Abstract
Background
Agrobacterium spp. are infrequent agents of bloodstream infections linked to healthcare-associated outbreaks. However, it is unclear if outbreaks also occur across larger geographic areas. Triggered by two local clusters from putative point sources, our aim was to detect potential additional clusters in Switzerland.
Methods
We performed a nationwide descriptive study of cases in Switzerland based on a prospective surveillance system (Swiss Centre for Antibiotic Resistance, anresis.ch), from 2008 to 2019. We identified patients with Agrobacterium spp. isolated from blood cultures and used a survey to collect clinical-epidemiological information and susceptibility testing results. We performed whole genome sequencing (WGS) of available clinical isolates and determined their relatedness by single nucleotide polymorphism (SNP) variant calling analysis.
Results
We identified a total of 36 cases of Agrobacterium spp. from blood samples over 10 years. Beyond previously known local clusters, no new ones were identified. WGS-based typing was performed on 22 available isolates and showed no clonal relationships between newly identified isolates or to those from the known clusters, with all isolates outside these clusters being at least 50 SNPs apart.
Conclusion and relevance
Agrobacterium spp. bacteraemia is infrequently detected and, given that it may be healthcare-associated and stem from a point source, occurrence of multiple episodes should entail an outbreak investigation. With the help of the national antimicrobial resistance surveillance system we identified multiple clinical cases of this rare pathogen but found no evidence by WGS that suggested a nation-wide outbreak.
Graphical abstract

Similar content being viewed by others
Introduction
The genus Agrobacterium is a group of gram-negative, aerobic and motile environmental bacteria. Agrobacterium species are recognized as rare opportunistic human pathogens, which affect mostly immunocompromised patients or patients with underlying diseases such as solid tumours or end-stage renal disease [1,2,3,4]. A. pusense has been described as the main human pathogen in the genus [5]. The majority of reported cases with Agrobacterium spp. are bloodstream infections related to the use of central venous catheters (CVC) [2,3,4, 6] or other permanent medical devices and foreign materials. In some cases, cure was only achieved by removal of the catheter [3, 4, 7]. This suggests that device colonisation plays an important role in the pathogenesis of bloodstream infection, which is supported by the ability of these bacteria to attach to silicone tubes [7] and the high colony counts found in catheter blood cultures [3]. Healthcare-associated cases have been reported [2, 6] and common infection sources for cases of Agrobacterium spp. bloodstream infections have been suggested previously [6]. On the other hand, community-acquired cases have been described as well [1, 3, 4] and in one study, a pulse-field gel electrophoresis of a cluster of cases showed distinct isolates, thus ruling out nosocomial spread [2]. Also, pseudo-bacteraemia with A. radiobacter (formerly named Rhizobium radiobacter [8]) due to contamination of blood cultures by an environmental source has been described [9]. A recent report from Brazil described strains of A. radiobacter as part of a three-species outbreak associated with the use of total parenteral nutrition and/or calcium gluconate [10].
From 2011–2017, a series of eight patients with Agrobacterium spp. bacteraemia was noted at Bern University Hospital [11]. All of these patients had previously undergone a CT scan and had received intravenous contrast medium. The relatedness of the corresponding isolates in two clusters (four Agrobacterium genomosp. 3 and two A. radiobacter) was confirmed by whole genome sequencing (WGS). This suggested a common transmission pathway with introduction from two different point sources. Despite an extensive outbreak investigation, the sources could not be identified [11].
Our aim was to expand the investigation on bacteraemia isolates to other Swiss healthcare institutions. The objective was to find Agrobacterium spp. bacteraemia cases in Switzerland and explore their relatedness by a molecular epidemiological approach.
Methods
Study design and setting
The study was designed as a nationwide, descriptive case series in Switzerland, based on a uniform case definition and a prospective surveillance system.
Preliminary data
Potential cases of Agrobacterium species and/or Rhizobium species isolated from blood cultures in Switzerland between January 2008 and December 2017 were identified via a query by the Swiss Centre for Antibiotic Resistance (anresis.ch). Anresis.ch is a national surveillance system that collects routine antibiotic resistance data as well as data on antibiotic usage. They maintain an antibiotic resistance database and inform the public about resistance trends on a regular basis. Currently, 30 microbiology laboratories across Switzerland and > 200 healthcare institutions contribute, covering approximately 80% of the annual hospitalisation days in Switzerland [12]. All participating laboratories performed antimicrobial susceptibility testing (AST) according to the Clinical Laboratory Standards Institute (CLSI) guidelines from 2004 to 2010. From 2011 to 2013, most of them switched to using the European Committee on Antimicrobial Susceptibility Testing (EUCAST) definitions [13,14,15]. Species identification was performed according to individual laboratory standard procedures, and these may have changed during the period covered in this analysis. For example, the microbiological laboratory at Bern University Hospital switched from 16S‐rRNA gene sequencing to matrix-assisted laser desorption ionisation-time-of- flight (MALDI‐TOF) mass‐spectrometry based identification during the study period.
The initial query included the following information: sample date, sample type, identification at the genus and species level, the responsible diagnostic microbiology laboratory, and (if available) the corresponding healthcare institution [16].
Survey and cases
Based on the query, a survey (Additional file 1: Fig. S1) was sent to the microbiology laboratories from which the Agrobacterium (Rhizobium) species had been reported. By means of the survey we collected antimicrobial susceptibility results and inquired about the availability of the isolates for further analysis. The case definition for inclusion was a first (non-duplicate within 30 days) patient isolate from a blood culture with Agrobacterium or Rhizobium spp. and a valid response to the survey. Cases identified through anresis.ch but without a valid laboratory response were excluded. Data from anresis.ch covered ten years, from January 2008 until December 2017. We also included cases not identified via the query but reported independently to us by the laboratories; these could also be from the years 2018 and 2019.
For the descriptive analysis, Agrobacterium und Rhizobium spp. were grouped together, due to changes in nomenclature and classification over the years [5, 17]. The “intermediate” susceptibility category was considered as resistant. For the evaluation, each healthcare institution was anonymized by an index number and each laboratory by an index letter.
Whole genome sequencing and SNP phylogeny
DNA was extracted from isolates using a DNA extraction robot (Qiacube, Qiagen). Libraries were prepared using NexteraFlex, sequenced PE150 on a NextSeq 500 Illumina sequencing platform. The mean coverage for each sample was in excess of 47x. Assemblies were generated using Unicycler v0.4.8 [18] and used in Genome-to-Genome Distance Calculator (GGDC) [19] for digital DNA:DNA hybridization (dDDH) species determination against the panel of reference isolates described [11] using 70% cutoff (formula 2). A comparison of genomes was generated in the Type (Strain) Genome Server (TYGS) [20]. For single nucleotide polymorphism (SNP) calling and phylogeny within species we used CLC workbench v12.0.3 with parameters that differed from the default as: variant calling with 10 × minimum coverage, 10 minimum count and 70% minimum frequency, and SNP tree creation with 10 × minimum coverage, 10% minimum coverage, 0 prune distance and including multi-nucleotide variants (MNVs). As a reference assembly for A. pusense, GCA_900013495 was downloaded from NCBI and fragmented into reads using SAMtools [21] wgsim. Mapping was also performed against plasmids pTi-SAKURA (accession number NC_002147.1), pRi1724 (NC_002575.1) and A. pusense assembly GCA_900013495. All data generated here was submitted to the European Nucleotide Archive under project number PRJEB37957 (https://www.ebi.ac.uk/ena/data/view/PRJEB37957).
Study size and potential bias
The study size was determined and restricted by the number of reported cases. To address potential reporting bias in case of an incomplete anresis.ch query, the contacted laboratories were encouraged to report additional cases not identified by the query. To prevent a selection bias due to differences in nomenclature, we included all Agrobacterium (Rhizobium) spp. that were reported to anresis.ch in the selected period.
Analysis and statistics
We used R for descriptive analysis and the graphs (R Foundation for Statistical Computing, Vienna, Austria) [22].
Results
Cases
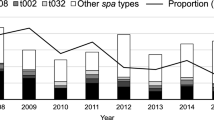
The initial nationwide query identified 39 cases of Agrobacterium or Rhizobium spp. from blood culture samples. Nine out of eleven contacted laboratories responded individually and reported nine additional cases not captured by the initial query. This resulted in 48 cases, from the years 2008 to 2019. We excluded twelve cases for not meeting the case definition; five with no response to the survey, three cases with Agrobacterium spp. from other sites (1 dialysate enriched in a blood culture bottle, 1 catheter tip, 1 conjunctival swab), two duplicate cases, and two quality control isolates. Our final dataset included 36 cases from ten healthcare institutions (Fig. 1).
An overview of the 36 cases according to annual cases per institution, initial species identification, and availability for WGS is shown in Fig. 2. According to the initial species identification by the originating microbiology laboratories, 22 (61%) were A. radiobacter, three were A. tumefaciens, and one was A. rhizogenes. In ten cases, the species was not identified beyond Agrobacterium spp..
Overview of the 36 cases. We plotted the number of annual cases (x-axis) for all healthcare institutions (y-axis; 1–10) of Agrobacterium spp. bacteraemia and indicated availability for whole genome sequencing. Indicated are the initial presumptive species identifications by the originating microbiology laboratories, which may differ from the definite identification by WGS as shown in Fig. 3
We saw a temporal-spatial accumulation of cases only in Institution 6 (Bern University Hospital, where the first outbreak was described; Fig. 2). Of the other institutions with two or more reported cases, only one (Institution 3) reported more than one case in the same year. Of note, the two reported cases from Institution 1 stemmed from the same patient, who had two A. radiobacter bacteraemia episodes within four years. Unfortunately, only the latter of these two isolates was available for sequencing.
WGS und SNP tree
22 isolates from five healthcare institutions were sequenced (Fig. 2): eight from the initial outbreak investigations [11] and 14 additional isolates as part of this study. This accounts for 61% (22/36) of the cases included. Definite species identification by genomic comparisons (dDDH) against reference genomes showed that 13 of the isolates are A. pusense, and one Agrobacterium genomospecies 1, in addition to the previously described Agrobacterium genomospecies 3 (n = 5), A. radiobacter (n = 2) and A. pusense (n = 1) isolates [11]. A genome comparison shows the relationships between isolates and reference genomes (Fig. 3). Of the isolates belonging to A. pusense, several pairs of isolates, from different institutes in all cases, clustered together. A SNP comparison within A. pusense shows all strains differ from each other by over 900 SNPs with the exceptions of pairs Inst3_Iso4_2015 / Inst2_Iso2_2017 (53 SNPs in this analysis) and Inst7_Iso1_2012 and Inst2_Iso1_2014 (63 SNPs). None of the newly analysed cases are related to those from the outbreak that was described first (AGRBE03_C, AGRBE04_D, AGRBE05_E and AGRBE06_F) [11]. Mapping against pRi and pTi plasmids shows that these are not present in any of the isolates (< 27% and < 18% of the references covered by mapping reads, respectively).
TYGS genome comparison of 20 of the 22 sequenced isolates compared to reference genomes. Isolates described in this paper are shown with blue + , including the previously described isolates [11] from the two clusters at Institution 6 (AGRBE03_C, AGRBE05_E & AGRBE06_F and ARGREBE01_A) with a green + . AGREBE02_B and AGRBE04_D were excluded from this analysis, as they are identical to AGREBE02_A and AGRBE04_C respectively. Species clusters are marked by colours, using 70% dDDH cutoffs
Susceptibility testing
Of the 36 cases included, AST data was available for 29. Most isolates were resistant to tobramycin (88%) and ceftazidime (43%), followed by trimethoprim-sulfamethoxazole (33%) and ampicillin (29%). The isolates were uniformly susceptible to carbapenems (imipenem and/or meropenem) and all but one were susceptible to fluoroquinolones (ciprofloxacin and/or levofloxacin). Further, some of the isolates with WGS-confirmed clonal relatedness showed variation in susceptibility. A summary of the susceptibility testing is shown in Table 1 susceptibility testing.
Discussion
Key results
We identified 36 cases of Agrobacterium spp. from blood samples. Besides the previously published clusters [11], six further healthcare institutions reported more than one case over the investigated time period. A WGS-based typing of 22 isolates showed no close relationship between any of these cases, besides the previously established clusters [11]. Further, none of the newly sequenced cases were related to the 2013–17 clusters from Bern. In studies on clinical isolates of Agrobacterium spp. in other parts of the world, pulse-field gel electrophoresis and/or multilocus sequence-based phylogeny was used to analyse the relatedness of the strains [2, 5, 6, 23], therefore our WGS approach is unique and novel.
Limitations
Our study size was restricted: not all healthcare institutions or microbiology laboratories report to anresis.ch, not all contacted laboratories responded to the survey, and not all the isolates were available for sequencing. Therefore, it is possible that not all cases that occurred in Switzerland during the study period were included.
Conventional methods (16S rDNA, API®, MALDI-TOF) used in clinical laboratories cannot reliably identify the species within the Agrobacterium genus and some commercial systems have predominantly or exclusively "Agrobacterium/Rhizobium radiobacter" in the database [11, 23]. Some isolates may thus have been initially missed and/or misidentified. Also, the database of the national surveillance does not include environmental samples and therefore such were not included in our study.
So far, no standardized AST breakpoints have been issued for Agrobacterium spp. EUCAST, however, publishes guidelines for groups of organisms, for which there are no established breakpoints. These guidelines state pharmacokinetic-pharmacodynamic (PK-PD) non species-related breakpoints should be used if available for the antimicrobial agent in question and that reporting “susceptible”, “intermediate” or “resistant” should be avoided for agents for which there are none; rather, the minimum inhibitory concentration (MIC) should be reported in those instances [24]. This lack of standardization complicates the comparison of susceptibility tests stemming from different laboratories. Further, the lack of standardization in susceptibility testing is a general limitation for outbreak investigations and an additional argument in favor of more sophisticated molecular analyses to assess relatedness of isolates.
Clustering and WGS
Seven out of the ten healthcare institutions reported more than one case during the covered time period, which could suggest local clusters derived from a common transmission pathway or source. Such a persistent point source may be plausible even though cases from different centres were separated by months or years in time. The WGS and the following SNP variant calling analysis were able to rule out the transmission of any of the newly sequenced cases, as most showed no close relationship to each other, or to the previously known clusters. Of those differing by < 100 SNPs, they were from different institutions and separated by two years. However, we cannot exclude relatedness and potential past outbreaks in the cases that were unavailable for sequencing, for example the three cases of presumptive "A. tumefaciens" from Institution 2 between 2009 and 2012 and the ten presumptive "A. radiobacter" isolates across seven institutions. Nevertheless, we have strong evidence that a cross-institutional outbreak is unlikely.
Antimicrobial susceptibility
The most frequent resistances we found were against tobramycin and ceftazidime. This matches case reports and case series of A. radiobacter bacteraemia published to date [3, 7, 25]. Like other studies, we also found variable susceptibilities to cephalosporins (ceftazidime and cefuroxime) [1, 2]. The almost uniform susceptibility of the isolates to carbapenems and fluoroquinolones is in line with published data [2,3,4, 7, 25].
Considering the lack in standardization in AST for this genus, the results of the susceptibility testing should be interpreted with caution, especially when comparing results from different laboratories or institutions as well as comparing them to findings from other studies. In addition, most laboratories switched from CLSI to EUCAST guidelines during the study period, so results from the same laboratory but from different years might also not be comparable.
The encountered variation in susceptibility between clonal isolates could also be explained with the technical difficulties in AST for these organisms. This underlines the limits of using susceptibility reporting to investigate relatedness of strains.
Conclusion
We identified 36 cases of Agrobacterium spp. bacteraemia in Switzerland from 2008 to 2019. The strains were mostly resistant to tobramycin and ceftazidime and susceptible to carbapenems and fluoroquinolones. Besides the two established clusters at Institution 6/Bern University Hospital [11], six further healthcare institutions reported multiple cases. A WGS-based typing of the 22 isolates available showed no close relatedness between any of the cases, besides the previously established outbreak [11]. Thus, we conclude that nosocomial outbreaks of Agrobacterium spp. bacteraemias from a point source may occur but remain the exception. If multiple cases of invasive Agrobacterium spp. with the same species occur at one healthcare institution this should prompt an outbreak investigation. Suspicion should be raised in particular if case patients underwent the same procedure in the same location, as common transmissions pathways with introduction from persistent point sources, which may remain unrecognized for years, are possible.
With the help of the nation-wide surveillance system, we identified multiple cases of a rare pathogen and WGS revealed they were all unrelated.
Availability of data and materials
All data generated was submitted to the European Nucleotide Archive under project number PRJEB37957 (https://www.ebi.ac.uk/ena/data/view/PRJEB37957).
Abbreviations
- Anresis.ch:
-
Swiss Centre for Antibiotic Resistance
- AST:
-
Antimicrobial susceptibility testing
- CLSI:
-
Clinical Laboratory Standards Institute
- CVC:
-
Central venous catheters
- dDDH:
-
Digital DNA:DNA hybridization
- EUCAST:
-
European Committee on Antimicrobial Susceptibility Testing
- GGDC:
-
Genome-to-Genome Distance Calculator
- MALDI‐TOF:
-
Matrix-assisted laser desorption ionisation-time-of- flight
- MIC:
-
Minimum inhibitory concentration
- MNVs:
-
Multi-nucleotide variants
- PK-PD:
-
Pharmacokinetic-pharmacodynamic
- SNP:
-
Single nucleotide polymorphism
- TYGS:
-
Type (Strain) Genome Server
- WGS:
-
Whole genome sequencing
References
Mastroianni A, Coronado O, Nanetti A, Manfredi R, Chiodo F. Agrobacterium radiobacter pneumonia in a patient with HIV infection. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 1996;15:960–3.
Lai C-C, Teng L-J, Hsueh P-R, et al. Clinical and microbiological characteristics of rhizobium radiobacter infections. Clin Infect Dis. 2004;38:149–53.
Paphitou NI, Rolston KVI. Catheter-related bacteremia caused by agrobacterium radiobacter in a cancer patient: case report and literaturereview. Infection. 2003;31:421–4.
Chen CY, Hansen KS, Hansen LK. Rhizobium radiobacter as an opportunistic pathogen in central venous catheter-associated bloodstream infection: case report and review. J Hosp Infect. 2008;68:203–7.
Aujoulat F, Marchandin H, Zorgniotti I, Masnou A, Jumas-Bilak E. Rhizobium pusense is the main human pathogen in the genus agrobacterium/rhizobium. Clin Microbiol Infect. 2015;21:472.
Giammanco GM, Pignato S, Santangelo C, Grimont PAD, Grimont F, Giammanco G. Molecular typing of agrobacterium species isolates from catheter-related bloodstream infections. Infect Control Hosp Epidemiol. 2004;25:885–7.
Alnor D, Frimodt-Moller N, Espersen F, Frederiksen W. Infections with the unusual human pathogens Agrobacterium species and Ochrobactrum anthropi. Clin Infect Dis Off Publ Infect Dis Soc Am. 1994;18:914–20.
Young JM, Kuykendall LD, Martinez-Romero E, Kerr A, Sawada H. A revision of Rhizobium Frank 1889, with an emended description of the genus, and the inclusion of all species of Agrobacterium Conn 1942 and Allorhizobium undicola de Lajudie et al. 1998 as new combinations: Rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis. Int J System Evolut Microbiol. 2001;51:89–103.
Rogues A-M, Sarlangue J, de Barbeyrac B, et al. Agrobacterium radiobacter as a cause of pseudobacteremia. Infect Control Hosp Epidemiol. 1999;20:345–7.
Pillonetto M, Arend L, Gomes SMT, et al. Molecular investigation of isolates from a multistate polymicrobial outbreak associated with contaminated total parenteral nutrition in Brazil. BMC Infect Dis. 2018;18:397.
Casanova C, Lo Priore E, Egli A, et al. Agrobacterium spp. nosocomial outbreak assessment using rapid MALDI-TOF MS based typing, confirmed by whole genome sequencing. Antimicrobial Resist Infect Control. 2019;8:171.
Gasser M, Schrenzel J, Kronenberg A. Aktuelle Entwicklung der Antibiotikaresistenzen in der Schweiz. Swiss Med Forum. 2018;18(46):943–9.
The European Committee on Antimicrobial Susceptibility Testing EUCAST. Available at: http://www.eucast.org/. Accessed 8 June 2020.
Clinical and Laboratory Standards Institute. Available at: http://clsi.org/standards/about-our-standards/standards-resources/. Accessed 7 February 2018.
Kronenberg A, Hilty M, Endimiani A, Muhlemann K. Temporal trends of extended-spectrum cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae isolates in in- and outpatients in Switzerland, 2004 to 2011. Euro Surveill. 2013;18:20484.
Balmer L. Rhizobium (Agrobacterium) Bacteraemias, Switzerland, January 2008 to December 2017, [Master Thesis, University of Bern] 2019.
Young JM, Kuykendall LD, Martinez-Romero E, Kerr A, Sawada H. Classification and nomenclature of Agrobacterium and Rhizobium. Int J Syst Evol Microbiol. 2003;53:1689–95.
Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13:e1005595.
Genome-to-Genome Distance Calculator 2.1. Available at: http://ggdc.dsmz.de/ggdc.php#. Accessed 23 Apr 2020.
Meier-Kolthoff JP, Göker M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat Commun. 2019;10:2182.
Li H, Handsaker B, Wysoker A, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–9.
The R Project for Statistical Computing. Available at: https://www.r-project.org/. Accessed 29 Nov 2017.
Aujoulat F, Jumas-Bilak E, Masnou A, et al. Multilocus sequence-based analysis delineates a clonal population of Agrobacterium (Rhizobium) radiobacter (Agrobacterium tumefaciens) of human origin. J Bacteriol. 2011;193:2608–18.
The European Committee on Antimicrobial Susceptibility Testing EUCAST, Clinical Breakpoints. Available at: http://www.eucast.org/clinical_breakpoints/. Accessed 8 June 2020.
Amaya RA, Edwards MS. Agrobacterium radiobacter bacteremia in pediatric patients: case report and review. Pediatr Infect Dis J. 2003;22:183–6.
Swiss Ethics Committees on research involving humans. Available at: https://swissethics.ch/assets/Themen/zustaendigkeit_d.pdf. Accessed 12 June 2020.
Acknowledgements
We would like to thank the Federal Office of Public Health (Dr. Daniel Koch, Dr. Céline Gardiol) for entrusting us with this outbreak investigation. We thank Elisabeth Schultheiss at University Hospital Basel for excellent technical assistance in WGS. Unicycler assemblies were performed at sciCORE (http://scicore.unibas.ch) scientific computing facility at the University of Basel. We would like to thank anresis for the collaboration (laboratories and group members listed on www.anresis.ch). We would like to thank the participating microbiological laboratories for their time and for sending their isolates for sequencing.
Funding
Institutional funding.
Author information
Authors and Affiliations
Contributions
All authors have seen and approved the manuscript. Contributions: Conception and Design: LB, RS. Acquisition of Data: LB, RS, AK, CC, JS, JM; Whole Genome Sequencing and analysis: HSS, AE. Interpretation: All authors; Writing of the first draft: LB, RS, HSS. Critical reviewing and approval of the final version: All authors. RS and LB are guarantors of the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
According to federal law, outbreak investigations are considered quality improvement projects and are therefore exempt from ethical approval [26]. The nationwide investigation was supported in part by the Federal Office of Public Health (www.bag.admin.ch).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Transparency declaration
None to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original article had the Corresponding Author’s email updated.
Supplementary Information
Additional file 1
. Survey.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Balmer, L., Seth-Smith, H.M.B., Egli, A. et al. Agrobacterium species bacteraemia, Switzerland, 2008 to 2019: a molecular epidemiological study. Antimicrob Resist Infect Control 11, 47 (2022). https://doi.org/10.1186/s13756-022-01086-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-022-01086-y