Abstract
Background
Tuberculosis (TB) is one of the leading causes of deaths in Africa, monitoring its treatment outcome is essential to evaluate treatment effectiveness. The study aimed to evaluate proportion of poor TB treatment outcomes (PTO) and its determinants during six-months of treatment at Kilifi County, Kenya.
Methods
We conducted a retrospective analysis of data from the TB surveillance system (TIBU) in Kilifi County, Kenya from 2012 to 2019. The outcome of interest was PTO (lost-to-follow-up (LTFU), death, transferred out, treatment failure, drug resistance) or successful treatment (cured or completed treatment). We performed time-stratified (at three months follow-up) survival regression analyses accounting for sub-county heterogeneity to determine factors associated with PTO.
Results
We included 14,706 TB patients, their median (IQR) age was 37 (28–50) years and 8,791 (60%) were males. A total of 13,389 (91%) were on first line anti-TB treatment (2RHZE/4RH), 4,242 (29%) were HIV infected and 192 (1.3%) had other underlying medical conditions. During 78,882 person-months of follow-up, 2,408 (16%) patients had PTO: 1,074 (7.3%) deaths, 776 (5.3%) LTFU, 415 (2.8%) transferred out, 103 (0.7%) treatment failure and 30 (0.2%) multidrug resistance. The proportion of poor outcome increased from 7.9% in 2012 peaking at 2018 (22.8%) and slightly declining to 20% in 2019 (trend test P = 0.03). Over two-thirds 1,734 (72%) poor outcomes occurred within first three months of follow-up. In the first three months of TB treatment, overweight ((aHR 0.85 (95%CI 0.73–0.98), HIV infected not on ARVS (aHR 1.72 (95% CI 1.28–2.30)) and year of starting treatment were associated with PTO. However, in the last three months of treatment, elderly age ≥50 years (aHR 1.26 (95%CI 1.02–1.55), a retreatment patient (aHR 1.57 (95%CI 1.28–1.93), HIV infected not on ARVs (aHR 2.56 (95%CI 1.39–4.72), other underlying medical conditions (aHR 2.24 (95%CI 1.41–3.54)) and year of starting treatment were positively associated with PTO while being a female (aHR 0.83 (95%CI 0.70–0.97)) was negatively associated with PTO.
Conclusions
Over two-thirds of poor outcomes occur in the first three months of TB treatment, therefore greater efforts are needed during this phase. Interventions targeting HIV infected and other underlying medical conditions, the elderly and retreated patients provide an opportunity to improve TB treatment outcome.
Similar content being viewed by others
Background
Tuberculosis (TB) remains a major global public health problem and is considered one of the leading life threatening conditions [1, 2]. It ranks high above HIV/AIDS as the leading cause of morbidity and is among the top 10 causes of mortality worldwide [3]. In 2019, approximately 10 million people developed the disease and a quarter of these cases were in Africa (25%) including Kenya that ranked among the 30 high TB burden countries accounting for 21% of the global cases [3].
Currently, the control and elimination of TB is a challenge due to microbial resistance to the available drug regimen (particularly Rifampicin and Isoniazid) [4, 5]. Anti-TB drug resistance is a major medical and public health concern worldwide [6,7,8]. In 2019, about 61% of people with TB were tested for Rifampicin resistance and a total of 206,030 found to have MDR/RR-TB [3]. Drug resistant mycobacterium strains are major challenge facing TB control because they require longer, expensive, complex, and more toxic treatments to cure [9, 10]. Often to minimise treatment noncompliance which leads to drug resistance and improve treatment outcomes among the drug resistant TB patients directly observed treatment, short course (DOTS) programs are implemented [11, 12]. The DOTS strategy was among the three (Stop TB and End TB) scaled-up essential strategies recommended by World Health Organization (WHO) to interrupt TB transmission and the period of infectiousness [12, 13]. While the DOTS strategy has been globally adopted, TB patients still end up with poor treatment outcomes [14,15,16,17].
Treatment outcomes, as recommended by World Health Organization (WHO) in 2013, are used to assess the effectiveness of TB control programs [18]. Cured, treatment completed, treatment failed, died, and defaulted were five exclusive groups of TB treatment results used as a benchmark for global TB data collection and treatment success assessment [12]. The number of TB patients who are cured or completed treatment are considered to have a good treatment outcome, while those who missed treatment, defaulted, or died are considered to have a poor treatment outcome [19, 20]. Monitoring TB care outcomes is important for evaluating the efficacy and improvement of TB treatments, as well as identifying possible barriers to TB control [21, 22].
We identified and reviewed three systematic reviews and meta-analysis pooling the proportional of successful TB treatment and evaluating predictors of poor treatment outcomes. Torres et al. [23] reviewed 151 studies published from January 2014 to November 2019 from 59 countries representing 5 continents. The pooled treatment success rate for adults was 80.1% but was lower among the 47 studies from Africa (success rate of 78.9%). The systematic review included one study from Kenya. However, the included study was limited to smear-positive pulmonary TB patients aged 15 to 49 years only [24]. Another systematic review included 31 studies from Sub-Saharan Africa published from July 2008 to June 2018. This systematic review reported a pooled treatment success of 76.2% and did not include any study from Kenya [20]. A more recent systematic review (published 12 Oct 2021) included 26 studies from Africa. The reported pooled treatment success was 79%. Again, the systematic review did not include any study from Kenya [19]. Of interest, all the pooled treatment success rates were below the 85% reported by WHO in 2020 and lagged behind the set target of 90% TB incidence rate reduction by 2035 [25]. Predictors of poor treatment outcomes reported were HIV infection, being a retreatment patient, elderly age (>65 years) and drinking alcohol [19, 23].
In 2016, the prevalence of TB in Kenya was 558cases per 100,000 adult population with the highest burden among the adults aged 25 to 34 years (prevalence of 716 cases per 100,000 adult population) [26]. In 2019, TB was the 6th leading cause of death in Kenya [27]. The estimated prevalence of TB in Kilifi County is 122/100,000 cases according to the national survey while that of HIV is 5% [28, 29]. There is limited data on TB treatment outcomes in Kenya, specifically among TB patients in Kilifi County [19, 23, 30,31,32]. Therefore, the aim of this study was to estimate proportion of TB patients on treatment who end with poor outcomes and identify the determinants associated with poor treatment outcomes among tuberculosis patients in Kilifi County, Kenya.
Methods
Study design
This was a retrospective secondary analysis of routine standard National Leprosy and Tuberculosis and Lung Disease (NTLD) register data of Kilifi County from January 2012 to December 2019. TB treatment outcomes were categorized into successful outcomes (cured or completed six months of treatment) or poor outcomes (lost-to-follow-up, death, transferred out, treatment failure or development of drug resistance). The exposures examined were demographic (age, sex), sub-county of resident, year of starting TB treatment, nutritional status (body mass index), nutritional support provided and clinical features (HIV status, underlying comorbidities, type of TB (pulmonary or extra-pulmonary), method of TB diagnosis (bacteriological confirmed TB or empirically treated), treatment regimen and direct observed treatment.
Setting
TB Electronic surveillance data was collected from health facilities in seven sub-counties including Kilifi North, Kilifi South, Malindi, Magarini, Kaloleni, Rabai, and Ganze in Kilifi County within the coast region of Kenya. The County had a population of 1.4 million people in 2019 census [33]. More than 70% of Kilifi County residents live in rural areas and are poor, lack formal education, and make a living from subsistence farming and fishing [34]. During the study period, there were only three health facilities with GeneXpert machines hence only ones with the capacity to diagnose TB. Not all rural health facilities have laboratory services to run sputum smear test for TB as the gold standard of TB diagnosis, nonetheless, facilities leverage the existing sputum sample referral to the high-volume health facilities for sample examination.
Participants
The study population was all adult TB patients (≥18 years) who were on anti-TB treatment from January 2012 to December 2019 within Kilifi County.
Variables
Pulmonary TB (PTB) was defined as any bacteriologically confirmed or clinically diagnosed case of TB involving the lung parenchyma or the tracheobronchial tree [35]. Extra-pulmonary TB (EPTB) were any bacteriologically confirmed or clinically diagnosed case of TB involving organs other than the lungs [35]. A patient was classified as transferred out if the treatment outcome was not known as a result of moving outside Kilifi County. Patients were classified as having poor treatment outcomes if they: (i) failed treatment (i.e., remaining smear-positive after 5 months of treatment), (ii) had defaulted or lost to follow-up (LTFU), (iii) died during treatment, (iv) transferred out or (v) developed drug resistance. Cured patients were those with PTB and bacteriologically confirmed at the beginning of treatment but had smear- or culture-negative test in the last one previous occasion. Deaths included all-cause mortality within the six months of follow-ups. TB patient who completed treatment without evidence of failure BUT with no record to show that sputum smear or culture results in the last month of treatment and on at least one previous occasion were negative, either because tests were not done or because results were unavailable, they were classified as having completed treatment. A TB patient whose sputum smear or culture was positive at month 5 or later during treatment was defined as treatment failure. A TB patient who did not start treatment or who started treatment but was interrupted for 2 consecutive months or more was defined as defaulted or LTFU. New TB cases were patients newly registered who had never been treated for TB before or had been on anti-TB treatment less than 4 weeks. Retreated patients were patients who had been treated for any form of TB before but had initiated treatment again following relapse, default or failure to cure after previous treatment.
Data sources/measurements
Data were extracted from the TB Electronic surveillance system known as Treatment Information from Basic Unit (TIBU) on 10th November 2020. This system stores individual patient episodes of TB including demographic characteristics, location, clinical details, laboratory results, and treatment outcomes [36]. De-identified data were extracted directly into a Microsoft Excel spreadsheet that was designed to capture the relevant variables. Data extractions were done by GGK (first author) in presence of the County TB Coordinator.
Study size
The study used all available eligible patient data from 2012 to 2019. A total of 14,706 patients were eligible. Assuming 14% probability of a poor outcome [37], a two-sided alpha level of 0.05, the study has power >90% to estimate a crude hazard ratio of at least 1.5 of HIV positive being associated with poor treatment outcome in the first three months of follow-up [37].
Quantitative variables
Bacteriological confirmed TB patients were those with positive smear microscopy, culture or GeneXpert MTB/RIF result. Empirically treated patients did not have any positive TB bacteriological test but had clinical signs suggestive of TB including abnormal chest radiograph, chronic cough, fever, night sweats, weight loss, suggestive histology or extrapulmonary cases.
We created four age groups:18 to 30, 31 to 40, 41 to 50 and 51+ years. Body Mass Index (BMI) was calculated as weight (Kg) divided by square of height (meters) and further recorded into three groups according to WHO guidelines: undernourished (BMI<18.5), normal (BMI 18.5 to 25) and overweight (BMI ≥25) [38].
We assumed the missing BMI and HIV status were not missing at random. To include all patients in the regression analysis, we added extra category (missing for BMI and unknown for HIV) and used the categorical variables in the analysis. No record was missing study outcome data.
Statistical methods
Statistical analyses were performed using STATA version 15.1 (StataCorp, College Station, TX, USA). All study patients’ characteristics were summarised using frequencies and percentages. We calculated the annual proportion of poor outcome and tested for trend across the years (from 2012 to 2019) [39].
To examine factors associated with poor treatment outcomes, we run single event survival analysis with time under observation starting from date of starting TB treatment up to 180 days later or date of any of the outcomes. All patients who completed treatment or were under treatment after 180 days were right censored at day 180. All other patients who did not complete treatment and experienced any of the poor outcomes were right censored at their last date seen alive or last follow-up. We tested the presence of heterogeneity across the seven sub-counties using likelihood ratio test in the final regression model. We found evidence for presence of sub-county heterogeneity (P < 0.001) and included the sub-county as random intercept in all the survival regression models using the shared frailty models [40]. We tested the Proportional-hazards (PH) assumption using the scaled Schoenfeld residuals in each independent variable and in the multivariable cox proportional hazard model with all the independent variables. Because of the violation of the PH assumption (P < 0.05), we performed time-stratified survival regression analyses. We chose to stratify the analysis at month three follow-up because this was the halfway of the follow-up time and from operational perspective, it would inform interventions targeting the early poor outcomes. However, we provided survival analyses results for the first three months and last three months of follow-up separately. We tested proportional-hazards assumption for the two time points and found no evidence of violation (the scaled Schoenfeld residuals global test for the first 3 months was P = 0.0936 and the last 3 months was P = 0.0656). We therefore used the Cox Proportional hazard regression model, running univariate model for each independent variable. To build the multivariable regression models, we used a backward stepwise approach retaining independent variables with a P < 0.1 and reported their hazard ratios and 95% confidence intervals. We assessed predictive values of the multivariable models using area under receiver operating curves (AUCs).
In sub-analysis, we repeated the multivariable regression models amongst bacteriological confirmed TB cases only and explored interaction between the year of starting TB treatment and various independent variables considered (age, gender, HIV status, TB diagnosis, underlying comorbidities, patient type) by comparing models with and without interaction terms using likelihood ratio test.
Results
Patient characteristics
During the study period, 14,706 patients were started on anti-TB treatment. Their median (Interquartile range (IQR)) age was 37 (28 to 50) years and 8,791 (60%) were male. About 13,027 (89%) were new TB patients while 12,975 (88%) had (PTB) and 1,731 (11%) had EPTB. Approximately half: 51% patients had normal BMI while 4,309 (29%) were undernourished. About 4,242 (29%) were HIV infected, of which 4,037/4,242 (95%) were on ARVs and 4,212/4,242 (99%) were on cotrimoxazole prophylaxis. More than three quarters: 78% of the patients were treated in a public health facility while 13,100 (89%) were on family-based direct observation treatment. A total of 13,389 (91%) patients were on Rifampicin (R), Isoniazid (H), Pyrazinamide (Z), Ethambutol (E) for the first two months and Rifampicin (R), Isoniazid (H) for the following four months (2RHZE/4RH) TB treatment regimen (Table 1). Approximately half of the patients (N = 7,293, 50%) were started on anti-TB empirically based on clinical signs. One hundred and ninety-two (1.3%) patients had underlying comorbidities: 128 (0.9%) were taking drugs (smoking for more than six months, drinking alcohol etc.), 5 (0.03%) had chronic obstructive pulmonary disease (COPD), 41 (0.3%) had hypertension, 25 (0.2%) had diabetes, 4 (0.03%) had cancer and 1 (0.01%) had asthma (Fig. 1).
Outcome
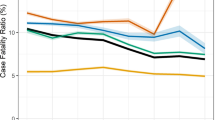
Of 14,706 patients on treatment, 2,408 had poor outcomes (16.3%, (95% CI 15.8 to 17.0)). The 2,408 poor outcomes were: deaths (n = 1,074, 7.3%), LTFU (n = 776, 5.3%), transferred out (n = 425, 2.8%), treatment failure (n = 103, 0.7%) and multidrug resistance (n = 30, 0.2%). The proportion of poor outcome increased from 7.9% in 2012 peaking at 2018 (22.8%) and slightly declining to 20% in 2019 (test for trend P = 0.03) Fig. 2.
Follow-up time
The patients were on follow-up for a total of 78,882 person-months: 73,788 and 5,094 person-months among the successful and poor outcomes patients respectively. The poor outcome incidence rate was 472 (95% CI 454–491) per 1,000 person-months. The patient with poor treatment outcomes were on follow-up for median (IQR) 55 (51 to 58) days. Of the 2,408 patients with poor treatment outcomes, 117 (4.9%) occurred on the day of starting TB treatment (70/117 were LTFU, 14/117 died and 33/117 transferred out). Most poor outcomes occurred early: 741/2,408 (31%), 1,282/2,408 (53%) and 1,734/2,408 (72%) occurring within the first one, two and three months respectively.
Factors associated with poor outcomes
In the univariate model of the first three months of TB treatment, HIV infected not on ARVs (Crude Hazard Ratio (CHR) 1.59 (95%CI 1.19–2.11)), patients empirically treated without bacteriological confirmation (CHR 1.16 (95%CI 1.05–1.28)) and the year of starting treatment were significantly associated with higher hazard of poor outcomes. However, overweight (CHR 0.84 (95%CI 0.72–0.98)) was negatively associated with hazard of poor outcome (Additional file 1).
In the univariate model of the last three months of TB treatment, elderly age (≥ 51years) (CHR 1.30 (95%CI 1.06–1.59)), a retreatment patient (CHR 1.69 (95%CI 1.38–2.06)), HIV infected on ARVs (CHR 1.48 (95%CI 1.26–1.73)), 2SRHZE/1RHZE/5RHE treatment regimen, underlying conditions and year of starting treatment were significantly associated with higher hazard of poor outcomes. However, females (CHR 0.84 (95%CI 0.71–0.98)) and patients empirically treated without bacteriological confirmation (CHR 0.81 (95%CI 0.69–0.94)) were negatively associated with hazard of poor outcomes (Additional file 1).
In the multivariable regression model of the first three months of TB treatment, HIV infected not on ARVs (adjusted Hazard Ratio (aHR) 1.72 (95%CI 1.28–2.30)) (Fig. 3a) and the year of starting treatment were significantly associated with higher hazard of poor outcomes. Being overweight (aHR 0.85 (95%CI 0.73–0.98)) was negatively associated with hazard of poor outcome (Table 2). We found evidence of interaction between being overweight and type of health facility (P = 0.02). Overweight was more common among patients recruited from private health facilities (14%) compared to those recruited from public health facilities (12%) and from prisoners (9.7%). The multivariable model AUC (95%CI) was 0.60 (95%CI 0.58–0.62).
In the multivariable regression model of the last three months of TB treatment, elderly age (≥ 51years) (aHR 1.26 (95%CI 1.02–1.55)), a retreatment patient (aHR 1.57 (95%CI 1.28–1.93)), HIV infected on ARVs and not on ARVs, underlying comorbidities (aHR 2.24 (95%CI 1.41–3.54)) (Fig. 3b) and year of starting treatment were significantly associated with higher hazard of poor outcomes. Being female (aHR 0.83 (95%CI 0.70–0.97)) and patients empirically treated without bacteriological confirmation (aHR 0.82 (95%CI 0.70–0.97)) were negatively associated with hazard of poor outcome (Table 2). The multivariable regression model AUC was 0.64 (95%CI 0.63–0.65).
Sub-analysis
In the sub-analysis, factors associated with poor outcomes in the multivariable regression models in the first and last three months of treatment including only TB confirmed cases, were approximately similar to the whole population (Additional file 2). We found no evidence of interaction between year of starting TB treatment and age (P = 0.56), sex (P = 0.62), HIV status (P = 0.70), type of TB diagnosis (P = 0.32), underlying comorbidities (P = 0.13) and patient type (P = 0.28) in the first three months of TB treatment. Similarly, there was no interaction between year of starting TB treatment and age (P = 0.54), sex (P = 0.92), HIV status (P = 0.15), type of TB diagnosis (P = 0.11), underlying comorbidities (P = 0.63) and patient type (P = 0.42) in the last three months of TB treatment.
Discussion
In this large study including >14,000 participants, poor treatment outcomes frequently (more than two thirds) occurred very early after starting TB treatment usually within the first three months. Characteristics of the patients with poor outcomes in the first three months and after three months were different suggesting different strategies to improve early and late treatment outcomes are needed. The 16% poor outcome in our study was similar with the findings reported in Southern Ethiopia, Somalia, India, and Russia [41,42,43,44]. However, our prevalence was higher than other studies in China (4.2%) and Europe (12.5%) [45, 46]. The low prevalence of poor outcome in China and Europe could be attributable to their more responsive health systems.
In the early phase of treatment where more than two-thirds of poor outcomes occurred, being HIV infected not on ARVs was associated with higher risk of poor outcome. This is an important phase of TB treatment where majority of the patients were on four drugs for the first two months and two drugs for the last four months [47]. The extra burden of taking ARVs and cotrimoxazole prophylaxis by the HIV infected patients and possible drug interactions with adverse effects might have negatively affected the patients impairing their TB treatment outcomes [48, 49]. TB and HIV diagnosis have also been associated with stigma, which might further have adverse effects on TB treatment outcomes [50, 51]. Like our study, a number of previous studies have found HIV association with poor TB treatment outcomes [52,53,54]. Surveillance systems like the one in place in our setting should play a key role in providing data for action. Owing to limited resources, our surveillance system is not able to actively monitor the patients in the community. However, evidence demonstrates simple and cheap strategies including digital technology (like reminders via short message services (SMSs) through mobile phones), use of community health volunteers to offer patient education and psychological support improves TB treatment outcomes [55, 56]. Therefore, our findings of poor outcomes majorly among the HIV coinfected patients should trigger the surveillance managers to explore strategies of integrating retention methods with the ones provided by HIV programs and possible adoption of cheap strategies like reminders via digital technology. This finding also highlights the need for systematic HIV screening among all presumptive TB patients and prompt treatment for those who test positive.
In the last three months of treatment, patients on retreatment, the elderly and those with underlying medical conditions including HIV were associated with poor outcomes. It is likely that patients on retreatment had poor outcomes because they developed drug resistant strains as reported elsewhere [57]. Also, studies show that patient’s behaviour influences unsuccessful treatment [30, 58]; a study looking at factors associated with poor outcomes indicated that patients who get lost to follow-up who then have to be retreated for TB are often reluctant to uptake and tend to interrupt treatment [41]. Studies conducted elsewhere have also shown the risk of poor TB treatment outcomes increases as age advances because old age comes with increased age-related immunosuppressant comorbidities such as diabetes mellitus that increase adverse effects of anti-TB drugs, cause drug resistance, mortality, and increases recurrence of TB in this group [49, 59, 60]. In addition to retreatment and old age complexities, underlying conditions such as HIV worsens treatment outcomes as reported in other studies by synergistically interacting with TB to alter its clinical manifestation, complicate the treatment follow-up process, and to cause death [61,62,63,64]. This finding suggests the need for strategies targeting patients with these features as a `high risk’ group for poor treatment outcome.
In the six months of follow-up, poor treatment outcome increased over the years. We found no evidence of interaction between the age of starting treatment and other exposures like HIV or age. Previous research in this cohort of patients had similar findings suggesting deteriorating TB treatment outcomes over years [37, 65]. This is a very worrying trend requiring further research. We hypothesis this could be driven by conditions such as diabetes mellitus that increase adverse effects of anti-TB drugs, cause drug resistance, mortality, and increases recurrence of TB in this group [66,67,68]. Given the increasing prevalence of diabetes mellitus in regions with a high TB burden, there is need for TB control programs to closely monitor and treat patients presenting with diabetes mellitus for improved treatment outcomes to be achieved [68].
Future studies should focus on factors not explored or collected in this study. Qualitative studies exploring patients experience with TB treatment and interaction with health workers every month would provide an opportunity for an in-depth understanding of the barriers to treatment success.
Study strengths and limitations
The main strength of the study is the large size and the robust analysis conducted. Being surveillance, our study was limited to the available data. We therefore did not have access to other variables such as other comorbidities and behavioural and socio-economic factors (like alcohol consumption, smoking, income, living conditions, education, and family size) which might affect treatment outcomes. The surveillance system lacks resources to implement active surveillance, thus the high proportion of TB poor treatment outcomes may not be generalizable in settings with resources to support active surveillance.
Conclusions
Poor treatment outcomes more frequently occur in the first three months following initiation of TB treatment and therefore greater efforts are needed during this phase. Our study findings suggest the need for different strategies to improve TB treatment outcomes during the first and last three months of treatment. Strategies targeting the elderly, retreated patients, HIV infected and those with underlying medical conditions provide an opportunity to improve TB treatment outcomes. The TB program team needs to offer more support to reverse the increasing annual poor TB treatment outcome trend.
Availability of data and materials
The study data are available from the corresponding author on reasonable request.
Abbreviations
- TB:
-
Tuberculosis
- PTO:
-
Poor TB Treatment Outcome
- IQR:
-
Inter-quartile Range
- HIV:
-
Human Immunodeficiency Virus
- LTFU:
-
Lost to Follow-up
- AIDS:
-
Acquired Immunodeficiency Syndrome
- MDR/RR-TB:
-
Multidrug- and Rifampicin-Resistant Tuberculosis
- DOTS:
-
Directly Observed Treatment Short Course
- WHO:
-
World Health Organization
- NTLD:
-
National Leprosy and Tuberculosis and Lung Disease
- PTB:
-
Pulmonary TB
- EPTB:
-
Extra-pulmonary TB
- TIBU:
-
Treatment Information from Basic Unit
- BMI:
-
Body Mass Index
- AUC:
-
Area Under the Curve
- RECORD:
-
Reporting of studies Conducted using Observational Routinely-collected health Data
- ARVs:
-
Antiretroviral Drugs
- COPD:
-
Chronic Obstructive Pulmonary Disease
- SMSs:
-
Short Message Services
References
Ramadiani NA, Hatta HR, Agus F, Ariffin Z, Azainil. Certain factor analysis for extra pulmonary tuberculosis diagnosis. Int Conf Electr Eng Comput Sci Informatics. 2017;4(September):138–44. Available from: https://ieeexplore.ieee.org/document/8239098.
Vachon J, Gallant V, Siu W. Tuberculosis in Canada, 2016. Canada Commun Dis Rep. 2018;44(3/4):75–81. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6449093/.
World Health Organization (WHO). Global tuberculosis report 2020. Geneva, Switzerland; 2020. Available from: https://www.who.int/publications/i/item/9789240013131.
Kurz SG, Furin JJ, Bark CM. Drug-Resistant Tuberculosis. Infect Dis Clin North Am. 2016;30(2):509–22. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0891552016300162.
Gygli SM, Borrell S, Trauner A, Gagneux S. Antimicrobial resistance in Mycobacterium tuberculosis: mechanistic and evolutionary perspectives. FEMS Microbiol Rev. 2017;41(3):354–73. Available from: https://doi.org/10.1093/femsre/fux011.
LaFreniere M, Hussain H, Vachon J. TB Drug resistance 2017. 2018.
Ullah I, Javaid A, Tahir Z, Ullah O, Shah AA, Hasan F, et al Pattern of Drug Resistance and Risk Factors Associated with Development of Drug Resistant Mycobacterium tuberculosis in Pakistan. Chatterji D, editor. PLoS One. 2016;11(1):e0147529. Available from: https://doi.org/dx.plos.org/10.1371/journal.pone.0147529.
Sinha P, Srivastava G, Gupta A, Anupurba S. Association of risk factors and drug resistance pattern in tuberculosis patients in North India. J Glob Infect Dis. 2017;9(4):139. Available from: http://www.jgid.org/text.asp?2017/9/4/139/220408.
Sturdy A, Goodman A, Jose RJ, Loyse A, O’Donoghue M, Kon OM, et al. Multidrug-resistant tuberculosis (MDR-TB) treatment in the UK: a study of injectable use and toxicity in practice. J Antimicrob Chemother. 2011;66(8):1815–20. Available from: https://academic.oup.com/jac/article-lookup/doi/10.1093/jac/dkr221.
Seung KJ, Keshavjee S, Rich ML. Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Cold Spring Harb Perspect Med. 2015;5(9):a017863. Available from: http://perspectivesinmedicine.cshlp.org/lookup/doi/10.1101/cshperspect.a017863.
Centers for Disease Control (CDC). Outbreak of multidrug-resistant tuberculosis--Texas, California, and Pennsylvania. MMWR Morb Mortal Wkly Rep. 1990;39(22):369–72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2111434.
Muluye AB, Kebamo S, Teklie T, Alemkere G. Poor treatment outcomes and its determinants among tuberculosis patients in selected health facilities in East Wollega, Western Ethiopia. Hasnain SE, editor. PLoS One. 2018;13(10):e0206227. Available from: https://doi.org/dx.plos.org/10.1371/journal.pone.0206227.
Sotgiu G, Sulis G, Matteelli A. Tuberculosis—a. World Health Organization Perspective. Schlossberg D, editor. Microbiol Spectr. 2017;5(1). Available from: https://journals.asm.org/doi/10.1128/microbiolspec.TNMI7-0036-2016.
Kibuule D, Rennie TW, Ruswa N, Mavhunga F, Thomas A, Amutenya R, et al. Effectiveness of community-based DOTS strategy on tuberculosis treatment success rates in Namibia. Int J Tuberc Lung Dis. 2019;23(4):441–9. Available from: https://www.ingentaconnect.com/content/10.5588/ijtld.17.0785.
Kibuule D, Aiases P, Ruswa N, Rennie TW, Verbeeck RK, Godman B, et al. Predictors of loss to follow-up of tuberculosis cases under the DOTS programme in Namibia. ERJ Open Res. 2020;6(1):00030–2019. Available from: http://openres.ersjournals.com/lookup/doi/10.1183/23120541.00030-2019.
Gebrezgabiher G, Romha G, Ejeta E, Asebe G, Zemene E, Ameni G. Treatment Outcome of Tuberculosis Patients under Directly Observed Treatment Short Course and Factors Affecting Outcome in Southern Ethiopia: A Five-Year Retrospective Study. Mistry N, editor. PLoS One. 2016;11(2):e0150560. Available from: https://doi.org/dx.plos.org/10.1371/journal.pone.0150560.
Ruru Y, Matasik M, Oktavian A, Senyorita R, Mirino Y, Tarigan LH, et al. Factors associated with non-adherence during tuberculosis treatment among patients treated with DOTS strategy in Jayapura, Papua Province, Indonesia. Glob Health Action. 2018;11(1):1510592. Available from: https://www.tandfonline.com/doi/full/10.1080/16549716.2018.1510592.
WHO. Global tuberculosis report 2016. Geneva; 2016. Available from: https://apps.who.int/iris/handle/10665/250441.
Teferi MY, El-Khatib Z, Boltena MT, Andualem AT, Asamoah BO, Biru M, et al. Tuberculosis Treatment Outcome and Predictors in Africa: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2021;18(20):10678. Available from: https://www.mdpi.com/1660-4601/18/20/10678.
Izudi J, Semakula D, Sennono R, Tamwesigire IK, Bajunirwe F. Treatment success rate among adult pulmonary tuberculosis patients in sub-Saharan Africa: a systematic review and meta-analysis. BMJ Open. 2019;9(9):e029400. Available from: https://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2019-029400.
Biruk M, Yimam B, Abrha H, Biruk S, Amdie FZ. Treatment Outcomes of Tuberculosis and Associated Factors in an Ethiopian University Hospital. Adv Public Heal. 2016;2016:1–9. Available from: http://www.hindawi.com/journals/aph/2016/8504629/.
Ayakaka I, Ackerman S, Ggita JM, Kajubi P, Dowdy D, Haberer JE, et al. Identifying barriers to and facilitators of tuberculosis contact investigation in Kampala, Uganda: a behavioral approach. Implement Sci. 2017;12(1):33. Available from: https://implementationscience.biomedcentral.com/articles/10.1186/s13012-017-0561-4.
Chaves Torres NM, Quijano Rodríguez JJ, Porras Andrade PS, Arriaga MB, Netto EM. Factors predictive of the success of tuberculosis treatment: A systematic review with meta-analysis. Ehtesham HS, editor. PLoS One. 2019;14(12):e0226507. Available from: https://doi.org/dx.plos.org/10.1371/journal.pone.0226507.
Kosgei RJ, Callens S, Gichangi P, Temmerman M, Kihara A-B, David G, et al. Gender difference in mortality among pulmonary tuberculosis HIV co-infected adults aged 15-49 years in Kenya. Kufa T, editor. PLoS One. 2020;15(12):e0243977. Available from: https://doi.org/dx.plos.org/10.1371/journal.pone.0243977.
WHO. WHO consolidated guidelines on tuberculosis. Module 4: treatment-drug-resistant tuberculosis treatment. Geneva, Switzerland: World Health Organization; 2020. Available from: https://www.who.int/publications/i/item/9789240007048.
Enos M, Sitienei J, Ong’ang’o J, Mungai B, Kamene M, Wambugu J, et al. Kenya tuberculosis prevalence survey 2016: Challenges and opportunities of ending TB in Kenya. Hozbor DF, editor. PLoS One. 2018;13(12):e0209098. Available from: https://doi.org/dx.plos.org/10.1371/journal.pone.0209098.
Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673620309259.
National AIDS Control Council (NACC). National AIDS Control Council (NACC). Kenya HIV County Profiles - 2016. 2016. Available from: http://nacc.or.ke/wp-content/uploads/2016/12/Kenya-HIV-County-Profiles-2016.pdf.
National TB and Lung Disease Program. National TB and Lung Disease Program. Annual Report 2016. Nairobi, Kenya; 2016. Available from: https://www.nltp.co.ke/annual-reports/.
Kimani E, Muhula S, Kiptai T, Orwa J, Odero T, Gachuno O. Factors influencing TB treatment interruption and treatment outcomes among patients in Kiambu County, 2016-2019. Ochodo E, editor. PLoS One. 2021;16(4):e0248820. Available from: https://doi.org/dx.plos.org/10.1371/journal.pone.0248820.
Muture BN, Keraka MN, Kimuu PK, Kabiru EW, Ombeka VO, Oguya F. Factors associated with default from treatment among tuberculosis patients in nairobi province, Kenya: A case control study. BMC Public Health. 2011;11(1):696. Available from: http://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-11-696.
Kosgei RJ, Sitienei JK, Kipruto H, Kimenye K, Gathara D, Odawa FX, et al. Gender differences in treatment outcomes among 15–49 year olds with smear-positive pulmonary tuberculosis in Kenya. Int J Tuberc Lung Dis. 2015;19(10):1176–81. Available from: http://openurl.ingenta.com/content/xref?genre=article&issn=1027-3719&volume=19&issue=10&spage=1176.
Kenya National Bureau of Statistics. 2019 Kenya Population and Housing Census. Nairobi K. 2019. Available from: https://www.knbs.or.ke/?wpdmpro=2019-kenya-population-and-housing-census-volume-iii-distribution-of-population-by-age-sex-and-administrative-units.
Scott JAG, Bauni E, Moisi JC, Ojal J, Gatakaa H, Nyundo C, et al. Profile: The Kilifi Health and Demographic Surveillance System (KHDSS). Int J Epidemiol. 2012;41(3):650–7. Available from: https://academic.oup.com/ije/article-lookup/doi/10.1093/ije/dys062.
World Health Organization (WHO). Treatment of tuberculosis: guidelines. Geneva, Switzerland: World Health Organization; 2010. Available from: http://apps.who.int/iris/bitstream/handle/10665/44165/9789241547833_eng.pdf;jsessionid=8E5B799E67619CC67B929EC0F9C0742E?sequence=1.
Sharma A, Ndisha M, Ngari F, Kipruto H, Cain KP, Sitienei J, et al. A review of data quality of an electronic tuberculosis surveillance system for case-based reporting in Kenya: Table 1. Eur J Public Health. 2015;25(6):1095–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26009610.
Abdullahi OA, Ngari MM, Sanga D, Katana G, Willetts A. Mortality during treatment for tuberculosis; a review of surveillance data in a rural county in Kenya. Dodd PJ, editor. PLoS One. 2019;14(7):e0219191. Available from: https://doi.org/dx.plos.org/10.1371/journal.pone.0219191.
WHO. BMI classification. Global Database on body mass index. World Heal Organ. 2006.
Cuzick J. A wilcoxon-type test for trend. Stat Med. 1985;4(1):87–90. Available from: http://doi.wiley.com/10.1002/sim.4780040112.
Aalen OO, Hjort NL. Frailty models that yield proportional hazards. Stat Probab Lett. 2002;58(4):335–42. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0167715202000901.
Muñoz-Sellart M, Cuevas LE, Tumato M, Merid Y, Yassin MA. Factors associated with poor tuberculosis treatment outcome in the Southern Region of Ethiopia. Int J Tuberc Lung Dis. 2010;14(8):973–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20626941.
Ali MK, Karanja S, Karama M. Factors associated with tuberculosis treatment outcomes among tuberculosis patients attending tuberculosis treatment centres in 2016-2017 in Mogadishu, Somalia. Pan Afr Med J. 2017;28:197. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29610635.
Jackson C, Stagg HR, Doshi A, Pan D, Sinha A, Batra R, et al. Tuberculosis treatment outcomes among disadvantaged patients in India. Public Heal action. 2017;7(2):134–40. Available from: http://www.ingentaconnect.com/content/https://doi.org/10.5588/pha.16.0107.
Jakubowiak WM, Bogorodskaya EM, Borisov SE, Borisov ES, Danilova ID, Danilova DI, et al. Risk factors associated with default among new pulmonary TB patients and social support in six Russian regions. Int J Tuberc Lung Dis. 2007;11(1):46–53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17217129.
Wen Y, Zhang Z, Li X, Xia D, Ma J, Dong Y, et al. Treatment outcomes and factors affecting unsuccessful outcome among new pulmonary smear positive and negative tuberculosis patients in Anqing, China: a retrospective study. BMC Infect Dis. 2018;18(1):104. Available from: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-018-3019-7.
Ditah IC, Reacher M, Palmer C, Watson JM, Innes J, Kruijshaar ME, et al. Monitoring tuberculosis treatment outcome: analysis of national surveillance data from a clinical perspective. Thorax. 2008;63(5):440–6. Available from: https://thorax.bmj.com/lookup/doi/10.1136/thx.2006.073916.
Nath H, Ryoo S. First– and Second–Line Drugs and Drug Resistance. In: Tuberculosis - Current Issues in Diagnosis and Management. InTech; 2013. Available from: https://www.intechopen.com/chapters/43723.
Tola A, Mishore KM, Ayele Y, Mekuria AN, Legese N. Treatment Outcome of Tuberculosis and Associated Factors among TB-HIV Co-Infected Patients at Public Hospitals of Harar Town, Eastern Ethiopia. A five-year retrospective study. BMC Public Health. 2019;19(1):1658. Available from: https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-019-7980-x.
Ncube RT, Takarinda KC, Zishiri C, van den Boogaard W, Mlilo N, Chiteve C, et al. Age-stratified tuberculosis treatment outcomes in Zimbabwe: are we paying attention to the most vulnerable? Public Heal Action. 2017;7(3):212–7. Available from: http://www.ingentaconnect.com/content/10.5588/pha.17.0024.
Mohammedhussein M, Hajure M, Shifa JE, Hassen TA. Perceived stigma among patient with pulmonary tuberculosis at public health facilities in southwest Ethiopia: A cross-sectional study. Hasnain SE, editor. PLoS One. 2020;15(12):e0243433. Available from: https://doi.org/dx.plos.org/10.1371/journal.pone.0243433.
Deribew A, Abebe G, Apers L, Jira C, Tesfaye M, Shifa J, et al. Prejudice and misconceptions about tuberculosis and HIV in rural and urban communities in Ethiopia: a challenge for the TB/HIV control program. BMC Public Health. 2010;10(1):400. Available from: https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-10-400.
Ali SA, Mavundla TR, Fantu R, Awoke T. Outcomes of TB treatment in HIV co-infected TB patients in Ethiopia: a cross-sectional analytic study. BMC Infect Dis. 2016;16(1):640. Available from: http://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-016-1967-3.
Izudi J, Tamwesigire IK, Bajunirwe F. Treatment success and mortality among adults with tuberculosis in rural eastern Uganda: a retrospective cohort study. BMC Public Health. 2020;20(1):501. Available from: https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-020-08646-0.
Engelbrecht MC, Kigozi NG, Chikobvu P, Botha S, van Rensburg HCJ. Unsuccessful TB treatment outcomes with a focus on HIV co-infected cases: a cross-sectional retrospective record review in a high-burdened province of South Africa. BMC Health Serv Res. 2017;17(1):470. Available from: http://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-017-2406-x.
Alipanah N, Jarlsberg L, Miller C, Linh NN, Falzon D, Jaramillo E, et al. Adherence interventions and outcomes of tuberculosis treatment: A systematic review and meta-analysis of trials and observational studies. PLoS Med. 2018;15(7):e1002595. Available from: https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002595.
Lukman M, MSi KI, Yani DI, Juniarti N. Exploring Strategies to Improve the Performance of Community Health Volunteers for Tuberculosis Care and Prevention: A Qualitative Study. Int J Community Based Nurs Midwifery. 2019;7(4):270. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6779918/.
Espinal MA, Kim SJ, Suarez PG, Kam KM, Khomenko AG, Migliori GB, et al. Standard Short-Course Chemotherapy for Drug-Resistant Tuberculosis. JAMA. 2000;283(19):2537. Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.283.19.2537.
Wanyonyi AW, Wanjala PM, Githuku J, Oyugi E, Kutima H. Factors associated with interruption of tuberculosis treatment among patients in Nandi County, Kenya 2015. Pan Afr Med J. 2017;28(Suppl 1). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6113692/.
Oshi DC, Oshi SN, Alobu I, Ukwaja KN. Profile and Treatment Outcomes of Tuberculosis in the Elderly in Southeastern Nigeria, 2011–2012. Mokrousov I, editor. PLoS One. 2014;9(11):e111910. Available from: https://dx.plos.org/10.1371/journal.pone.0111910.
Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120(4):435–46. Available from: http://doi.wiley.com/10.1111/j.1365-2567.2007.02555.x.
Tanue EA, Nsagha DS, Njamen TN, Assob NJC. Tuberculosis treatment outcome and its associated factors among people living with HIV and AIDS in Fako Division of Cameroon. Haider MR, editor. PLoS One. 2019;14(7):e0218800. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0218800.
Tesfaye B, Alebel A, Gebrie A, Zegeye A, Tesema C, Kassie B. The twin epidemics: Prevalence of TB/HIV co-infection and its associated factors in Ethiopia; A systematic review and meta-analysis. Wilkinson KA, editor. PLoS One. 2018;13(10):e0203986. Available from: https://doi.org/dx.plos.org/10.1371/journal.pone.0203986.
Gebremariam G, Asmamaw G, Hussen M, Hailemariam MZ, Asegu D, Astatkie A, et al Impact of HIV Status on Treatment Outcome of Tuberculosis Patients Registered at Arsi Negele Health Center, Southern Ethiopia: A Six Year Retrospective Study. Spigelman M, editor. PLoS One. 2016;11(4):e0153239.
Mayer KH, Dukes Hamilton C. Synergistic Pandemics: Confronting the Global HIV and Tuberculosis Epidemics. Clin Infect Dis. 2010;50(s3):S67–70. Available from: https://academic.oup.com/cid/article/50/Supplement_3/S67/321450.
Abdullahi O, Moses N, Sanga D, Annie W. The effect of empirical and laboratory-confirmed tuberculosis on treatment outcomes. Sci Rep. 2021;11(1):14854. Available from: http://www.nature.com/articles/s41598-021-94153-0.
Crevel R van, Critchley JA. The Interaction of Diabetes and Tuberculosis: Translating Research to Policy and Practice. Trop Med Infect Dis. 2021;6(1):8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7838867/.
Yorke E, Atiase Y, Akpalu J, Sarfo-Kantanka O, Boima V, Dey ID. The Bidirectional Relationship between Tuberculosis and Diabetes. Tuberc Res Treat. 2017;2017:1702578. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5705893/.
Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lönnroth K, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9(1):81. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3155828/.
Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4595218/.
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. 2007;85:867–72. Available from: https://www.bmj.com/content/335/7624/806.
Acknowledgements
Authors would like to acknowledge all participating sub-counties, hospital staff working at the respective TB clinics and the Kilifi County TB Control Programme for their contribution in this study. We would like to acknowledge Professor Jameela Hassanali for her guidance and technical support in the manuscript writing.
Funding
The study was not funded.
Author information
Authors and Affiliations
Contributions
GK, MN, and OA conceived the idea. DS, GK, and MN performed data curation. MN conducted formal analysis. TM and MN developed the first draft which was further developed, reviewed and approved by all authors. The author(s) read and approved the final manuscript.
Author information
GK holds an MPH and is a public health officer at the Kilifi County Department of Public Health and a part-time lecturer Department of Public Health, Pwani University, Kilifi, Kenya.
MN holds Dphil in Epidemiology and works as Programme statistician at KEMRI Wellcome Trust Research Programme and is an adjunct lecturer Department of Public Health, Pwani University, Kilifi, Kenya.
TM holds MPH and currently is a part-time lecturer Department of Public Health, Pwani University, Kilifi County, Kenya.
DS is the coordinator of the Kilifi County TB Control Program, Kilifi, Kenya.
OA holds PhD in Epidemiology and is currently the Chair of Department of Public Health, Pwani University, Kilifi County, Kenya.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was obtained from Pwani University Ethics Review Committee (Reference No. ERC/MSc/049/2014). Permission to access patient’s data was sought from the Kilifi County Health research committee and TB Control Programme Coordinators of participating sub-counties before data were extracted. The study was conducted following the Reporting of studies Conducted using Observational Routinely-collected health Data (RECORD) guidelines [69] and Strengthening the Reporting of Observational studies in Epidemiology (STROBE) [70].
Consent for publication
Not applicable.
Competing interests
We declare that we have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Katana, G.G., Ngari, M., Maina, T. et al. Tuberculosis poor treatment outcomes and its determinants in Kilifi County, Kenya: a retrospective cohort study from 2012 to 2019. Arch Public Health 80, 48 (2022). https://doi.org/10.1186/s13690-022-00807-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13690-022-00807-4