Abstract
Introduction
Shock-induced endotheliopathy (SHINE), defined as a profound sympathoadrenal hyperactivation in shock states leading to endothelial activation, glycocalyx damage, and eventual compromise of end-organ perfusion, was first described in 2017. The aggressive resuscitation therapies utilised in treating shock states could potentially lead to further worsening endothelial activation and end-organ dysfunction.
Objective
This study aimed to systematically review the literature on resuscitation-associated and resuscitation-induced endotheliopathy.
Methods
A predetermined structured search of literature published over an 11-year and 6-month period (1 January 2011 to 31 July 2023) was performed in two indexed databases (PubMed/MEDLINE and Embase) per PRISMA guidelines. Inclusion was restricted to original studies published in English (or with English translation) reporting on endothelial dysfunction in critically ill human subjects undergoing resuscitation interventions. Reviews or studies conducted in animals were excluded. Qualitative synthesis of studies meeting the inclusion criteria was performed. Studies reporting comparable biomarkers of endothelial dysfunction post-resuscitation were included in the quantitative meta-analysis.
Results
Thirty-two studies met the inclusion criteria and were included in the final qualitative synthesis. Most of these studies (47%) reported on a combination of mediators released from endothelial cells and biomarkers of glycocalyx breakdown, while only 22% reported on microvascular flow changes. Only ten individual studies were included in the quantitative meta-analysis based on the comparability of the parameters assessed. Eight studies measured syndecan-1, with a heterogeneity index, I2 = 75.85% (pooled effect size, mean = 0.27; 95% CI − 0.07 to 0.60; p = 0.12). Thrombomodulin was measured in four comparable studies (I2 = 78.93%; mean = 0.41; 95% CI − 0.10 to 0.92; p = 0.12). Three studies measured E-selectin (I2 = 50.29%; mean = − 0.15; 95% CI − 0.64 to 0.33; p = 0.53), and only two were comparable for the microvascular flow index, MFI (I2 = 0%; mean = − 0.80; 95% CI − 1.35 to − 0.26; p < 0.01).
Conclusion
Resuscitation-associated endotheliopathy (RAsE) refers to worsening endothelial dysfunction resulting from acute resuscitative therapies administered in shock states. In the included studies, syndecan-1 had the highest frequency of assessment in the post-resuscitation period, and changes in concentrations showed a statistically significant effect of the resuscitation. There are inadequate data available in this area, and further research and standardisation of the ideal assessment and panel of biomarkers are urgently needed.
Similar content being viewed by others
Introduction
Shock, defined as the clinical expression of circulatory failure, leads to a mismatch in the oxygen demand and delivery to tissue that is initially reversible [1]. However, progressive cellular and tissue hypoxia rapidly becomes irreversible [2], leading to a cascade of multi-organ failure and, ultimately, death if untreated [3]. Endothelial dysfunction is the putative underlying common pathway leading to this cascade. Johansson et al. recently described shock-induced endotheliopathy (SHINE), a profound sympathoadrenal hyperactivation in shock states that leads to endothelial stimulation, glycocalyx damage, and eventual compromise of end-organ perfusion [4].
The breakdown and shedding of the glycocalyx layer in shock, termed endotheliopathy, trigger a cascade of inflammatory and coagulation responses that can lead to uncoupling of the macro- and microcirculation [5]. Invasive arterial access for pressure monitoring and acute resuscitative interventions for shock, including venous lines for rapid volume expansion and fluid bolus administration, non-pulsatile blood flow (such as mechanical circulatory support with extracorporeal membrane oxygenation (ECMO), and other extracorporeal support therapies, such as renal replacement therapy (RRT), have traditionally targeted restoring well-validated macro-circulatory endpoints such as improving mean arterial blood pressure and urine output. Microcirculatory endpoints, however, have been difficult to quantify objectively, particularly in critical illness [6,7,8], yet recent data has shown that these strongly correlate with patient outcomes [9, 10].
Despite several direct and indirect techniques for assessing endothelial integrity and microcirculatory flow, they have yet to be standardised and adopted for evaluating microcirculation during acute resuscitation [11]. Therefore, inference on endothelial dysfunction is usually obtained from a singular or a combination of different techniques of microcirculatory assessment.
While SHINE provides a biological plausible concept for endotheliopathy observed in shock, it is incomplete and needs to be further expanded to include haemodynamic resuscitation. Resuscitative interventions may either cause endothelial dysfunction by themselves, augment the dysfunction initially instigated by shock, and may inhibit the natural defensive mechanisms that repair endothelial integrity. We coined the term resuscitation-associated endotheliopathy (RAsE) to encompass this phenomenon beyond SHINE.
Some literature supports this theory and has explored the mechanistic effects of aggressive resuscitation protocols, including rapidly administered fluid boluses for volume expansion [12]. In addition, increased circulating volume and pressure within blood vessels may generate shearing forces, leading to glycocalyx shedding and subsequent endothelial dysfunction [13, 14]. Therefore, it is plausible that aggressive volume-expansion resuscitation exacerbates endotheliopathy, predisposing to progressive worsening of end organs and ultimately adverse outcomes in the context of a shock state.
This comprehensive review aimed to systematically examine the literature for studies describing endothelial dysfunction following resuscitation therapies administered in shock states. We sought to synthesise recommendations for reporting standards in this fast-expanding research are by quantifying published literature.
Methods
A predetermined systematic search, registered in the prospective international register of systematic reviews—PROSPERO (ID: CRD42022349074), was performed. Two online indexed medical databases, PubMed/MEDLINE and Excerpta Medica Database (Embase), were searched per the PRISMA guidelines [15] (Fig. 1).
All studies that reported on endothelial biomarkers data from critically ill humans who underwent resuscitation interventions were searched. The search terms used in [MeSH Terms] or [All Fields] were the keywords [‘resuscitation’] AND [‘endothelial dysfunction’ OR ‘endotheliopathy’ OR ‘endothelial damage’ OR ‘endothelial activation’]. The initial search was conducted from 1 January 2011 to 31 December 2021 and updated until 31 July 2023. A detailed description of the search strategy is included in Supplementary Table S1. All abstracts retrieved from the searches were filtered for duplicates, compiled in EndNote® (Thomson Reuters), and screened for relevance.
Eligible studies for inclusion were original clinical studies (including randomised controlled clinical trials, observational studies, case series, and case reports) published in English (or with an English translation). In these studies, the population was patients in shock states, and the intervention was administration of resuscitation fluid for treatment of the shock. Shock typically presents with a reduction in blood pressure, and most clinical guidelines recommend administration of volume expansion resuscitation to restore the microcirculatory parameters such as blood pressure and urine output. Therefore, some studies did not specify a comparator population, and it was assumed that all patients presenting in shock states were treated in accordance with the clinical resuscitation guidelines. The outcome of interest was a description of endotheliopathy following resuscitation for shock states by the following: (a) direct imaging for assessment of the microcirculatory function, measurement and quantification of (b) glycocalyx-breakdown biomarkers, and (c) mediators released from endothelial cells circulating in plasma. Relevant studies had their full manuscripts retrieved and reviewed by two independent reviewers in duplicate (NGO, DPS, assisted by RR, BS) (Supplementary Table S2). Assessment for risk of bias was performed based on the Cochrane risk of bias for randomised controlled trials [16] and the Newcastle–Ottawa scale (NOS) for observational studies [17] (Supplementary Table S3). Disagreements were resolved by consensus and additional senior review (SR, LESH). Reference lists and citations of the retrieved articles were also screened for relevance. The review articles and studies excluded did not describe endothelial dysfunction following resuscitation for circulatory shock or were conducted in animals.
Data analysis
Meta-analysis of eligible studies presenting means and standard deviations with comparable microcirculatory and endothelial assessments was performed. Transformational analysis based on the method by Wan et al. [18] was performed for comparison of studies assessing similar markers of endothelial and microvascular dysfunction but reporting medians and interquartile ranges. A random-effects meta-analysis model was used, and all analysis was performed using STATA (ver. 17).
Results
One-hundred and ninety-five articles were identified from the database searches, reference lists of key publications, and contact with authors. After an initial screening to remove duplicates and articles of no relevance, 102 studies were screened for eligibility, of which 68 full-text articles were accessed and reviewed. Thirty-two studies met the inclusion criteria and were included in the final qualitative synthesis as shown in Fig. 1. The details of these studies, including the population, intervention, control, and endothelial assessment, are presented in Table 1 below (additional details in Supplementary Table 2).
Of these thirty-two studies examining resuscitation and associated endotheliopathy included, there were 11 (34%) each on patients with trauma and haemorrhagic shock [19,20,21,22,23,24,25,26,27,28,29], and septic shock [30,31,32,33,34,35,36,37,38,39,40], and 6 (19%) on cardiogenic shock patients [41,42,43,44,45,46]. Two studies were case reports on systemic capillary leak syndrome (SCLS) [47, 48]. One study reported post-resuscitation endothelial dysfunction in acute respiratory failure [49] and another in dengue shock syndrome [50].
Twenty-four of the included studies (75%) were conducted in adults ≥ 18 years old (75%, i.e. 24/32) [20, 22, 23, 25,26,27,28, 30, 32,33,34,35,36,37,38, 40,41,42,43,44,45,46,47,48]. One study (3%) reported enrolment of a mix of adults and children [31].
Endotheliopathy assessment
A combination of mediators released from endothelial cells and biomarkers of glycocalyx breakdown was reported in 47% [15/32] of studies [19,20,21,22,23,24,25,26, 34, 37, 39,40,41, 44, 45]. The number of studies reporting only endothelial cell mediators in plasma was 25% (8/32) [28, 29, 33, 35, 42, 43, 49, 50], while those that exclusively reported biomarkers of glycocalyx shedding were 12.5% (4/32) [30, 36, 38, 48] (Supplementary Table 2).
The microcirculatory flow was assessed in 22% (7/32) of the studies, 57% (4/7) of which used orthogonal polarisation spectroscopy (OPS) [23, 31, 46], with one study only reporting the perfused boundary region (PBR) rather than microvascular flow [39]. One study used laser Doppler flowmetry [32] and flow-mediated dilatation (FMD) [27], while another study did not clearly describe the technique used to evaluate microvascular flow [47].
Meta-analysis
Ten unique studies were included in the quantitative meta-analysis. However, it was only possible to analyse comparative endothelial assessments performed. For the glycocalyx biomarker syndecan-1, eight studies were included [20, 24, 31, 34, 36, 38,39,40], with a heterogeneity index I2 = 75.85% and pooled effect size mean = 0.27 (95% CI − 0.07 to 0.60; p = 0.12) (Fig. 2a). Four studies were included for the endothelial cell mediator thrombomodulin [20, 24, 34, 42], with a heterogeneity index I2 = 78.93% and pooled effect size mean = 0.41 (95% CI − 0.10 to 0.92; p = 0.12) (Fig. 2b). Comparable data was available for three studies for the endothelial cell mediator E-selectin [20, 35, 40] (I2 = 50.29%; mean = − 0.15; 95% CI − 0.64 to 0.33; p = 0.53) (Fig. 2c) and only two studies for the microvascular flow index (MFI) [31, 32] (I2 = 0%; mean = − 0.80; 95% CI − 1.37 to − 0.24; p < 0.01) (Fig. 2d). Graphical summaries of the meta-analyses and publication bias are presented as Funnel plots (Supplementary Fig. 1a–d) and Galbraith plots (Supplementary Fig. 2a–d), respectively.
a Forest plot showing meta-analysis of eight studies that described syndecan-1 release post-resuscitation. Despite the high heterogeneity index, I2 = 75.87%, resuscitation caused a statistically significant release of syndecan-1 (pooled effect size; mean = 0.27; 95% CI - 0.07 to 0.60; p = 0.12). b Forest plot showing meta-analysis of four studies that described thrombomodulin release post-resuscitation. There was a high heterogeneity index between the studies with no significant effect of resuscitation on thrombomodulin release (I2 = 78.93%; mean = 0.41; 95% CI − 0.10 to 0.92; p = 0.12). c Forest plot of e-selectin release post-resuscitation. Only three studies described e-selectin release with high homogeneity but no significant effect of resuscitation on e-selectin release (I2 = 50.29%; mean = − 0.15; 95% CI − 0.64 to 0.33; p = 0.53). d Forest plot of microvascular flow index (MFI) post-resuscitation. Only two-studies described MFI with high homogeneity but showed resuscitation significantly reduced the MFI (I.2 = 0%; mean = − 0.80; 95% CI − 1.37 to − 0.24; p < 0.01)
Discussion
Since the description of the SHINE phenomenon [4], this study systematically reviews post-resuscitation endotheliopathy in different types of circulatory shock. In summary, two-thirds of the studies included were published in 2017 or later, with equal numbers reporting on septic and haemorrhagic-trauma-related shock forming the bulk of the studies. While there are several biomarkers and techniques for assessing and quantifying endothelial and microcirculatory dysfunction, there are no standardised criteria for use in critically ill patients in shock. Only a few studies yielded comparable measurements for inclusion in the meta-analysis. Eight studies quantitatively compared syndecan-1, and four compared thrombomodulin that were included in the meta-analysis. However, these had relatively elevated heterogeneity indices indicative of underlying variability in the original studies. E-selectin had three comparable studies, while microvascular flow index (MFI) had only two comparable studies with high homogeneity. In the meta-analysis, only MFI reached the statistical threshold of significance. Four other studies meeting the inclusion criteria describing endothelial dysfunction post-resuscitation were not included in the meta-analysis as they did not fit within the framework of SHINE described by Johanssen et al. Two of these studies were case reports on systemic capillary leak syndrome: one on acute respiratory failure and another on dengue shock syndrome.
Knowledge gap in endothelial biomarker research
While there are several biomarkers and techniques for assessing and quantifying endothelial and microcirculatory dysfunction, there are no standardised clinical criteria. Therefore, clinical assessment and quantification of endothelial dysfunction in critical illness and during resuscitation need a consistent approach. Despite the previous description of microvascular dysfunction in critical illness [13], only a few studies yielded comparable measurements for inclusion in the meta-analysis. Injury to the endothelium and shedding of the glycocalyx trigger the inflammatory-coagulopathy cascade leading to progressive microvascular dysfunction [13, 51,52,53]. Most of the studies included in this review used a combination of biomarkers, including glycocalyx breakdown products and mediators released from endothelial cells [19,20,21,22,23,24,25,26, 34, 37, 39,40,41, 44, 45]. Syndecan was the most described glycocalyx breakdown product in the reviewed studies. It is a transmembrane proteoglycan that undergoes cytokine-mediated release during inflammation [54], with levels circulating in plasma increasing during shock states [4]. Of the syndecans classified, syndecan-1 is the most common in shock-induced inflammation and has been extensively described in a recent literature review [55]. Our results highlight the variability in reporting practices. Future reporting guidelines need to be more prescriptive to enable progress in this field of research.
Relationship between biomarkers and resuscitation practices
The typical clinical presentation of shock states is reduced blood pressure, indicative of impaired perfusion to match tissue requirements. Tissue hypoxia activates neutrophils in microvessels, and the subsequent neutrophil accumulation induces endothelial damage [56, 57].
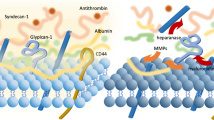
Different shock aetiologies could impact the endothelium differently (Supplementary Fig. 3a–c). For instance, in septic shock, diverse pathogens may cause varied profiles of endotheliopathy [51]. Despite these differences, shock types share similar phenotypic features as the shock progresses, including sympatho-adrenal activation, catecholamine-induced glycocalyx damage, and pro-coagulant profile [4]. A recent review highlighted contradictions between basic, preclinical, and clinical studies on the significance of glycocalyx damage as a marker of vascular permeability [58].
Figure 3 shows the hypothesised exacerbation of endotheliopathy during resuscitation for septic, haemorrhagic, and cardiogenic shock. Preclinical evidence has demonstrated that aggressive volume expansion in acute critical illness resuscitation leads to the progression and exacerbation of microcirculatory and endothelial dysfunction of endotoxaemic shock [12]. Based on this conceptual framework, it is plausible that initial damage to the endothelial-glycocalyx layer from the underlying shock could be further exacerbated by subsequent resuscitative interventions, thus predisposing to additional end-organ injury. Currently, there is limited clinical evidence for variation in endothelial injury following aggressive resuscitation for different shock states.
A summary of the pathophysiological mechanisms associated with the three different types of shock discussed are presented in Supplementary Fig. 3a–c. In septic shock, there is a relative reduction in the effective circulating volume due to vasodilatation and leakage into interstitial tissue. In contrast, in haemorrhagic shock, the decrease in the intravascular volume is due to blood loss. Volume replacement is the current standard of resuscitation for both these shock types [59,60,61]. In cardiogenic shock, global tissue hypoxia is secondary to poor perfusion, inducing a systemic inflammatory response syndrome (SIRS) comparable to sepsis [53, 62]. A similar SIRS response is seen with the initiation of extracorporeal membrane oxygenation (ECMO) [63]. The endothelial damage during shock and its subsequent exacerbation following resuscitation and reperfusion could have potential implications on clinical outcomes. It has been shown that disruption of the endothelial glycocalyx in cardiogenic shock is associated with worse patient outcomes [53, 64, 65]. Tsai et al. (2019) reported significantly higher levels of circulating vascular endothelial growth factor (VEGF), an endothelial survival factor associated with angiogenesis, at 72 h in patients who survived compared to those who died.
While higher resuscitation fluid volumes have been reported to correlate with higher levels of biomarkers such as syndecan-1 circulating in plasma [38], the clinical utility of syndecan-1 measurements during resuscitation remains limited. Thus, more research is required to correlate endothelial dysfunction biomarkers with the progression of end-organ dysfunction in shock states and during resuscitation [66]. Additionally, attainment of uniformity in analysing biomarkers of endotheliopathy requires some degree of standardisation of the time when they are measured since their relative abundance circulating in blood will vary over time. A proposed framework showing the major domains for the assessment and quantification of endotheliopathy in clinical studies is presented in Supplementary Table 4. With a better description of the endothelial injury biomarkers in the various resuscitation scenarios, investigators might be able to delineate the ‘epiphenomenon’ from real correlation and even risk factors.
This study has some limitations. One major limitation is the paucity of literature on resuscitation-associated endothelial dysfunction. Therefore, in order to understand which markers have been used clinically, studies were included on the basis of their reporting of endothelial markers post-resuscitation. Assessment of bias was performed in light of the fact that different study designs have been considered. As expected, the risk of bias was much lower in the randomised controlled studies than in the observational studies as reported in Supplementary Table 3. Funnel plots have been included to highlight the effects of smaller and non-randomised studies. Further description of the heterogeneity seen is provided by Galbraith plots that show the potential outliers mainly being non-randomised studies. However, the studies included in the meta-analysis are few, and more randomised studies investigating resuscitation-associated endothelial dysfunction are therefore required to address the knowledge gaps in this field.
Another limitation of this study was the lack of consistency in reporting of results in the studies that were reviewed. The lack of comparable biomarkers led to a reduction in the final number of studies that could be included in the quantitative meta-analysis resulting in high heterogeneity indices. Additionally, the application of transformations for the estimation of means and standard deviations from medians and interquartile ranges in the original publications were based on methods described by Wan et al. and are subject to mathematical assumptions [18]. Therefore, clearer reporting guidelines are necessary to achieve comparable and scientifically reproducible outcomes.
Conclusion
In this review, we conceptualise the term resuscitation-associated endotheliopathy (RAsE) in relation to worsening endothelial dysfunction resulting from acute resuscitative therapies administered in shock states described as shock-induced endotheliopathy (SHINE). Unfortunately, there is neither consensus nor consistency in the definition of microvascular biomarkers in critically ill patients. Thus, additional research and standardisation of the ideal assessment and panel of biomarkers are urgently needed.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- ANGPT2:
-
Angiopoietin 2
- ATIII:
-
Antithrombin III
- ECMO:
-
Extracorporeal membrane oxygenation
- ECVVH:
-
Early-initiated continuous venovenous haemofiltration
- EoT:
-
Endotheliopathy of trauma
- FMD:
-
Flow-mediated dilatation
- FFP:
-
Fresh-frozen plasma
- GCS:
-
Glasgow Coma Scale score
- HES:
-
Hydroxyethyl starch
- MAP:
-
Mean arterial pressure
- MeSH:
-
Medical Subject Headings
- MFI:
-
Microvascular flow index
- NOS:
-
Newcastle-Ottawa scale
- OHCA:
-
Out-of-hospital cardiac arrest
- OPS:
-
Orthogonal polarisation spectroscopy
- PAI-1:
-
Plasminogen activator inhibitor-1
- PALICC:
-
Paediatric Acute Lung Injury Consensus Conference
- PBR:
-
Perfused boundary region
- RAsE:
-
Resuscitation-associated endotheliopathy
- ROSC:
-
Return of spontaneous circulation
- RRT:
-
Renal replacement therapy
- sCD40L:
-
Soluble CD40 ligand
- SCLS:
-
Systemic capillary leak syndrome
- sE-selectin:
-
Soluble endothelial leucocyte adhesion molecule
- sFLT-1:
-
Soluble vascular endothelial growth factor receptor-1
- SHINE:
-
Shock-induced endotheliopathy
- sI-CAM:
-
Soluble intercellular adhesion molecule
- SIRS:
-
Systemic inflammatory response syndrome
- sP-selectin:
-
Soluble platelet adhesion molecule
- sTM:
-
Soluble thrombomodulin
- sVCAM-1:
-
Soluble vascular cell adhesion molecule-1
- sVE-cadherin:
-
Soluble vascular endothelial cadherin
- sVEGF:
-
Soluble vascular endothelial growth factor
- Tpa:
-
Tissue-type plasminogen activator
- VEcad:
-
Vascular endothelial cadherin
- vWF:
-
Von Willebrand factor
References
Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726–34.
Gaieski DF MM. Definition, classification, etiology, and pathophysiology of shock in adults https://www.medilib.ir/uptodate/show/1594.
Standl T, Annecke T, Cascorbi I, Heller AR, Sabashnikov A, Teske W. The nomenclature, definition and distinction of types of shock. Dtsch Arztebl Int. 2018;115(45):757–68.
Johansson PI, Stensballe J, Ostrowski SR. Shock induced endotheliopathy (SHINE) in acute critical illness - a unifying pathophysiologic mechanism. Crit Care. 2017;21(1):25.
Moore JP, Dyson A, Singer M, Fraser J. Microcirculatory dysfunction and resuscitation: why, when, and how. Br J Anaesth. 2015;115(3):366–75.
Erdem O, Ince C, Tibboel D, Kuiper JW. Assessing the microcirculation with handheld vital microscopy in critically ill neonates and children: evolution of the technique and its potential for critical care. Front Pediatr. 2019;7:273.
Dilken O, Ergin B, Ince C. Assessment of sublingual microcirculation in critically ill patients: consensus and debate. Ann Transl Med. 2020;8(12):793.
De Backer D, Ospina-Tascon G, Salgado D, Favory R, Creteur J, Vincent JL. Monitoring the microcirculation in the critically ill patient: current methods and future approaches. Intensive Care Med. 2010;36(11):1813–25.
Schaid TR Jr, Hansen KC, Sauaia A, Moore EE, DeBot M, Cralley AL, et al. Postinjury complement C4 activation is associated with adverse outcomes and is potentially influenced by plasma resuscitation. J Trauma Acute Care Surg. 2022;93(5):588–96.
Johansson PI, Soe-Jensen P, Bestle MH, Clausen NE, Kristiansen KT, Lange T, et al. Prostacyclin in intubated patients with COVID-19 and severe endotheliopathy: a multicenter, randomized clinical trial. Am J Respir Crit Care Med. 2022;205(3):324–9.
Chia PY, Teo A, Yeo TW. Overview of the assessment of endothelial function in humans. Front Med (Lausanne). 2020;7: 542567.
Byrne L, Obonyo NG, Diab SD, Dunster KR, Passmore MR, Boon AC, et al. Unintended consequences: fluid resuscitation worsens shock in an ovine model of endotoxemia. Am J Respir Crit Care Med. 2018;198(8):1043–54.
Ince C, De Backer D, Mayeux PR. Microvascular dysfunction in the critically ill. Crit Care Clin. 2020;36(2):323–31.
Obad A, Marinovic J, Ljubkovic M, Breskovic T, Modun D, Boban M, et al. Successive deep dives impair endothelial function and enhance oxidative stress in man. Clin Physiol Funct Imaging. 2010;30(6):432–8.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7): e1000097.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928.
GA Wells BS, D O'Connell, J Peterson, V Welch, M Losos, P Tugwell. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2021 [Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Welling H, Henriksen HH, Gonzalez-Rodriguez ER, Stensballe J, Huzar TF, Johansson PI, et al. Endothelial glycocalyx shedding in patients with burns. Burns. 2020;46(2):386–93.
Peng HT, Nascimento B, Rhind SG, da Luz L, Beckett A. Evaluation of trauma-induced coagulopathy in the fibrinogen in the initial resuscitation of severe trauma trial. Transfusion. 2021;61(Suppl 1):S49–57.
Lopez E, Peng Z, Kozar RA, Cao Y, Ko TC, Wade CE, et al. Antithrombin III contributes to the protective effects of fresh frozen plasma following hemorrhagic shock by preventing syndecan-1 shedding and endothelial barrier disruption. Shock. 2020;53(2):156–63.
Gruen DS, Brown JB, Guyette FX, Vodovotz Y, Johansson PI, Stensballe J, et al. Prehospital plasma is associated with distinct biomarker expression following injury. JCI Insight. 2020;5(8):e135350. https://doi.org/10.1172/jci.insight.135350.
Naumann DN, Hazeldine J, Bishop J, Midwinter MJ, Harrison P, Nash G, et al. Impact of plasma viscosity on microcirculatory flow after traumatic haemorrhagic shock: a prospective observational study. Clin Hemorheol Microcirc. 2019;71(1):71–82.
Naumann DN, Hazeldine J, Davies DJ, Bishop J, Midwinter MJ, Belli A, et al. Endotheliopathy of trauma is an on-scene phenomenon, and is associated with multiple organ dysfunction syndrome: a prospective observational study. Shock. 2018;49(4):420–8.
Gonzalez Rodriguez E, Cardenas JC, Cox CS, Kitagawa RS, Stensballe J, Holcomb JB, et al. Traumatic brain injury is associated with increased syndecan-1 shedding in severely injured patients. Scand J Trauma Resusc Emerg Med. 2018;26(1):102.
Stensballe J, Ulrich AG, Nilsson JC, Henriksen HH, Olsen PS, Ostrowski SR, et al. Resuscitation of endotheliopathy and bleeding in thoracic aortic dissections: the VIPER-OCTA randomized clinical pilot trial. Anesth Analg. 2018;127(4):920–7.
Turk E, Caliskan M, Karagulle E, Aydogan C, Oguz H, Kulaksizoglu S, et al. A prospective clinical study of flow-mediated dilatation in burn injury. J Burn Care Res. 2014;35(2):169–75.
Tang N, Yin S, Sun Z, Pan Y. Time course of soluble P-selectin and von Willebrand factor levels in trauma patients: a prospective observational study. Scand J Trauma Resusc Emerg Med. 2013;21:70.
Junger WG, Rhind SG, Rizoli SB, Cuschieri J, Shiu MY, Baker AJ, et al. Resuscitation of traumatic hemorrhagic shock patients with hypertonic saline-without dextran-inhibits neutrophil and endothelial cell activation. Shock. 2012;38(4):341–50.
Saoraya J, Wongsamita L, Srisawat N, Musikatavorn K. Plasma syndecan-1 is associated with fluid requirements and clinical outcomes in emergency department patients with sepsis. Am J Emerg Med. 2021;42:83–9.
Rovas A, Seidel LM, Vink H, Pohlkotter T, Pavenstadt H, Ertmer C, et al. Association of sublingual microcirculation parameters and endothelial glycocalyx dimensions in resuscitated sepsis. Crit Care. 2019;23(1):260.
Bourcier S, Joffre J, Dubee V, Preda G, Baudel JL, Bige N, et al. Marked regional endothelial dysfunction in mottled skin area in patients with severe infections. Crit Care. 2017;21(1):155.
Meng JB, Lai ZZ, Xu XJ, Ji CL, Hu MH, Zhang G. Effects of early continuous venovenous hemofiltration on E-selectin, hemodynamic stability, and ventilatory function in patients with septic-shock-induced acute respiratory distress syndrome. Biomed Res Int. 2016;2016:7463130.
Muller RB, Ostrowski SR, Haase N, Wetterslev J, Perner A, Johansson PI. Markers of endothelial damage and coagulation impairment in patients with severe sepsis resuscitated with hydroxyethyl starch 130/0.42 vs Ringer acetate. J Crit Care. 2016;32:16–20.
K G H Katundu LTH, L M Davids, I A Joubert, M G A Miller, J L Piercy, W L Michell. An observational study on the relationship between plasma vitamin C, blood glucose, oxidative stress, endothelial dysfunction and outcome in patients with septic shock. South African J Crit Care. 2016;32(1):21. https://doi.org/10.7196/SAJCC.2016.v32i1.270.
Saoraya J, Wongsamita L, Srisawat N, Musikatavorn K. The effects of a limited infusion rate of fluid in the early resuscitation of sepsis on glycocalyx shedding measured by plasma syndecan-1: a randomized controlled trial. J Intensive Care. 2021;9(1):1.
Hippensteel JA, Uchimido R, Tyler PD, Burke RC, Han X, Zhang F, et al. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit Care. 2019;23(1):259.
Wu X, Hu Z, Yuan H, Chen L, Li Y, Zhao C. Fluid resuscitation and markers of glycocalyx degradation in severe sepsis. Open Med (Wars). 2017;12:409–16.
Fernandez-Sarmiento J, Salazar-Pelaez LM, Acevedo L, Nino-Serna LF, Florez S, Alarcon-Forero L, et al. Endothelial and glycocalyx biomarkers in children with sepsis after one bolus of unbalanced or balanced crystalloids. Pediatr Crit Care Med. 2023;24(3):213–21.
Macdonald S, Bosio E, Keijzers G, Burrows S, Hibbs M, O’Donoghue H, et al. Effect of intravenous fluid volume on biomarkers of endothelial glycocalyx shedding and inflammation during initial resuscitation of sepsis. Intensive Care Med Exp. 2023;11(1):21.
Meyer ASP, Johansson PI, Kjaergaard J, Frydland M, Meyer MAS, Henriksen HH, et al. “Endothelial dysfunction in resuscitated cardiac arrest (ENDO-RCA): safety and efficacy of low-dose iloprost, a prostacyclin analogue, in addition to standard therapy, as compared to standard therapy alone, in post-cardiac-arrest-syndrome patients.” Am Heart J. 2020;219:9–20.
Grand J, Meyer AS, Kjaergaard J, Wiberg S, Thomsen JH, Frydland M, et al. A randomised double-blind pilot trial comparing a mean arterial pressure target of 65 mm Hg versus 72 mm Hg after out-of-hospital cardiac arrest. Eur Heart J Acute Cardiovasc Care. 2020;9(4_suppl):S100–9.
Ohbe H, Kudo D, Yamanouchi S, Kushimoto S. Decreased a disintegrin-like and metalloprotease with thrombospondin type 1 motif 13 activity and neurologic outcome in patients with successful resuscitation of out-of-hospital cardiac arrest: a prospective observational study. J Crit Care. 2017;37:13–8.
Bro-Jeppesen J, Johansson PI, Kjaergaard J, Wanscher M, Ostrowski SR, Bjerre M, et al. Level of systemic inflammation and endothelial injury is associated with cardiovascular dysfunction and vasopressor support in post-cardiac arrest patients. Resuscitation. 2017;121:179–86.
Bro-Jeppesen J, Johansson PI, Hassager C, Wanscher M, Ostrowski SR, Bjerre M, et al. Endothelial activation/injury and associations with severity of post-cardiac arrest syndrome and mortality after out-of-hospital cardiac arrest. Resuscitation. 2016;107:71–9.
Omar YG, Massey M, Andersen LW, Giberson TA, Berg K, Cocchi MN, et al. Sublingual microcirculation is impaired in post-cardiac arrest patients. Resuscitation. 2013;84(12):1717–22.
Case R, Ramaniuk A, Martin P, Simpson PJ, Harden C, Ataya A. Systemic capillary leak syndrome secondary to coronavirus disease 2019. Chest. 2020;158(6):e267–8.
Boe OW, Sveen K, Borset M, Druey KM. Raised serum levels of syndecan-1 (CD138), in a case of acute idiopathic systemic capillary leak syndrome (SCLS) (Clarkson’s disease). Am J Case Rep. 2018;19:176–82.
Monteiro ACC, Flori H, Dahmer MK, Sim MS, Quasney MW, Curley MAQ, et al. Thrombomodulin is associated with increased mortality and organ failure in mechanically ventilated children with acute respiratory failure: biomarker analysis from a multicenter randomized controlled trial. Crit Care. 2021;25(1):271.
Somasetia DH, Setiati TE, Sjahrodji AM, Idjradinata PS, Setiabudi D, Roth H, et al. Early resuscitation of dengue shock syndrome in children with hyperosmolar sodium-lactate: a randomized single-blind clinical trial of efficacy and safety. Crit Care. 2014;18(5):466.
Chang JC. Sepsis and septic shock: endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb J. 2019;17:10.
Naumann DN, Hazeldine J, Midwinter MJ, Hutchings SD, Harrison P. Poor microcirculatory flow dynamics are associated with endothelial cell damage and glycocalyx shedding after traumatic hemorrhagic shock. J Trauma Acute Care Surg. 2018;84(1):81–8.
Jung C, Fuernau G, Muench P, Desch S, Eitel I, Schuler G, et al. Impairment of the endothelial glycocalyx in cardiogenic shock and its prognostic relevance. Shock. 2015;43(5):450–5.
Xian X, Gopal S, Couchman JR. Syndecans as receptors and organizers of the extracellular matrix. Cell Tissue Res. 2010;339(1):31–46.
Gopal S. Syndecans in inflammation at a glance. Front Immunol. 2020;11:227.
Gando S, Nanzaki S, Morimoto Y, Kobayashi S, Kemmotsu O. Out-of-hospital cardiac arrest increases soluble vascular endothelial adhesion molecules and neutrophil elastase associated with endothelial injury. Intensive Care Med. 2000;26(1):38–44.
McBride A, Chanh HQ, Fraser JF, Yacoub S, Obonyo NG. Microvascular dysfunction in septic and dengue shock: pathophysiology and implications for clinical management. Glob Cardiol Sci Pract. 2020;2020(2): e202029.
Dull RO, Hahn RG. The glycocalyx as a permeability barrier: basic science and clinical evidence. Crit Care. 2022;26(1):273.
Weiss SL, Peters MJ, Alhazzani W, Agus MSD, Flori HR, Inwald DP, et al. Surviving Sepsis Campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020;21(2):e52–106.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063–143.
Spahn DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care. 2019;23(1):98.
Langeland H, Damas JK, Mollnes TE, Ludviksen JK, Ueland T, Michelsen AE, et al. The inflammatory response is related to circulatory failure after out-of-hospital cardiac arrest: a prospective cohort study. Resuscitation. 2022;170:115–25.
Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care. 2016;20(1):387.
Senthil S, Veerappan RM, Ramakrishna Rao M, Pugalendi KV. Oxidative stress and antioxidants in patients with cardiogenic shock complicating acute myocardial infarction. Clin Chim Acta. 2004;348(1–2):131–7.
Cosgun ZC, Fels B, Kusche-Vihrog K. Nanomechanics of the endothelial glycocalyx: from structure to function. Am J Pathol. 2020;190(4):732–41.
Pierce RW, Giuliano JS, Whitney JE, Ouellette Y, Pediatric organ dysfunction information update mandate C. Endothelial dysfunction criteria in critically ill children: the PODIUM Consensus Conference. Pediatrics. 2022;149(1 Suppl 1):S97–102.
Funding
Critical Care Research Group (CCRG) and Queensland Cardiovascular Research Network (QCVRN).
Author information
Authors and Affiliations
Contributions
NGO, JYS, and JFF conceived the study and developed the concept with additional input from SR, LESH, JPF, GLB, and KM. NGO, DPS, RR, and BS conducted the review and assessment for bias. NGO, DPS, and JPF performed the statistical analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Disclosure
NGO has a Postdoctoral Research Fellowship at The Prince Charles Hospital Foundation.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary figures: S1.
Funnel plots. S2. Galbraith plots. S3. Shock pathophysiology. Supplementary tables: S1. Search terms. S2.All studies meeting the inclusion criteria. S3. Risk of bias assessment. S4.Framework for the assessment and quantification of endotheliopathy in clinical studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Obonyo, N.G., Sela, D.P., Raman, S. et al. Resuscitation-associated endotheliopathy (RAsE): a conceptual framework based on a systematic review and meta-analysis. Syst Rev 12, 221 (2023). https://doi.org/10.1186/s13643-023-02385-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-023-02385-0