Abstract
Background
We investigated the trend change in vancomycin-intermediate Staphylococcus aureus (VISA)/heterogeneous VISA (hVISA) prevalence among methicillin-resistant S. aureus (MRSA) bacteremia strains and antistaphylococcal antibiotic use together with mutation studies of vancomycin resistance-related gene loci to evaluate the impact of changes in antibiotic use after new antistaphylococcal antibiotics became available.
Methods
Among 850 healthcare-associated MRSA isolates from 2006 to 2019 at a tertiary hospital in South Korea, hVISA/VISA was determined by modified PAP/AUC analysis, and the identified hVISA/VISA strains were genotyped. Gene mutations at vraSR, graSR, walKR, and rpoB were studied by full-length sequencing. Antistaphylococcal antibiotic use in 2005–2018 was analyzed.
Results
Two VISA and 23 hVISA strains were identified. The prevalence rate ratio of hVISA/VISA carrying mutations at the two-component regulatory systems among MRSA was 0.668 (95% CI 0.531–0.841; P = 0.001), and the prevalence rate ratio of hVISA/VISA carrying rpoB gene mutations was 1.293 (95% CI 0.981–1.702; 174 P = 0.068). Annual vancomycin use density analyzed by days of therapy (DOT) per 1,000 patient-days did not decrease significantly, however the annual average length of time analyzed by the number of days vancomycin was administered for each case showed a significantly decreasing trend.
Conclusions
During the 14-year period when the average length of vancomycin therapy decreased every year with the availability of alternative antibiotics, the prevalence of hVISA/VISA did not decrease significantly. This seems to be because the resistant strains carrying the rpoB mutations increased despite the decrease in the strains carrying the mutations at the two-component regulatory systems.
Similar content being viewed by others
Introduction
Since Staphylococcus aureus with reduced vancomycin susceptibility has emerged, successful treatment with vancomycin for methicillin-resistant S. aureus (MRSA) infection has been challenging [1, 2]. Reduced vancomycin susceptibility could present in the whole MRSA population (vancomycin-intermediate S. aureus, VISA) or subpopulations (heterogeneous VISA, hVISA), and VISA/hVISA infections frequently have been associated with vancomycin failure or persistent infection [2,3,4,5].
The VISA/hVISA phenotypes are associated with mutations in the vraSR (vancomycin resistance-associated sensor/regulator), graSR (glycopeptide resistance-associated sensor/regulator), and walKR (sensor protein kinase/regulator) genes of two-component systems that function during cell-wall synthesis [6, 7]. Mutations in the rifampin resistance-determining region of rpoB have also been reported to be associated with emergence of VISA/hVISA [8, 9]. Additionally, prolonged exposure to vancomycin was associated with VISA/hVISA phenotype and cell-wall thickening caused by these mutations [2, 10, 11].
The prevalence of VISA and hVISA over the past decade has increased [5, 12, 13]; however, prevalence varies by region, country, and medical institution [13,14,15]. Furthermore, antistaphylococcal agents that can replace vancomycin, including linezolid, daptomycin, and tigecycline, have been available since 2002, and studies on how reduction in vancomycin use affected the prevalence of VISA/hVISA are lacking.
In this study, we investigated the trend change in VISA/hVISA prevalence among MRSA bacteremia strains and antistaphylococcal antibiotic use together with mutation studies of vancomycin resistance-related gene loci to evaluate the impact of changes after new antistaphylococcal antibiotics became available.
Materials and methods
Bacterial strains and susceptibility testing
We collected all MRSA blood isolates from 2006 to 2019 at Samsung Medical Center (SMC, Seoul, Korea), a large tertiary referral hospital at which more than 70% of patients were referred from other regions across the country. If MRSA were isolated multiple times from one patient, the first MRSA isolate was collected. A total of 984 non-duplicate MRSA isolates were collected. Hospital-onset strain was defined as strain collected from a positive blood culture taken at least 48 h after admission to hospital. Community-onset strain was further classified as SMC-specific healthcare-associated (HCA), HCA (other facilities), or community-associated. SMC-specific HCA infection was defined as an infection that meets any of the following: hospitalization or surgery at SMC within the year preceding the admission; history of hemodialysis at SMC within 30 days before admission; history of catheterization at SMC within 30 days before admission. Finally 850 SMC-associated MRSA strains were included (Additional file 1: Fig. S1).
In vitro antimicrobial susceptibility testing was performed by a broth microdilution method according to Clinical and Laboratory Standards Institute (CLSI) guidelines [16]. S. aureus ATCC 29213 and Enterococcus faecium ATCC 29212 were used as control strains. This study was approved by the Institutional Review Board (IRB) of SMC.
VISA/hVISA screening and confirmation
All isolates were screened for VISA or hVISA strains as previously described [17]. Four 10-µl droplets from 0.5 McFarland suspensions were dropped onto brain heart infusion (BHI) agar supplemented with 4 µg/ml (BHI-V4 medium) vancomycin. Plates were incubated for 24–48 h at 37ºC, and individual colonies in each droplet were counted. An isolate was considered VISA/hVISA if at least one droplet contained two or more colonies.
Modified population analysis profile/area under the curve (PAP/AUC) analysis was performed as described previously [17, 18]. Briefly, overnight cultures were grown in trypton soy broth and diluted in saline to 10–2 and 10–5. Each dilution was aliquoted using a spiral dispenser (Interscience, St. Nom, France) onto BHI agar plates containing 0, 0.5, 1, 2, 3, and 4 mg/L vancomycin. Colonies were counted after a 48-h incubation at 37ºC. Bacterial colony counts were determined using the Scan 500 (Interscience). PAP/AUC was calculated using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA). The AUCtest/AUCMu3 ratio was calculated, and strains were determined to be vancomycin-susceptible S. aureus (VSSA), hVISA, or VISA according to ratio: VSSA, < 0.9; hVISA, 0.9–1.3; VISA, > 1.3. Mu3 strain (hVISA, ATCC700698), Mu50 (VISA, ATCC700699), and S. aureus ATCC29213 (VSSA) were used as controls.
Genotypic characterization of hVISA/VISA
The SMC-associated hVISA/VISA strains were characterized by multilocus sequence typing (MLST), Staphylococcal Cassette Chromosome mec (SCCmec) typing, spa typing, agr typing, and pulsed-field gel electrophoresis (PFGE). MLST was carried out, and sequence types (STs) were assigned by reference to the S. aureus MLST website (http://saureus.mlst.net) [19].The SCCmec type was profiled by multiplex PCR assay [20]. The spa type was determined by PCR sequencing of the repeat region of the S. aureus protein, as described previously [21]. The agr specificity group was determined by PCR using specific primers as described previously [22]. Clonal relationships were determined by PFGE after DNA digestion by SmaI, as previously described [23]. PFGE patterns were analyzed with GelCompar version 6.6 (Applied Maths, Austin, Texas, USA) using the Dice coefficient and were represented by the unweighted pair-grouping method using arithmetic averages (UPGMA) with 1.7 tolerance and 1.2% optimization settings. Results were interpreted using a cut-off point of 80%.
Mutation detection in two-component regulatory systems and rpoB gene
Full-length forward and reverse sequences were obtained for vraSR, graSR, walKR, and rpoB from PCR-amplified fragments using the primers shown in Additional file 2: Table S1 [10, 24, 25]. Sequences were aligned and compared to the reference genome N315 (GenBank accession number BA000018). Sequence data were analyzed using EDITSEQ and MEGALIGN software (DNASTAR, Inc., Madison, WI, USA).
Analysis of antibiotic use
Antibiotic use density rates of vancomycin, teicoplanin, linezolid, and tigecycline for the period from 2005–2018 were calculated annually as days of therapy (DOT)/1,000 patient-days, an index provided by the hospital’s data warehouse for antibiotic use monitoring: SMC Antibiotic Use Guard (SMC ANTIBUG). Additionally, the number of consecutive prescription days or length of therapy (LOT) of vancomycin per prescription event was calculated to estimate the burden of continuous vancomycin exposure. Because patients with decreased creatinine clearance were not administered vancomycin daily, an interval of ≤ 4 days between doses of vancomycin was considered as the same prescription event.
Statistical analyses
Annual prevalence rates of hVISA/VISA among MRSA were analyzed using Poisson regression. Prevalence rates of hVISA/VISA strains carrying mutations at the two-component systems among MRSA and the strains carrying rpoB gene mutation were also determined, respectively. Linear regression analysis was performed to evaluate trend change in antimicrobial use over time, with year as the independent variable. All P-values were two-tailed, and P < 0.05 was considered statistically significant. All statistical analyses were performed using R software version 3.4.4 (Vienna, Austria; http://www.R-project.org).
Results
Changes in prevalence of hVISA/VISA over time
When all 984 MRSA strains were included for analysis, 4 (0.4%) were VISA and 27 (2.7%) were hVISA. The annual prevalence rate of hVISA/VISA decreased by 10% {prevalence rate ratio 0.907 [95% confidence interval (CI) 0.823–0.997; P = 0.042]} (Fig. 1a). The prevalence rate ratio of hVISA/VISA carrying mutations at the two-component regulatory systems among MRSA was 0.732 (95% CI 0.619–0.868; P < 0.001), and the prevalence rate ratio of hVISA/VISA carrying rpoB gene mutations among MRSA was 1.066 (95% CI 0.890–1.276; P = 0.488) (Fig. 1b, c). When only 850 SMC-associated MRSA strains were analyzed, the annual prevalence rate ratio of hVISA (23 strains)/VISA (two strains) among MRSA was 0.923 (95% CI 0.830–1.027; P = 0.142) (Fig. 1d). The prevalence rate ratio of hVISA/VISA carrying mutations at the two-component systems among MRSA was 0.668 (95% CI 0.531–0.841; P = 0.001), and the prevalence rate ratio of hVISA/VISA carrying rpoB gene mutations among MRSA was 1.293 (95% CI 0.981–1.702; P = 0.068) (Fig. 1e, f).
Annual trend in number of hVISA/VISA and hVISA/VISA proportions among MRSA blood isolates. a Analysis of all 984 MRSA strains of bacterial collection. b hVISA/VISA carrying mutations at the two-component regulatory systems among 984 MRSA strains. c hVISA/VISA carrying mutations at the rpoB gene loci among 984 MRSA strains. d analysis of 850 strains of MRSA related to the Samsung Medical Center. e hVISA/VISA carrying mutations at the two-component systems among 850 MRSA strains. f hVISA/VISA carrying mutations at the rpoB gene loci among 850 MRSA strains. VISA, vancomycin-intermediate Staphylococcus aureus; hVISA, heterogeneous VISA; MRSA, methicillin-resistant S. aureus
None of the 25 hVISA/VISA strains were resistant to linezolid and tigecycline, and the rates of resistance to ciprofloxacin, clindamycin, erythromycin, tetracycline, and gentamicin were 92.0%, 80.0%, 92.0%, 84.0%, and 76.0%, respectively. Resistance rates to rifampin and trimethoprim-sulfamethoxazole were 24.0% and 24.0%, respectively (Additional file 2: Table S2).
Genotypic characteristics of hVISA/VISA strains
Among 25 SMC-associated hVISA/VISA strains from 2006–2019, the most frequent genotype was ST5-SCCmec II (n = 20, 80.0%), followed by ST72-SCCmec IVA (n = 4, 16.0%) and ST239-SCCmec III (n = 1, 4.0%; Table 1, Additional file 3: Fig. S2). Among ST5 hVISA/VISA strains, the most frequent spa type was t2460 (n = 15, 75.0%), followed by t002 (n = 3, 15.0%) and t9353 (n = 2, 10.0%). There were three spa types in ST72-SCCmec IVA: t324 (n = 2, 50.0%), t148 (n = 1, 25.0%), and t664 (n = 1, 25.0%). Analysis of the PFGE band patterns for the 25 strains revealed 11 pulsotypes, with a Dice coefficient cut off of 80% (Additional file 3: Fig. S2). Even strains of the same pulsotype did not belong to cases that occurred at the same time in the same ward.
Frequency of single-nucleotide polymorphism (SNP) in vraSR, graSR, walKR, and rpoB
Multiple SNPs were identified in vraSR, graSR, walKR, and rpoB (Table 1). Mutations of the two-component regulatory systems or rpoB loci were confirmed in 16 (64.0%) of 25 hVISA/VISA strains. One of two VISA strains showed distinct mutations in vraR (E59D, E127K), graS (T224I), and rpoB (H481Y). Among hVISA, 10 of 23 strains (43.5%) showed distinct mutations in vraR (A113V, E59D, E87A), vraS (E99K, E117G), graR (D148Q), graS (I59L, L26F, T224I), and walK (A468T, A400V). Mutations in rpoB (A477D, H481Y, P475S, T518S) were found in 6 of 23 hVISA strains (26.1%). Mutations were observed most frequently in vraSR loci, followed by rpoB and graSR loci. Multiple mutations of the vraSR, graSR, and walKR loci were observed only in hVISA/VISA strains until 2011, whereas mutations in rpoB were observed only in hVISA/VISA strains collected in 2011 and later (Fig. 2).
Time trends of antimicrobial use
During the study period (2005–2018), annual vancomycin use density in DOT/1,000 patient-days did not show any trend (Fig. 3a). Although annual teicoplanin use showed a significantly decreasing trend (P = 0.001), annual glycopeptide use did not. In contrast, annual linezolid and tigecycline use, which could be prescribed alternatively for infections by Gram-positive bacteria including MRSA, showed a significantly increasing trend during the study period (Fig. 3b). In contrast to the vancomycin use density calculated by DOT/1,000 patient-days, annual average length of vancomycin therapy in individual cases showed a significantly decreasing trend over time (Fig. 4).
Discussion
Our study revealed that annual hVISA/VISA prevalence rates among MRSA bacteremia strains significantly decreased during the 14-year period, however the prevalence rates did not show a significant decrease when limited to the strains only from the SMC-specific HCA infections, except for cases transferred from the other hospitals/chronic care facilities and community-associated cases. Instead, subgroup analysis showed that only the hVISA/VISA strains showing the mutations in the two-component regulatory systems showed a significant decrease, whereas the rpo-B mutant strain tended to increase. In particular, there was no VISA strain after 2011 and rpo-B mutant strain was only found since 2011. Analysis of antibiotic use data showed that the average length of vancomycin therapy during the study period significantly decreased every year, suggesting that this decrease might have affected the decrease of hVISA/VISA, especially strains with mutations in the two-component systems.
The previous multicenter study in South Korea reported that hVISA prevalence among MRSA from six medical centers in South Korea decreased from 25.0% during 2006–2007 to 2.2% during 2011–2013 [15]. One tertiary hospital in South Korea reported 37.7% hVISA prevalence during 2008–2010 [14]; however, the high prevalence here might be associated with clonal dissemination. In contrast, we posit that our findings were not affected by clonal dissemination because the PFGE analysis of hVISA/VISA strains showed various pulsotypes. Although some strains belonged to the same pulsotype, the time of bacteremia was different or infection occurred in a different ward, suggesting that it was not a case due to direct transmission or outbreak.
As vancomycin substitutes became available, it was predicted that vancomycin use would decrease; however, the annual vancomycin use density analyzed by DOT/1,000 patient-days in our study did not decrease significantly. Although teicoplanin use density showed a significant decrease, the amount of teicoplanin used was relatively lower than that of vancomycin, so the trend did not show a significant change when analyzed by overall use density of glycopeptide. On the other hand, linezolid and tigecycline use showed a significantly increasing trend. Daptomycin was not available in South Korea until 2020, and was not included in this study. The results of our study in which vancomycin use density did not decrease, but the annual average LOT analyzed by the number of days vancomycin was administered for each case showed a significantly decreasing trend are very interesting. This was a convincing result considering that prolonged exposure to vancomycin is the most important risk factor for emergence of VISA or hVISA [2, 3].
The possible reasons for the results that the annual vancomycin use density (DOT/1,000 patient-days) did not decrease despite the decrease in annual average LOT of vancomycin are as follows. First, improved hand hygiene compliance and enhanced infection control have contributed to reducing the incidence of MRSA infection in hospitals, but MRSA remains the major causative organism of healthcare-associated infections in Korean hospitals [26, 27]. Second, despite the introduction of an alternative antibiotics to vancomycin, the standard treatment recommended for MRSA infection is still vancomycin or teicoplanin in South Korea, which has a universal health insurance system. For these reasons, annual vancomycin use density does not appear to have decreased significantly. On the other hand, the decrease in the average LOT in individual cases is thought to be due to antimicrobial stewardship activities by infection specialists who recommends changing to an alternative antibiotic early in case that shows poor clinical response to vancomycin or adverse drug reactions. In addition, SMC Computerized Antimicrobial Stewardship System (SMC COMPASS) which was designed to automatically stop prescription if it does not receive approval by an infection specialist within 2 days after prescribing vancomycin, may also have contributed to the decrease in LOT of vancomycin. Teicoplanin was more likely to be used as an alternative to vancomycin in Korea [28], but as linezolid became available, teicoplanin use decreased.
Considering that the hVISA/VISA phenotype is associated with mutations at the vraSR, graSR, and walKR loci due to prolonged exposure to vancomycin [2, 10, 11], our findings that the annual prevalence rate of hVISA/VISA carrying mutations at these two-component systems among MRSA showed a significantly decreasing trend, and multiple mutations of these loci were observed only in hVISA/VISA strains until 2011 could be explained by the finding that the length of vancomycin therapy decreased following the availability of vancomycin-replacing antibiotics in the hospital.
Mutations at the rpoB locus were found in six hVISA/VISA strains, and mutations (A477D, H481Y) were confirmed in four of six rifampin-resistant hVISA/VISA strains. Interestingly, the rpoB gene mutation continued to be frequently observed in contrast to the mutations at the two-component systems were rarely observed in the later study period. Analysis of the annual prevalence rate ratio of hVISA/VISA carrying rpoB gene mutations among SMC-associated MRSA also tended to increase. Although this study did not include an analysis of rifampin use, these findings are corroborated by the increasing need to administer rifampin for MRSA infections. Because the occurrence of device-related infections is increasing, and rifampin, which has excellent biofilm penetration, has been recommended as an adjunctive therapy for S. aureus infection [29], it is possible that mutation of the rpoB locus and induction of rifampin resistance can lead to hVISA/VISA development [30]. Despite the decline in hVISA/VISA prevalence due to reduced exposure to long-term use of vancomycin, this appears to have led to low but continued hVISA prevalence in our study.
The main strength of this study is that it is, to our knowledge, the first to analyze the trend change in hVISA/VISA prevalence among MRSA bacteremia strains and antistaphylococcal antibiotic use together with mutation studies of vancomycin resistance-related gene loci. In order to properly determine the impact of changes in the use of antibiotics in our hospital on the trend in hVISA/VISA prevalence rates, only cases associated with our hospital were included, and all cases that were transferred from other hospitals or chronic care facilities and that were community-associated were excluded. Additionally, high-quality antibiotic use data from the hospital data warehouse were analyzed to identify trends in hVISA/VISA occurrence in connection with antibiotic use over a long study period to evaluate the impact of changes in antibiotic use after new antistaphylococcal antibiotics became available.
Our study has some limitations. First, this was a retrospective study from a single medical center, which limits the generalizability of our findings. Second, because we only investigated hVISA/VISA phenotypes among MRSA bacteremia cases, hVISA/VISA strains that could emerge from non-bacteremic cases were not included. Third, since no mutation in the vraSR, graSR, walKR, and rpoB loci was found in the 9 of 25 hVISA/VISA strains, there may be other vancomycin resistance mechanisms, which may also limit the interpretation of this study. Fourth, because the analysis of antistaphylococcal antibiotic use was limited to a few specific antibiotics, the effect of changes in other antibiotics was not considered. In particular, analysis of trend change in rifampin use could have provided valuable information, but these data were excluded from the analysis because it represented an overall amount used to treat tuberculosis as well as the amount used to treat S. aureus infection. Lastly, in this study, improvement of hand hygiene of the healthcare workers and reinforcement of infection control strategies in hospital which may also have influenced the change in the prevalence of hVISA/VISA were not considered. However, the investigation of 25 hVISA/VISA strains did not show cases suspected of a small outbreak or direct transmission.
Conclusions
Annual prevalence rates of hVISA/VISA among healthcare-associated MRSA bacteremia strains did not decrease during the 14-year period in a tertiary care hospital in South Korea. However, the subgroup analysis revealed that annual prevalence rates of hVISA/VISA carrying mutations at the two-component systems among healthcare-associated MRSA bacteremia strains significantly decreased during the study period. The average length of vancomycin therapy decreased every year as alternative antibiotics became available, which may have had an effect on the decrease in the hVISA/VISA prevalence. The increase in hVISA carrying rpoB mutation in the later study period offset the decrease in hVISA/VISA prevalence as the vancomycin use decreased. The increasing need to treat MRSA infection with rifampin and the fact that hVISA/VISA can emerge from rpoB mutation require continued surveillance for hVISA/VISA phenotypes and antibiotic use monitoring.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–6.
Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev. 2010;23:99–139.
Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis. 2004;38:448–51.
Bae IG, Federspiel JJ, Miró JM, Woods CW, Park L, Rybak MJ, et al. Heterogeneous vancomycin-intermediate susceptibility phenotype in bloodstream methicillin-resistant Staphylococcus aureus isolates from an international cohort of patients with infective endocarditis: prevalence, genotype, and clinical significance. J Infect Dis. 2009;200:1355–66.
van Hal SJ, Paterson DL. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2011;55:405–10.
Turner AM, Lee JYH, Gorrie CL, Howden BP, Carter GP. Genomic insights into last-line antimicrobial resistance in multidrug-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Front Microbiol. 2021;12:637656.
Dereskinski S. The multiple paths to heteroresistance and intermediate resistance to vancomycin in Staphylococcus aureus. J Infect Dis. 2013;208:7–9.
Cui L, Isii T, Fukuda M, Ochiai T, Neoh HM, Camargo IL, et al. An RpoB mutation confers dual heteroresistance to daptomycin and vancomycin in Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54:5222–33.
Guérillot R, Gonçalves da Silva A, Monk I, Giulieri S, Tomita T, Alison E, et al. Convergent evolution driven by rifampin exacerbates the global burden of drug-resistant Staphylococcus aureus. mSphere. 2018;3:e00550-17.
Wang Y, Li X, Jiang L, Han W, Xie X, Jin Y, et al. Novel mutation sites in the development of vancomycin- intermediate resistance in Staphylococcus aureus. Front Microbiol. 2016;7:2163.
Rose WE, Knier RM, Hutson PR. Pharmacodynamic effect of clinical vancomycin exposures on cell wall thickness in heterogeneous vancomycin-intermediate Staphylococcua aureus. J Antimicrob Chemother. 2010;65:2149–54.
Zhang S, Sun X, Chang W, Dai Y, Ma X. Systematic review and meta-analysis of the epidemiology of vancomycin-intermediate and heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. PLoS ONE. 2015;10:e0136082.
Shariati A, Dadashi M, Moghadam MT, van Belkum A, Yaslianifard S, Darban-Sarokhalil D. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: a systematic review and meta-analysis. Sci Rep. 2020;10:12689.
Park KH, Kim ES, Kim HS, Park SJ, Bang KM, Park HJ, et al. Comparison of the clinical features, bacterial genotypes and outcomes of patients with bacteraemia due to heteroresistant vancomycin-intermediate Staphylococcus aureus and vancomycin-susceptible S. aureus. J Antimicrob Chemother. 2012;67:1843–9.
Kang YR, Chung DR, Baek JY, Kim SH, Cho SY, Ha YE, et al. Decreasing prevalence of heterogeneous vancomycin-intermediate Staphylococcus aureus among blood isolates in Korean hospitals. Diagn Microbiol Infect Dis. 2016;86:464–6.
CLSI. Clinical and Laboratory Standards Institute (CLSI), Performance standards for antimicrobial susceptibility testing; 31st Edition, 2021; (M100-S25) Wayne, PA.
Satola SW, Farley MM, Anderson KF, Patel JB. Comparison of detection methods for heteroresistant vancomycin-intermediate Staphylococcus aureus, with the population analysis profile method as the reference method. J Clin Microbiol. 2011;49:177–83.
Wootton M, Howe RA, Hillman R, Walsh TR, Bennett PM, MacGowan AP. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J Antimicrob Chemother. 2001;47:399–403.
Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38:1008–15.
Oliveira DC, de Lencastre H. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2002;46:2155–61.
Harmsen D, Claus H, Witte W, Rothgänger J, Claus H, Turnwald D, Vogel U. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol. 2003;41:5442–8.
Gilot P, Lina G, Cochard T, Poutrel B. Analysis of the genetic variability of genes encoding the RNA III-activating components Agr and TRAP in a population of Staphylococcus aureus strains isolated from cows with mastitis. J Clin Microbiol. 2002;40:4060–7.
Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–9.
Kato Y, Suzuki T, Ida T, Maebashi K. Genetic changes associated with glycopeptide resistance in Staphylococcus aureus: predominance of amino acid substitutions in YvqF/VraSR. J Antimicrob Chemother. 2010;65:37–45.
Hafer C, Lin Y, Kornblum J, Lowy FD, Uhlemann AC. Contribution of selected gene mutations to resistance in clinical isolates of vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2012;56:5845–51.
Kim H, Kim ES, Lee SC, Yang E, Kim HS, Sung H, et al. Decreased incidence of methicillin-resistant Staphylococcus aureus bacteremia in intensive care units: a 10-year clinical, microbiological, and genotypic analysis in a tertiary hospital. Antimicrob Agents Chemother. 2020;64:e01082-20.
Chiang CH, Pan SC, Yang TS, Matsuda K, Kim HB, Choi YH, et al. Healthcare-associated infections in intensive care units in Taiwan, South Korea, and Japan: recent trends based on national surveillance reports. Antimicrob Resist Infect Control. 2018;7:129.
Yoon YK, Park DW, Sohn JW, Kim HY, Kim YS, Lee CS, et al. Multicenter prospective observational study of the comparative efficacy and safety of vancomycin versus teicoplanin in patients with health care-associated methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2014;58:317–24.
Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–55.
Maudsdotter L, Ushijima Y, Morikawa K. Fitness of spontaneous rifampicin-resistant Staphylococcus aureus isolates in a biofilm environment. Front Microbiol. 2019;10:988.
Acknowledgements
Not applicable.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant, funded by the Korean government (MSIT) (No. 2020R1F1A1067794) and by a government-wide R&D fund for research about infectious diseases in Korea (No. HG18C0062).
Author information
Authors and Affiliations
Contributions
Conceptualization, DRC, and S-HK; Data curation, YRK, and S-HK Genetic analysis, YRK; Formal analysis, S-HK, and SYC; Methodology, DRC and KH; Visualization, DRC, YRK, and S-HK; Writing—original draft; DRC, YRK, S-HK, J-HK, KH, SYC, C-IK, and KRP; Writing—review and editing, all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the local institutional ethics board (SMC 2020-01-014). The need for informed consent was waived because this study used anonymized clinical data form novel hospital`s data warehouse. The study was performed in accordance with the guidelines followed as per the Declaration of Helsinki.
Consent for publication
The manuscript is approved by all authors for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
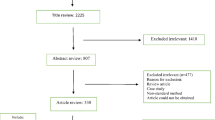
Additional file 1: Fig. S1
. Flow diagram showing strategies for selection of bacterial strains.
Additional file 2. Table S1
. Primer sequences for amplification of two-component systems and rpoB gene loci. Table S2. Phenotypic characterization of 25 S. aureus strains with reduced vancomycin susceptibility.
Additional file 3: Fig. S2
. Genotypic characteristics of 25 hVISA/VISA strains associated with the Samsung Medical Center. VISA, vancomycin-intermediate Staphylococcus aureus; hVISA, heterogeneous VISA; CO-HCA, community-onset healthcare-associated; HO, hospital-onset; F, floor; H, hospital; ICU, intensive care unit; MICU, medical ICU; SICU, surgical ICU.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kang, Y.R., Kim, SH., Chung, D.R. et al. Impact of vancomycin use trend change due to the availability of alternative antibiotics on the prevalence of Staphylococcus aureus with reduced vancomycin susceptibility: a 14-year retrospective study. Antimicrob Resist Infect Control 11, 101 (2022). https://doi.org/10.1186/s13756-022-01140-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-022-01140-9