Abstract
Background
In intensive care unit (ICU) settings, the transmission risk of carbapenem-resistant, gram-negative bacteria (CRGNB) is high. There is a paucity of data regarding the effectiveness of interventions, including active screening, preemptive isolation, and contact precautions, to reduce transmission of CRGNB.
Methods
We conducted a pragmatic, cluster-randomized, non-blinded cross-over study in 6 adult ICUs in a tertiary care center in Seoul, South Korea. ICUs were randomly assigned to perform active surveillance testing with preemptive isolation and contact precautions (intervention) or standard precautions (control) during the initial 6-month study period, followed by a 1-month washout period. During a subsequent 6-month period, departments that used standard precautions switched to using interventional precautions and vice versa. The incidence rates of CRGNB were compared between the two periods using Poisson regression analysis.
Results
During the study period, there were 2268 and 2224 ICU admissions during the intervention and control periods, respectively. Because a carbapenemase-producing Enterobacterales outbreak occurred in a surgical ICU (SICU), we excluded admissions to the SICU during both the intervention and control periods and performed a modified intention-to-treat (mITT) analysis. In mITT analysis, a total of 1314 patients were included. The acquisition rate of CRGNB was 1.75 cases per 1000 person-days during the intervention period versus 3.33 cases per 1000 person-days during the control period (IRR, 0.53 [95% confidence interval (CI) 0.23–1.11]; P = 0.07).
Conclusions
Although this study was underpowered and showed borderline significance, active surveillance testing and preemptive isolation could be considered in settings with high baseline prevalence of CRGNB.
Trial registration Clinicaltrials.gov Identifier: NCT03980197.
Similar content being viewed by others
Introduction
Carbapenem-resistant, gram-negative bacteria (CRGNB), including Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterobacterales, have been leading causes of healthcare-associated infections and intensive care unit (ICU)-acquired infections [1]. In Korea, the proportion of carbapenem resistance rates in A. baumannii (CRAB) and P. aeruginosa (CRPA) have increased; in a 2015 surveillance program by the Korea Centers for Disease Control and Prevention, 85% of A. baumannii and 35% of P. aeruginosa were carbapenem-resistant [2]. In addition, carbapenem-resistant Enterobacterales (CRE) and carbapenemase-producing Enterobacterales have also increased exponentially [3]. Transmission of CRGNB is a great burden in hospitals because there are limited treatment options for CRGNB infections, and it has high morbidity and mortality. To prevent transmission of CRGNB, infection-control measures, including promotion of hand hygiene, environmental cleaning, and screening for carriers, have been implemented. However, there is limited evidence that screening for identification of CRGNB carriers is useful. For methicillin-resistant Staphylococcus aureus (MRSA), several studies found that screening and isolation were not effective for reducing its transmission [4] with good hand hygiene compliance and daily chlorhexidine-bathing. Thus, we aimed to evaluate the effectiveness of active surveillance testing for identifying CRGNB carriers to reduce its transmission in ICUs in the chlorhexidine-bathing era.
Methods
Study design
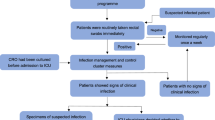
We conducted a pragmatic, cluster-randomized, nonblinded cross-over study in the included randomized ICUs between June 2019 and June 2020. We included 6 adult ICUs in a tertiary care hospital, Seoul, South Korea: two medical ICUs (23 beds), two surgical ICUs (26 beds), a cardiac ICU (16 beds), and a cardiothoracic surgery ICU (15 beds) in a tertiary care hospital. The study was approved by the physicians and nurse team leaders of each ICU and the institutional review board (IRB no. 2019–0274). The requirements for informed consent were waived. ICUs were randomly assigned to perform active surveillance testing (intervention) or use standard precautions (control) during the initial 6-month study period (period 1), followed by a 1-month washout period, and alternative during the second 6-month period (period 2). Randomization of ICU was performed by SPSS for Windows software, version 21 (SPSS Inc., Chicago, IL, USA). The microbiology laboratory processed surveillance specimens using standard culture-based identification of CRGNB. Patients with histories of CRGNB colonization or infection were placed under contact precautions at the time of admission.
Active surveillance and contact precautions
In the intervention period, stool or perirectal swabs for CRPA, CRAB, and CRE surveillance cultures and sputum, or endotracheal cultures for CRPA or CRAB, were obtained from patients within 2 days of their admission to the ICU and weekly thereafter. In the intervention period, preemptive isolation and contact precautions were implemented at admission, and if the initial surveillance test was negative, contact precautions were ceased, and standard precautions were continued. If the initial surveillance test or subsequent surveillances or clinical culture tests were positive for CRGNB, isolation and contact precautions were continued until 3 negative consecutive test results were obtained. In the control period, surveillance testing was not performed, and if clinical specimens were positive for CRGNB, contact precautions were implemented. During both the intervention and control periods, daily chlorhexidine-bathing was performed in all ICUs, and contact precautions were required in patients with MRSA and VRE colonization or infection. In period 2 (from April to June 2020), universal use of personal protective equipment (PPE) (gown, glove, KF94 mask, and face shield or goggle) was implemented for response to COVID-19 pandemic when caring patients in ICUs. During the whole study period, hand hygiene compliance was observed 4 times by a year by the infection control team staff, and the results by units were disclosed to all hospital staffs. Promotions for improving the compliance of hand hygiene included frequent monitoring and real-time feedback by infection control leader in ICU nursing team, and hospital-wide rewards given to the units with high hand hygiene compliance.
If outbreaks of CRGNB occurred, surveillance and post-outbreak surveillance in the control period were permitted.
Definition
An event was defined as a positive result for CRGNB from a clinical culture. The event date was the date of the earliest positive clinical culture. A patient was classified as having a new event if they had stayed in the ICU > 2 days, had no history of colonization or infection during the previous year, had no positive clinical culture within 2 days after admission to the ICU, and if admitted to an intervention ICU, a negative surveillance culture was obtained within 2 days of admission. Days at risk were calculated from the date of the third day in the ICU through the event date or the date two days after discharge from the ICU, whichever was later.
The primary outcome was the ICU-level incidence of new events per 1000 ICU patient-days at risk. Secondary ICU-level outcomes were the incidences of new events with CRPA, CRAB, or CRE calculated separately and the incidences of hospital-acquired bloodstream infections, catheter-related bloodstream infections, urinary tract infections, catheter-associated urinary tract infections, pneumonia, ventilator-associated pneumonia, and in-ICU mortality. We also performed subgroup analysis of individual ICUs for new events per 1000 ICU patient-days at risk. In addition, we compared new events per 1000 ICU patient-days at risk between periods 1 and 2. For the evaluation of economic impacts, we also compared the lengths of hospital and ICU stays and the costs of hospitalization between the intervention and control periods.
Outbreak was defined as ≥ 3 cases of acquisition of CRGNB within 2 weeks. If surveillance and post-outbreak surveillance were performed in the control period because of a CRGNB outbreak, we excluded the ICU in the modified intention-to-treat (mITT) analysis.
Statistical analysis
Based on the acquisition rate of CRGNB from 2016 to 2018 in ICUs of our hospital, we assumed a mean baseline incidence of CRGNB colonization or infection of 8 per 1000 patient-days; between-cluster variance would be 0.4, and the average amount of time a patient spent in the ICU would be 10 days. This study was designed to achieve 80% power for detecting a reduction in acquisition of 40% in the intervention period with a 2-sided type I error of 5%. According to these assumptions, the estimated sample size was 2400 patients (200 per cluster; a total of 12 clusters with one cross-over of 6 ICUs) [5].
Categorical variables were analyzed using the chi-square or Fisher’s exact test, as appropriate. Normally and non-normally distributed continuous variables were analyzed by Student’s t test and the Mann–Whitney U test, respectively. The primary analysis was a comparison of the primary outcomes between the intervention and control periods using an unadjusted Poisson regression model according to the mITT. All statistical analyses were performed using SPSS for Windows software, version 21 (SPSS Inc., Chicago, IL, USA) and MedCalc Statistical Software version 18.10.2 (MedCalc Software bvba, Sotend, Belgium) with P < 0.05 considered statistically significant.
Results
Characteristics of ICUs and patients
A total of 4492 admissions to the 6 ICUs occurred during the study period, and 1884 (42%) with ICU stays ≥ 3 days were enrolled in this study (Fig. 1). Two hundred and sixty patients in the intervention period and 98 patients in the control period were excluded for ITT analysis, respectively. A CRE outbreak occurred in SICU2 during the intervention period, and post-outbreak surveillance of CRE was performed in the control period; thus, we excluded the 212 patients admitted to SICU2 from the mITT analysis. The original and revised study designs are shown in Additional file 1: Figure S1. There were no significant differences in characteristics between patients in the intervention period and those in the control period (Table 1). During the total study period, the observed hand hygiene compliance was 96%. The number of clinical specimens submitted to the laboratory was not different between intervention and control periods in mITT analysis (mean [IQR], 3634 [2824–5568] in intervention period vs. 2767 [1902–4378] in control period; P = 0.35).
Results of the acquisition rate of CRGNB according to clinical culture
In the mITT analysis, the acquisition rate of CRGNB was 1.75 cases per 1,000 person-days in the intervention period versus 3.33 cases per 1000 person-days in the control period (incidence rate ratio [IRR], 0.53; 95% confidence interval [CI] 0.23–1.11; P = 0.07) (Table 2).
Secondary outcomes
There were no significant differences in the acquisition rates of CRPA, CRAB, and CRE in the intervention and control periods (CRPA, 0.32 vs. 1.07 per 1,000 person-days; IRR, 0.30 [95% CI 0.03–1.50]; P = 0.10; CRAB, 0.80 vs. 1.73 per 1000 person-days; IRR, 0.46 [95% CI 0.13–1.37]; P = 0.13; CRE, 0.80 vs. 0.93; IRR, 0.85 [95% CI 0.21–3.12]; P = 0.79) (Table 2). In addition, there were no significant differences in the rates of hospital-acquired bloodstream infections, catheter-related bloodstream infections, urinary tract infections, catheter-associated urinary tract infections, pneumonia, ventilator-associated pneumonia, and in-ICU mortality (Table 3).
The subgroup analysis of the CRGNB acquisition rate by ICU is shown in Additional file 1: Table S1. The acquisition rates of CRGNB were significantly higher in the control period than in the intervention period in MICU2, SICU1, and the cardiac ICU, while the rates were higher in the intervention period than in the control period in MICU1 and SICU2; there was no difference between the rates in the intervention and control periods in the cardiothoracic surgery ICU. In ITT analysis, the acquisition rate of CRGNB was 2.94 cases per 1000 person-days in the intervention period versus 3.46 cases per 1000 person days in the control period (IRR, 0.85; 95% CI 0.46–1.54; P = 0.56) (Additional file 1: Table S2).
The acquisition rate of CRGNB was significantly higher in period 1 than in period 2 (3.68 cases per 1000 person-days vs. 0.52 cases per 1000 person-days; P < 0.001) (Additional file 1: Table S3).
Of 104 patients who admitted to ICU during the intervention period but did not perform surveillance culture within 2 days after ICU admission, 15 were admitted to SICU2. We compared the baseline characteristics of the remaining 89 patients and those enrolled in intervention group of mITT analysis (n = 590) (Additional file 1: Table S4). Solid organ transplant recipient (16.9% vs. 5.8%, P < 0.001) and patients with end-stage renal disease (12.4% vs. 5.6%, P = 0.01) were more common in patients without surveillance culture than in intervention group of mITT analysis. Patients with solid cancer was less common in those without surveillance culture than in intervention group of mITT analysis (12.4% vs. 24.2%, P = 0.01). The type of ICU was significantly different between two groups (P < 0.001), which reflects the difference of compliance of study protocol by ICUs.
In mITT analysis, 39 (7%) cases in intervention period were detected in surveillance culture. Of these, 31 were detected in surveillance culture only, and 8 were detected in both surveillance and clinical culture. Five were detected in surveillance culture earlier than in clinical culture. Therefore, 36 (6%) were actually detected in surveillance culture (only or earlier than in clinical culture).
Evaluation of the economic impact of active surveillance testing
For the economic impact evaluation of active surveillance testing of CRGNB, we compared the lengths of hospital and ICU stays and the total cost of hospitalization between the intervention and control periods in the mITT population (Table 4). The mean length of hospital stays was 0.9 day shorter in the intervention period than in the control period, but the difference was not statistically significant (P = 0.73). Although there was an additional cost of $5666 for hospitalization in the intervention period than in the control period, the cost difference was also not statistically significant (P = 0.43).
Discussion
This pragmatic, cluster-randomized, cross-over study showed that active surveillance testing to identify patients colonized with CRGNB was associated with non-statistically significant decrease in the acquisition of CRGNB in clinical specimens.
Early detection of patients colonized or infected with CRGNB is important for implementing timely interventions to prevent subsequent spread. However, active surveillance testing is a complicated and resource-intensive intervention that has the potential for several adverse consequences, including reduced contact between healthcare workers and patients due to contact precautions [6]. Previous studies have reported that active surveillance testing in combination with contact precautions for colonized patients contributed to the decline of MRSA or vancomycin-resistant Enterococcus [7,8,9]. However, there is limited evidence that active surveillance testing is associated with reducing CRGNB transmission, but many hospitals have implemented active surveillance testing for identifying CRGNB. Recent studies showed that screening and isolation of colonized patients do not reduce multidrug-resistant bacteria, especially MRSA, when compliance with hand hygiene and chlorhexidine-bathing is high [10,11,12,13]. We conducted this study to provide evidence of the effectiveness of active surveillance in the chlorohexidine-bathing and high hand hygiene compliance era. Our study had low power because only 79% (1884/2400) of patients were enrolled in the target sample size, and the baseline acquisition rate was lower than expected. Although it had borderline significance, we showed that about half of CRGNB acquisition can be prevented through active surveillance testing. Therefore, this strategy may be beneficial in high-baseline-prevalence settings. A large, multicenter study is needed to confirm our findings.
The incidence of CRGNB acquisition was higher in the period 1 (before COVID-19 pandemic) than in period 2 (after COVID-19 pandemic). The lower acquisition rate in period 2 may be associated with additional infection prevention measure to respond COVID-19 pandemic, especially universal donning of gown and glove. Previous cluster randomized study showed that universal glove and gown use was associated with decrease in acquisition of antibiotic-resistant gram-negative bacteria, although it was not statistically significant [14]. As we excluded SICU2 in the analysis, more patients were allocated to the control group in the first period, and we may overestimate the positive effect of the intervention. Further study for evaluating the effectiveness of active surveillance in the setting of identical infection prevention measures between intervention and control period is needed.
In our subgroup analysis, the CRGNB acquisition rate was higher in the intervention period than in the control period in MICU1 and SICU2. Although a CPE outbreak occurred in SICU2, there was no outbreak of CRGNB in MICU1, and the reason for this unanticipated finding is unclear. It may be a seasonal effect, or more enhanced environmental cleaning might have been performed during the control period due to the COVID-19 pandemic, which occurred in the latter 6-month period (Additional file 1: Table S3).
The acquisition rates of CRPA, CRAB, and CRE did not differ between the intervention and control periods. Because this trial was designed to identify the effectiveness of reducing the total CRGNB acquisition rate, further study is needed to identify the effectiveness of active surveillance testing for each organisms.
Our study has some limitations. First, it was a single-center study with a low prevalence of CRGNB. A multicenter study with variable prevalences of CRGNB is warranted to generalize our findings. Despite this limitation, well-monitored infection control practices and policies to minimize unmeasured confounding factors by different centers during the study period is a strength of our study. Second, as this study was not blinded, the difference in the number of clinical specimens submitted to the laboratory may be present, and this may have affected the chance to detect CRGNB. However, the number of clinical specimen was not different between intervention and control periods in mITT analysis. Third, we did not perform surveillance testing during the control period, which may have biased our findings. However, we performed a pragmatic trial that reflects actual clinical practices, and we evaluated the outcomes of CRGNB acquisition in clinical specimens. Fourth, data regarding immunosuppressant use was absent. Use of immunosuppressant is associated with exposure to antimicrobials and acquisition of MDR gram-negative organism [15,16,17]. However, there was no significant difference of the recent antibiotics exposure between the intervention period and the control period. Therefore, this limitation may not substantially affect our main findings. Finally, we performed a conventional culture method for active surveillance, and the turnaround time is longer than rapid PCR testing. Therefore, further study to evaluate active surveillance testing using PCR testing is needed.
Conclusions
In conclusion, active surveillance testing for CRGNB may reduce its acquisition in clinical specimens in the ICU without additional costs. Individual hospitals should consider the cost-effectiveness of the intervention based on the baseline acquisition rate of CRGNB and the cost of intervention when they decide whether to adopt active surveillance testing.
Availability of data and materials
Data and materials are not available.
References
Tacconelli E, Cataldo MA, Dancer SJ, De AG, Falcone M, Frank U, et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014;20(S1):1–55.
Kim D, Ahn JY, Lee CH, Jang SJ, Lee H, Yong D, et al. Increasing resistance to extended-spectrum cephalosporins, fluoroquinolone, and carbapenem in gram-negative bacilli and the emergence of carbapenem non-susceptibility in Klebsiella pneumoniae: analysis of Korean Antimicrobial Resistance Monitoring System (KARMS) data from 2013 to 2015. Ann Lab Med. 2017;37(3):231–9.
Lee E, Lee S, Bahk H, Kim S, Lee H. Analysis of carbapenemase-producing Enterobacteriaceae (CPE) surveillance results for 2017 in Korea: Comparison with the surveillance results of the previous 5 years (2012–2016). Korea Disease Control and Prevention Agency. Public Health Weekly Report. Available at: http://www.kdca.go.kr/board/board.es?mid=a20602010000&bid=0034&list_no=141909&act=view. Accessed 22 Nov 2018.
Fätkenheuer G, Hirschel B, Harbarth S. Screening and isolation to control meticillin-resistant Staphylococcus aureus: sense, nonsense, and evidence. Lancet. 2015;385(9973):1146–9.
Reich NG, Myers JA, Obeng D, Milstone AM, Perl TM. Empirical power and sample size calculations for cluster-randomized and cluster-randomized crossover studies. PLoS ONE. 2012;7(4): e35564.
Diekema DJ, Edmond MB. Look before you leap: active surveillance for multidrug-resistant organisms. Clin Infect Dis. 2007;44:1101–7.
Jernigan JA, Clemence MA, Stott GA, Titus MG, Alexander CH, Palumbo CM, et al. Control of methicillin-resistant Staphylococcus aureus at a university hospital: one decade later. Infect Control Hosp Epidemiol. 1995;16:686–96.
Muto CA, Jernigan JH, Ostrowsky BE, Richet HM, Jarvis WR, Boyce JM, et al. SHEA guideline Active surveillance cultures are essential to identify the reservoir for spread of MRSA and VRE infections and make control possible using the CDC’s long-recommended contact precautions. Infect Control Hosp Epidemiol. 2003;24(5):362–86.
Ostrowsky BE, Trick WE, Sohn AH, Quirk SB, Holt S, Carson LA, et al. Control of vancomycin-resistant enterococcus in health care facilities in a region. N Engl J Med. 2001;344:1427–33.
Troche G, Joly LM, Guibert M, Zazzo JF. Detection and treatment of antibiotic-resistant bacterial carriage in a surgical intensive care unit: a 6-year prospective survey. Infect Control Hosp Epidemiol. 2005;26:161–5.
Nijssen S, Bonten MJ, Weinstein RA. Are active microbiological surveillance and subsequent isolation needed to prevent the spread of methicillin-resistant Staphylococcus aureus? Clin Infect Dis. 2005;40:405–9.
Derde LPG, Cooper BS, Goossens H, Malhotra-Kumar S, Willems RJL, Gniadkowski M, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomised trial. Lancet Infect Dis. 2014;14:31–9.
Huskins WC, Huckabee CM, O’Grady NP, Murray P, Kopestskie H, Zimmer L, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011;364:1407.
Harris AD, Morgan DJ, Pineles L, Magder L, O’Hara LM, Johnson JK. Acquisition of antibiotic-resistant gram-negative bacteria in the Benefits of Universal Glove and Gown (BUGG) Cluster randomized trial. Clin Infect Dis. 2021;72(3):431–7.
Pouch SM, Patel G, AST Infectious Disease Community of Practice. Multidrug-resistant Gram-negative bacterial infections in solid organ transplant recipients—Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13594.
Aguilar-Guisado M, Espigado I, Martin’Pena A, Gudiol C, Royo-Cebrecos C, Falantes J, et al. Optimisation of empirical antimicrobial therapy in patients with haematological malignancies and febrile neutropenia (How Long study): an open-label, randomized, controlled phase 4 trial. Lancet Haematol. 2017;4(12):e573-83.
Anderson DJ, Jenkins TC, Evans SR, Harris AD, Weinstein RA, Tamma PD, Han JH, et al. The role of stewardship in addressing antibacterial resistance: stewardship and infection control committee of the antibacterial resistance leadership group. Clin Infect Dis. 2017;64:S36-40.
Funding
This work was supported by grants from the research fund donation for COVID-19 research to Asan Medical Center by Kyu-Kang Cho.
Author information
Authors and Affiliations
Contributions
JJ and SHK designed the study. JJ, JHP, and HY collected and analyzed the data. JJ wrote the main manuscript text and JHP prepared figure. YJL, EOK, CML, MNK, MWJ, SCY, and SHK reviewed and revised the manuscript. This study was presented in part in ASM Microbe, 2022, Jun 12, Washington DC (Abstract number CIV-2578). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the physicians and nurse team leaders of each ICU and the institutional review board (IRB no. 2019–0274). The requirements for informed consent were waived.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests as defined by BMC, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Supplemental Figure and Tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jung, J., Park, J.H., Yang, H. et al. Active surveillance testing to reduce transmission of carbapenem-resistant, gram-negative bacteria in intensive care units: a pragmatic, randomized cross-over trial. Antimicrob Resist Infect Control 12, 16 (2023). https://doi.org/10.1186/s13756-023-01222-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-023-01222-2