Abstract
Background
Setting large- and medium-sized wild mammal (≥ 2 kg) restoration goals is important due to their role as ecosystem engineers and generalized numeric reductions. However, determining wild mammal restoration goals is very challenging due to difficulties in obtaining data on current mammal density and due to unclear information on what mammal density values should be used as a reference. Here we chose a 154 ha conservation area within one of the last remnants of the mountainous Chaco from central Argentina. We suspected that extensive and unreported defaunation had occurred due to past human pressure and the introduction of non-native mammals. To conduct the analyses, we used a simplified technique that integrates methods used in rangeland and ecological sciences.
Results
Eight native mammal species including only one herbivore species, and four non-native mammal species including three herbivore species were detected during 6113 camera trap days. We used known cattle densities as estimated by droppings and direct counts, together with the relative abundance indexes obtained from camera trap photos to calculate the densities of the other species, correcting for mammal size. Densities for the least and most abundant native species were 0.2 and 1.33 individuals km−2, respectively; and for non-native species, 0.03 and 5.00 individuals km−2, respectively. Native and non-native species represented 0.8% and 99.2%, respectively, of the biomass estimates. Reference values for native herbivore biomass, as estimated from net primary productivity, were 68 times higher than values estimated for the study area (3179 vs. 46.5 kg km−2).
Conclusions
There is an urgent need to increase native mammals, with special emphasis on herbivore biomass and richness, while non-native mammal numbers must be reduced. As cattle are widespread in large portions of the globe and there is a lot of experience estimating their abundances, the ratio method we used extrapolating from cattle to other large- and medium-sized mammals could facilitate estimating mammal restoration goals in other small and defaunated areas, where traditional methods are not feasible when target mammal densities get very low.
Similar content being viewed by others
Background
Large- and medium-sized mammals play a key role in ecosystem structure and functioning (Ripple et al. 2015). Herbivores modify vegetation composition and structure through grazing, browsing and trampling, accelerate nutrient recycling and modify the water cycle, whereas carnivores regulate the abundances and habitat selection patterns of their prey (Ripple and Beschta 2003; Cingolani et al. 2014; Periago et al. 2015). Many mammals such as foxes (Lycalopex gymnocercus) and wild boars (Sus scrofa) are good seed dispersers, whereas burrowing mammals provide habitat for a large range of organisms that do not dig burrows but use active or abandoned burrows (Bodmer 1991; Torres and Tamburini 2018; Varela et al. 2008; Davidson et al. 2012).
A balanced and functional ecosystem must have most or all of its components; however, large native wild mammal populations, and biodiversity in general, are being lost due to the rapid habitat transformation, climate change, over exploitation, and invasion by non-native species driven by the increasing world human population (Ripple et al. 2015; Johnson et al. 2017). Instead, a few mammal species have spread over large parts of the globe, often causing ecosystem dysfunction in their non-native ranges (Benitez et al. 2013; Novillo and Ojeda 2008). For example, dogs (Canis familiaris) have been responsible for the decline of native carnivore mammals (Zapata-Ríos and Branch 2018) and livestock (Bos taurus) have caused drastic soil losses, reductions in forage availability for native herbivores, and changes in carnivore prey composition (e.g., Pia et al. 2003, 2013; Hofgaard et al. 2009; Cingolani et al. 2013).
To manage wild mammal populations to help restore a balanced and functional ecosystem we need: (1) basic information on mammal present-day composition, density and biomass; and (2) a description of the desired future trajectory that the mammal community should undergo to meet restoration targets and goals. The desired restoration goals are set using primary sources of information from undamaged reference areas, or secondary sources of information on the areas before they were impaired (Clewell and Aronson 2017). Unfortunately, undamaged reference areas are increasingly rare, and past species composition and biomass are difficult to estimate in the case of mammals (Fløjgaard et al. 2022). An interesting way out for the case of herbivores is obtaining reference values from long-term average estimates of wild herbivore biomass in protected areas and relating to aerial net primary productivity (McNaughton et al. 1989, 1991; Oesterheld et al. 1992) or net primary productivity (Fløjgaard et al. 2022). For other mammal trophic guilds, the only alternative may be to rely on the scarce literature available and local knowledge.
Based on a literature review, species lists are the only available information on mammal composition, density and biomass values for the mountain Chaco of South America (e.g., Fariñas Torres et al. 2019), but no studies indicating at least the relative abundances, densities or biomass of large mammals are yet available. Thus, a quantitative study reporting mammal species richness with a rough estimate of their densities and biomass is indispensable to complete the process of assessing restoration goals. Density and distribution patterns are not well-known for most of the wild mammals inhabiting central Argentina (Torres and Tamburini 2018) and improved knowledge would contribute to the development and planning of better conservation strategies (Bruner et al. 2001). The vegetation of the mountain Chaco in central Argentina consists of a seasonally dry forest that has partially escaped the great expansion of agriculture during the last decades due to its rugged topography. However, recurrent wildfires, the presence of domestic livestock, forestry activities, forest fragmentation, displacement by invasive species and urbanization are rapidly transforming the mountain Chaco ecosystem (Giorgis et al. 2017; Aguilar et al. 2018; Cingolani et al. 2022).
Our objectives were to: (1) generate the first density and biomass estimates for large- and medium-sized mammal species (≥ 2 kg, hereafter, mammals) for one of the few remaining mountain Chaco woodlands; (2) compare the relative importance of native and non-native species for each of the main trophic guilds; (3) Inform possible restoration goals by estimating reference biomass values of native herbivores, as expected based on net primary productivity. We hypothesized that we would find an impoverished mammal community due to centuries of human activities and the introduction of several non-native species, such as cattle and dogs.
Methods
Study area
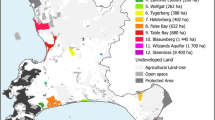
The mountain Chaco (also called Chaco Serrano) is part of the Chaco Biogeographic Province and extends from the mountains of central Argentina to northwest Argentina and southern Bolivia, forming complex ecotones with upper mountain ecosystems and woody communities and agricultural landscapes dominating the lower Chaco plains (Prado 1993; Fig. 1).
The mountains of central Argentina (latitude 31.4716° S, longitude 64.6125° W, elevation 500 to 2770 m) have a seasonally dry subtropical to temperate climate. Mean annual temperatures at 500 m asl vary between 19 °C in the northwest and 16 °C in the south and decrease with elevation to 7 °C at 2700 m. Average annual rainfall varies between 650 and 984 mm, as recorded from 1992 to 2020; rainfall is concentrated in the warm months, between September and March (CIRSA 2014–2020). The area is characterized by a mosaic of physiognomic vegetation types: (1) grasslands (2) shrublands; and (3) a mix of open and closed woodlands of variable height (Giorgis et al. 2017; Cingolani et al. 2022).
Fieldwork was carried out in a private land managed for nature conservation called “El Mollar” (154 ha; elevation 800 to 1120 m). The landscape consists of a mosaic of grasslands (10%), shrublands (50%), and forests dominated by Lithraea molleoides trees (40%); the forest proportion is well above the average for the mountain Chaco of central Argentina (5.5%, Cingolani et al. 2022). According to recollections of the first author, who has lived in the area for the past 40 years, in the 1980s one wildfire affected 7% of the area; thus, annual fire incidence could be estimated as 0.17%, which is similar to the average fire incidence reported for the mountains of central Argentina (Argañaraz et al. 2020). The land is situated within a patch of 275 km2 of fairly well-preserved mountain Chaco vegetation, which has not been fragmented by roads or urbanization (Cingolani et al. 2022).
The land was managed for traditional livestock rearing, since at least the 1920s, but we did not find records of stocking densities for the 1900s. Traditional livestock rearing for the area involves continuous stocking rates of free ranging cattle which feed on natural vegetation and are not supplemented with extra feed, From 2008 to 2012, livestock densities were 40 cattle km−2 (personal communication from previous land tenants). Most livestock were removed in 2013, with a low density of cattle and horses remaining in the area due to herding difficulties in the rugged topography. New landowners devoted the land to nature conservation in 2015. Direct cattle counts and estimates based on dung count suggest a density of 5 cattle km−2 for the 2015–2016 period (Barberá et al. 2018) due to the presence of feral cattle; this situation persisted to the end of the present study, according to direct counts (Renison, personal observations). From 2015 to the end of this study, the conservation management consisted of weekly rounds to reduce poaching (mainly of herbal plants) and mechanical control of non-native plants, which occupy less than 5% of the area but increasing.
Besides past livestock rearing, present-day feral cattle presence, and invasions by non-native plants, such as Rubus ulmifolius, Lantana camara and Pyracantha angustifolia, the study area does not have other evident signs of human impact. The closest road and village are 2 km away in a straight line and represent a 1 h walking distance. A nearby river and adjacent stream are visited by tourists during the warm season.
Camera traps and settings
To enable comparison with other studies, we follow the advice of Meek et al. (2014) to report camera trap settings, survey design, and camera deployment. We used a total of 12 cameras, which were incorporated progressively during the study. We started the formal study with one camera in September 2015, added three in November 2016, four in January 2017, two in February 2018, and two more in March 2019, all used until October 2019, totaling a period of 4 years when the cameras were deployed continuously. The cameras were of three models: Denver WCT-810 (six cameras), Acorn LTL 5210A (four cameras), and Browning Trail Camera Model BTC-6HDP (two cameras). All three models use passive infrared sensors to trigger the cameras and had red light flashes for nocturnal pictures.
Camera settings were selected based on trials conducted for 6 months before the start of the study, where we tried different settings (data not reported). To avoid excess photos on windy days, we set the cameras to medium sensitivity and to wait 10 min between each camera trigger. All other settings were left in default.
Survey design and camera deployment
Camera trap locations were determined using Google Earth by haphazardly choosing tentative coordinates of trap stations separated by at least 100 m, including all sections of the study area except operator inaccessible areas such as steep rock cliffs and sites invaded by Rubus ulmifolius thickets. In the field, we located the tentative trapping coordinates using a GPS and from there we selected the closest site that we considered adequate for the trap station.
We chose trap stations, where the camera could be placed away from direct sunlight and where the cameras view included at least 12 m2 of ground cover, which was achieved by clipping interfering vegetation when necessary. In addition, we selected station locations that, taken together, were representative of the vegetation of the whole area. In other words, the station-day weighted average for four main land-cover variables was similar to the average for the whole study area, calculated from a vegetation map and the mean values of those cover variables in each map class (Cingolani et al. 2022). Camera traps were left out in the same trap station for at least 2 months and then re-positioned. This setup resulted in 74 trap stations, where cameras were active for 20 days or more, and 6113 camera trap days.
Data extraction
We constructed a first matrix, where for each camera-trap station, we recorded a unique ID number, coordinates, elevation, start and finish date and species identification. We identified mammal species following Torres and Tamburini (2018), discarding data from the few individuals we were not able to identify to the species level. We only considered a new record if 4 h had elapsed between two consecutive photographs of the same species. We selected 4 h and not the more traditional 0.5 or 1 h, because these smaller time intervals artificially augmented feral cattle numbers due to their habit of staying at the same place for rumination. This procedure seldom reduced counts of other species.
We extracted mass values and diet types of the native photo-trapped species from Torres and Tamburini (2018). We used maximum mass values for all species, because we did not find mean values for several species, and we preferred to optimize comparability between species. For feral cattle, horses, and dogs, we used maximum mass for the photo-trapped breeds of each species as reported by local informants, taking into account that for feral cattle 25% of the animals were bulls. The mass values we used for each species are shown in Table 1. Diets were categorized into mainly herbivore, omnivore, insectivore and carnivore. We lumped into mainly herbivorous all grazers, browsers and root eaters when their diets consisted mainly of one or more of these components. Our study area had almost no large (< 1 cm in diameter) fruit bearing plants so we did not consider frugivory when categorizing diets. As a consequence, we considered the pampas fox (Lycalopex gymnocercus) to be an omnivore even when in other regions it could be considered mainly a frugivore (Varela et al. 2008).
Reference biomass
To estimate reference wild herbivore biomass for our study area, we re-analysed the herbivore biomass global data base (for herbivores larger than 5 kg) reported by Fløjgaard et al. (2022) as Suppl. Material. We obtained net primary productivity (NPP) values for each observation from a 30-arc seconds resolution satellite-derived global map (Zhao et al. 2005, 2006). From these data, we obtained Eqs. (1, 2 and 3) to estimate global wild herbivore reference values and values for Africa and the Americas, respectively (see details in Additional file 1: Appendix S1). We estimated those continents separately, because they widely differ in the wild mammal biomass they hold, even when primary productivity is controlled, possibly due to Pleistocene human induced extinctions in the Americas (Fløjgaard et al. 2022):
where BiomassG is the global herbivore biomass (kg km–2) estimation, BiomassAf is the herbivore biomass estimation for Africa, BiomassAm is the herbivore estimation for the Americas, and NPP is the net primary productivity (kg ha–1) obtained from the global map.
From the same map, we obtained NPP for our study area and, using the above equations, we estimated the three values of possible reference biomass and their confidence intervals (see Additional file 1: Appendix S1).
Data processing and analysis
To check for the completeness of our study regarding species richness, we generated a rarefaction curve implemented in the vegan package of R (Oksanen et al. 2020). To provide density estimates for the camera trapped species we were unable to use traditional data processing and analysis, such as site-structured models, unmarked spatial capture–recapture, random encounter model, time or space-to-event models and distance sampling. The reason was that their assumptions were not met: individual animals were often camera trapped at the same or other trapping stations, and we had a relatively high proportion of trapping stations with no or few photo trapped individuals for most of the species (Gilbert et al 2020). Because of the very low densities of wild animals inhabiting the study area, cameras had to be placed for long time periods and trapping stations had to be relatively packed together to fit them all. This scenario is likely to happen in most remaining small fragments of wild-land that hold few mammals, precluding the possibility of using standard methods of calculating densities. To circumvent this caveat we adopted a novel approach that consisted of extrapolating the density of feral cattle, which was known from dung counts and direct counts, to other species. This technique has its precursors in a study of white-tailed deer (Odocoileus virginianus) performed by Jacobson et al. (1997) who from known densities of individually identifiable branch-antlered bucks and, from photo ratios, extrapolated to estimate densities of spike bucks, does, and fawns of the same species. The ratio technique functions adequately when individual animals are repeatedly photo-trapped, but is biased at low densities of camera trapping stations when movement patterns are different between studied categories (or species) (Jacobson et al. 1997). As no method of estimating densities is error free (Santini et al. 2022) we judged the ratio approach as the best option given the circumstances.
In addition to the ratio approach, we applied a correction for body mass of the species, accounting for the fact that smaller individuals are less likely to be detected by the camera in comparison with larger individuals. From data in Tobler et al. (2008) we estimated a linear function to calculate the probability that a camera is triggered by a passing animal (hereafter “probability of detection”), as a function of body size. These authors used paired camera traps and empirically estimated, for each species, the probability of being detected with both cameras against the overall detection (one or two cameras) as the ratio between both values. They plotted that probability against the log of body weight, and the relationship was strongly positive (Spearman S = 0.811, N = 14, p < 0.0001, Fig. 2 in Tobler et al. 2008). We obtained the data from the plot and estimated the regression equation, since it was not reported in the study:
where BS is the species’ body size (kg) and Y is the probability of being detected with both cameras against the overall detection (at least one camera). From this equation we calculated Y for each of our species, and then the probability of detection by one camera (PD), considering a binomial distribution, as
We corrected the number of camera trapped individuals by dividing the detected number by the detection probability (PD).
Finally, we calculated the species relative abundance (SRA) as
To estimate species population densities, we extrapolated the ratio of feral cattle density (known from Barberá et al. 2018) to other species using the following equation:
where Di = estimated density of species i (individuals km−2), 5 is cattle density (individuals km−2), and 1117 is the corrected number of photo-trapped cattle individuals in the present study (see Table 1). We estimated the relative proportion of native mammals as
To estimate biomass index we made similar calculations as above, but using camera-trapped mass instead of photo-trapped individuals. Mass per unit area (kg km−2) was calculated for each species by multiplying its maximum mass value by the density of each species.
Results
Field surveys
Our camera trap efforts revealed 12 mammal species with masses of 2 kg or more. Of the 12 recorded species, eight were native and four were non-native. The species accumulation curves as a function of time and non-corrected number of camera-trapped mammals show a relatively long flat plateau, suggesting a low probability of more species being recorded if the camera trap efforts were to continue (Figs. 2 and 3). All native species are categorized as of least concern according to the International Union for Nature Conservation; at the local level, however, one species is categorized as endangered, three as vulnerable, one as near threatened and three as least concern (Table 1).
Native mammal species recorded in the study. A Pecari tajacu. B Lycalopex gymnocercus. C Conepatus chinga. D Leopardus geoffroyi. E Didelphis albiventris. F Puma concolor. G Herpailurus yagouaroundi. H Galictis cuja. Enlarged image of the animal is displayed in the yellow insets for the case of animals that are difficult to identify at the scale of this image
The only photo-trapped native species with a mainly herbivore diet was the collared peccary (Pecari tajacu), with an estimated abundance of 1.33 individuals km−2 and an estimated biomass of 46.5 kg km−2 (Table 1). Non-herbivore photo-trapped species in our study were the white-eared opossum (Didelphis albiventris) and the pampas fox (Lycalopex gymnocercus), with an omnivore diet, the lesser grison (Galictis cuja), the Geoffroy’s cat (Leopardus geoffroyi), the Jaguarundi (Herpailurus yagouaroundi—both dark grey and reddish coloration phenotypes), and the puma (Puma concolor), with a mainly carnivore diet, and the Molina’s hog-nosed skunk (Conepatus chinga) with a mainly insectivore diet. The four species of non-native mammals were feral cattle (Bos taurus), the horse (Equus caballus) and the wild boar (Sus scrofa) with mainly herbivore diets, and the domestic dog (Canis familiaris) with a mainly carnivore diet. Densities and biomass estimations are shown in Table 1.
The number of camera-trapped native and non-native mammals represented 38% and 62%, respectively, of the corrected photo-trapped mammals (out of 2384). However, because of the relatively large mass of the non-native cattle, horses, wild boars, and dogs as compared to the native fauna, the natives and non-natives represented 1.5% and 98.5%, respectively, of the camera-trapped biomass. When we performed the same calculations by main diet type, we found that native herbivores represented 19% and 1.1% of the corrected total photo-trapped mammals and biomass, respectively. The calculations for carnivores indicate 36.1% and 37.3%, respectively. Species with mainly omnivore and insectivore diets were 100% native (see Table 1).
Reference values
Estimated herbivore biomass reference values for the net primary productivity of the mountain Chaco of our study area using relationships at the global level, for the Americas and for Africa were 3179 kg km−2 (2679–3772), 255 kg km−2 (389–345) and 4477 kg km−2 (3391–5909), respectively (see Additional file 1: Appendix S1 for details of estimations). Thus, the estimated reference value was 68, 5.5 and 96 times higher than the biomass value estimated for native herbivores in our study area when using global, Americas and African relationships, respectively. When we included both native and non-native biomass estimations for our study area, target reference values were lower than or similar to herbivore biomass estimations from our study (global, Americas and Africa data sets, respectively, 3179, 255 and 4477 kg km−2 as compared to 4403 kg km−2 for our study areas). The ratio of reference to study site herbivore biomass is a robust estimation regarding changes in cattle density, cattle mass values and correction factors (see simulations in Additional file 2: Appendix S2).
Discussion
Our results support the hypothesis that, across the study area, the mammal community is impoverished presumably due to centuries of human activities and the introduction of several non-native species. Thus, an apparently well-preserved forest as judged by plant composition and structure is not necessarily well-preserved as judged by large and medium-sized mammal composition.
Possibly more species than those detected were present in our study area, since the failure to detect a species does not mean that the species was absent, even with a large trapping effort as ours (Rovero et al. 2010). However, the species accumulation curve suggests it is unlikely that missing species, if present, have numbers large enough to invalidate our main findings. Six mammal species were not photo-trapped in our study area and could be present according to the potential distribution maps of Torres and Tamburini (2018). Three of these missing mammals are herbivores: the gray brocket deer (Mazama gouazoubira), the vizcacha (Lagostomus maximus) and the coypu (Myocastor coypus). The last gray brocket deer were seen in the area during the 1980s (Renison, personal observations), and may have disappeared due to past hunting pressure, competition for pastures with cattle and the presence of dogs (Nanni 2015; Zapata-Ríos and Branch 2016). Abandoned vizcacha burrows were still present in the 1970s according to local informants. Vizcachas are regarded as ecosystem engineers because of their social burrowing habits; therefore, they should be a priority for ongoing re-introduction efforts (Villareal et al. 2008; Davidson et al. 2012; Renison et al. 2023). The coypu is still rarely seen in streams and rivers near our study area and was probably not captured, because we did not focus on streams. Other missing species were the carnivore Pampas cat (Leopardus colocola) which is rare all across its distribution in the Argentine Chaco (Nanni et al. 2020), the omnivore big hairy armadillo (Chaetophractus villosus) that started its decline in the last 1000 years (Soibelzon et al. 2013) and the insectivore southern tamandua (Tamandua tetradactyla) that was first cited in the region 25 years ago and is occasionally but increasingly reported (Tamburini 2018).
More mammal species were likely present in our study area during the past but were not reported in the potential distribution maps of Torres and Tamburini (2018), because their maps were elaborated using presence records from 20 years before their publication. For example, central Argentina was inhabited by jaguars (Panthera onca) up to at least the 1740s and guanacos (Lama guanicoe) up to the 1920s (Barri 2016; Cuyckens et al. 2017).
Even when the ratio method we used to calculate densities has its drawbacks (Jacobson et al. 1997), its errors and biases are very unlikely to invalidate our main findings of extensive defaunation. Wild herbivore reference densities, as calculated using relationships at the global scale, ranged from 2679 to 3772 kg km−2. Even the lower limit of 2679 kg km−2 is more than one order of magnitude higher than the biomass index of 46.5 kg km−2 here estimated for native herbivores in our study area, whereas the upper limit of 3772 kg km−2 is almost 100 times higher. Moreover, even if we consider estimated herbivore biomass for sites with similar productivity in the Americas, which were already defaunated in the Pleistocene, our field estimates are comparatively very low, although we corrected the numbers by the detection probability, and considered the maximum biomass values for native herbivores. Besides having a very low native herbivore biomass in our study area as compared to our reference estimations, herbivores are represented by only one species (the collared peccary). We are thus relatively close to losing all large- and medium-sized native mammal herbivores. The estimated density of 1.33 collared peccary individuals km−2 in our study area is lower than that reported for the Atlantic forests in Brazil, which is also considered defaunated (2.8 to 8.9 individuals km−2 in Keuroghlian et al 2004; 3.35 to 13.55 individuals km−2 in Galetti et al. 2017).
In contrast to the scarcity of native herbivores, our estimation of non-native herbivore biomass (4356 kg km−2) is similar to wild herbivore reference biomass, as calculated using data from Africa, and higher when calculated using global data or data from the American continent. In our study area, cattle and horses were the main exotic mammals, and their known negative effects include delaying forest regeneration (Torres and Renison 2016; Barberá et al. 2018; Torres et al. 2021) and reducing other mammal densities due to direct competition for resources or changes in woody vegetation (Nanni 2015; Periago et al. 2015; Puechagut et al. 2018). The wild boar is an invasive species whose negative global impacts are well-known (Barrios-Garcia and Ballari 2012), and possibly a direct competitor to the only remaining native herbivore, the collared peccary (Barrios-Garcia and Ballari 2012). So far, the density of wild boars in our study area is low as compared to a global assessment (0.02 and 7 individuals km−2, respectively, Sanguinetti and Pastore 2016). In our opinion, management of mountain Chaco protected areas should focus on a reduction in non-native herbivores that allows time for increases in native herbivore numbers. This said, we advocate more studies to determine whether a certain amount of non-native herbivores should be left or not. When native mammals are ecologically extinct, non-natives may partially replace their functions and have positive effects on ecosystem functioning, through modification of land cover patterns that increase native biodiversity, through shared predator avoidance or by providing prey for native carnivores (Schlaepfer et al. 2011; Schieltz and Rubenstein 2016; Buenavista and Palomares 2018).
The native carnivore assemblage, consisting of four camera-trapped species in our study area, appeared to be more resistant to human disturbances than the herbivore assemblage of only one species. Pumas may have survived due to their wide trophic niche and diet adaptations to exotic species (Pessino et al. 2001; Pia et al. 2013). Supporting this statement, non-native prey represented 81% of the biomass consumed by pumas in central Argentina (Zanón-Martínez et al. 2016a), and between 54% and 99% of the diet consumed by the native carnivore assemblage in north-western Patagonia (Novaro et al. 2000). Furthermore, Geoffroy’s cat is not affected or sometimes is even favored by modified habitats (Caruso et al. 2016). Our carnivore density estimations are similar to those reported in other studies. For example, puma density estimation of 0.08 individuals km−2 in our study area was similar to values reported for protected areas in central Argentina, ranging from 0.05 to 0.09 individuals km−2 (Zanón-Martínez et al. 2016b), and within the range of estimations for northern Patagonia and the Chaco plains (0.03 and 0.75 individuals km−2, respectively, Quiroga et al. 2016; Gelin et al. 2017). Geoffroy’s cat density estimations of 0.38 individuals km−2 is within the range of values reported for the Bolivian dry forests (0.09 to 0.4 individuals km−2, Cuellar et al. 2006), and lower than those reported in the Monte ecoregion of central Argentina, ranging from 1.2 to 2.9 individuals km−2 (Pereira et al. 2010). We did not find density estimations for the jaguarundi cat and the lesser grison, but our estimations coincide with other studies that mention these species as very scarce (e.g., Poo-Muñoz et al. 2014; da Silva et al. 2016; Luengos Vidal et al. 2016). Even when the carnivore assemblage appeared to be more resistant than the herbivore assemblage, at the local scale all four carnivores recorded in our study are considered of conservation concern (Torres and Tamburini 2018). In the light of the penetration of introduced dogs into the remaining forests, we recommend the implementation of responsible pet ownership regulations, as well as ethically approved control actions for feral dogs (e.g., Contardo et al. 2021). Dogs are known to prey upon all the native species recorded in our study (Zamora-Nasca et al. 2021).
Associations between our photo-trapped mammal species and land cover variables were only found for the non-native feral cattle. The association between cattle and herbaceous plants usually found in grasslands agrees with previous studies suggesting high cattle selectivity for short palatable grasslands (von Müller et al. 2017), which in our study area were intermingled with taller tussocks. The eight native species that we detected in our study are habitat generalists, as suggested by our results and previous reports (Periago et al. 2017; Torres and Tamburini 2018). Instead, specialists like the southern tamandua and the Pampas cat were absent, possibly because habitat specialists are often more susceptible to anthropogenic changes (e.g., Newbold et al. 2014).
Conclusions
When extreme defaunation has occurred, large- and medium-sized mammal densities can be estimated as a ratio using the species relative abundance index corrected for body mass as estimated from camera traps and comparison to known densities of feral cattle. Large- and medium-sized mammal restoration goals can partly be estimated from primary productivity values. Using these methods we conclude that the present state of our study area is a highly defaunated scenario regarding native large- and medium-sized mammals, a scenario that is likely to be occurring in all the remaining mountain Chaco of central Argentina. The future prospects of the native fauna are bleak unless the defaunated situation is reversed. Management should include active restoration of herbivores increasing the abundance of the only native species present at the moment and reintroducing locally extinct species. Culling non-native species to reduce competition is also necessary. To the best of our knowledge, this is the first quantitative mammal restoration goal proposal for a protected area in South America that calculated reference values, as suggested by Fløjgaard et al. (2022); the ratio method we used to estimate densities could contribute to the determination of mammal restoration goals in other small and very defaunated areas.
Availability of data and materials
Data available at http://hdl.handle.net/11336/187690 or on request to the authors.
References
Aguilar R, Calviño A, Ashworth L, Aguirre-Acosta N, Carbone LM, Albrieu-Llinás G, Nolasco M, Ghilardi A, Cagnolo L (2018) Unprecedented plant species loss after a decade in fragmented subtropical Chaco Serrano forests. PLoS ONE 13:e0206738. https://doi.org/10.1371/journal.pone.0206738
Argañaraz JP, Cingolani AM, Bellis LM, Giorgis MA (2020) Fire incidence along an elevation gradient in the mountains of central Argentina. Ecol Austral 30:268–281. https://doi.org/10.25260/EA.20.30.2.0.1054
Barberá I, Renison D, Torres RC (2018) Regeneration of Sebastiania commersoniana (Euphorbiaceae) in relation to livestock and distance from forest in the mountains of central Argentina. Boletín De La Sociedad Argentina De Botánica 53:405–420
Barri FR (2016) Reintroducing guanaco in the upper belt of central Argentina: using population viability analysis to evaluate extinction risk and management priorities. PLoS ONE 11:e0164806. https://doi.org/10.1371/journal.pone.0164806
Barrios-Garcia MN, Ballari SA (2012) Impact of wild boar (Sus scrofa) in its introduced and native range: a review. Biol Invasions 14:2283–2300
Benitez VV, Almada Chavez S, Gozzi AC, Messetta NL, Guichón ML (2013) Invasion status of Asiatic red-bellied squirrels in Argentina. Mamm Biol 78:164–170
Bodmer RE (1991) Strategies of seed dispersal and seed predation in Amazonian ungulates. Biotropica 23:255–261. https://doi.org/10.2307/2388202
Bruner AG, Gullison RE, Rice RE, da Fonseca GAB (2001) Effectiveness of parks in protecting tropical biodiversity. Science 291:125–128
Buenavista S, Palomares F (2018) The role of exotic mammals in the diet of native carnivores from South America. Mammal Rev 48:37–47. https://doi.org/10.1111/mam.12111
Caruso N, Lucherini M, Fortin D, Casanave EB (2016) Species-specific responses of carnivores to human-induced landscape changes in central Argentina. PLoS ONE 11:e0150488. https://doi.org/10.1371/journal.pone.0150488
Cingolani AM, Vaieretti MV, Giorgis MA, La Torre N, Whitworth-Hulse JI, Renison D (2013) Can livestock and fires convert the sub-tropical mountain rangelands of central Argentina into a rocky desert? Rangeland J 35:285–297
Cingolani AM, Vaieretti MV, Giorgis MA, Poca M, Tecco PA, Gurvich DE (2014) Can livestock grazing maintain landscape diversity and stability in an ecosystem that evolved with wild herbivores? Perspect Plant Ecol Evol Syst 16:143–153
Cingolani AM, Giorgis MA, Hoyos LE, Cabido M (2022) La vegetación de las montañas de Córdoba (Argentina) a comienzos del siglo XXI: un mapa base para el ordenamiento territorial. Boletín De La Sociedad Argentina De Botánica 57:65–100. https://doi.org/10.31055/1851.2372.v57.n1.34924
CIRSA (2014–2020) Anuarios pluviométricos, Cuenca del Río San Antonio. Sistema del Río Suquía - Provincia de Provincia de Córdoba. Instituto Nacional del Agua y Centro de Investigaciones de la Región Semiárida (CIRSA), Córdoba, Argentina
Clewell AF, Aronson J (2017) Ecological restoration: principles, values, and structure of an emerging profession, 2nd edn. Island Press, Washington, DC
Contardo J, Grimm-Seyfarth A, Cattan PE, Schüttler E (2021) Environmental factors regulate occupancy of free-ranging dogs on a sub-Antarctic island, Chile. Biol Invasions 23:677–691. https://doi.org/10.1007/s10530-020-02394-3
Cuellar E, Maffei L, Arispe R, Noss A (2006) Geoffroy’s cats at the northern limit of their range: activity patterns and density estimates from camera trapping in Bolivian dry forests. Stud Neotrop Fauna Environ 41:169–177
Cuyckens GAE, Perovic PG, Herrán M (2017) Living on the edge: regional distribution and retracting range of the jaguar (Panthera onca). Anim Biodivers Conserv 40:71–86
da Silva LG, Oliveira TG, Kasper CB, Cherem JJ, Moraes EA Jr, Paviolo A, Eizirik E (2016) Biogeography of polymorphic phenotypes: mapping and ecological modelling of coat colour variants in an elusive Neotropical cat, the jaguarundi (Puma yagouaroundi). J Zool 299:295–303. https://doi.org/10.1111/jzo.12358
Davidson AD, Detling JK, Brown JH (2012) Ecological roles and conservation challenges of social, burrowing, herbivorous mammals in the world’s grasslands. Front Ecol Environ 10:477–486. https://doi.org/10.1890/110054
Fariñas Torres T, Ríos A, Schiappacasse E, Mañez M, Beruhard J, Morales R (2019) Relevamiento de fauna para la puesta en valor de la Reserva Natural de Usos Múltiples Cerro del Cóndor, Departamento Pocho, Córdoba, Argentina. Nótulas Faunísitica 265:1–10
Fløjgaard C, Birkefeldt Møller Pedersen P, Svenning JC, Ejrnæs R (2022) Exploring a natural baseline for large-herbivore biomass in ecological restoration. J Appl Ecol 59:18–24. https://doi.org/10.1111/1365-2664.14047
Galetti M, Brocardo CR, Begotti RA, Hortenci L, Rocha-Mendes F, Bernardo CSS, Bueno RS, Nobre R, Bovendorp RS, Marques RM, Meirelles F, Gobbo SK, Beca G, Schmaedecke G, Siquiera T (2017) Defaunation and biomass collapse of mammals in the largest Atlantic forest remnant. Anim Conserv 20:270–281. https://doi.org/10.1111/acv.12311
Gelin ML, Branch LC, Thornton DH, Novaro AJ, Gould MJ, Caragiulo A (2017) Response of pumas (Puma concolor) to migration of their primary prey in Patagonia. PLoS ONE 12:e0188877. https://doi.org/10.1371/journal.pone.0188877
Gilbert NA, Clare JDJ, Stenglein JL, Zuckerberg J (2020) Abundance estimation of unmarked animals based on camera-trap data. Conserv Biol 35:88–100. https://doi.org/10.1111/cobi.13517
Giorgis MA, Cingolani AM, Gurvich DE, Tecco PA, Chiapella J, Chiarini F, Cabido M (2017) Changes in floristic composition and physiognomy are decoupled along elevation gradients in central Argentina. Appl Veg Sci 20:558–571
Hofgaard A, Dalen L, Hytteborn H (2009) Tree recruitment above the treeline and potential for climate-driven treeline change. J Veg Sci 20:1133–1144
Jacobson HA, Kroll JC, Browning RW, Koerth BH, Conway MH (1997) Infrared-triggered cameras for censusing white-tailed deer. Wildl Soc Bull 25:547–556
Johnson CN, Balmford A, Brook BW, Buettel JC, Galetti M, Guangchun L, Wilmshurst JM (2017) Biodiversity losses and conservation responses in the Anthropocene. Science 356:270–275. https://doi.org/10.1126/science.aam9317
Keuroghlian A, Eaton DP, Longland WS (2004) Area use by white-lipped and collared peccaries (Tayassu pecari and Tayassu tajacu) in a tropical forest fragment. Biol Conserv 120:411–425
Luengos Vidal EM, Castillo DF, Caruso NC, Casanave EB, Lucherini M (2016) Field capture, chemical immobilization, and morphometrics of a little-studied South American carnivore, the Lesser Grison. Wildl Soc Bull 40:400–405
McNaughton SJ, Oesterheld M, Frank DA, Williams KJ (1989) Ecosystem-level patterns of primary productivity and herbivory in terrestrial habitats. Nature 341:142–144
McNaughton SJ, Oesterheld M, Frank DA, Williams KJ (1991) Primary and secondary production in terrestrial ecosystems. In: Cole J, Lovett G, Findlay S (eds) Comparative analyses of ecosystems patterns, mechanisms, and theories. Springer, New York
Meek PD, Ballard G, Claridge A, Kays R, Moseby K, O’Brien T, O’Connell A, Sanderson J, Swann DE, Tobler M, Townsend S (2014) Recommended guiding principles for reporting on camera trapping research. Biodivers Conserv 23:2321–2343
Nanni AS (2015) Dissimilar responses of the gray brocket deer (Mazama gouazoubira), crab-eating fox (Cerdocyon thous) and pampas fox (Lycalopex gymnocercus) to livestock frequency in subtropical forests of NW Argentina. Mammal Biol 80:260–264
Nanni AS, Castro L, Cuyckens GAE, Barri FR, Giordano AJ, Lucherini M (2020) New records of the pampas cat, Leopardus colocola (Molina, 1782) (Mammalia, carnivora, felidae), from the Chaco ecoregion raise questions about its status in Argentina. Check List 16:729–735
Newbold T, Hudson LN, Phillips HRP, Hill SLL, Contu S, Lysenko I, Blandon A, Butchart SHM, Booth HL, Day J, De Palma A, Harrison MLK, Kirkpatrick L, Pynegar E, Robinson A, Simpson J, Mace GM, Scharlemann JPW, Purvis A (2014) A global model of the response of tropical and sub-tropical forest biodiversity to anthropogenic pressures. Proc R Soc B 281:1371. https://doi.org/10.1098/rspb.2014.1371
Novaro AJ, Funes MC, Walker SR (2000) Ecological extinction of native prey of a carnivore assemblage in Argentine Patagonia. Biol Conserv 92:25–33
Novillo A, Ojeda RA (2008) The exotic mammals of Argentina. Biol Invasions 10:1333–1344
Oesterheld M, Sala OE, McNaughton SJ (1992) Effects of animal husbandry on herbivore-carrying capacity at a regional scale. Nature 356:234–236
Oksanen JF, Blanchet G, Friendly M, Kindt R, Legendre P, Mcglinn D, Minchin PR, Hara RBO, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2020) Vegan: community ecology package. R package version 2.5-7 2395–96
Pereira JA, Di Bitetti MS, Fracassi NG, Paviolo A, De Angelo CD, Di Blanco YE, Novaro AJ (2010) Population density of Geoffroy’s cat in scrublands of central Argentina. J Zool 283:37–44. https://doi.org/10.1111/j.1469-7998.2010.00746.x
Periago ME, Chillo V, Ojeda RA (2015) Loss of mammalian species from the South American Gran Chaco: empty savanna syndrome? Mamm Rev 45:41–53
Periago ME, Tamburini DM, Ojeda RA, Cáceres DM, Díaz S (2017) Combining ecological aspects and local knowledge for the conservation of two native mammals in the Gran Chaco. J Arid Environ 147:54–62. https://doi.org/10.1016/j.jaridenv.2017.07.017
Pessino MEM, Sarasola JH, Wander C, Besoky N (2001) Respuesta a largo plazo del puma (Puma concolor) a una declinación poblacional de la vizcacha (Lagostomus maximus) en el desierto del Monte, Argentina. Ecol Austral 11:61–67
Pia MV, López MS, Novaro AJ (2003) Effects of livestock on the feeding ecology of endemic culpeo foxes (Pseudalopex culpaeus smithersi) in central Argentina. Rev Chil Hist Nat 76:313–321
Pia MV, Renison D, Mangeaud A, Angelo CD, Haro JG (2013) Occurrence of top carnivores in relation to land protection status, human settlements and rock outcrops in the high mountains of central Argentina. J Arid Environ 91:31–37. https://doi.org/10.1016/j.jaridenv.2012.11.004
Poo-Muñoz DO, Escobar LE, Townsend Peterson A, Astorga F, Organ JF, Medina-Vogel G (2014) Galictis cuja (Mammalia): an update of current knowledge and geographic distribution. Iheringia Série Zoologia 104:341–346. https://doi.org/10.1590/1678-476620141043341346
Prado DE (1993) What is the Gran Chaco vegetation in South America? II. A redefinition. Contribution to the study of the flora and vegetation of the Chaco. VII. Candollea 48:615–629
Puechagut PB, Politi N, Ruiz de los Llanos E, Lizarraga L, Bianchi CL, Bellis LM, Rivera LO (2018) Association between livestock and native mammals in a conservation priority area in the Chaco of Argentina. Mastozool Neotrop 25:407–418
Quiroga VA, Noss AJ, Paviolo A, Boaglio GI, Di Bitetti MS (2016) Puma density, habitat use and conflict with humans in the Argentine Chaco. J Nat Conserv 31:9–15
Renison D, Cingolani AM, Contarde C, Guzmán D (2023) Asistiendo a la reintroducción de vizcachas (Lagostomus maximus): ¿Cómo aumentar el área de pastoreo seguro? Ecol Austral 33:20–29. https://doi.org/10.25260/EA.23.33.1.0.1961
Ripple WJ, Beschta RL (2003) Wolf reintroduction, predation risk, and cottonwood recovery in Yellowstone National Park. For Ecol Manag 184:299–313
Ripple WJ, Newsome TM, Wolf C, Dirzo R, Everatt KT, Galetti M, Hayward MW, Kerley GHI, Levi T, Lindsey PA, Macdonald DW, Malhi Y, Painter LE, Sandom CJ, Terborgh J, Van Valkenburgh B (2015) Collapse of the world’s largest herbivores. Sci Adv 1:e1400103. https://doi.org/10.1126/sciadv.1400103
Rovero F, Toble M, Sanderson J (2010) Camera trapping for inventorying terrestrial vertebrates (Chapter 6). In: Eymann J, Degreef J, Häuser C, Monje JC, Samyn Y, van denSpiegel D (eds) Manual on field recording techniques and protocols for all taxa biodiversity inventories. p 653
Sanguinetti J, Pastore H (2016) Abundancia poblacional y manejo del jabalí (Sus scrofa): Una revisión global para abordar su gestión en la Argentina. Mastozool Neotrop 23:305–323
Santini G, Abolaffio M, Ossi F, Franzetti B, Cagnacci F, Focardi S (2022) Population assessment without individual identification using camera-traps: a comparison of four methods. Basic Appl Ecol 61:68–81. https://doi.org/10.1016/j.baae.2022.03.007
Schieltz JM, Rubenstein DI (2016) Evidence based review: positive versus negative effects of livestock grazing on wildlife. What do we really know? Environ Res Lett 11:113003
Schlaepfer MA, Sax DF, Olden JD (2011) The potential conservation value of non-native species. Conserv Biol 25:428–437
Soibelzon E, Medina M, Abba AM (2013) Late Holocene armadillos (Mammalia, Dasypodidae) of the Sierras of Córdoba, Argentina: zooarchaeology, diagnostic characters and their paleozoological relevance. Quatern Int 299:72–79. https://doi.org/10.1016/j.quaint.2012.09.009
Tamburini D (2018) Orden Pilosa. In: Torres R, Tamburini D (eds) Mamíferos de Córdoba y su estado de conservación. Editorial de la UNC. p 81–86
Tobler MW, Carrillo-Percastegui SE, Leite Pitman R, Mares R, Powell G (2008) An evaluation of camera traps for inventorying large-and medium-sized terrestrial rainforest mammals. Anim Conserv 11:169–178. https://doi.org/10.1111/j.1469-1795.2008.00169.x
Torres RC, Renison D (2016) Indirect facilitation becomes stronger with seedling age in a degraded seasonally dry forest. Acta Oecol 70:138–143. https://doi.org/10.1016/j.actao.2015.12.006
Torres R, Tamburini D (2018) Mamíferos de Córdoba y su estado de conservación. Editorial de la UNC, Córdoba
Torres RC, Pollice J, Valfré-Giorello TA, Herrero ML, Navarro-Ramos SE, Ibarra-Grellet I, Renison D (2021) Effects of forest preservation, livestock exclusion, and use of shrubs as potential nurses on planting success of an endangered tree. Restor Ecol 29:e13427. https://doi.org/10.1111/rec.13427
Varela O, Cormenzana-Méndez A, Krapovickas L, Bucher EH (2008) Seasonal diet of the pampas fox (Lycalopex gymnocercus) in the Chaco dry woodland, Northwestern Argentina. J Mammal 89:1012–1019
Vidal EL, Guerisoli M, Caruso N, Lucherini M (2017) Updating the distribution and population status of jaguarundi, Puma yagouaroundi (É. Geoffroy, 1803) (Mammalia: Carnivora: Felidae), in the southernmost part of its distribution range. Check List 13:75–79
Villareal D, Clark KL, Branco LC, Hierro JL, Machicote M (2008) Alteration of ecosystem structure by a burrowing herbivore, the plains Vizcacha (Lagostomus maximus). J Mammal 89:700–711. https://doi.org/10.1644/07-MAMM-A-025R1.1
von Müller AR, Renison D, Cingolani AM (2017) Cattle landscape selectivity is influenced by ecological and management factors in a heterogeneous mountain range land. Rangeland J 39:1–14. https://doi.org/10.1071/RJ15114
Zamora-Nasca LB, di Virgilio A, Lambertucci SA (2021) Online survey suggests that dog attacks on wildlife affect many species and every ecoregion of Argentina. Biol Conserv 256:109041
Zanón-Martínez JI, Santillán MA, Sarasola JH, Travaini A (2016a) A native top predator relies on exotic prey inside a protected area: the puma and the introduced ungulates in Central Argentina. J Arid Environ 134:17–20. https://doi.org/10.1016/j.jaridenv.2016.06.015
Zanón-Martínez JI, Kelly MJ, Mesa-Cruz JB, Sarasola JH, DeHart C, Travaini A (2016b) Density and activity patterns of pumas in hunted and non-hunted areas in central Argentina. Wildl Res 43:449–460. https://doi.org/10.1071/WR16056
Zapata-Ríos G, Branch LC (2016) Altered activity patterns and reduced abundance of native mammals in sites with feral dogs in the high Andes. Biol Conserv 193:9–16. https://doi.org/10.1016/j.biocon.2015.10.016
Zapata-Ríos G, Branch LC (2018) Mammalian carnivore occupancy is inversely related to presence of domestic dogs in the high Andes of Ecuador. PLoS ONE 13:e0192346. https://doi.org/10.1371/journal.pone.0192346
Zhao M, Heinsch FA, Nemani RR, Running SW (2005) Improvements of the MODIS terrestrial gross and net primary production global data set. Remote Sens Environ 95:164–176. https://doi.org/10.1016/j.rse.2004.12.011
Zhao M, Running SW, Nemani RR (2006) Sensitivity of moderate resolution imaging spectroradiometer (MODIS) terrestrial primary production to the accuracy of meteorological reanalyses. J Geophys Res Biogeosci 111:G01002. https://doi.org/10.1029/2004JG000004
Acknowledgements
Pablo Friedlander who made a gift to first author of a camera trap and thus triggered the study. Sebastian Stanganelli, Gabriela Hermitte and Duilio Schinner helped with field work. Daniela Tamburini, Joaquín Piedrabuena and Fernando Barri helped identify species when difficult. Jorgelina Brasca checked our English. This work was supported by CONICET—Argentina under Grant PIP # 11220170100143C which funded 4 of the camera traps and by CONCYTEC-Peru under grant contract No. 187-2019-FONDECYT which funded the stay of HRQM in Córdoba, Argentina.
Funding
CONICET—Argentina under Grant PIP # 11220170100143C which funded 4 of the camera traps and CONCYTEC-Peru under grant contract No. 187-2019-FONDECYT which funded the stay of HRQM in Córdoba, Argentina.
Author information
Authors and Affiliations
Contributions
DR: study design, field work, writing of original draft, review and editing of subsequent drafts. HRQM: photo processing and data entry, data analysis and interpretation, review and editing of drafts. GAEC: data analysis, review and editing of drafts. AMC: study design, data analysis, review and editing of drafts. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Appendix S1
. Estimation of reference values.
Additional file 2: Appendix S2
. Simulations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Renison, D., Quispe-Melgar, H.R., Erica Cuyckens, G.A. et al. Setting large- and medium-sized mammal restoration goals in a last mountain Chaco remnant from central Argentina. Ecol Process 12, 21 (2023). https://doi.org/10.1186/s13717-023-00434-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13717-023-00434-z