Abstract
Background
The burden of antimicrobial resistance is high in solid organ transplant (SOT) recipients. Among Swiss SOT recipients, we assessed temporal trends of ESBL-producing Enterobacterales (ESBL-E), identified risk factors for ESBL-E, and assessed the impact of resistance on patient outcome.
Methods
Data from the Swiss Transplant Cohort Study (STCS), a nationwide prospective cohort of SOT-recipients, were analysed. Temporal trends were described for ESBL-detection among Escherichia coli and non-Escherichia coli. In a nested case–control study, cases with ESBL-E infection were 1:1 matched (by time since transplantation, organ transplant, pathogen) to controls infected with non-ESBL-E. Factors associated with resistance and with unfavourable 30-day outcome (death, infection relapse, graft loss) were assessed.
Results
From 2012 to 2018, we identified 1′212 infection episodes caused by Enterobacterales in 1′074 patients, thereof 11.4% (138/1′212) caused by ESBL-E. The proportion of ESBL-production among Escherichia coli remained stable over time (p = 0.93) but increased for non-E. coli (p = 0.02) Enterobacterales. In the case–control study (n = 102), antibiotic pre-treatment was independently associated with ESBL-production (aOR = 2.6, 95%-CI: 1.0–6.8, p = 0.046). Unfavourable outcome occurred in 24/51 (47%) cases and 9/51 (18%) controls (p = 0.003). Appropriate empiric antibiotic therapy was the only modifiable factor associated with unfavourable outcome.
Conclusions
In Swiss SOT-recipients, proportion of infections with ESBL-producing non-E. coli Enterobacterales increased in recent years. Antibiotic pre-treatment represents a risk factor for ESBL-E. Improving appropriateness of empiric antibiotic treatment might be an important measure to reduce unfavourable outcome, which was observed in almost half of SOT-recipients with ESBL-E infections.
Similar content being viewed by others
Introduction
Antimicrobial resistance is a major threat to the achievements of modern medicine. Solid organ transplant (SOT) recipients are at particular risk for acquisition of resistant pathogens, most likely due to increased healthcare and antibiotic exposure [1]. Specifically in SOT-recipients, donor-derived infections due to resistant pathogens are associated with significant burden of disease [2]. Over the last decade, Gram-negative bacteria have become the focus of attention regarding antibiotic resistance for both the general hospital population and SOT-recipients. A recent systematic review reported that 20% of SOT-recipients are colonized with ESBL-producing Escherichia coli [3]. Colonization by ESBL-producing isolates is an important risk factor for subsequent infection [4]; about one in 10 renal transplant recipients (RTR) colonized by ESBL-producing bacteria experiences a urinary tract infection (UTI) caused by these pathogens [5]. Compared to infections with non-resistant pathogens, those caused by resistant bacteria are associated with an increased risk for recurrent infection, allograft dysfunction and excess mortality [5, 6]. Case-fatality infection with resistant pathogens is high (up to 50% in the case of bacteremia due to carbapenem-resistant Enterobacterales) [7,8,9].
Among Swiss SOT recipients, most infections in the first year post-transplantation are caused by Enterobacterales. Among E. coli and Klebsiella pneumoniae, ESBL-production was observed in 15%, whereas no carbapenemase-producing Enterobacterales (CPE) were identified in our cohort [10]. Here, we aimed to assess temporal trends of ESBL-producing Enterobacterales (ESBL-E), to identify risk factors for infections with ESBL-E, and to assess the impact of ESBL-production on patient outcome.
Methods
Data source
The Swiss Transplant Cohort Study (STCS) is a nationwide, multi-centre, open, prospective cohort and has enrolled all SOT-recipients in Switzerland since May 2008 [11]. Clinical and laboratory data are prospectively collected at the time of transplantation, at 6 and 12 months, and annually thereafter. Infectious episodes are identified by transplant infectious disease (ID) physicians on a regular basis using electronic patient records, according to definitions developed by the STCS infectious diseases working group [10]. Six participating transplantation centres (Basel, Bern, Geneva, Lausanne, St. Gallen and Zurich) undergo regular data monitoring and in-depth data quality audits.
Study design and participants
We included SOT-recipients of heart, liver, kidney, and kidney-pancreas grafts aged 18 or older enrolled in the STCS. Lung transplant recipients were excluded because of the particular challenge to distinguish colonisation from infection in this population. From August 2012 onwards, information about ESBL production has been available in the STCS database. For the analysis of temporal trends, we thus retrieved all infection episodes diagnosed between August 2012 and December 2018. Participants contributed a maximum of one episode per year. Those with episodes caused by resistant and susceptible pathogens were counted as having had one resistant episode and were not eligible as controls. Patients with episodes caused by ESBL-producing E. coli and non-E. coli Enterobacterales in the same year were counted in both groups.
A nested case–control study was performed to assess risk factors for infection by ESBL-E and its effect on 90-day outcome afterwards. As opposed to the analysis for temporal trends (episode level) this analysis was performed on the patient-level (i.e. only one episode per patient considered). We included all patients with infections due to ESBL-producing (or MDR and extended-spectrum cephalosporine-resistant, see below) Enterobacterales, diagnosed between August 2012 and December 2016. In case of multiple episodes caused by a resistant pathogen, only the patients’ first episode after transplantation was considered. Cases were matched to controls in a 1:1 fashion, applying incidence density sampling according to time to first infectious episode after transplantation; type of transplanted organ and bacterial pathogen were used as further matching criteria. Controls with previous colonization by ESBL-E were excluded. Detailed information about infections in cases and controls were additionally collected via chart review and recorded in an electronic database (SecuTrial®). These included administration of antibiotics within 30 days before infection (both therapeutic and prophylactic), travel history, urinary obstruction (only for RTR), admission to acute or intensive care, involvement of ID specialist, type, duration and effectiveness of empiric and definite antibiotic treatment, and 90-day outcome.
Microbiology
Pathogen identification and resistance testing was performed on a routine basis in the microbiology laboratories serving the participating centres. Since 2012, information about infections caused by ESBL-E is being recorded. Also, the presence of multidrug-resistance (MDR) is recorded according to the European Centre for Disease Prevention and Control (ECDC) definitions [12].
For the analysis of temporal trends, the variable “ESBL-production” as reported in the database was used to classify resistant and susceptible pathogens. For the case–control study, this definition was extended to also include MDR pathogens with resistance to extended-spectrum cephalosporins (ESC), i.e. 3rd or 4th generation cephalosporins. This approach was chosen because for some bacterial isolates ESBL-production was not tested or reported.
Definitions
In brief, proven infection was defined as the presence of clinical signs or symptoms, detection of a bacterial pathogen, and given treatment [10]. Effectiveness of antibiotic treatment was assessed according to locally performed susceptibility tests. Beta-lactam/beta-lactamase inhibitor combinations were considered inadequate against ESBL-E irrespective of the reported minimal inhibitory concentration. Unfavourable outcome was defined as any of the following: microbiological relapse (i.e. infection with the same pathogen at same body site as the initial infectious episode), graft failure (defined as dialysis post renal transplantation; or re-transplantation post heart or liver transplantation; or recurrence of insulin-dependence following pancreas transplant) or death, all within 90 days after infection.
Statistical analysis
A temporal trend analysis was performed to detect a pattern in occurrence of infectious episodes caused by ESBL-producing E. coli and non-E. coli Enterobacterales between 2012 and 2018, using Chi-squared test for trends in proportion. In the matched subpopulation (including episodes which occurred between 2012 and 2016), a descriptive analysis was done followed by univariate and multivariable conditional logistic regression to evaluate risk factors for infections caused by ESBL-E. Age and gender, as well as baseline characteristics associated with ESBL-E infection in univariate analysis (p < 0.1) were included in the multivariable model. Multicollinearity was assessed calculating the variance inflation factor (cut-off > 10).
For the analysis regarding impact of resistance on patient outcome, infection by ESBL-E itself was considered a key predictor. We used the change in estimate method as screening method for selection of co-variables into the multivariable logistic regression model (i.e. change of key predictor estimate > 10% after adding the co-variable to the model) [13]. R software version 3.6.1 was used for all statistical analyses; a p value of < 0.05 was considered statistically significant.
Results
Temporal trends
Between 2012 and 2018, we registered 1′212 infectious episodes caused by Enterobacterales among 1′074 patients, mostly among RTR (784/1′074, 73%). Among all isolates, ESBL-production was reported in 138/1′212 isolates (11.4%). ESBL rates for episodes of kidney, heart, and liver transplant patients were 13.2% (168/1271), 12.4% (11/89), and 13.6% (30/221), respectively; across the six participating centres, the proportion of ESBL-producing isolates ranged from 8.3 to 18.3%. The proportion of ESBL E. coli remained stable over time (p = 0.93) whereas an increasing trend (p = 0.024) was observed for non-E. coli Enterobacterales (Fig. 1). No temporal trend was observed when ESBL E. coli and non-E. coli were combined (p = 0.36).
Case–control study: population and infection characteristics
Between 2012 and 2016, we identified 88 case patients which were matched to 88 controls. After chart review and revision of the original susceptibility test results, 51 matching pairs remained for the analysis. For the 37 excluded patients, reasons for exclusion were mostly limited or missing susceptibility testing (15/37) and revision of case status (14/37). Of the 51 cases and controls, 33 (65%) were RTR, 4 (8%) kidney-pancreas, 9 (18%) liver or kidney-liver, and 5 (10%) heart transplant patients, respectively. The urinary tract was the most common site of infection (75%). E. coli was the most frequent pathogen found in infections (75.5%), followed by K. pneumoniae (15.7%) and other pathogens (8.8%).
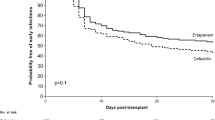
Baseline characteristics of cases and controls were summarized in Table 1. Infections occurred a median of 69 days after transplant (interquartile range [IQR]: 25–232), mostly (68%) within the first 6 months after transplantation (Fig. 2). Patients were predominantly outpatients at time of diagnosis (63% for ESBL-E, 61% for non-ESBL-E infections). ESBL-E and non-ESBL-E infections were evenly distributed among centres and years, as were infection sites and comorbidities. Travel history was not available for most patients and therefore dropped from further analyses.
Risk factors for ESBL infection
Male gender (p = 0.04) and antibiotic treatment within 30 days prior to infection (p = 0.05) were significantly associated with ESBL-E infection (Table 2). Antibiotic pre-treatment remained as independent risk factor for ESBL-E infection in multivariable analysis after correction for age, gender and underlying metabolic/endocrinologic disease (adjusted OR = 2.6, 95% CI: 1.0–6.8, p = 0.046).
Infection management and outcome
Patients with susceptible and resistant pathogens were similar in terms of proportion of hospital admission (16/51 vs. 14/51), intensive care admission (9/51 vs. 8/51), or involvement of an ID physician for choice of treatment (22/51 vs. 27/51) (Table 3). Length of stay was longer for those with resistant (median 18 days, IQR 8.2–30 days) vs. those with susceptible pathogens (median 9, IQR 3–24 days), but did not reach statistical significance (p = 0.10). Patients with susceptible pathogens were more likely to receive appropriate empiric antibiotic therapy (36/51, 76%) vs. those with resistant pathogens (16/51, 36%) (p < 0.001). Inappropriate therapy was mostly due to late initiation (i.e. > 2 days after infection) of antibiotic therapy (similar in both groups: 8/51 vs. 11/51, p = 0.44), or due to administration of beta-lactam/beta-lactamase inhibitors against resistant pathogens (1/51 vs. 10/51, p = 0.004).
Unfavourable outcome occurred in 9/51 (18%) controls with non-ESBL-E infections as compared to 24/51 (47%) patients with ESBL-E infections (p < 0.003). This difference was mainly due to relapse of infection (5/51 vs. 20/51, p = 0.001), whereas graft loss (4/51 vs. 1/51) and mortality (3/51 vs. 4/51) were similar in both groups.
Evaluating the impact of resistance on unfavourable outcome, infection with ESBL-E was associated with unfavourable (OR = 4.0, 95%-CI: 1.7–10.5, p = 0.003) and adequate empiric therapy (OR = 0.3, 95%-CI: 0.1–0.9, p = 0.03) with favourable outcome in univariate analysis (Table 4). After adjusting for modifiable (adequate empiric therapy) and non-modifiable (gender and need for ICU stay after infection) factors, the effect of ESBL-E infection was still large, but not anymore significantly associated with unfavourable outcome (adjusted OR = 3.1, 95%-CI: 0.8–12.5, p = 0.10).
Discussion
In this study based on data from a prospective national cohort representing all SOT-recipients in Switzerland, we show that ESBL-producing non-E. coli infections have been increasing over the last years and that antibiotic pre-treatment is independently associated with infection caused by ESBL-producing pathogens. Almost half of patients with ESBL-E had a relapsing infection compared to only 18% in those with non-ESBL E infections. Adequate empiric therapy, being less common among those with ESBL-E infection, was the only modifiable factor associated with unfavourable outcome. The comprehensive dataset and the thorough revision of the original data are particular strengths of the study.
The three last decades have witnessed a global dissemination of ESBL-producing Enterobacterales into healthcare systems and healthy populations alike [14]. From an epidemiological perspective, their emergence is propelled by various factors. Important drivers of community-acquisition (mostly ESBL-E. coli carrying the plasmid-encoded blaCTX-M gene) are travel to/healthcare in endemic countries or household crowding [15, 16]. In contrast, ESBL-non E. coli (mostly Klebsiella spp.) are often hospital-acquired, mainly as a result of clonal expansion due to person-to-person transmission [17]. In our cohort of SOT-recipients, we found stable numbers or ESBL-E. coli, but an increase in ESBL-non E. coli over time, a worrisome finding which has also been observed in other European SOT cohorts [18,19,20]. We can only speculate as to the reason for this trend. Increased in-hospital transmission of these pathogens is one possible explanation, given the substantial differences in the prevalence of ESBL-producing Enterobacterales among participating centres in our study and given the many reports of resistant K. pneumoniae (and particularly high-risk clones such as ST11 or ST147) as a cause of nosocomial outbreaks among transplant and non-transplant patients [21,22,23,24].
In both community and hospital settings, antibiotic treatment—mainly cephalosporins but also quinolones—is associated with ESBL-colonization or infection, probably due to selection (or co-selection in the case of quinolones) of ESBL-E in the gut flora of colonized patients [15, 25]. Razazi et al. found treatment with 3rd generation cephalosporins to be predictive of ESBL-E colonization in patients admitted to intensive care [26]. In a Canadian cohort of RTR, antibiotic pre-treatment was the strongest risk factor for detection of resistant Gram-negative bacteria in the urine [27]; in a recent study on enterobacterial bloodstream infections among SOT-recipients in the United States, antibiotic exposure to trimethoprim-sulfamethoxazole and again 3rd generation cephalosporins were strong risk factors for infections with ESBL-producers [28]. We could confirm this important finding in our cohort of SOT-recipients. Due to the small sample size of the subgroups in our cohort, it is difficult to tell which antibiotic substances contributed mainly to this effect. It is important to note that not only cephalosporins or quinolones, but also beta-lactam/beta-lactamase inhibitor combinations have been independently associated with ESBL-Klebsiella spp., at least in hospitalized non-transplant patients [29]. Consequently, we think that antibiotic stewardship programs aiming at reducing the overall antibiotic use in this population is key in lowering the selection pressure for ESBL-E and other resistant pathogens. In this context it is important to note that—according to a recent survey among European transplant centres—a majority of transplant physicians uses antibiotics including quinolones and cephalosporins for asymptomatic bacteriuria in RTR despite the uncertain benefit of this intervention [30, 31].
In general, SOT-recipients infected with resistant pathogens fare worse than those infected with susceptible bacteria. Delmas-Frenette et al. found resistance to be associated with a longer duration of antibiotic treatment and a higher rate of hospitalization [27]. Other studies have even reported higher case fatality rates in those with resistant bacterial infections [6, 7]. In our univariable analysis, ESBL-production was strongly associated with unfavourable outcome, which was mostly recurrent infection among RTR. Similarly, data from a systematic review showed that UTI recurrence was clearly more common among RTR infected with ESBL-E compared to non-ESBL-E [5]. There are different reasons which could explain the higher recurrence rate in those with ESBL-E infections. First, as shown above, ESBL-infection develops more often in those with previous antibiotic treatment. This is probably the first step in a vicious circle, as antibiotic treatment itself might increase the risk for UTI recurrence which then again exposes the patient to antibiotic treatment [32]. Breaking this circle could be achieved by a reduction of antibiotic use. At least for the prevention of UTIs with non-antibiotic substances studies have shown promising results for transplant and non-transplant patients [33, 34]. Second, there have been suggestions that E. coli strains like ST131, a hyperendemic clone often associated with ESBL-production, come along with increased virulence compared to susceptible pathogens [35]. However, in a study among healthy young women ST131 was not associated with recurrence [36]. Third, patients with resistant infections are less likely to receive appropriate empiric antibiotic therapy, as shown in our results and by others [7]. In a recent study on community-acquired UTI among non-transplant patients, the higher recurrence rate among ESBL-E infections was primarily driven by inappropriate antibiotic treatment [37]. This is in line with results from our multivariable analysis, showing a reduction of the association between ESBL and unfavourable outcome after adjusting for inappropriate empiric therapy. Improving appropriateness of empiric therapy in this population without universally administering carbapenems represents a challenge. However, using clinical prediction tools which identify patients at high risk for ESBL-E infection could be an option [38]. Also, shortening the turnaround time of resistance results might mitigate the deleterious effect of inappropriate empiric therapy.
Limitations of our study are the retrospective design and the lack of travel history, which in the healthy population is among the most important risk factors for ESBL-E colonization and infection. However, travel is unlikely to have a major impact in this particular patient population. Because most of our study participants were RTR, the results might not be applicable to other SOT recipients. The sample size for the analysis of unfavourable outcome might have been too small to draw valid conclusions. In particular, our study might have been underpowered to evaluate the impact of being a RTR (compared to other SOT recipients), of infections with K. pneumoniae (vs. E. coli), or of treatment with carbapenems, which were all marginally associated with unfavourable outcome in univariate analysis. Also, molecular analysis of causing pathogens, which could shed light on the molecular epidemiology of ESBL-E in our geographic area including the presence of E. coli ST131 or healthcare-associated K. pneumoniae clones, was not performed. Further, using ESC-resistance as a proxy for ESBL-production is debatable. However, in the antibiotic resistance report of the ECDC from 2016, 89% of ESC-resistant E. coli were ESBL-producers [39]. Last, appropriateness of antibiotic therapy was defined in a rather conservative way, categorizing treatment with piperacillin/tazobactam as inappropriate also in non-bacteremic urinary tract infections.
Conclusions
To conclude, ESBL-production among non-E. coli Enterobacterales has steadily been increasing among Swiss SOT-recipients in recent years. The role of resistant high-risk clones in this worrisome trend remains unknown. The only modifiable factor associated with the occurrence of ESBL-producing pathogens in our study was antibiotic pre-treatment, calling for action to strengthen antibiotic stewardship programs in this setting. Also, improving appropriateness of empiric antibiotic treatment might be an important measure to reduce unfavourable outcome, which occurred in almost half of SOT-recipients with ESBL-E infections.
Availability of data and materials
The datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- CPE:
-
Carbapenemase-producing Enterobacterales
- ECDC:
-
European Centre for Disease Prevention and Control
- ESBL:
-
Extended-spectrum beta-lactamase
- ESBL-E:
-
ESBL-producing Enterobacterales
- ESC:
-
Extended-spectrum cephalosporin
- ICU:
-
Intensive Care Unit
- ID:
-
Infectious diseases
- IQR:
-
Interquartile range
- MDR:
-
Multidrug-resistance
- RTR:
-
Renal transplant recipient
- OR:
-
Odds ratio
- SOT:
-
Solid organ transplant
- STCS:
-
Swiss Transplant Cohort Study
- UTI:
-
Urinary tract infection
References
Rodriguez-Bano J, Picon E, Gijon P, Hernandez JR, Cisneros JM, Pena C, et al. Risk factors and prognosis of nosocomial bloodstream infections caused by extended-spectrum-lactamase-producing Escherichia coli. J Clin Microbiol. 2010;48:1726–31.
Mularoni A, Bertani A, Vizzini G, Gona F, Campanella M, Spada M, et al. Outcome of transplantation using organs from donors infected or colonized with carbapenem-resistant gram-negative bacteria. Am J Transplant. 2015;15:2674–82.
Alevizakos M, Kallias A, Flokas ME, Mylonakis E. Colonization with extended-spectrum beta-lactamase-producing Enterobacteriaceae in solid organ transplantation: a meta-analysis and review. Transpl Infect Dis. 2017;19:e12718.
Wilkowski P, Gajko K, Marczak M, Hryniewiecka E, Wojtowicz M, Dobrzaniecka K, et al. Clinical significance of gastrointestinal carriage of Klebsiella pneumonia—producing extended-spectrum beta-lactamases in kidney graft recipients. Transpl Proc. 2018;50:1874–7.
Alevizakos M, Nasioudis D, Mylonakis E. Urinary tract infections caused by ESBL-producing Enterobacteriaceae in renal transplant recipients: a systematic review and meta-analysis. Transpl Infect Dis. 2017;19:e12759.
Linares L, Cervera C, Cofán F, Ricart MJ, Esforzado N, Torregrosa V, et al. Epidemiology and outcomes of multiple antibiotic-resistant bacterial infection in renal transplantation. Transpl Proc. 2007;39:2222–4.
Bodro M, Sabé N, Tubau F, Lladó L, Baliellas C, Roca J, et al. Risk factors and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in solid-organ transplant recipients. Transplantation. 2013;96:843–9.
Bergamasco MD, Barroso Barbosa M, de Oliveira GD, Cipullo R, Moreira JCM, Baia C, et al. Infection with Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae in solid organ transplantation. Transpl Infect Dis. 2012;14:198–205.
Clancy CJ, Chen L, Shields RK, Zhao Y, Cheng S, Chavda KD, et al. Epidemiology and molecular characterization of bacteremia due to carbapenem-resistant Klebsiella pneumoniae in transplant recipients. Am J Transplant. 2013;13:2619–33.
van Delden C, Stampf S, Hirsch HH, Manuel O, Meylan P, Cusini A, et al. Burden and timeline of infectious diseases in the first year after solid organ transplantation in the Swiss Transplant Cohort Study. Clin Infect Dis. 2020;71:e159–69.
Koller MT, van Delden C, Müller NJ, Baumann P, Lovis C, Marti H-P, et al. Design and methodology of the Swiss Transplant Cohort Study (STCS): a comprehensive prospective nationwide long-term follow-up cohort. Eur J Epidemiol. 2013;28:347–55.
Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81.
Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–37.
Pitout JDD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8:159–66.
Karanika S, Karantanos T, Arvanitis M, Grigoras C, Mylonakis E. Fecal colonization with extended-spectrum beta-lactamase-producing Enterobacteriaceae and risk factors among healthy individuals: a systematic review and metaanalysis. Clin Infect Dis. 2016;63:310–8.
Otter JA, Natale A, Batra R, Tosas Auguet O, Dyakova E, Goldenberg SD, et al. Individual- and community-level risk factors for ESBL Enterobacteriaceae colonization identified by universal admission screening in London. Clin Microbiol Infect. 2019;25:1259–65.
Freeman JT, Rubin J, McAuliffe GN, Peirano G, Roberts SA, Drinković D, et al. Differences in risk-factor profiles between patients with ESBL-producing Escherichia coli and Klebsiella pneumoniae: a multicentre case–case comparison study. Antimicrob Resist Infect Control. 2014;3:27.
Origüen J, Fernández-Ruiz M, López-Medrano F, Ruiz-Merlo T, González E, Morales JM, et al. Progressive increase of resistance in Enterobacteriaceae urinary isolates from kidney transplant recipients over the past decade: narrowing of the therapeutic options. Transpl Infect Dis. 2016;18:575–84.
Oriol I, Sabé N, Simonetti AF, Lladó L, Manonelles A, González J, et al. Changing trends in the aetiology, treatment and outcomes of bloodstream infection occurring in the first year after solid organ transplantation: a single-centre prospective cohort study. Transpl Int. 2017;30:903–13.
Korth J, Kukalla J, Rath P-M, Dolff S, Krull M, Guberina H, et al. Increased resistance of gram-negative urinary pathogens after kidney transplantation. BMC Nephrol. 2017;18:164.
Kassis-Chikhani N, Saliba F, Carbonne A, Neuville S, Decre D, Sengelin C, et al. Extended measures for controlling an outbreak of VIM-1 producing imipenem-resistant Klebsiella pneumoniae in a liver transplant centre in France, 2003–2004. Euro Surveill. 2010;15:19713.
Matsumura Y, Tanaka M, Yamamoto M, Nagao M, Machida K, Ito Y, et al. High prevalence of carbapenem resistance among plasmid-mediated AmpC β-lactamase-producing Klebsiella pneumoniae during outbreaks in liver transplantation units. Int J Antimicrob Agents. 2015;45:33–40.
Pena I, Picazo JJ, Rodríguez-Avial C, Rodríguez-Avial I. Carbapenemase-producing Enterobacteriaceae in a tertiary hospital in Madrid, Spain: high percentage of colistin resistance among VIM-1-producing Klebsiella pneumoniae ST11 isolates. Int J Antimicrob Agents. 2014;43:460–4.
Damjanova I, Tóth A, Pászti J, Hajbel-Vékony G, Jakab M, Berta J, et al. Expansion and countrywide dissemination of ST11, ST15 and ST147 ciprofloxacin-resistant CTX-M-15-type beta-lactamase-producing Klebsiella pneumoniae epidemic clones in Hungary in 2005—the new “MRSAs”? J Antimicrob Chemother. 2008;62:978–85.
Houard M, Rouzé A, Ledoux G, Six S, Jaillette E, Poissy J, et al. Relationship between digestive tract colonization and subsequent ventilator-associated pneumonia related to ESBL-producing Enterobacteriaceae. PLoS ONE. 2018;13:e0201688.
Razazi K, Derde LPG, Verachten M, Legrand P, Lesprit P, Brun-Buisson C. Clinical impact and risk factors for colonization with extended-spectrum β-lactamase-producing bacteria in the intensive care unit. Intensive Care Med. 2012;38:1769–78.
Delmas-Frenette C, Dorais M, Tavares-Brum A, Frenette C, Yang B, Medani S, et al. Epidemiology and outcome of antimicrobial resistance to gram-negative pathogens in bacteriuric kidney transplant recipients. Transpl Infect Dis. 2017;19:e12722.
Anesi JA, Lautenbach E, Tamma PD, Thom KA, Blumberg EA, Alby K, et al. Risk factors for extended-spectrum β-lactamase–producing enterobacterales bloodstream infection among solid-organ transplant recipients. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa190/5764495.
Wener KM, Schechner V, Gold HS, Wright SB, Carmeli Y. Treatment with fluoroquinolones or with beta-lactam-beta-lactamase inhibitor combinations is a risk factor for isolation of extended-spectrum-beta-lactamase-producing Klebsiella species in hospitalized patients. Antimicrob Agents Chemother. 2010;54:2010–6.
Coussement J, Maggiore U, Manuel O, Scemla A, López-Medrano F, Nagler EV, et al. Diagnosis and management of asymptomatic bacteriuria in kidney transplant recipients: a survey of current practice in Europe. Nephrol Dial Transplant. 2018;33:1661–8.
Origüen J, López-Medrano F, Fernández-Ruiz M, Polanco N, Gutiérrez E, González E, et al. Should asymptomatic bacteriuria be systematically treated in kidney transplant recipients? Results from a randomized controlled trial. Am J Transplant. 2016;16:2943–53.
Cai T, Mazzoli S, Mondaini N, Meacci F, Nesi G, D’Elia C, et al. The role of asymptomatic bacteriuria in young women with recurrent urinary tract infections: to treat or not to treat? Clin Infect Dis. 2012;55:771–7.
Pagonas N, Hörstrup J, Schmidt D, Benz P, Schindler R, Reinke P, et al. Prophylaxis of recurrent urinary tract infection after renal transplantation by cranberry juice and L-methionine. Transplant Proc. 2012;44:3017–21.
Albrecht U, Goos K-H, Schneider B. A randomised, double-blind, placebo-controlled trial of a herbal medicinal product containing Tropaeoli majoris herba (Nasturtium) and Armoraciae rusticanae radix (Horseradish) for the prophylactic treatment of patients with chronically recurrent lower urinary tract infections. Curr Med Res Opin. 2007;23:2415–22.
Nicolas-Chanoine M-H, Bertrand X, Madec J-Y. Escherichia coli ST131, an intriguing clonal group. Clin Microbiol Rev. 2014;27:543–74.
Ismail MD, Ali I, Hatt S, Salzman EA, Cronenwett AW, Marrs CF, et al. Association of Escherichia coli ST131 lineage with risk of urinary tract infection recurrence among young women. J Glob Antimicrob Resist. 2018;13:81–4.
Anesi JA, Lautenbach E, Nachamkin I, Garrigan C, Bilker WB, Omorogbe J, et al. The role of extended-spectrum cephalosporin-resistance in recurrent community-onset Enterobacteriaceae urinary tract infections: a retrospective cohort study. BMC Infect Dis. 2019;19:163.
Wang R, Lautenbach E, Han J, Blumberg E, Tamma P, Thom K, et al. 1588. Clinical prediction tool for extended-spectrum Β lactamase-producing Enterobacteriaceae as the etiology of bacteremia in solid organ transplant recipients. Open Forum Infect Dis. 2018;5:S497–S497.
ECDC Surveillance Report. Surveillance of antimicrobial resistance in Europe. https://ecdc.europa.eu/sites/portal/files/documents/AMR-surveillance-Europe-2016.pdf. Last accessed 17 Dec 2020.
Acknowledgements
None.
The members of the Swiss Transplant Cohort Study are: Patrizia Amico, Andres Axel, John-David Aubert, Vanessa Banz, Beckmann Sonja, Guido Beldi, Christian Benden, Christoph Berger, Isabelle Binet, Pierre-Yves Bochud, Sanda Branca, Heiner Bucher, Thierry Carrel, Emmanuelle Catana, Yves Chalandon, Sabina de Geest, Olivier de Rougemont, Michael Dickenmann, Joëlle Lynn Dreifuss, Michel Duchosal, Thomas Fehr, Sylvie Ferrari-Lacraz, Christian Garzoni, Paola Gasche Soccal, Christophe Gaudet, Emiliano Giostra, Déla Golshayan, Karine Hadaya, Jörg Halter, Dimitri Hauri, Dominik Heim, Christoph Hess, Sven Hillinger, Hans Hirsch, Patricia Hirt, Günther Hofbauer, Uyen Huynh-Do, Franz Immer, Michael Koller (Head of the data center), Bettina Laesser, Brian Lang, Roger Lehmann, Alexander Leichtle, Christian Lovis, Oriol Manuel, Hans-Peter Marti, Pierre Yves Martin, Michele Martinelli, Katell Mellac, Aurélia Merçay, Karin Mettler, Pascal Meylan, Nicolas Mueller (Chairman Scientific Committee), Antonia Müller, Thomas Müller, Ulrike Müller-Arndt, Beat Müllhaupt, Mirjam Nägeli, Manuel Pascual (Executive office), Klara Posfay-Barbe, Juliane Rick, Anne Rosselet, Simona Rossi, Silvia Rothlin, Frank Ruschitzka, Urs Schanz, Stefan Schaub, Aurelia Schnyder, Macé Schuurmans, Federico Simonetta, Katharina Staufer, Susanne Stampf, Jürg Steiger (Head, Excecutive office), Guido Stirniman, Christian Toso, Christian Van Delden (Executive office), Jean-Pierre Venetz, Jean Villard, Madeleine Wick (STCS coordinator), Markus Wilhelm, Patrick Yerly
Funding
The Swiss Transplant Cohort Study is supported by the Swiss National Science Foundation (SNSF, http://www.snf.ch), Unimedsuisse (https://www.unimedsuisse.ch) and the participating Transplant Centers. PK received an Ambizione Career Grant from the SNSF (Grant Number 179919). PWS is supported by the academic career program “Filling the Gap” of the Medical Faculty of the University of Zurich.
Author information
Authors and Affiliations
Consortia
Contributions
Participated in research design (PK, AW, CvD, SK, OM, PS, NM); Participated in the writing of the paper (all authors); Participated in the performance of the research (data collection: PK, AW, AB, KB, MF, CH, OM, DN, SR, LW); Participated in data analysis (PK, AW, SS, AB, NM). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from all patients; ethics approval was obtained in all participating centres.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kohler, P., Wolfensberger, A., Stampf, S. et al. Temporal trends, risk factors and outcomes of infections due to extended-spectrum β-lactamase producing Enterobacterales in Swiss solid organ transplant recipients between 2012 and 2018. Antimicrob Resist Infect Control 10, 50 (2021). https://doi.org/10.1186/s13756-021-00918-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-021-00918-7