (Fig. 1). First, inclusion was based on title and abstract reading. Selected articles were then accessed in full text to determine eligibility and extract the data. The reference lists of eligible publications were screened for additional articles. The scientific publications had to meet all the following criteria for inclusion: reported data had to include the number of VRE isolates and/or cases, and studies had to be conducted in a hospital. The following data were extracted from the selected publications: the first author’s name, country of origin, province of where the study was conducted, time frame for conducting the study, study methodology (outbreak report, surveillance report, prevalence/incidence study), hospital type, ward/ICU type, number of cases/samples involved in the study, the number and prevalence, incidence or proportion of VRE, and presence of resistance genes when available.
In addition, the national surveillance data from the two countries were reviewed by extracting information from NethMap in the Netherlands and the National Antimicrobial Resistance Surveillance (ARS) database established by the Robert Koch-Institute (RKI) in Germany and for both countries from EARS-Net data [31,32,33].
Results
Study inclusion and characteristics
The initial search yielded 156 potentially relevant publications, 80 of which were excluded based on title and abstract reading (Fig. 1). A further 32 publications were excluded after full-text evaluation. The reference lists of the eligible studies were screened, and four additional studies were included. Ultimately, 46 publications were included (Figs. 1, 2). Of the selected publications, 12 were conducted in the Netherlands, and 32 in Germany. Two further studies were cross-border studies that included data from both countries. In total, there were one ecological, one pre-post study, one longitudinal study, four cohort studies, 14 outbreak reports, and 25 cross-sectional studies.
Outbreaks due to vancomycin-resistant E. faecium (VREfm)
Of the 12 studies conducted in the Netherlands, eight were outbreak reports (Table 1) [34,35,36,37,38,39,40,41]. Of the 32 German publications, six were outbreak reports (Table 2) [42,43,44,45,46,47]. All outbreaks in both countries were caused by VREfm. In three of eight outbreaks observed in Dutch hospitals and in four of six outbreaks observed in German hospitals, VREfm infections were reported alongside patients colonized with VREfm [34, 37, 40, 42, 45,46,47]. One common factor observed in these reports was that colonization played a pivotal role in the occurrence of outbreaks in both countries.
Summary on the epidemiology of VRE
Thirty-two studies reported prevalence or incidence of VRE among inpatients: 17 studies reported the prevalence of VRE colonization, three studies reported the proportion of VRE in nosocomial infections, 11 studies reported the frequency of VRE among all clinical and screening cultures, and one study reported both the proportion of VRE in nosocomial infections and the frequency of VRE among clinical and screening cultures. Table 1, Table 2 and Table 3, which provide detailed epidemiological data, indicate whether the numbers presented correspond to VRE isolates or to the total number of patients diagnosed with VRE.
Studies reporting on the prevalence of VRE colonization
Of the 17 studies that reported on the prevalence of VRE colonization, three were from Dutch hospitals and 14 were from German hospitals. One cohort study and two cross-sectional studies investigated VRE colonization in different patient groups in Dutch hospitals (Table 1). In the cohort study, the prevalence of vancomycin-resistant E. faecalis colonization was 1.1% in surgical patients from three university hospitals [48]. In the cross-sectional studies, the prevalence of VRE colonization (E. faecalis and E. faecium) was 12.9% in the study conducted in hematology patients of the university hospital in Leiden in 1991 [49] and 1.3% in the study involving intensive care and hematology-oncology patients from nine different hospitals between 1995 and 1998 [50].
The prevalence of VRE colonization in different patient groups was investigated in nine cross-sectional studies, two cohort studies and one pre-post study in German hospitals. The prevalence ranged between 1.2% and 27.7% (Table 2) [51,52,53,54,55,56,57,58,59,60,61,62]. All studies reported the prevalence of VREfm colonization, except for three studies, one that did not specify the species and the other two that reported both E. faecalis and E. faecium [51,52,53,54,55,56,57,58,59,60,61,62]. The highest prevalence was reported in studies among hematology-oncology patients (23.8%), geriatric patients (15.2%), and patients at high risk (27.7%) for VREfm colonization [52, 54, 58, 59]. The lowest VREfm colonization (1.2% and 1.6%) prevalence was reported in two hospital-wide studies, which did not include intensive care unit (ICU) patients [55, 56]. One of these studies was carried out in six university hospitals throughout Germany and found an increase in VREfm colonization prevalence (0.8% in 2014, 1.2% in 2015, 1.3% in 2016, 1.5% in 2017, 2.6% in 2018) in inpatients over the years between 2014 to 2018 [56].
Two cross-border studies compared the prevalence of VRE colonization among hospitalized patients (Table 3). In one of the studies conducted at two university hospitals in the Northern Dutch-German cross-border region between 2012 and 2013, the prevalence of VRE colonization in the German hospital (3.9%) was three times higher than in the Dutch hospital (1.3%) [63]. The difference was even more significant in the study carried out in 23 hospitals’ ICUs (8 Dutch and 15 German) in the cross-border region between 2017 and 2018: VRE colonization prevalence was almost 30 times higher in the German hospitals (2.7%) than in the Dutch hospitals (0.1%) [64].
Studies reporting the proportion of VRE in nosocomial infections and the incidence of VREfm in bloodstream infections (BSIs)
The studies that reported the proportion of nosocomial, invasive VRE were all conducted in German hospitals and presented an increase in VRE infections in Germany over the years (Table 2) [65,66,67,68]. Two studies analyzed data from the German National Nosocomial Surveillance System (KISS, Krankenhaus-Infektions-Surveillance-System, https://www.nrz-hygiene.de/kiss/kiss-module) and reported the proportion of VRE (E. faecium and E. faecalis) in nosocomial infections. The first study analyzed the proportion of VRE in nosocomial infections in ICUs and surgical departments between 2007 and 2012 [67]. This study found not only an increasing trend of VRE (from 2007 to 2012: in SSI, 0.87% to 4.58%; in BSI, 4.91% to 12.99%; in UTI, 2.23% to 6.19%) in Germany in general, but also a diversity between federal states including a “VRE belt” in the middle of the country, ranging from the West (North Rhine-Westphalia) to East (Saxony) [67]. The second study described a continuous increase in nosocomial infections caused by VRE in German ICUs and surgical wards from 1.4% in 2007 to 10% in 2016 [65].
The remaining two studies reported the incidence density of VREfm in bloodstream infections (BSI). The first study was a prospective longitudinal study in 31 laboratories in North Rhine-Westphalia, Germany [66]. This study found an increase in the incidence density (per 100,000 inhabitants) of VREfm BSI from 0.52 in 2016 to 1.48 in 2019 [66]. The second study analyzed the ARS surveillance system, which reported an increasing estimated incidence density (per 100,000 inhabitants) of VREfm BSI from 1.4 in 2015 to 2.9 in 2020 across the country [68].
Studies reporting the frequency of VRE among all clinical and screening cultures
All 12 studies (one conducted in a Dutch hospital and 11 in German hospitals) analyzed microbiology data without distinguishing between VRE infection or VRE colonization. Unless otherwise stated, the reported numbers represent the combined rate of VRE in both E. faecium and E. faecalis isolates. The study conducted at the Dutch hospital (University Hospital Groningen) was a cross-sectional study, reporting a prevalence of 0.3% VRE in ICU patients [69].
Of the 11 German studies, nine were cross-sectional studies, one was a cohort study, and one was an ecologic study (investigating the impact of antibiotic use on VRE prevalence). An international surveillance study, including data from 169 German hospitals between 2000 and 2002, reported a VRE prevalence of 4.8% for E. faecium and 0.3% for E. faecalis [70] and a study that analyzed MDR-KISS data between 2005 and 2006 reported a VRE prevalence of 0.1% in ICU patients [71]. The ecologic study that was conducted at the university hospital Berlin in 2012 reported a VRE prevalence of 0.7% [72]; in a point prevalence study conducted in 37 acute-care hospitals in Munich in 2012 a VREfm prevalence of 0.38% was recorded in inpatients, including ICU patients [73].
Three of the cross-sectional studies reported an increasing incidence of VRE over several years. In one of the studies that was conducted at four university hospitals across different regions in Germany (East, North, Southwest, Southeast), an increase in the incidence (with rates rising from 5 to 9 to 14 per 10,000 patients) of VREfm was observed between 2007 and 2009 [74]. Two studies that analyzed the data from KISS and the Surveillance of Antibiotic use and Resistance in ICUs (SARI) project also recorded an increase in VRE in German hospitals [67, 75]. The incidence of VRE cases (per 100 admitted patients) in ICUs rose from 0.11 in 2007 to 0.31 in 2012 [67], whereas the resistance density of VRE in German ICUs increased from 0.1 in 2001 to 1.1 per 1000 patient days in 2015 in the other study, which included the SARI cohort [75]. In contrast, three nationwide one-day point prevalence studies conducted in 2010, 2012, and 2014 using the same study protocol but with different numbers of participating hospitals did not show an increase in VRE colonization or infection among hospitalized patients [76,77,78]. In addition to these national studies, a regional study was conducted to identify regional trends of AMR in Lower Saxony. In this study, the data of the Antimicrobial Resistance Monitoring in Lower Saxony (ARMIN) project in the period 2006–2010 were analyzed, and strikingly, this study reported a decreasing proportion of VREfm cases within those years in Lower Saxony from 13.6% in 2006 to 5.6% in 2010 [79].
Molecular epidemiology of VRE over time
Data from outbreaks in both Dutch and German hospitals revealed that the molecular epidemiology of VREfm causing outbreaks has changed from a predominance of vanA towards vanB over the years (Table 1–2) [34, 37, 39,40,41,42,43,44,45,46,47].
In the Netherlands, of the eight reported outbreaks, six reported the vanA/B status of isolates. In an outbreak in 1999 at the university hospital in Amsterdam, all VREfm isolates were vanA-positive [34], and in another outbreak at a non-university hospital in Utrecht between 2012 and 2014, the majority of the VREfm isolates were vanA-positive [37]. In contrast, two outbreaks at the university hospital in Groningen in 2014 and 2017 were predominantly caused by vanB-VREfm [39, 40]. Similarly, in an outbreak at a tertiary hospital in Tilburg in 2018, all VREfm isolates were vanB-positive [41].
In Germany, all reported VREfm outbreaks provided molecular data. The outbreaks at the university pediatric hospital in Hamburg (1993–1997), at the university hospital in Halle (1999–2001), and at the university hospital in Tübingen (2001) were all caused solely by vanA VRE [42,43,44]. In another outbreak at the university hospital in Tübingen in 2004, most VREfm isolates were vanA-positive [45]. In a hospital-wide outbreak at a university hospital in south-west Germany in 2015 [46] and in a VREfm outbreak in two regional hospitals in southern Germany between 2015 and 2019, vanB was most frequently detected [47].
Apart from the above-mentioned outbreak reports, no other studies from the Netherlands reported molecular data of VRE. However, a shift from vanA to vanB over time was also observed in German non-outbreak studies (Table 2). In a cross-sectional study at two hospitals in Berlin in 1995 [51] and another at the university hospital in Cologne between 2012 and 2013, most isolates were vanA-positive [61]. In contrast, most studies after 2013 reported a predominance of vanB, including a study at a tertiary care hospital in southern Germany (2014–2015) [54], a cohort study at the university hospitals in Cologne, Freiburg, Hamburg, and Tübingen (2016) [62], and a cross-sectional study at six university hospitals throughout Germany (2014–2018) [56]. In a longitudinal study in 31 microbiology laboratories in North Rhine-Westphalia, vanA was predominant in 2016, while vanB was most prevalent in 2017–2019 in VRE BSIs [66]. Similarly, in a study in 2018–2019 at the university hospital in Hannover [59] and another study in 2019 in Munich [60], vanB was more frequent than vanA.
VRE surveillance data reports on the national level
Both countries have their own national antibiotic resistance surveillance systems, including VREfm, and both submit their results to EARS-Net.
The Netherlands
Microbiological data of all isolates from medical microbiology laboratories in the Netherlands are collected in the Infectious Diseases Surveillance Information System for Antimicrobial Resistance (ISIS-AR) [80]. Based on these data and in collaboration with the Dutch Working Group on Antibiotic Policy of the Dutch Society of Medical Microbiology, a SWAB/RIVM report (NethMap) has been published annually to monitor AMR since 2003 [81]. Data regarding VRE from clinical isolates have been available since 2003 in NethMap reports (Fig. 3).
Summary of the number of VREfm outbreaks (blue boxes) and VREfm proportion (orange boxes) in clinical isolates in Dutch hospitals between 2003 and 2021 (NethMap reports) [81]. The data in the boxes represent the temporal distribution of VRE data over the years. (VREF: vancomycin resistant E. faecalis, VREfm: vancomycin- resistant E. faecium)
According to the NethMap reports, there was a significant increase (from 0.1–0.8% to 1.5%) in the proportion of vanB-positive VREfm in hospitals between 2008 and 2011. This increase was attributed to VREfm outbreaks, particularly occurring in hospitals in the northern region of the country [81]. As Fig. 3 shows, numerous VREfm outbreaks have been reported in the Netherlands over the years. However, the proportion of VREfm in clinical isolates of E. faecium in hospitals remained below 1% and has not changed in the last decade. To manage and prevent large-scale outbreaks of AMR in healthcare facilities and contain its spread to other institutions at an early stage, the Early Warning and Intervention Meeting for Nosocomial Infections and Antimicrobial Resistance (SO-ZI/AMR), was established in the Netherlands in 2012 [82]. Participating hospitals have voluntarily committed to the SO-ZI/AMR system, which includes reporting obligations and regular updates until the outbreak is resolved. Of all VREfm outbreaks in the last decade, the lowest numbers were recorded in 2020 and 2021. This decrease could potentially be influenced by multiple factors such as the implementation of enhanced infection control measures during the COVID-19 pandemic or a potential decrease in reporting due to the burden of the pandemic, as reporting is voluntary.
There is currently no nationwide surveillance of the molecular epidemiology of VRE in the Netherlands. Centrally collected national data on VREfm molecular typing were available only between 2012 and 2018, and vanA was always more frequent than vanB during this period [81].
Germany
Microbiological data of all isolates from participating medical microbiology laboratories and hospitals in Germany are collected in the ARS database established by the RKI since 2008 [33]. Pre-2008 national data are available in so-called Epidemiology Bulletins, which have been periodically published by the RKI. According to these reports, there was an increase in the number of VREfm isolates observed in 2003 and 2004 (both screening and clinical samples) compared to the previous years [83]. Following a short decrease in the following two years, numbers increased again in 2007 [84]. The ARS database, available since 2008, provides data regarding the proportion (%) of VREfm in all E. faecium isolates obtained from inpatient blood cultures (Fig. 4). Since 2009 an overall increasing trend of the VREfm proportion could be observed.
VREfm as the proportion (%) of all E. faecium isolates from inpatients’ blood cultures between 2008 and 2021 in Germany (ARS-RKI Statistics) [85]. (VREfm: vancomycin- resistant E. faecium)
A National Reference Center (NRC) for staphylococci and enterococci was assigned by RKI in 2012 [86]. According to the NRC, significantly more vanB-VREfm than vanA-VREfm isolates were sent to the NRC for the first time in 2017, and the situation has remained the same since then [87, 88].
EARS-net
The national AMR data represented in EARS-Net are obtained from the RIVM and RKI in the Netherlands and Germany, respectively [24]. In 2021, the population coverage in the EARS-Net surveillance data was 68% for the Netherlands and 35% for Germany [89]. Throughout the years, the coverage percentages have remained relatively stable, with the Netherlands consistently having higher coverage compared to Germany [90]. The Netherlands is among 13 out of 30 countries that have maintained a VRE rate below 5% in clinical E. faecium isolates over the course of several years. In contrast, in Germany, the percentage increased continuously between 2016 (11.9%) and 2019 (26.3%) and surpassed the European average since 2017 (Fig. 5) [24]. Interestingly, this percentage (22.3%) decreased in 2020 for the first time since 2014 [91].
The percentage of VREfm in clinical (invasive) E. faecium isolates in the Netherlands and Germany between 2001 and 2021. EU/EAA average was only reported between 2013 and 2020. Data from the ECDC Surveillance Atlas [92]
Discussion
Given the limited treatment options and increasing prevalence of VRE in Europe, VRE remains a severe problem in healthcare [5, 24]. Despite this overall increase, large variations have been reported between countries [24]. To the best of our knowledge, we provide the first comparative overview of the epidemiology of VRE in hospital settings in the Netherlands and Germany, covering 102 million EU inhabitants, by reviewing the literature and national surveillance data.
In this review, the studies from the two countries did not only differ in number but also in the type of design. While most of the studies in the Netherlands were outbreak reports, cross-sectional prevalence studies were predominant in Germany. The larger number of cross-sectional prevalence studies in German hospitals may indicate that VRE is a more pertinent problem in German than in Dutch hospitals.
Analysis of outbreak reports revealed that all outbreaks in both countries were caused by VREfm. This is not surprising because of the high tenacity of E. faecium to survive in the hospital environment [93]. Although the rate of infections differed within and between countries, colonization was a common cause of VREfm outbreaks in both countries. Studies on prevalence or incidence of VRE varied considerably depending on the patient population and time. Generally, high VRE prevalences were reported in high-risk wards such as haemato-oncology and geriatric wards in both countries [49, 52,53,54, 59]. This finding is consistent with previous studies, which have identified age and haemato-oncological malignancies as risk factors for both VRE colonization and infection [94,95,96].
The most prominent difference between the two countries was that the German studies showed an increasing trend of VRE prevalence in German hospitals, yet such a trend was not observed in the Dutch studies. It is important to acknowledge that the smaller number of Dutch studies restricts the ability to draw conclusive observations regarding this matter. Cross-border studies have also demonstrated this difference when applying the same screening strategy for hospitalized patients [63, 64]. This observation is supported by the national data of both countries and EARS-Net data. EARS-Net data shows that the proportion of VREfm in clinical E. faecium isolates from patients with invasive infections has remained stable, with slight fluctuations below 1% in the Netherlands over the past decade, while in Germany, it has risen to over 25% with an increasing trend [24, 65].
In the following paragraphs, we will elaborate on some points that may explain the difference in epidemiology of VRE between these two neighboring countries.
Healthcare system
The inherent differences in healthcare structures could serve as a primary explanation for this difference [25, 28]. Both Germany and the Netherlands have well-established healthcare systems, however, they differ in important aspects [28]. Firstly, the density of inpatient care (number of cases), the average length of hospital stay, and bed occupancy rate were found to be significantly higher in Germany-all factors that could increase the risk of VRE transmission through increased patient-to patient and patient-to-healthcare professional (HCP) contact [28]. As the hospital environment is one of the key factors for VRE transmission via surfaces, a high occupancy rate in hospitals would also facilitate the spread of VRE [16]. In addition, high bed occupancy rates result in fewer single rooms available to isolate patients with VRE, making it challenging to implement adequate IPC rules in German hospitals [97]. In contrast, even pre-emptive isolation is implemented for at risk patients upon admission in Dutch hospitals [28, 98]. Secondly, despite the high number of hospitalizations and longer hospital stays, German hospitals suffer more compared to Dutch hospitals from a shortage of HCPs, resulting in understaffing, particularly in nursing care [28]. The interaction between patients and HCPs has a crucial role in VRE transmission, which may be one of the factors contributing to the high VRE prevalence in German hospitals, due to the low nurse-to-patient ratio [99].
Infection control guidelines
In addition to the differences in healthcare structure, there are also variations in the national German and Dutch IPC guidelines for the prevention of VRE in hospitals [25]. The frequency of MDROs in hospitals could serve as an indicator of the effectiveness of IPC measures. In Germany, the Commission for Hospital Hygiene and Infection Prevention (KRINKO, Kommission für Krankenhaushygiene und Infektionsprävention), and in the Netherlands the Infection Prevention Working Group (WIP, Werkgroep Infectie Preventie, Samenwerkingsverband Richtlijnen Infectiepreventie), issue these national IPC guidelines [98, 100, 101]. In general, while the application of IPC rules in the German guideline varies according to the epidemiological situation of the hospital and region, there is no such exception in the Dutch guideline. The KRINKO guidelines primarily focus on prevention of infections requiring antibiotic therapy, classifying patient groups according to their risk of evolving VRE infection, whereas the WIP guidelines recommend a search and detect strategy. For instance, in the WIP guidelines, there is no distinction between high-risk wards and normal-care wards in VRE screening, whereas the KRINKO guidelines recommend VRE screening only on patients in high-risk wards. The management of VRE carriers also differs in the two guidelines; the WIP guidelines recommend contact isolation without exception, but the KRINKO guidelines leaves the decision to clinicians, based on the patient's risk assessment. Thus, the stricter infection control rules applied in Dutch hospitals could contribute to the lower prevalence of VRE.
Antibiotic consumption
In addition to well-established IPC measures and the level of compliance with these measures, appropriate use of antibiotics plays a significant role in preventing colonization with VRE and, hence, infection [102]. For instance, the use of broad-spectrum cephalosporins has been linked to an increased VRE prevalence, both by facilitating the acquisition of VRE and by exerting high selective pressure on the gastrointestinal flora [103,104,105,106]. Data from the European Surveillance of Antimicrobial Consumption Network (ESAC-Net) from 1997 to 2020 indicate that the use of broad-spectrum cephalosporins in the community in Germany was higher than in the Netherlands [107]. Given this difference in the use of this particular antibiotic group between the two countries, it is possible that this will also have an impact on the difference in VRE prevalence observed between them.
Diagnostics
Apart from the aforementioned differences that have been outlined between the Netherlands and Germany, it is important to consider that variations in the diagnostic laboratory protocols, guidelines, and availability of resources for detecting VRE may also play a role in influencing the reported VRE cases in each country [64]. Variations in diagnostic protocols, including sample collection, culturing techniques, and antimicrobial susceptibility testing, can impact VRE detection. For example, variances in media and selective agents used for VRE isolation affect sensitivity and specificity [108]. Differences in the adoption and implementation of surveillance guidelines can also affect VRE detection and reporting, particularly in screening frequency and extent for VRE colonization in specific patient populations [98, 100]. Additionally, the availability of resources (financial, technological, and human) plays a significant role in a laboratory's capacity to detect VRE, with advanced technologies like PCR assays improving sensitivity and speed [109]. These factors can potentially impact the accuracy and thoroughness of VRE detection and reporting, thus contributing to variations in the reported number of VRE cases between the two countries.
Commonalities
Even though the general development in VRE epidemiology in the Netherlands and Germany differed substantially in the last decades, two common trends have emerged. The first trend is the potential impact of the COVID-19 pandemic on VRE epidemiology. Data from EARS-Net reports for 2020 and 2021 indicate that the number of VRE outbreaks and the proportion of VRE among all E. faecium isolates from clinical isolates have decreased in both countries compared to the previous year [110]. This decline could be due to an increased awareness of IPC measures among healthcare professionals and the disruption of healthcare services due to the COVID-19 pandemic. However, it is also possible that deprioritization of AMR surveillance in hospitals and less engagement to national surveillance systems may have led to an underestimation of actual situation.
The second trend is the change in the molecular epidemiology of VRE over time. In Germany, molecular typing analyses have been performed on all enterococci submitted to the NRC, while in the Netherlands, such analyses were only available for centrally collected enterococci between 2012 and 2018. Apart from the national surveillance data, identified publications illustrated that vanB began to be reported as the leading cluster both in the Netherlands and in Germany, since 2014 [39,40,41, 54, 56, 59]. This shift in molecular epidemiology has led to debate about whether this change is a result of an actual rise in the circulation of vanB strains or limitations in the detection of vanB-VRE in the laboratory [111]. Comparative studies have revealed that gradient strip assays and automated antibiotic susceptibility testing methods commonly used in the routine laboratory setting fail to detect vanB-mediated vancomycin resistance [112, 113]. EUCAST has also acknowledged these issues and revised recommendations to reduce the error rate in detecting vanB-VRE [114].
Limitations
There are limitations to this study. Firstly, a meta-analysis was not possible due to the heterogeneity in study design, patient populations, timeframes, and outcome definitions across the publications. Secondly, comparing the national surveillance data might cause biases owing to the changing number of participating hospitals and laboratories and different data collection compliance in the two countries. Thirdly, a comprehensive comparison of implementation and compliance to the national IPC guidelines at the hospital level was beyond the scope of the current study, disallowing us to compare the real-life records of hospital practice.
Conclusion
In conclusion, this review has provided an overview of the epidemiology of VRE in the hospital setting in the Netherlands and Germany, highlighting the potential causes for the difference in VRE prevalence between these neighboring countries. Given the increasing prevalence of VRE in Europe, we demonstrate that VRE remains a serious problem in healthcare and call for further research to understand the underlying factors driving the difference in VRE prevalence between countries to develop effective strategies to control the spread of VRE.
Availability of data and materials
Not applicable.
Abbreviations
- AMR:
-
Antimicrobial resistance
- BSI:
-
Blood-stream infection
- ARMIN:
-
Antimicrobial Resistance Monitoring in Lower Saxony
- ARS:
-
National Antimicrobial Resistance Surveillance
- CHARE-GD:
-
Comparison of healthcare structures, processes and outcomes in the Northern German and Dutch cross-border region
- EARS-Net:
-
European Antimicrobial Resistance Surveillance Network
- ESAC-Net:
-
European Surveillance of Antimicrobial Consumption Network
- HCP:
-
Healthcare professional
- ICU:
-
Intensive care unit
- IPC:
-
Infection prevention and control
- ISIS-AR:
-
Infectious Diseases Surveillance Information System for Antimicrobial Resistance
- MDRO:
-
Multidrug-resistant microorganism
- NICU:
-
Neonatal intensive care unit
- KISS:
-
Krankenhaus-Infektions-Surveillance-System (Hospital Infection Surveillance System from Germany)
- KRINKO:
-
Kommission für Krankenhaushygiene und Infektionsprävention (Commission for Hospital Hygiene and Infection Prevention in Germany)
- PICU:
-
Pediatric intensive care unit
- RIVM:
-
Rijksinstituut voor Volksgezondheid en Milieu (National Institute for Public Health and the Environment in the Netherlands)
- RKI:
-
Robert Koch Institute
- SARI:
-
Antibiotic use and Resistance in ICUs
- SSI:
-
Surgical site infection
- SO-ZI/AMR:
-
Signaleringsoverleg Zorginstellingen en Antimicrobiële Resistentie (The Early Warning and Intervention Meeting for Nosocomial Infections and Antimicrobial Resistance in the Netherlands)
- UTI:
-
Urinary tract infection
- VRE:
-
Vancomycin-resistant enterococci
- VREfm:
-
Vancomycin-resistant E. faecium
- VSE:
-
Vancomycin-susceptible enterococci
- WIP:
-
Werkgroep Infectie Preventie (Infection Prevention Working Group in the Netherlands)
- WHO:
-
World Health Organization
References
Cattoir V. The multifaceted lifestyle of enterococci: genetic diversity, ecology and risks for public health. Curr Opin Microbiol. 2021;65:73–80. https://doi.org/10.1016/j.mib.2021.10.013.
Gilmore MS, Lebreton F, van Schaik W. Genomic transition of enterococci from gut commensals to leading causes of multidrug-resistant hospital infection in the antibiotic era. Curr Opin Microbiol. 2013;16(1):10–6. https://doi.org/10.1016/j.mib.2013.01.006.
Hollenbeck BL, Rice LB. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence. 2012;3(5):421–33. https://doi.org/10.4161/viru.21282.
Faron ML, Ledeboer NA, Buchan BW. Resistance mechanisms, epidemiology, and approaches to screening for vancomycin-resistant enterococcus in the health care setting. J Clin Microbiol. 2016;54(10):2436–47. https://doi.org/10.1128/jcm.00211-16.
Werner G, Coque TM, Hammerum AM, Hope R, Hryniewicz W, Johnson A, et al. Emergence and spread of vancomycin resistance among enterococci in Europe. Euro Surveill. 2008;13(47):52.
Uttley AH, Collins CH, Naidoo J, George RC. Vancomycin-resistant enterococci. Lancet. 1988;1(8575–6):57–8. https://doi.org/10.1016/s0140-6736(88)91037-9.
Leclercq R, Derlot E, Duval J, Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988;319(3):157–61. https://doi.org/10.1056/nejm198807213190307.
Arthur M, Courvalin P. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob Agents Chemother. 1993;37(8):1563–71. https://doi.org/10.1128/aac.37.8.1563.
García-Solache M, Rice LB. The Enterococcus: a model of adaptability to its environment. Clin Microbiol Rev. 2019;32(2):522. https://doi.org/10.1128/cmr.00058-18.
Bender JK, Cattoir V, Hegstad K, Sadowy E, Coque TM, Westh H, et al. Update on prevalence and mechanisms of resistance to linezolid, tigecycline and daptomycin in enterococci in Europe: towards a common nomenclature. Drug Resist Updat. 2018;40:25–39. https://doi.org/10.1016/j.drup.2018.10.002.
Klare I, Fleige C, Geringer U, Thürmer A, Bender J, Mutters NT, et al. Increased frequency of linezolid resistance among clinical Enterococcus faecium isolates from German hospital patients. J Glob Antimicrob Resist. 2015;3(2):128–31. https://doi.org/10.1016/j.jgar.2015.02.007.
Schulte B, Heininger A, Autenrieth IB, Wolz C. Emergence of increasing linezolid-resistance in enterococci in a post-outbreak situation with vancomycin-resistant Enterococcus faecium. Epidemiol Infect. 2008;136(8):1131–3. https://doi.org/10.1017/s0950268807009508.
Krull M, Klare I, Ross B, Trenschel R, Beelen DW, Todt D, et al. Emergence of linezolid- and vancomycin-resistant Enterococcus faecium in a department for hematologic stem cell transplantation. Antimicrob Resist Infect Control. 2016;5:31. https://doi.org/10.1186/s13756-016-0131-6.
Werner G, Gfrörer S, Fleige C, Witte W, Klare I. Tigecycline-resistant Enterococcus faecalis strain isolated from a German intensive care unit patient. J Antimicrob Chemother. 2008;61(5):1182–3. https://doi.org/10.1093/jac/dkn065.
Monteserin N, Larson E. Temporal trends and risk factors for healthcare-associated vancomycin-resistant enterococci in adults. J Hosp Infect. 2016;94(3):236–41. https://doi.org/10.1016/j.jhin.2016.07.023.
Top J, Willems R, Bonten M. Emergence of CC17 Enterococcus faecium: from commensal to hospital-adapted pathogen. FEMS Immunol Med Microbiol. 2008;52(3):297–308. https://doi.org/10.1111/j.1574-695X.2008.00383.x.
Ford CD, Gazdik MA, Lopansri BK, Webb B, Mitchell B, Coombs J, et al. Vancomycin-resistant enterococcus colonization and bacteremia and hematopoietic stem cell transplantation outcomes. Biol Blood Marrow Transpl. 2017;23(2):340–6. https://doi.org/10.1016/j.bbmt.2016.11.017.
Bonten MJ, Willems R, Weinstein RA. Vancomycin-resistant enterococci: why are they here, and where do they come from? Lancet Infect Dis. 2001;1(5):314–25. https://doi.org/10.1016/s1473-3099(01)00145-1.
DiazGranados CA, Zimmer SM, Klein M, Jernigan JA. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin Infect Dis. 2005;41(3):327–33.
Kramer TS, Remschmidt C, Werner S, Behnke M, Schwab F, Werner G, et al. The importance of adjusting for enterococcus species when assessing the burden of vancomycin resistance: a cohort study including over 1000 cases of enterococcal bloodstream infections. Antimicrob Resist Infect Control. 2018;7:133. https://doi.org/10.1186/s13756-018-0419-9.
Salgado CD, Farr BM. Outcomes associated with vancomycin-resistant enterococci: a meta-analysis. Infect Control Hosp Epidemiol. 2003;24(9):690–8.
Carmeli Y, Eliopoulos G, Mozaffari E, Samore M. Health and economic outcomes of vancomycin-resistant enterococci. Arch Intern Med. 2002;162(19):2223–8.
Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–27. https://doi.org/10.1016/s1473-3099(17)30753-3.
European Centre for Disease Prevention and Control. Antimicrobial resistance in the EU/EEA (EARS-Net)-Annual Epidemiological Report 2019. Stockholm: ECDC; 2020
Gunnink LB, Arouri DJ, Jolink FEJ, Lokate M, de Jonge K, Kampmeier S, et al. Compliance to screening protocols for multidrug-resistant microorganisms at the emergency departments of two academic hospitals in the Dutch-German cross-border region. Trop Med Infect Dis. 2021;6(1):522. https://doi.org/10.3390/tropicalmed6010015.
Müller J, Voss A, Köck R, Sinha B, Rossen JW, Kaase M, et al. Cross-border comparison of the Dutch and German guidelines on multidrug-resistant Gram-negative microorganisms. Antimicrob Resist Infect Control. 2015;4:7. https://doi.org/10.1186/s13756-015-0047-6.
Dik JW, Sinha B, Friedrich AW, Lo-Ten-Foe JR, Hendrix R, Köck R, et al. Cross-border comparison of antibiotic prescriptions among children and adolescents between the north of the Netherlands and the north-west of Germany. Antimicrob Resist Infect Control. 2016;5:14. https://doi.org/10.1186/s13756-016-0113-8.
Köck R, Becker K, Idelevich EA, Jurke A, Glasner C, Hendrix R, et al. Prevention and control of multidrug-resistant bacteria in The Netherlands and Germany-the impact of healthcare structures. Int J Environ Res Public Health. 2020;17(7):522. https://doi.org/10.3390/ijerph17072337.
Keizer J, Braakman-Jansen LMA, Kampmeier S, Köck R, Al Naiemi N, Te Riet-Warning R, et al. Cross-border comparison of antimicrobial resistance (AMR) and AMR prevention measures: the healthcare workers’ perspective. Antimicrob Resist Infect Control. 2019;8:123. https://doi.org/10.1186/s13756-019-0577-4.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73. https://doi.org/10.7326/m18-0850.
NethMap: Report about consumption of antimicrobial agents and antimicrobial resistance among medically important bacteria in the Netherlands.
Epidemiologisches Bulletin. Aktuelle Daten und Informationen zu Infektionskrankheiten und Public Health. Robert Koch Institute.
ARS. Antibiotika-Resistenz-Surveillance in Deutschland. Available from: https://ars.rki.de/.
Timmers GJ, van der Zwet WC, Simoons-Smit IM, Savelkoul PH, Meester HH, Vandenbroucke-Grauls CM, et al. Outbreak of vancomycin-resistant Enterococcus faecium in a haematology unit: risk factor assessment and successful control of the epidemic. Br J Haematol. 2002;116(4):826–33. https://doi.org/10.1046/j.0007-1048.2002.03339.x.
van der Steen LF, Bonten MJ, van Kregten E, Harssema-Poot JJ, Willems R, Gaillard CA. Vancomycin-resistant Enterococcus faecium outbreak in a nephrology ward. Ned Tijdschr Geneeskd. 2000;144(53):2568–72.
Mascini EM, Troelstra A, Beitsma M, Blok HE, Jalink KP, Hopmans TE, et al. Genotyping and preemptive isolation to control an outbreak of vancomycin-resistant Enterococcus faecium. Clin Infect Dis. 2006;42(6):739–46.
Frakking FNJ, Bril WS, Sinnige JC, Klooster JEV, de Jong BAW, van Hannen EJ, et al. Recommendations for the successful control of a large outbreak of vancomycin-resistant Enterococcus faecium in a non-endemic hospital setting. J Hosp Infect. 2018;100(4):e216–25. https://doi.org/10.1016/j.jhin.2018.02.016.
Weterings V, van Oosten A, Nieuwkoop E, Nelson J, Voss A, Wintermans B, et al. Management of a hospital-wide vancomycin-resistant Enterococcus faecium outbreak in a Dutch general hospital, 2014–2017: successful control using a restrictive screening strategy. Antimicrob Resist Infect Control. 2021;10(1):38. https://doi.org/10.1186/s13756-021-00906-x.
Zhou X, Chlebowicz MA, Bathoorn E, Rosema S, Couto N, Lokate M, et al. Elucidating vancomycin-resistant Enterococcus faecium outbreaks: the role of clonal spread and movement of mobile genetic elements. J Antimicrob Chemother. 2018;73(12):3259–67. https://doi.org/10.1093/jac/dky349.
Lisotto P, Couto N, Rosema S, Lokate M, Zhou X, Bathoorn E, et al. Molecular characterisation of vancomycin-resistant Enterococcus faecium isolates belonging to the lineage ST117/CT24 causing hospital outbreaks. Front Microbiol. 2021;12(2741):52. https://doi.org/10.3389/fmicb.2021.728356.
Gast KB, van Oudheusden AJG, Murk JL, Stohr J, Buiting AG, Verweij JJ. Successful containment of two vancomycin-resistant Enterococcus faecium (VRE) outbreaks in a Dutch teaching hospital using environmental sampling and whole-genome sequencing. J Hosp Infect. 2021;5:63. https://doi.org/10.1016/j.jhin.2021.02.007.
Elsner HA, Sobottka I, Feucht HH, Harps E, Haun C, Mack D, et al. Nosocomial outbreak of vancomycin-resistant Enterococcus faecium at a German university pediatric hospital. Int J Hyg Environ Health. 2000;203(2):147–52. https://doi.org/10.1078/s1438-4639(04)70020-6.
Knoll M, Daeschlein G, Okpara-Hofmann J, Klare I, Wilhelms D, Wolf HH, et al. Outbreak of vancomycin-resistant enterococci (VRE) in a hematological oncology ward and hygienic preventive measures A long-term study. Onkologie. 2005;28(4):187–92. https://doi.org/10.1159/000084061.
Borgmann S, Niklas DM, Klare I, Zabel LT, Buchenau P, Autenrieth IB, et al. Two episodes of vancomycin-resistant Enterococcus faecium outbreaks caused by two genetically different clones in a newborn intensive care unit. Int J Hyg Environ Health. 2004;207(4):386–9. https://doi.org/10.1078/1438-4639-00304.
Borgmann S, Schulte B, Wolz C, Gruber H, Werner G, Goerke C, et al. Discrimination between epidemic and non-epidemic glycopeptide-resistant E. faecium in a post-outbreak situation. J Hosp Infect. 2007;67(1):49–55. https://doi.org/10.1016/j.jhin.2007.06.002.
Liese J, Schüle L, Oberhettinger P, Tschörner L, Nguyen T, Dörfel D, et al. Expansion of vancomycin-resistant Enterococcus faecium in an academic tertiary hospital in Southwest Germany: a large-scale whole-genome-based outbreak investigation. Antimicrob Agents Chemother. 2019. https://doi.org/10.1128/aac.01978-18.
Bender JK, Hermes J, Zabel LT, Haller S, Mürter N, Blank HP, et al. Controlling an unprecedented outbreak with vancomycin-resistant Enterococcus faecium in Germany, October 2015 to November 2019. Microorganisms. 2022;10(8):258. https://doi.org/10.3390/microorganisms10081603.
Nys S, Bruinsma N, Filius PM, van den Bogaard AE, Hoffman L, Terporten PH, et al. Effect of hospitalization on the antibiotic resistance of fecal Enterococcus faecalis of surgical patients over time. Microb Drug Resist. 2005;11(2):154–8. https://doi.org/10.1089/mdr.2005.11.154.
Guiot HF, Peetermans WE, Sebens FW. Isolation of vancomycin-resistant enterococci in haematologic patients. Eur J Clin Microbiol Infect Dis. 1991;10(1):32–4. https://doi.org/10.1007/bf01967094.
van den Braak N, Ott A, van Belkum A, Kluytmans JA, Koeleman JG, Spanjaard L, et al. Prevalence and determinants of fecal colonization with vancomycin-resistant Enterococcus in hospitalized patients in The Netherlands. Infect Control Hosp Epidemiol. 2000;21(8):520–4. https://doi.org/10.1086/501797.
Wendt C, Krause C, Xander LU, Löffler D, Floss H. Prevalence of colonization with vancomycin-resistant enterococci in various population groups in Berlin, Germany. J Hosp Infect. 1999;42(3):193–200. https://doi.org/10.1053/jhin.1999.0597.
Gruber I, Heudorf U, Werner G, Pfeifer Y, Imirzalioglu C, Ackermann H, et al. Multidrug-resistant bacteria in geriatric clinics, nursing homes, and ambulant care–prevalence and risk factors. Int J Med Microbiol. 2013;303(8):405–9. https://doi.org/10.1016/j.ijmm.2013.05.002.
Liss BJ, Vehreschild JJ, Cornely OA, Hallek M, Fätkenheuer G, Wisplinghoff H, et al. Intestinal colonisation and blood stream infections due to vancomycin-resistant enterococci (VRE) and extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBLE) in patients with haematological and oncological malignancies. Infection. 2012;40(6):613–9. https://doi.org/10.1007/s15010-012-0269-y.
Neumann B, Bender JK, Maier BF, Wittig A, Fuchs S, Brockmann D, et al. Comprehensive integrated NGS-based surveillance and contact-network modeling unravels transmission dynamics of vancomycin-resistant enterococci in a high-risk population within a tertiary care hospital. PLoS One. 2020;15(6):e0235160.
Bui MT, Rohde AM, Schwab F, Märtin N, Kipnis M, Boldt AC, et al. Prevalence and risk factors of colonisation with vancomycin-resistant Enterococci faecium upon admission to Germany’s largest university hospital. GMS Hyg Infect Control. 2021;16:52. https://doi.org/10.3205/dgkh000377.
Xanthopoulou K, Peter S, Tobys D, Behnke M, Dinkelacker AG, Eisenbeis S, et al. Vancomycin-resistant Enterococcus faecium colonizing patients on hospital admission in Germany: prevalence and molecular epidemiology. J Antimicrob Chemother. 2020;75(10):2743–51. https://doi.org/10.1093/jac/dkaa271.
Sommer L, Hackel T, Hofmann A, Hoffmann J, Hennebach E, Köpke B, et al. Multi-resistant bacteria in patients in hospitals and medical practices as well as in residents of nursing homes in saxony-results of a prevalence study 2017/2018. Gesundheitswesen. 2020. https://doi.org/10.1055/a-1138-0489.
Heininger A, Zimmermann S, Bootsveld C, Boutin S, Nurjadi D. Low prevalence of combined linezolid- and vancomycin-resistant Enterococcus faecium from hospital admission screening in an endemic region in Germany. J Glob Antimicrob Resist. 2020;22:646–50. https://doi.org/10.1016/j.jgar.2020.05.003.
Chhatwal P, Ebadi E, Thol F, Koenecke C, Beutel G, Ziesing S, et al. Prospective infection surveillance and systematic screening for vancomycin-resistant enterococci in hematologic and oncologic patients-findings of a German tertiary care center. J Glob Antimicrob Resist. 2020;22:102–5. https://doi.org/10.1016/j.jgar.2020.02.012.
Trautmannsberger I, Kolberg L, Meyer-Buehn M, Huebner J, Werner G, Weber R, et al. Epidemiological and genetic characteristics of vancomycin-resistant Enterococcus faecium isolates in a University Children’s Hospital in Germany: 2019 to 2020. Antimicrob Resist Infect Control. 2022;11(1):48. https://doi.org/10.1186/s13756-022-01081-3.
Messler S, Klare I, Wappler F, Werner G, Ligges U, Sakka SG, et al. Reduction of nosocomial bloodstream infections and nosocomial vancomycin-resistant Enterococcus faecium on an intensive care unit after introduction of antiseptic octenidine-based bathing. J Hosp Infect. 2019;101(3):264–71. https://doi.org/10.1016/j.jhin.2018.10.023.
Biehl LM, Higgins PG, Stemler J, Gilles M, Peter S, Dörfel D, et al. Impact of single-room contact precautions on acquisition and transmission of vancomycin-resistant enterococci on haematological and oncological wards, multicentre cohort-study, Germany, January-December 2016. Euro Surveill. 2022;27(2):522. https://doi.org/10.2807/1560-7917.Es.2022.27.2.2001876.
Zhou X, García-Cobos S, Ruijs G, Kampinga GA, Arends JP, Borst DM, et al. Epidemiology of extended-spectrum β-lactamase-producing E. coli and vancomycin-resistant Enterococci in the northern Dutch-German cross-border region. Front Microbiol. 2017;8:1914. https://doi.org/10.3389/fmicb.2017.01914.
Glasner C, Berends MS, Becker K, Esser J, Gieffers J, Jurke A, et al. A prospective multicentre screening study on multidrug-resistant organisms in intensive care units in the Dutch-German cross-border region, 2017 to 2018: the importance of healthcare structures. Euro Surveill. 2022. https://doi.org/10.2807/1560-7917.Es.2022.27.5.2001660.
Remschmidt C, Schröder C, Behnke M, Gastmeier P, Geffers C, Kramer TS. Continuous increase of vancomycin resistance in enterococci causing nosocomial infections in Germany- 10 years of surveillance. Antimicrob Resist Infect Control. 2018;7:54. https://doi.org/10.1186/s13756-018-0353-x.
Correa-Martínez CL, Jurke A, Schmitz J, Schaumburg F, Kampmeier S, Mellmann A. Molecular epidemiology of vancomycin-resistant Enterococci bloodstream infections in germany: a population-based prospective longitudinal study. Microorganisms. 2022. https://doi.org/10.3390/microorganisms10010130.
Gastmeier P, Schröder C, Behnke M, Meyer E, Geffers C. Dramatic increase in vancomycin-resistant enterococci in Germany. J Antimicrob Chemother. 2014;69(6):1660–4. https://doi.org/10.1093/jac/dku035.
Brinkwirth S, Martins S, Ayobami O, Feig M, Noll I, Zacher B, et al. Germany’s burden of disease of bloodstream infections due to vancomycin-resistant Enterococcus faecium between 2015–2020. Microorganisms. 2022. https://doi.org/10.3390/microorganisms10112273.
Aardema H, Arends JP, de Smet AM, Zijlstra JG. Burden of highly resistant microorganisms in a Dutch intensive care unit. Neth J Med. 2015;73(4):169–74.
Jones ME, Draghi DC, Thornsberry C, Karlowsky JA, Sahm DF, Wenzel RP. Emerging resistance among bacterial pathogens in the intensive care unit-a European and North American Surveillance study (2000–2002). Ann Clin Microbiol Antimicrob. 2004;3:14. https://doi.org/10.1186/1476-0711-3-14.
Kohlenberg A, Schwab F, Meyer E, Behnke M, Geffers C, Gastmeier P. Regional trends in multidrug-resistant infections in German intensive care units: a real-time model for epidemiological monitoring and analysis. J Hosp Infect. 2009;73(3):239–45. https://doi.org/10.1016/j.jhin.2009.07.017.
Remschmidt C, Behnke M, Kola A, Peña Diaz LA, Rohde AM, Gastmeier P, et al. The effect of antibiotic use on prevalence of nosocomial vancomycin-resistant enterococci- an ecologic study. Antimicrob Resist Infect Control. 2017;6:95. https://doi.org/10.1186/s13756-017-0253-5.
Hübner NO, Wegner C, Gleich S. Multidrug-resistant organisms and C. difficile in Munich acute-care clinics: results from a point prevalence study of clinical routine data. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58(11–12):1306–13. https://doi.org/10.1007/s00103-015-2248-9.
Meyer E, Ziegler R, Mattner F, Schwab F, Gastmeier P, Martin M. Increase of patients co-colonised or co-infected with methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium or extended-spectrum β-lactamase-producing Enterobacteriaceae. Infection. 2011;39(6):501–6. https://doi.org/10.1007/s15010-011-0154-0.
Remschmidt C, Schneider S, Meyer E, Schroeren-Boersch B, Gastmeier P, Schwab F. Surveillance of antibiotic use and resistance in intensive care units (SARI). Dtsch Arztebl Int. 2017;114(50):858–65. https://doi.org/10.3238/arztebl.2017.0858.
Kramer A, Ryll S, Wegner C, Jatzwauk L, Popp W, Hübner NO. One-day point prevalence of emerging bacterial pathogens in four secondary and five tertiary care German hospitals - results from a pilot study of the German Society for Hospital Hygiene (Deutsche Gesellschaft für Krankenhaushygiene, DGKH). GMS Krankenhhyg Interdiszip. 2011. https://doi.org/10.3205/dgkh000177.
Wegner C, Hübner NO, Gleich S, Thalmaier U, Krüger CM, Kramer A. One-day point prevalence of emerging bacterial pathogens in a nationwide sample of 62 German hospitals in 2012 and comparison with the results of the one-day point prevalence of 2010. GMS Hyg Infect Control. 2013. https://doi.org/10.3205/dgkh000212.
Huebner NO, Dittmann K, Henck V, Wegner C, Kramer A. Epidemiology of multidrug resistant bacterial organisms and Clostridium difficile in German hospitals in 2014: results from a nationwide one-day point prevalence of 329 German hospitals. BMC Infect Dis. 2016;16(1):467. https://doi.org/10.1186/s12879-016-1756-z.
Scharlach M, Wagner D, Dreesman J, Pulz M. Antimicrobial resistance monitoring in Lower Saxony (ARMIN): first trends for MRSA, ESBL-producing Escherichia coli and VRE from 2006 to 2010. Gesundheitswesen. 2011;73(11):744–7. https://doi.org/10.1055/s-0031-1291265.
Infectious Diseases Surveillance Information System for Antimicrobial Resistance (ISIS-AR) [October, 2021]. Available from: https://www.rivm.nl/isis-ar.
The Dutch Working Party on Antibiotic Policy (SWAB) [October, 2021]. Available from: https://swab.nl/en/nethmap-pvid369.
Signaleringsoverleg ziekenhuisinfecties en antimicrobiële resistentie (SO-ZI/AMR) [October, 2021]. Available from: https://www.rivm.nl/surveillance-van-infectieziekten/signalering-infectieziekten/signaleringsoverleg-zi-amr.
Zum Auftreten und zur Verbreitung glycopeptidresistenter Enterokokken-Update 2003/2004. Epidemiologisches Bull. 2005(17): 149–155.
Vancomycin-resistente Enterokokken in deutschen Krankenhäusern 2006/2007. Epidemiologisches Bull. 2008(23): 179–188.
ARS (Antibiotika-Resistenz-Surveillance in Deutschland), Datenbank: Resistenzübersicht E. faecium, E. faecalis; Blutkulturen bzw. ambulanter Bereich, 2000–2020 (https://ars.rki.de/).
Nationales Referenzzentrum (NRZ) für Staphylokokken und Enterokokken. Available from: https://www.rki.de/DE/Content/Infekt/NRZ/Staphylokokken/staphylo_node.html;jsessionid=81576F122A8322FA38F6904EFEE588CE.internet062.
Klare I, Bender JK, Werner G, Marktwart R, Reuss A, Sin MA, et al. Eigenschaften, Häufigkeit und Verbreitung von Vancomycin-resistenten Enterokokken in Deutschland. Epidemiologisches Bull. 2019;35:365–72.
Weber RE, Bender JK, Werner G, Noll I, Abu Sin M, Eckmanns T. Eigenschaften, Häufigkeit und Verbreitung von Vancomycin-resistenten Enterokokken (VRE) in Deutschland-update 2019/2020. Epidemiologisches Bulletin. 2021;27:32–42.
European Centre for Disease Prevention and Control. Antimicrobial resistance in the EU/EEA (EARS-Net) - Annual Epidemiological Report 2021. Stockholm: ECDC; 2022.
European Centre for Disease Prevention and Control, Annual surveillance reports on antimicrobial resistance. https://www.ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-disease-data/report.
European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe, 2020 data. Stockholm: 2021. Report No.
European Centre for Disease Prevention and Control (ECDC). Surveillance Atlas of Infectious Diseases. [March, 2023]. Available from: https://www.ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-disease-data/data-ecdc.
Werner G, Coque TM, Franz CM, Grohmann E, Hegstad K, Jensen L, et al. Antibiotic resistant enterococci-tales of a drug resistance gene trafficker. Int J Med Microbiol. 2013;303(6–7):360–79. https://doi.org/10.1016/j.ijmm.2013.03.001.
Boeing C, Correa-Martinez CL, Schuler F, Mellmann A, Karch A, Kampmeier S. Development and validation of a tool for the prediction of vancomycin-resistant Enterococci colonization persistence-the PREVENT score. Microbiol Spectr. 2021;9(2):e0035621. https://doi.org/10.1128/Spectrum.00356-21.
Weinstock DM, Conlon M, Iovino C, Aubrey T, Gudiol C, Riedel E, et al. Colonization, bloodstream infection, and mortality caused by vancomycin-resistant enterococcus early after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2007;13(5):615–21. https://doi.org/10.1016/j.bbmt.2007.01.078.
Alevizakos M, Gaitanidis A, Nasioudis D, Tori K, Flokas ME, Mylonakis E. Colonization with vancomycin-resistant enterococci and risk for bloodstream infection among patients with malignancy: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4(1):ofw246.
Zhou X, Willems RJL, Friedrich AW, Rossen JWA, Bathoorn E. Enterococcus faecium: from microbiological insights to practical recommendations for infection control and diagnostics. Antimicrob Resist Infect Control. 2020;9(1):130. https://doi.org/10.1186/s13756-020-00770-1.
Werkgroep Infectiepreventie. WIP-Richtlijn BRMO (Bijzonder Resistente Micro-Organismen) [ZKH]; RIVM: Bilthoven, The Nether- lands, 2013.
Jackson SS, Harris AD, Magder LS, Stafford KA, Johnson JK, Miller LG, et al. Bacterial burden is associated with increased transmission to health care workers from patients colonized with vancomycin-resistant Enterococcus. Am J Infect Control. 2019;47(1):13–7. https://doi.org/10.1016/j.ajic.2018.07.011.
Empfehlung der Kommission für Krankenhaushygiene und Infektionspr ̈avention (KRINKO) beim Robert Koch Institut. Hygienemaßnahmen zur Prävention der Infektion durch Enterokokken mit speziellen Antibiotikaresistenzen. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. (61):1310–61. https://doi.org/10.1007/s00103-018-2811-2.
Samenwerkingsverband Richtlijnen Infectiepreventie (SRI) [May, 2022]. Available from: https://www.sri-richtlijnen.nl/nieuws/kom-naar-kick-off-van-sri.
Austin DJ, Bonten MJ, Weinstein RA, Slaughter S, Anderson RM. Vancomycin-resistant enterococci in intensive-care hospital settings: transmission dynamics, persistence, and the impact of infection control programs. Proc Natl Acad Sci U S A. 1999;96(12):6908–13. https://doi.org/10.1073/pnas.96.12.6908.
McKinnell JA, Kunz DF, Chamot E, Patel M, Shirley RM, Moser SA, et al. Association between vancomycin-resistant Enterococci bacteremia and ceftriaxone usage. Infect Control Hosp Epidemiol. 2012;33(7):718–24.
Harbarth S, Cosgrove S, Carmeli Y. Effects of antibiotics on nosocomial epidemiology of vancomycin-resistant enterococci. Antimicrob Agents Chemother. 2002;46(6):1619–28. https://doi.org/10.1128/aac.46.6.1619-1628.2002.
Fridkin SK, Edwards JR, Courval JM, Hill H, Tenover FC, Lawton R, et al. The effect of vancomycin and third-generation cephalosporins on prevalence of vancomycin-resistant enterococci in 126 U.S. adult intensive care units. Ann Intern Med. 2001;135(3):175–83. https://doi.org/10.7326/0003-4819-135-3-200108070-00009.
Kritsotakis EI, Christidou A, Roumbelaki M, Tselentis Y, Gikas A. The dynamic relationship between antibiotic use and the incidence of vancomycin-resistant Enterococcus: time-series modelling of 7-year surveillance data in a tertiary-care hospital. Clin Microbiol Infect. 2008;14(8):747–54. https://doi.org/10.1111/j.1469-0691.2008.02026.x.
ECDC. European Surveillance of Antimicrobial Consumption Network (ESAC- Net) - Distribution of antimicrobial consumption by antimicrobial group. Available from: https://www.ecdc.europa.eu/en/antimicrobial-consumption/database/distribution-by-antimicrobial-group.
Boschert AL, Arndt F, Hamprecht A, Wolke M, Walker SV. Comparison of five different selective agar for the detection of vancomycin-resistant Enterococcus faecium. Antibiotics (Basel). 2023;12(4):558. https://doi.org/10.3390/antibiotics12040666.
Seo JY, Kim PW, Lee JH, Song JH, Peck KR, Chung DR, et al. Evaluation of PCR-based screening for vancomycin-resistant enterococci compared with a chromogenic agar-based culture method. J Med Microbiol. 2011;60:945–9. https://doi.org/10.1099/jmm.0.029777-0.
European Centre for Disease Prevention and Control (ECDC). Surveillance of antimicrobial resistance in Europe, 2020 data [February, 2022]. Available from: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2020.
Werner G, Neumann B, Weber RE, Kresken M, Wendt C, Bender JK. Thirty years of VRE in Germany-expect the unexpected: the view from the National Reference Centre for Staphylococci and Enterococci. Drug Resist Updat. 2020;53:100732. https://doi.org/10.1016/j.drup.2020.100732
Klare I, Bender JK, Fleige C, Kriebel N, Hamprecht A, Gatermann S, et al. Comparison of VITEK® 2, three different gradient strip tests and broth microdilution for detecting vanB-positive Enterococcus faecium isolates with low vancomycin MICs. J Antimicrob Chemother. 2019;74(10):2926–9.
Walker SV, Wolke M, Plum G, Weber RE, Werner G, Hamprecht A. Failure of Vitek2 to reliably detect vanB-mediated vancomycin resistance in Enterococcus faecium. J Antimicrob Chemother. 2021;76(7):1698–702.
EUCAST. Vancomycin susceptibility testing in Enterococcus faecalis and E. faecium using MIC gradient tests–a modified warning 21 May, 2019.
Acknowledgements
We gratefully acknowledge the support and cooperation with the CHARE-GD (Comparison of healthcare structures, processes and outcomes in the Northern German and Dutch cross-border region) Study Group. This study was conducted in partnership with the CrossBorder Institute of Healthcare Systems and Prevention (CBI), Groningen/Oldenburg.
Funding
This project is funded by the Ministry of Science and Culture of Lower Saxony (MWK) as part of the Niedersachsen ‘Vorab’ Program. (Grant Agreement No. ZN3831).
Author information
Authors and Affiliations
Contributions
CC, AH and CG designed the study. CC and MSB performed literature screening independently, study selection and data extraction. CC wrote the manuscript, which was critically reviewed and revised by MSB, AH, CG, EB, ML, AV, and AWF. All authors approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for Publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. The final applied search term.
Additional file 2
. Dataset presenting the extracted data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cimen, C., Berends, M.S., Bathoorn, E. et al. Vancomycin-resistant enterococci (VRE) in hospital settings across European borders: a scoping review comparing the epidemiology in the Netherlands and Germany. Antimicrob Resist Infect Control 12, 78 (2023). https://doi.org/10.1186/s13756-023-01278-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-023-01278-0