Abstract
Background
More than 1.7 billion people are affected by neglected tropical diseases (NTDs) worldwide. Forty percent of the NTD-affected people live in Africa with the poorest, most vulnerable, and hard to reach geographical areas. The NTDs cause significant social and economic burden and deepen marginalization and stigmatization. The World Health Organization’s current roadmap for NTD aims to prevent, control, eliminate, or eradicate 20 tropical diseases. Ethiopia experiences a high burden of these diseases, but current access to diagnostics, medicine, and/or care has been little explored to inform the country’s NTD strategic plan. The overall purpose of the scoping review was to map and characterize the burden of NTDs and challenges in access to diagnostics, medicine, and/or care in Ethiopia.
Methods
A systematic search of evidence was conducted in PubMed, Cochrane Library, and Google Scholar from January 2000 until May 2022, without restrictions of language or study design. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Review was followed for screening of studies. Key findings were extracted and narrated qualitatively.
Results
The search resulted in 4532 articles, of which 105 met the inclusion criteria and were included in the scoping review under three themes: burden of NTDs, access to diagnostics, medicine and/or care, and key barriers. Although gains have been made in the prevention and control of NTDs in Ethiopia, the burden remains high, and progress in access to diagnostics, medicine/drugs, and/or care is very slow. Poverty, poor quality of life, and underfunding of NTD programs decelerate the process of NTD elimination program in the country.
Conclusions
The scoping review identified a considerable number of studies on the burden of NTDs in Ethiopia and strategies for diagnosis, treatment, and/or care; however, there is a paucity of evidence on the suitability and potential benefits of novel diagnostic technologies and medicines in the country. A regular review and analysis of such country-level evidence is important to inform the country NTDs roadmap and local implementation strategies.
Similar content being viewed by others
Background
Neglected tropical diseases (NTDs) are a group of more than twenty preventable and treatable infectious diseases in the tropics [1], which affect around 1.7 billion people in 149 countries worldwide [2,3,4]. Of these, 40% live in Africa with the poorest and most vulnerable people living in hard to reach geographical areas [3]. NTDs cause significant social and economic burden and deepen marginalization and stigmatization [5]. NTDs are also associated with huge costs and losses to productivity [6]. The World Health Organization’s (WHO) new roadmap for NTDs (2021–2030) is looking ahead to prevent, control, eliminate, or eradicate 20 tropical diseases [7]. The roadmap is aimed at reducing morbidities and mortalities from vector-borne diseases and achieving integrated coverage of preventive chemotherapy for NTDs [2, 7]. Ending the epidemics of infectious diseases including NTDs is one of the United Nations (UN) Sustainable Development Goals (SDG:3) under target 3.3 [8]. In response to this, the WHO urged countries to develop national roadmaps on NTDs by 2020 to sustain enhanced and equitable access to high-quality healthcare coverage against NTDs by 2030. More than 74 countries [2], including Ethiopia [5, 9, 10], are ready to implement national NTDs roadmap, stimulating increased demand for program implementation and donated medicines crucial to reach the roadmap’s targets.
Almost all regions of Ethiopia have been affected by at least three NTDs. The burden is higher in the central, western, and northwestern parts of the country [11], and more than 75 million people were at risk of infection by at least one NTD in Ethiopia [12]. Over a third of those in need (27 million) had not received treatment in 2016 [3]. In response to this, increasing attention has been given to NTDs over the last couple of decades in Ethiopia [2, 5, 12]. The ambitious goals of ending NTDs have to be matched by strategies tailored to local settings, evidence-based decision-making, and sufficient access to therapeutics [13]. Likewise, there is an urgent need for effective and efficient data monitoring and national surveillance systems to enable early detection and mitigation of the spread of NTDs [7, 14].
Quality evidence is required to inform NTD policymaking and implementation Ethiopia. However, there is a paucity of synthesized evidence relating to disease burden, challenges around access to diagnostics, drugs/therapeutics, and/or care to inform NTD policymaking and program implementation in Ethiopia. The main aim of this scoping review was to map the available research undertaken on the burden of NTDs and implementation key challenges on access to diagnostic and treatment services in Ethiopia.
Scoping review question(s)
-
▪
What is the scope and volume of literature on NTDs in Ethiopia?
-
▪
What is the burden of NTDs in Ethiopia?
-
▪
What are the available NTD diagnostics, medicines, and/or care for in Ethiopa?
-
▪
What are the challenges to NTD diagnostics, medicine, and/or care in Ethiopia?
Methods
The methodology of this scoping review was developed based on the recommendation of Preferred Reporting Items for Systematic Review and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) statement [15, 16], and items in the PRISMA-ScR checklist are completed [16] (Additional file 1). The type of NTDs to be considered in this scoping review was informed by the revised national NTDs master plans of Ethiopia [5, 10].
Search and identification of studies
Studies were searched from major electronic databases that include both published and unpublished (gray literature) and available from January 2000 to May 2022. The year 2000 was selected to capture comprehensive evidence since the inception of the Millennium Development Goals (MDGs) [17, 18]. Systematic search was carried out to retrieve studies indexed in PubMed, Cochrane Library, and direct search from Google Scholar (Additional file 2). Medical Subject Headings (MeSHs) were used to search studies from the databases that were designed considering the participants, concepts, and contexts (PCC) of the research questions. Terms included NTDs prioritized in Ethiopia (onchocerciasis, trachoma, lymphatic filariasis, podoconiosis, soil transmitted helminthiasis, schistosomiasis, leishmaniasis, scabies, and dracunculiasis/guinea-worm), poverty-related disease, key challenges, barriers, associated factors, determinants, constraints, prevention and control, and Ethiopia (Table 1). Generally, MeSH terms were combined according to the databases interface compatibility and recommendation. All the searches were imported into EndNote citation manager, and duplicates were removed.
Eligibility criteria
Existing evidence was included into the scoping review using the following eligibility criteria:
-
▪
Studies focused on prioritized NTD in Ethiopia according to the WHO definition.
-
▪
Any type of study — observational, interventional, evaluation study designs, and reviews
-
▪
Both facility- and community-based studies conducted in Ethiopia
-
▪
Both published and unpublished (gray literature) studies
-
▪
Year of publication: January 2000 to May 2022
Selection process
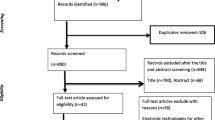
Studies selection was carried out in a stepwise process. Initially, screening of studies was performed based on their title and abstract by two authors (A. S. and E. G.). Studies with title and abstract that clearly stated one or more of the prioritized NTDs in Ethiopia, associated factors, access to diagnostics, medicine, and/or care were considered for further evaluation. Second, the same two authors (A. S. and E. G.) performed full-text assessment of included studies. The full-text assessment involved evaluation of the study design, sample size, participants recruitment, data analysis, presentation of key findings, conclusions, and recommendations. Third, any uncertainty or disagreement between the two full-text assessors was resolved by consensus through consultation of senior authors (A. F. and T. M.). Study screening and selection were guided by the PRISMA-ScR flowchart [15] (Fig. 1).
Measurement of outcome and exposure
The burden of the neglected tropical diseases, access to diagnostics, medicine (drug/therapeutics), and/or care were considered as primary outcomes. The key barriers to access to diagnostic, medicine and/or care, and identified key policy recommendation to prevent and control NTDs in Ethiopia were consider as exposure.
Data charting and summary of results
The selection process was guided by existing methodology recommendations [19]. Data charting (extraction) template was designed in Microsoft Word to record key findings of included studies (Additional file 3). The main characteristics of individual studies such as author, publication year, study area (specific geographic, administration site and regional state, national), aim of the study, design, sampling method, and type of NTDs considered in the study, burden of NTDs, diagnostic method used, medicine (drug/therapeutics) used and/or care, and other key findings were recorded in the data abstraction template. The content of the studies was grouped into three thematic areas: burden of NTDs (theme 1), studies on diagnostic (theme 2), and access to medicine (drug/therapeutic) services (theme 3).
Results
Description of characteristics of studies
A total of 4532 articles related to NTDs in Ethiopia were retrieved. Of these, 1518 duplicates were removed. Next, documents were screened by title, abstract, and full text to decide their eligibility for the scoping review. Finally, 105 articles were included into the scoping review. Details of the screening and selection process are depicted using a PRISMA-ScR flowchart on Fig. 1, and the detail profile of the included article is presented as an additional file (Additional file 3).
Evidence on burden of NTDs in Ethiopia
We identified 31 articles that report the burden of prioritized NTDs in Ethiopia [3, 10, 11, 20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. Most were systematic reviews and meta-analyses on trachoma [20], leishmaniasis [21,22,23,24,25], soil-transmitted helminthiasis (STH) [26,27,28,29,30], schistosomiasis [30,31,32,33], scabies [34], lymphatic filariasis (LF) and podoconiosis [35,36,37,38], review on onchocerciasis [40], and historical review of the overall NTDs in Ethiopia (1941–2019) [39]. Trachoma, podoconiosis and leishmaniasis [3, 11], onchocerciasis, LF, schistosomiasis, STH, and scabies [3] are three common NTDs in Ethiopia. The government of Ethiopia has prioritized nine NTDs for intervention [10]. The STHs are common among both adults and school children in Ethiopia [26, 39, 41,42,43,44]. One-third of infants in Ethiopia are affected by STHs (specifically ascariasis) [11]. Podoconiosis and trachoma are also common in Ethiopia, mainly in Amhara, Oromia, and Southern Nation, Nationalities and Peoples (SNNP) regions [45]. The distributions of NTDs at country level have been mapped and endemic areas identified and prioritized [36, 46, 47].
Podoconiosis and lymphatic filariasis (LF)
Both podoconiosis and LF are among the prioritized NTDs in Ethiopia [10]. Thirteen relevant articles were identified [10, 35, 37, 48,49,50,51,52,53,54,55,56], which report the burden of podoconiosis and/or LF in Ethiopia [35, 52, 53]. The burden of podoconiosis is widespread in Africa which ranged from 0.1 to 8.1%, where Ethiopia registered the second highest prevalence rate (7.5%) next to Cameroon (8.1%) [10, 37, 38, 48, 50]. Podoconiosis has gained attention recently in Ethiopia after evidence emerged that it is a preventable noninfectious geochemical form of lymphedema caused by exposure of bare feet to irritant alkalic clay soil [49, 50]. The prevalence of podoconiosis in Ethiopia shows marked regional variation, as high as 8.3% in SNNP, 4.0% in Oromia, and 3.9% in Amhara region [10]. In addition, there was disparity in the burden of podoconiosis by gender where prevalence was higher among women (3.7%) than men (2.4%) [51]. Leg lymphedema has a significant negative socio-economic impact on people affected by podoconiosis and LF and their caregivers in co-endemic districts of Ethiopia [56] and also increases mortality [55].
Trachoma
Five studies were identified on the burden of trachoma in Ethiopia [20, 57,58,59,60]. The overall prevalence of active trachoma among children in Ethiopia was 26.9%, and was highest in SNNP (35.8%) followed by 30.2% in Amhara and 20.2% in Oromia [20]. Studies conducted in Amhara region (152–160) have shown that 28% of districts had trachoma, with prevalence ranging from 8.5 to 36% [57, 58]. A high burden of trachoma was also reported in studies conducted in Wolayita Zone [59, 60].
Soil-transmitted helminthiasis (STH)
Systematic reviews and meta-analyses identified STH burden [27,28,29,30, 41, 43, 61, 62]. In five studies, the prevalence of STH in Ethiopia ranged from 13 to 52.4% [27,28,29,30, 39, 41]. Of these, Ascaris lumbricoides (8.8 to 14.0%) [27, 28, 30, 41, 43], hookworm (9.5 to 12.5%) [28,29,30, 41], Trichuris trichiura [30, 41], and Strongyloides stercoralis (5.6%) [29] were the most common STHs in Ethiopia. The burden of parasite among pregnant women was 29% in Ethiopia [43]. Furthermore, the prevalence of Strongyloides stercoralis in Ethiopia ranged from 1.8 to 11.1% in adults [61, 62], from 0.3 to 20.7% in children, and 1.5 to 17.3% in HIV-positive adults [62].
Schistosomiasis
Overall, the prevalence of schistosomiasis varies across regions of Ethiopia from 39.8 to 41.5% in SNNP [31, 32], 41% in Amhara, 31.4% in Tigray, 28.9% in Oromia [32], and 15% in Afar [31]. In four studies, the prevalence of schistosomiasis was as high as 73.9% in some districts of Ethiopia and with lower figures 2% [31,32,33, 39]. Among school-age children, males were 58% more likely to be infected with schistosomiasis than females [31]. Hepatosplenic schistosomiasis is another ignored morbidity in Ethiopia [63].
Leishmaniasis
Leishmaniasis is another prioritized NTD with a report of high morbidity and mortality in Ethiopia [10]. Five studies reported the prevalence of leishmaniasis [21,22,23,24,25]. The prevalence of leishmaniasis in high burden areas of Ethiopia was between 9.1% and 19% [21, 25]. The prevalence of leishmaniasis was 39.1% in Amhara region, followed by 23% in Tigray region [21]. Visceral leishmaniasis was one of the common HIV co-infections (5.2%) [24]. Being male was associated with an increased risk of leishmaniasis diagnosis [21].
Scabies
The prevalence of scabies is documented in several studies in Ethiopia [34, 64,65,66,67]. In one systematic review, the overall prevalence of scabies infestation in Ethiopia was 14.5% [34]. Scabies was more common among people who have frequent contact with people with active scabies at home, who do not use soap/detergent for hand-washing, and move from non-endemic to endemic areas [68]. Scabies has a strong relationship with malnutrition among orphan children in Ethiopia [69].
Onchocerciasis
Onchocerciasis has been known in Ethiopia since 1939 and listed as a priority NTD in 2013 [9, 10, 70]. Although onchocerciasis affects millions of people in wide geographic area, and the transmission of onchocerciasis in many districts has remained persistent, Ethiopia envisaged to interrupt onchocerciasis transmission by 2020 and to be certified free from onchocerciasis by 2025 [40]. In one study, the mean microfilaremia (mf) of onchocerciasis in Ethiopia was 4.7% (village range 1.1–11.0%) [71].
Evidence on access to diagnostics and its challenges
Leishmaniasis
Peripheral blood microscopy [72], kinetoplast deoxyribonucleic acid (kDNA) polymerase chain reaction (PCR) method [73], quantitative real-time kinetoplast deoxyribonucleic acid (qRT-kDNA) PCR [74], diagnosis of leishmaniasis based on rK39 immunochromatographic (rK39-ICT) [75, 76], in-house liquid direct agglutination test (AQ-DAT) antigen [77], microculture method (MCM), traditional culture method (TCM) and smear microscopy [78], and molecular diagnosis [25] are available diagnostic procedures for leishmaniasis in Ethiopia. Visceral leishmaniasis can be ruled in with peripheral blood microscopy in a substantial number of suspected cases and may reduce the number of tissue aspirations performed. However, more sensitive and logistically feasible methods than light microscopy are needed to detect the parasites in the blood [72]. The kDNA PCRs showed excellent performance for diagnosis of Leishmania aethiopica. The dry blood sample (DBS) can be used for PCR in microscopy is negative, where kDNA PCR method is available [73].
The qRT-kDNA PCR is a highly sensitive test [74], but the sensitivity of rK39-ICT is low, and its specificity is poor in Ethiopia as compared with splenic aspiration as a gold standard test. So the rK39-ICT needs improvement for clinical use for visceral leishmaniasis in Ethiopia [75, 76]. In-house AQ-DAT is an accessible diagnostic method that minimizes intermittent stock outs and could strengthen the national visceral leishmaniasis control program [77]. In addition, the microculture method (MCM) is a more sensitive and rapid culturing method for the isolation of Leishmania aethiopica than traditional culture method (TCM) and smear microscopy [78]. The molecular diagnostic method has significantly lower prevalence than microscopic examination [25].
Trachoma
In addition to clinical evaluation, three studies reported diagnostic methods for trachoma [79,80,81]. Photo evaluation (standard 3D images) is the chief diagnostic approach for trachomatous trichiasis (TT) [79], while Chlamydia trachomatis deoxyribonucleic acid (DNA) [80] and putative attractants [81] were the diagnostic methods for active trachoma. Trachoma can also be detected using Chlamydia trachomatis DNA on the hands, faces, or clothing of the individuals living with ocular-positive household members [80]. In addition, putative attractants may play a role in identifying short-chain fatty acids and aromatic compounds that are detected by the antennae of M. sorbens. Further work is required to optimize chemical blends and release rates, to produce a synthetic lure to which the behavioral responses of M. sorbens can be investigated [81].
Soil-transmitted helminthiasis (STH)
Combinations of formol-ether concentration, Baermann concentration, and molecular methods, Kato-Katz, direct saline microscopy, and formol ether concentration methods [61] are available in Ethiopia. Kato-Katz, McMaster, and Mini Parasep® SF were used to diagnose schistosomiasis [82], while quantitative polymerase chain reaction (qPCR) [83], serology, and PCR are diagnostic test for strongyloidiasis [62]. Kato-Katz thick smear, Kato-Katz thick smear and formol-ether concentration, and triple urine-circulating cathodic antigen (CCA)-cassette are the diagnostic test for S. mansoni [32]. Direct wet mount microscopy (DWMM) Kato-Katz, McMaster, and Mini-FLOTAC are used to diagnose both trichuris and hookworms [84].
The sensitivity of Mini Parasep® SF, Kato-Katz, and McMaster tests for detecting at least one species of parasites was 90.2%, 62.4%, and 80.0%, respectively. The specificity of these tests was 44.5%, 55.2%, and 26.5%, respectively. The Mini Parasep® SF fecal parasite concentrator technique showed better performance than Kato-Katz and McMaster techniques in detecting STHs in stool samples, particularly for S. mansoni and A. lumbricoides. Hence, Mini Parasep® SF could be used as one of the suitable fecal examination methods for surveillance, monitoring, and evaluation of preventive chemotherapy of schistosomiasis [82].
Likewise, qPCR is the method used in the monitoring and evaluation to confirm cessation of program [83]. Serology and PCR diagnostic tests have four times the capacity for diagnostics of strongyloidiasis than microscopy techniques [62]. Kato-Katz thick smear; Kato-Katz thick smear and formol-ether concentration, and triple urine-CCA cassette are the commonly used diagnostic methods for S. mansoni among children in Ethiopia [32]. The diagnostic sensitivity of DWMM was compared to a composite reference standard (CRS) consisting of Kato-Katz, McMaster, and Mini-FLOTAC for the diagnosis of STHs. The sensitivity of DWMM was 73.8% for Ascaris but was around 17% for both trichuris and hookworms [84].
Podoconiosis and LF
Clinical history, physical examination, and tests to rule out other forms of lymphedema together make up the important diagnosis algorithm that has been validated for identification of podoconiosis [85]. In addition, a new tool (3D imaging) for mapping LF was piloted in Ethiopia and found to benefit national LF programs by confirming where LF is endemic, therefore saving time and resources by preventing mass drug administration (MDA) where there is no evidence of ongoing LF transmission [86].
Evidence on access to medicine and care for NTDs in Ethiopia
Leishmaniasis
Available evidence shows that sodium stibogluconate (SSG), liposomal-amphotericin B (L-AMB), a combination of SSG with paromomycin [22], antimonials with paromomycin in combination or pentamidine [87], and a combination of AmBisome and miltefosine [88] were available treatments for leishmaniasis. The overall treatment success rate was 82.6%. The treatment success rates using SSG were 81.5%, that of multiple doses of liposomal-amphotericin B (L-AMB) was 96.7%, and the combination of SSG with paromomycin (90.1%) [22]. Antimonials with paromomycin in combination or pentamidine are an effective treatment options for diffused cutaneous leishmaniasis [87]. In addition, the combination of AmBisome and miltefosine is effective strategy to treat visceral leishmaniasis in HIV-co-infected patients in Ethiopia [88].
Trachoma
A blended approach of latrine promotion and MDA with azithromycin has been proven to prevent trachoma in Ethiopia [89]. Mass drug administrations of azithromycin are the predominant approach to prevent trachoma in Ethiopia [90,91,92,93]. Other intervention includes doxycycline for postoperative trichiasis cases [94], TT surgery [94,95,96], and hygiene measures including facial cleanliness and environmental improvement (SAFE) [57]. The overall MDA coverage of azithromycin ranged from 79.5 to 93.3% in Ethiopia, which is higher than the minimum WHO set criteria of 80% [91, 92]. Misconceptions and poor mobilization were common challenges [91].
Soil-transmitted helminthiasis (STH)
Chemotherapy using mebendazole [97, 98] and MDA campaigns [92, 99] are used to prevent and treat STHs. Chemotherapy against STH is crucial, and its coverage was 71% in Ethiopia [97]. MDA mobilization and awareness creation campaigns targeted head of household, those in poorer health, and older age groups [92]. Access to water sanitation and hygiene (WASH) program is an important public health intervention for STHs [27, 99]. Access to WASH reduced infestation of parasites by 54% [27].
Schistosomiasis
Overall treatment coverage of praziquantel against schistosomiasis was 75.5% in Ethiopia [100]. Praziquantel was a drug of choice for the treatment for Schistosoma mansoni in Ethiopia [100,101,102].
Podoconiosis and LF care
In terms of prevention, foot hygiene in areas of irritant soil is [49]. Shoe-wearing norms are progressively changing due to secular change in Ethiopia [103]. Ivermectin MDA coverage for LF was 81.5% which is higher than the minimum recommended level of coverage (65%) [104]. Nevertheless, 75 Woredas were newly identified endemic for LF, and only 3.4% of the LF patients had received treatment [52]. A simple and inexpensive package of lymphedema self-care comprising information about foot hygiene, skin care, bandaging, exercises to improve lymph drainage, and use of socks and shoes has shown effect to reduce occurrence of acute attacks [105]. Minor surgical intervention (nodulectomy) [49, 105,106,107], foot hygiene and footwear [37, 49, 103, 108, 109], economic empowerment and life skill programs [49, 53, 107,108,109,110,111,112], transformation of rigid inequity gender norms [110, 111, 113, 114], awareness creation, and psychosocial support for stigma minimization [53, 109, 111, 113, 115,116,117] were crucial component of care for people affected by podoconiosis and LF. Several articles have documented the integrated morbidity management for podoconiosis and LF [49, 104, 106, 108, 109, 118]. Family-based intervention had played a key role in preventing impairments and reducing stigma through self-management of disabilities and improving family quality of life [112]. Among 363 health facilities surveyed, podoconiosis and LF were the major causes of lymphedema in Ethiopia, but only 24% of LF and 12% of podoconiosis patients had received care from the health facilities [106].
Onchocerciasis
Onchocerciasis is targeted for elimination, and the coverage of community-directed treatment (ivermectin) coverage of onchocerciasis has been around 80% and up to 85.9% in some districts in Ethiopia [40, 119,120,121,122]. Vector control was also an important prevention and control intervention [40].
Challenges in access to medicine and care in Ethiopia
Lack of adequate resource for drug discovery, and/or low-purchasing capacity, were common challenge for access to medicine for leishmaniasis treatment in Ethiopia [123]. These were compounded by very poor access to diagnosis and, consequently, significantly delayed access to treatment for visceral leishmaniasis [124]. Similarly, the podoconiosis elimination program has been affected by lack of necessities including footwear and health education [109]. Social stigma has an immense impact on podoconiosis care and support in Ethiopia [114]. The MDA with ivermectin monotherapy did not interrupt LF transmission, but adding albendazole and improving treatment coverage are recommended to improve LF prevention [44, 71].
Gender inequity has significant impact on women’s healthcare seeking and access to medicine and/or care in Ethiopia [113]. In addition, stigmatized attitudes towards patients with podoconiosis influence patients’ seeking care. More than half (52.7%) of youths had stigmatizing attitudes towards patients with podoconiosis. Of these, 59.3% of them were female [117]. Furthermore, social and financial pressures placed on podoconiosis cases affected families and caregivers [111].
MDA intervention has been used to control scabies for many years [122]. Scabies MDA that recently employed in response to a massive outbreak in Amhara region was affected by failure to follow-up, shortage of medicine, and lack of leadership effective prioritization [122]. Likewise, MDA program coverage for onchocerciasis was significantly high among in-school adolescents with treatment offering and swallowing status [119]. Hence, school absenteeism was the main reason for not being offered ivermectin (40.9%) and not knowing about MDA (25.3%) [119].
Strategies to improve access to medicine
The national plan for NTD prevention and control has been put forward through three consecutive national strategic documents or master plans, the first covering 2013–2015, second 2016–2020, third roadmap 2021–2025 [9, 10, 70], and NTD elimination district-level coordination toolkit [12]. Comprehensive prevention strategies, promotion of footwear, and personal hygiene are highly needed interventions [37]. Raising community awareness about NTDs, particularly podoconiosis and LF, to transform inequitable gender norms is vital to improve access to healthcare [113, 116]. Integration of the care package into routine healthcare in Ethiopia may be effective in improving health-related quality of life and disability and reducing time out of economic activity due to podoconiosis and LF [115]. Sustainable awareness creation will have a crucial contribution to minimize stigma and make patients resilient through psychosocial and life skill interventions [53]. Similarly, community engagement is essential in the success of MDA program, alongside strong political commitment, and guideline adherence [122]. In one systematic review, vaccine development was identified as an emerging science for the prevention of schistosomiasis, but this has not yet been realized. A vaccination strategy would be an ideal tool for a significant and sustainable reduction in the transmission and disease burden of schistosomiasis [125].
Discussion
This scoping review mapped the volume of available evidence on the burden of the priority NTDs in Ethiopia, access to diagnostic, and treatment gaps. It covered studies undertaken on burden, access to diagnostic, medicine/drug and/or care for NTDs, and key program implementation challenges in Ethiopia. We found systematic reviews, geospatial mapping, national surveys, scoping reviews, primary studies, and national strategic plan or roadmaps on NTDs. The geographical coverage of NTD prevention and control has increased over time [44], with bolder strategies. Nevertheless, the evidence suggests that, despite high burden of NTDs in Ethiopia, the implementation of prevention and control programs still lags.
This scoping review was guided by the prioritized NTDs in the three master plans of Ethiopia [5, 9, 10]. Other evidence has documented that up-to-date diagnostic data is highly valuable for programmatic decision and reframing of diagnostic methods. Nonetheless, there is limited availability of diagnostic tool for many NTDs. Therefore, creation guiding framework is critical to stimulate investment on research and diagnostic support for NTDs [13]. Strong private–public partnerships, donation by pharmaceutical companies, and increased budget allocation for the NTD program are very crucial to improve access chemotherapies to eliminate NTD [3]. Nevertheless, lack of service integration and WASH are still the challenges of NTD prevention and control programs [44]. The fact that strengthening stakeholder engagement, service integration and coordination of evidence-informed interventions are crucial strategies to synergize NTD elimination programs in Ethiopia [44].
In addition, integrated NTD control, mapping, rapid scale-up of interventions, and shifting from operational research into implementation of intervention packages are very crucial to eliminate NTDs in Ethiopia [11]. Interventions such as deworming for school children, access to improved personal and environmental hygiene in school [99], community mobilization and uniform training campaign for trachoma prevention and control [91], poverty reduction to attitude transformation to minimize stigma and discriminations, and mental distress among podoconiosis-affected people [109] should be emphasized to interrupt disease transmissions and improve quality of life of NTD-affected people.
We used comprehensive evidence, both published and unpublished literature including policy documents related to NTD burden, access to medicine, and its barriers in Ethiopia, which is the strength of this scoping review. This scoping review considers all NTDs prioritized by the government of Ethiopia that made the findings very broad which is the known weakness of scoping review. Therefore, this scoping review contributes to inform the NTD elimination global policy in the Sustainable Development Goals by 2030 [126, 127] and evidence uptake for NTD policy and program design in Ethiopia. Furthermore, the findings from this scoping review are crucial to organize stakeholder consultative meetings to put a foundation for evidence to health policymaking translation platform and suggest future health care, health policy research direction, and evidence to policy strategies in Ethiopia.
Conclusions
The scoping review has found substantial level of evidence to inform neglected tropical diseases policymaking and practice in Ethiopia. Articles identified have shown the burden of NTDs, diagnostic approaches, and treatment coverage in Ethiopia. There is relatively strong evidence on podoconiosis, trachoma, soil-transmitted helminthiasis, and leishmaniasis but much less on scabies, guinea-worm, and onchocerciasis. It is vital that available research findings are taken up to inform policymaking and practice. Although some primary studies reported diagnostic methods for the NTDs, there is little high-quality evidence on the sensitivity and specificity of these diagnostic methods. Similarly, preventive chemotherapy coverage of mass drug administration is widespread, but access to medicine and/or care for intensive diseases management is very limited. Access to diagnostics, medicine, and/or care has been affected by gender inequality, attitude towards footwear, stigma and discrimination, and economic status of the most vulnerable group of population. Evidence on access to timely and proven diagnostics, medicine, and/or care for NTD is very scarce in Ethiopia. We suggest that available evidence from nationwide surveys, mapping exercise, and systematic review and meta-analyses should be regularly reviewed and used to inform policymaking and implementation strategies or practice in Ethiopia in the future.
Availability of data and materials
We will make data available once completed the main scoping review and upon request to the corresponding author.
Abbreviations
- AQ-DAT:
-
In-house liquid direct agglutination test
- CCA:
-
Circulating cathodic antigen
- CDT-Africa:
-
Centre for Innovative Drug Development and Therapeutic Trials for Africa
- DWMM:
-
Direct wet mount microscopy
- kDNA:
-
Kinetoplast deoxyribonucleic acid
- LF:
-
Lymphatic filariasis
- MDA:
-
Mass drug administration
- MDG:
-
Millennium Development Goal
- NTDs:
-
Neglected tropical diseases
- PCR:
-
Polymerase chain reaction
- qPCR:
-
Quantitative polymerase chain reaction
- qRT-kDNA:
-
Quantitative real-time kinetoplast deoxyribonucleic acid
- PRISMA-ScR:
-
Preferred Reporting Items for Systematic Review and Meta-Analysis extension for Scoping Review
- rK39-ICT:
-
RK39 immunochromatographic
- SDG:
-
Sustainable development goal
- SNNP:
-
Southern Nation, Nationality and People
- STH:
-
Soil-transmitted helminthiasis
- SSG:
-
Sodium stibogluconate
- WHO:
-
World Health Organization
References
WHO. Countries NTD masterplan 2021 - 2025 framework for development. Brazzaville: World Health Organization office for African Region; 2020. Available from: https://espen.afro.who.int/system/files/content/resources/NTDMasterPlan_Guidelines_WHOAfrRegion_Version3_160321.pdf.
WHO. Investing to overcome the global impact of neglected tropical siseases; third Wolrd Health Orgnazation (WHO) report on neglected tropical diseases. Geneva: World Health Organization; 2015.
UNITING to COMBAT. Ethiopia and neglected tropical diseases. Mass treatment coverage for NTDS - 2016. Grey Literature. 2016. Available from: www.unitingtocombatntds.org OR info@unitingtocombatntds.org.
WHO. Neglected tropical diseases. World NTDs Day on 30th January 2022. Geneva: World Health Organization; 2022. Available from: https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_2.
MOH. Federal Democratic Republic Ethiopia Ministry of Health; Second Edition of National Neglected Tropical Disease Masterplan; 2015/16 – 2019/20 (2008–2012 EFY). 2nd ed. Addis Ababa: Federal Ministry of Health (FMOH); 2016. http://repository.iifphc.org/handle/123456789/1333.
De Neve JW, Andriantavison RL, Croke K, Krisam J, Rajoela VH, Rakotoarivony RA, et al. Health, financial, and education gains of investing in preventive chemotherapy for schistosomiasis, soil-transmitted helminthiases, and lymphatic filariasis in Madagascar: a modeling study. PLoS Negl Trop Dis. 2018;12(12):e0007002.
WHO. The road map targets for 2030; ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases 2021–2030. Geneva: World Health Organization; 2020. Available from: https://www.who.int/teams/control-of-neglected-tropical-diseases/ending-ntds-together-towards-2030/targets.
UN. Transforming our world: the 2030 agenda for sustainable development. 2015. Available from: https://sdgs.un.org/2030agenda.
FMOH. National Masterplan for Neglected Tropical Diseases (NTDs) (2013–2015). Addis Ababa: Federal Democratic Republic of Ethiopia Ministry of Health; 2013.
MoH. The Third National Neglected Tropical Diseases Strategic Plan 2021–2025 (2013/14 – 2017/18 E.C.). Addis Ababa: Ministry of Health-Ethiopia; 2021. Available from: https://espen.afro.who.int/system/files/content/resources/Third%20NTD%20national%20Strategic%20Plan%202021-2025.pdf.
Tamiru HF, Mashalla YJ, Mohammed R, ThupayagaleTshweneagae G. Cutaneous leishmaniasis a neglected tropical disease: community knowledge, attitude and practices in an endemic area, northwest Ethiopia. Parasites Vectors. 2019;19:855.
FMOH, JDC, NALA, Sightsavers, IDC, Sightsavers. Elimination of neglected tropical diseases (NTDs) in Ethiopia; Woreda Level coordination toolkit for the WASH and NTD sectors. 2018. https://www.susana.org/_resources/documents/default/3-3709-7-1571741955.pdf.
Taylor EM. NTD diagnostics for disease elimination: a review. Diagnostics. 2020;10:375.
Akinsolu FT, Nemieboka PO, Njuguna DW, Ahadji MN, Dezso D, Varga O. Emerging resistance of neglected tropical diseases: a scoping review of the literature. Int J Environ Res Public Health. 2019;16:1925.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Tricco A, Zarin LE, O’Brien K, Colquhoun H, Levac D. Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist. Ann Intern Med. 2018;169:467–73. Available from: http://www.prisma-statement.org/Extensions/ScopingReviews.
UN. The Millennium Development Goals Report. Zero time for Global Action for people and planet. United Nations. New York; 2015. p. 72. Available from: https://www.un.org/millenniumgoals/2015_MDG_Report/pdf/MDG%202015%20rev%20(July%201).pdf.
WHO. Health and the millennium development goals, keep the pomise. World Health Organization. Geneva: WHO Library Cataloguing-in-Publication Data; 2005. Available from: https://www.who.int/hdp/publications/mdg_en.pdf.
Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143.
Gebrie A, Alebel A, Zegeye A, Tesfaye B, Wagnew F. Prevalence and associated factors of active trachoma among children in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2019;19(1073):1–12.
Haftom M, Petrucka P, Gemechu K. Prevalence and risk factors of human leishmaniasis in Ethiopia : a systematic review and meta-analysis. Infect Dis Ther. 2020;10(1):47–60. https://doi.org/10.1007/s40121-020-00361-y.
Gebreyohannes EA, Bhagvathula AS, Abegaz TM, Seid MA. Treatment outcomes of visceral leishmaniasis in Ethiopia from 2001 to 2017: a systematic review and. Infect Dis Poverty. 2018;7(108):1–9.
Abongomera C, van Henten S, Vogt F, Buyze J, Verdonck K, van Griensven J. Prognostic factors for mortality among patients with visceral leishmaniasis in East Africa: systematic review and meta-analysis. PLoS Negl Trop Dis. 2020;14(5):1–19. https://doi.org/10.1371/journal.pntd.0008319.
Alemayehu M, Wubshet M, Mesfin N. Magnitude of visceral leishmaniasis and poor treatment outcome among HIV patients: meta-analysis and systematic review. HIV/AIDS - Res Palliat Care. 2016;8:75–81. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4809333/pdf/hiv-8-075.pdf.
Assefa A. Leishmaniasis in Ethiopia: a systematic review and meta-analysis of prevalence in animals and humans. Heliyon. 2018;4(8):e00723. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6082994/pdf/main.pdf.
Hailegebriel T, Nibret E, Munshea A. Prevalence of soil-transmitted helminth infection among school-aged children of Ethiopia: a systematic review and meta-analysis. Infect Dis Res Treat. 2020;13:117863372096281.
Yimam Y, Woreta A, Mohebali M. Intestinal parasites among food handlers of food service establishments in Ethiopia: a systematic review and meta-analysis. BMC Public Health. 2020;20(73):1–12.
Assemie MA, Shitu Getahun D, Hune Y, Petrucka P, Abebe AM, Telayneh AT, et al. Prevalence of intestinal parasitic infection and its associated factors among primary school students in ethiopia: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2021;15(4):1–16. https://doi.org/10.1371/journal.pntd.0009379.
Alemu A, Bitew ZW, Worku T. Intestinal parasites co-infection among tuberculosis patients in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2020;20(1):510. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7362415/pdf/12879_2020_Article_5237.pdf.
Maddren R, Phillips A, Ower A, Landeryou T, Mengistu B, Anjulo U, et al. Soil-transmitted helminths and schistosome infections in Ethiopia: a systematic review of progress in their control over the past 20 years. Parasit Vectors. 2021;14(97):1–15. https://doi.org/10.1186/s13071-021-04600-0.
Woldeyohannes D, Sahiledengle B, Tekalegn Y, Hailemariam Z. Prevalence of schistosomiasis ( S . mansoni and S . haematobium ) and its association with gender of school age children in Ethiopia : a systematic review and meta-analysis. Parasite Epidemiol Control. 2021;13:e00210. https://doi.org/10.1016/j.parepi.2021.e00210.
Bisetegn H, Eshetu T, Erkihun Y. Prevalence of Schistosoma mansoni infection among children in Ethiopia : a systematic review and meta-analysis. Trop Dis Travel Med Vaccines. 2021;7(30):1–15.
Ponpetch K, Erko B, Bekana T, Richards L, Liang S. Biogeographical characteristics of Schistosoma mansoni endemic areas in Ethiopia: a systematic review and meta analysis. J Parasitol Res. 2021;10:83. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8185935/pdf/40249_2021_Article_864.pdf.
Azene AG, Aragaw AM, Wassie GT. Prevalence and associated factors of scabies in Ethiopia: systematic review and meta-analysis. BMC Infect Dis. 2020;20(1):1–10.
Yimer M, Hailu T, Mulu W, Abera B. Epidemiology of elephantiasis with special emphasis on podoconiosis in Ethiopia : a literature review. J Vector Borne Dis. 2015;52:111–5.
Tilahun B, Gashu KD, Mekonnen ZA, Endehabtu BF, Angaw DA. Mapping the role of digital health technologies in the case detection, management, and treatment outcomes of neglected tropical diseases: a scoping review. Trop Med Health. 2021;49(17):1–10.
Deribe K, Cano J, Trueba ML, Newport MJ, Davey G. Global epidemiology of podoconiosis: a systematic review. PLoS Negl Trop Dis. 2020;14(8):e0008616. Available from: https://storage.googleapis.com/plos-corpus-prod/10.1371/journal.pntd.0006324/2/pntd.0006324.pdf?X-Goog-Algorithm=GOOG4-RSA-SHA256&X-Goog-Credential=wombat-sa%40plos-prod.iam.gserviceaccount.com%2F20210611%2Fauto%2Fstorage%2Fgoog4_request&X-Goog-Date=20210.
Berhe B, Legese H, Mardu F, Tesfay K, Adhanom G, Kahsay T, et al. Epidemiology and sex differences of podoconiosis in Ethiopia: a systemic review and meta-analysis. Heliyon. 2021;7(4):e05446. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8082554/pdf/main.pdf.
Ngowi HA. Prevalence and pattern of waterborne parasitic infections in eastern Africa: a systematic scoping review. Food Waterborne Parasitol. 2020;20:e00089. https://doi.org/10.1016/j.fawpar.2020.e00089.
Meribo K, Kebede B, Feleke SM, Mengistu B. Review of Ethiopian onchocerciasis elimination programme. Ethiop Med J. 2017;55(Suppl 1):55–63. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5582636/pdf/emss-73813.pdf.
Boltena MT, El-Khatib Z, Kebede AS, Asamoah BO, Tadesse Boltena A, Yeshambaw M, et al. Comorbidity of geo-helminthes among malaria outpatients of the health facilities in Ethiopia: systematic review and meta-analysis. Int J Env Res Public Heal. 2021;18(3):862. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7908091/pdf/ijerph-18-00862.pdf.
Chelkeba L, Melaku T, Emana D, Jimma W. A systematic review and metanalyis of soil transmitted helminths infections among preschool and school-age children in Ethiopia: evaluation of neglected tropica diseases (NTDS) elimination program by 2020. 2020. p. 1–30.
Chelkeba L, Melaku T, Lemma D, Mekonnen Z. Burden of intestinal parasitic infections among pregnant women in Ethiopia: a systematic review and meta-analysis. 2021. Available from: https://link.springer.com/article/10.1007%2Fs15010-021-01635-4.
Worku K. Neglected tropical diseases program in Ethiopia, progress and challenges. Ethiop Med J. 2017;55(Supp. 1):1–2.
Burn H, Aweke S, Wondie T, Habtamu E, Deribe K, Rajak S, et al. Podoconiosis, trachomatous trichiasis and cataract in northern Ethiopia: a comparative cross sectional study. PLoS Negl Trop Dis. 2017;11(2):1–14.
Deribe K, Cano J, Newport MJ, Golding N, Pullan RL, Sime H, et al. Mapping and modelling the geographical distribution and environmental limits of podoconiosis in Ethiopia. PLoS Negl Trop Dis. 2015;9(7):1–18.
Leta GT, Mekete K, Wuletaw Y, Gebretsadik A, Sime H, Mekasha S, et al. National mapping of soil-transmitted helminth and schistosome infections in Ethiopia. Parasites Vectors. 2020;13(1):1–13. https://doi.org/10.1186/s13071-020-04317-6.
Deribe K, Cano J, Trueba ML, Newport MJ, Davey G. Global epidemiology of podoconiosis: a systematic review. PLoS Negl Trop Dis. 2018;12(3):e0006324. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5849362/pdf/pntd.0006324.pdf.
Davey G, Tekola F, Newport MJ. Podoconiosis : non-infectious geochemical elephantiasis. Trans R Soc Trop Med Hyg. 2007;101:1175–80.
Deribe K, Brooker SJ, Pullan RL, Hailu A, Enquselassie F, Reithinger R, et al. Spatial distribution of podoconiosis in relation to environmental factors in Ethiopia: a historical review. PLoS One. 2013;8(7):e68330.
Bekele K, Deribe K, Amberbir T, Tadele G, Davey G, Samuel A. Burden assessment of podoconiosis in Wayu Tuka woreda, East Wollega zone, western Ethiopia: a community-based cross-sectional study. BMJ Open. 2016;6:e012308. Available from: https://bmjopen.bmj.com/content/bmjopen/6/9/e012308.full.pdf.
Rebollo MP, Sime H, Assefa A, Cano J, Deribe K, Gonzalez-Escalada A, et al. Shrinking the lymphatic filariasis map of Ethiopia: reassessing the population at risk through nationwide mapping. PLoS Negl Trop Dis. 2015;9(11):1–15.
Cremers AL, Visser BJ, Getahun Z, Borku M, Meskele E, Ahmed J, et al. Using ethnographic film in tackling podoconiosis. Trans R Soc Trop Med Hyg. 2020;114(12):896–8.
Tekola Ayele F, Adeyemo A, Finan C, Hailu E, Sinnott P, Burlinson ND, et al. HLA class II locus and susceptibility to podoconiosis. N Engl J Med. 2012;366(13):1200–8.
Masraf H, Azemeraw T, Molla M, Jones CI, Bremner S, Ngari M, et al. Excess mortality among people with podoconiosis: secondary analysis of two Ethiopian cohorts. Trans R Soc Trop Med Hyg. 2020;114(12):1035–7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7738655/pdf/traa150.pdf.
Caprioli T, Martindale S, Mengiste A, Assefa D, H/Kiros F, Tamiru M, et al. Quantifying the socio-economic impact of leg lymphoedema on patient caregivers in a lymphatic filariasis and podoconiosis co-endemic district of Ethiopia. PLoS Negl Trop Dis. 2020;14(3):e0008058. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7069637/pdf/pntd.0008058.pdf.
Sata E, Nute AW, Astale T, Gessese D, Ayele Z, Zerihun M, et al. Twelve-year longitudinal trends in trachoma prevalence among children aged 1–9 years in Amhara, Ethiopia, 2007–2019. Am J Trop Med Hyg. 2021;104(4):1278–89.
Astale T, Ebert CD, Nute AW, Zerihun M, Gessese D, Melak B, et al. The population-based prevalence of trachomatous scarring in a trachoma hyperendemic setting: results from 152 impact surveys in Amhara, Ethiopia. Comput Math Methods Med. 2021;21(1):213. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8120834/pdf/12886_2021_Article_1972.pdf.
Alambo MM, Lake EA, Workie SB, Wassie AY. Prevalence of active trachoma and associated factors in Areka town, South Ethiopia, 2018. Interdiscip Perspect Infect Dis. 2020;2020:8635191.
Melkie G, Azage M, Gedamu G. Prevalence and associated factors of active trachoma among children aged 1–9 years old in mass drug administration graduated and non-graduated districts in northwest Amhara region, Ethiopia: a comparative crosssectional study. PLoS ONE. 2020;15(12):1–16. https://doi.org/10.1371/journal.pone.0243863.
Hailu T, Nibret E, Amor A, Munshea A. Strongyloides stercoralis infection in Ethiopia: systematic review and meta-analysis on prevalence and diagnostic methods. Helminthol. 2021;58(1):17–27.
Terefe Y, Ross K, Whiley H. Strongyloidiasis in Ethiopia: systematic review on risk factors, diagnosis, prevalence and clinical outcomes. Infect Dis Poverty. 2019;8(1):53.
Abdela SG, Hassen NG, Hussien FM, Yesufa AM, van Griensven J, van Henten S. Hepatosplenic schistosomiasis, the ignored morbidity: experience from a referral hospital in Ethiopia. Trans R Soc Trop Med Hyg. 2021;2(115):57–62.
Dagne H, Dessie A, Destaw B, Yallew WW, Gizaw Z. Prevalence and associated factors of scabies among schoolchildren in Dabat district, northwest Ethiopia, 2018. Environ Health Prev Med. 2019;24(1):1–8.
Walker SL, Lebas E, De Sario V, Deyasso Z, Doni SN, Marks M, et al. The prevalence and association with health-related quality of life of tungiasis and scabies in schoolchildren in southern Ethiopia. PLoS Negl Trop Dis. 2017;11(8):1–11.
Wochebo W, Haji Y, Asnake S. Scabies outbreak investigation and risk factors in Kechabira district, Southern Ethiopia: Unmatched case control study. BMC Res Notes. 2019;12(1):12–7. https://doi.org/10.1186/s13104-019-4317-x.
Yassin ZJ, Dadi AF, Nega HY, Derseh BT, Asegidew W. Scabies outbreak investigation among “Yekolo Temaris” in Gondar town, North Western Ethiopia, November 2015. Electron J Biol. 2017;13(3):203–9.
Worku ED, Asemahagn MA, Endalifer ML. Determinants of scabies out- break in Takusa district of Amhara Region, northwest Ethiopia. J Public Health Afr. 2020;11(1325):1502–5. Available from: https://www.publichealthinafrica.org/index.php/jphia/article/download/1325/603.
Arega B, Diro E, Zewude T, Getahun T, Agunie A, Owiti P, et al. High levels of scabies and malnutrition amongst orphans referred to a hospital in Addis Ababa, Ethiopia. J Infect Dev Ctries. 2020;14(6.1):48s–52s. Available from: https://jidc.org/index.php/journal/article/download/32614796/2270.
FMOH. Federal Democratic Republic of Ethiopia Ministry of Health: Second Edition of National Neglected Tropical Diseases Master Plan. Addis Ababa: Federal Ministry of Health (FMOH); 2016. p. 1–78. http://repository.iifphc.org/handle/123456789/1333.
Endeshaw T, Taye A, Tadesse Z, Katabarwa MN, Shafi O, Seid T, et al. Presence of Wuchereria bancrofti microfilaremia despite 7 years of annual ivermectin monotherapy mass drug administration for onchocerciasis control: a study in North-West Ethiopia. Pathog Glob Heal. 2015;109(7):344–51. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4768627/pdf/ypgh-109-344.pdf.
Diro E, Yansouni CP, Takele Y, Mengesha B, Lynen L, Hailu A, et al. Diagnosis of visceral leishmaniasis using peripheral blood microscopy in Ethiopia: a prospective phase-III study of the diagnostic performance of different concentration techniques compared to tissue aspiration. Am J Trop Med Hyg. 2017;96(1):190. Available from: https://www.ajtmh.org/downloadpdf/journals/tpmd/96/1/article-p190.pdf.
Merdekios B, Pareyn M, Tadesse D, Eligo N, Kassa M, Jacobs BKM, et al. Evaluation of conventional and four real-time PCR methods for the detection of Leishmania on field-collected samples in Ethiopia. PLoS Negl Trop Dis. 2021;15(1):e0008903. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7802924/pdf/pntd.0008903.pdf.
Abbasi I, Aramin S, Hailu A, Shiferaw W, Kassahun A, Belay S, et al. Evaluation of PCR procedures for detecting and quantifying Leishmania donovani DNA in large numbers of dried human blood samples from a visceral leishmaniasis focus in Northern Ethiopia. BMC Infect Dis. 2013;13:153. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3621070/pdf/1471-2334-13-153.pdf.
Kiros YK, Regassa BF. The role of rk39 serologic test in the diagnosis of visceral leishmaniasis in a tertiary hospital, northern Ethiopia. BMC Res Notes. 2017;10(1):169. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5407002/pdf/13104_2017_Article_2490.pdf.
Kassa M, Abdellati S, Cnops L, Bremer Hinckel BC, Yeshanew A, Hailemichael W, et al. Diagnostic accuracy of direct agglutination test, rK39 ELISA and six rapid diagnostic tests among visceral leishmaniasis patients with and without HIV coinfection in Ethiopia. PLoS Negl Trop Dis. 2020;14(12):e0008963. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7774845/pdf/pntd.0008963.pdf.
Ayelign B, Jemal M, Negash M, Genetu M, Wondmagegn T, Zeleke AJ, et al. Validation of in-house liquid direct agglutination test antigen: the potential diagnostic test in visceral leishimaniasis endemic areas of northwest Ethiopia. BMC Microbiol. 2020;20(1):90. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7158028/pdf/12866_2020_Article_1780.pdf.
Aberra L, Abera A, Belay T, Kebede A, Gadisa E, Tasew G. Evaluation of microcapillary culture method for the isolation of Leishmania aethiopica parasites from patients with cutaneous lesions in Ethiopia. 2019;3:4. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6460804/pdf/41512_2019_Article_51.pdf.
Hoffman JJ, Habtamu E, Rono H, Tadesse Z, Wondie T, Minas T, et al. 3D images as a field grader training tool for trachomatous trichiasis: a diagnostic accuracy study in Ethiopia. PLoS Negl Trop Dis. 2019;13(1):e0007104. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6363231/pdf/pntd.0007104.pdf.
Last A, Versteeg B, Abdurahman OS, Robinson A, Dumessa G, Aga MA, et al. Detecting extra-ocular Chlamydia trachomatis in a trachoma-endemic community in Ethiopia: identifying potential routes of transmission. PLoS Negl Trop Dis. 2020;14(3):e0008120. https://doi.org/10.1371/journal.pntd.0008120.
Bayissasse B, Sullivan KM, Merbs SL, Munoz B, Keil A, Sisay A, et al. Maximising trichiasis surgery success (MTSS) trial: rationale and design of a randomised controlled trial to improve trachomatous trichiasis surgical outcomes. PLoS Negl Trop Dis. 2020;10(3):e036327. Available from: https://storage.googleapis.com/plos-corpus-prod/10.1371/journal.pntd.0007719/2/pntd.0007719.pdf?X-Goog-Algorithm=GOOG4-RSA-SHA256&X-Goog-Credential=wombat-sa%40plos-prod.iam.gserviceaccount.com%2F20210812%2Fauto%2Fstorage%2Fgoog4_request&X-Goog-Date=20210.
Adugna S, Kebede T, Mekonnen Z, Degarege A, Liang S, Erko B. Diagnostic performance of Mini Parasep® solvent-free faecal parasite concentrator relative to Kato-Katz and McMaster for the diagnosis of intestinal parasitic infections. Trans R Soc Trop Med Hyg. 2017;111(12):572–8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6543883/pdf/try010.pdf.
Cools P, Vlaminck J, Albonico M, Ame S, Ayana M, Jose BAP, et al. Diagnostic performance of a single and duplicate Kato-Katz, Mini-FLOTAC, FECPAKG2 and qPCR for the detection and quantification of soil-transmitted helminths in three endemic countries. PLoS Negl Trop Dis. 2019;13(8):e0007446. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6675048/pdf/pntd.0007446.pdf.
Dana D, Vlaminck J, Mekonnen Z, Ayana M, Vogt F, Verdonck K, et al. Diagnostic sensitivity of direct wet mount microscopy for soil-transmitted helminth infections in Jimma Town, Ethiopia. J Infect Dev Ctries. 2020;14(6.1):66s–71s. Available from: https://jidc.org/index.php/journal/article/download/32614799/2273.
Deribe K, Florence L, Kelemework A, Getaneh T, Tsegay G, Cano J, et al. Developing and validating a clinical algorithm for the diagnosis of podoconiosis. Trans R Soc Trop Med Hyg. 2020;114(12):916–25. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7738664/pdf/traa074.pdf.
Sime H, Gass KM, Mekasha S, Assefa A, Woyessa A, Shafi O, et al. Results of a confirmatory mapping tool for lymphatic filariasis endemicity classification in areas where transmission was uncertain in Ethiopia. PLoS Negl Trop Dis. 2018;12(3):e0006325. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5886699/pdf/pntd.0006325.pdf.
van Griensven J, Gadisa E, Aseffa A, Hailu A, Abate BM, Diro E. Treatment of cutaneous Leishmaniasis caused by Leishmania aethiopica: a systematic review. PLoS Negl Trop Dis. 2016;10(3):1–20.
Diro E, Blesson S, Edwards T, Ritmeijer K, Fikre H, Admassu H, et al. A randomized trial of AmBisome monotherapy and AmBisome and miltefosine combination to treat visceral leishmaniasis in HIV co-infected patients in Ethiopia. PLoS Negl Trop Dis. 2019;13(1):e0006988. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6336227/pdf/pntd.0006988.pdf.
Stoller NE, Gebre T, Ayele B, Zerihun M, Assefa Y, Habte D, et al. Efficacy of latrine promotion on emergence of infection with ocular Chlamydia trachomatis after mass antibiotic treatment: a cluster-randomized trial. Int Health. 2011;3(2):75–84. https://doi.org/10.1016/j.inhe.2011.03.004.
Keenan JD, Tadesse Z, Gebresillasie S, Shiferaw A, Zerihun M, Emerson PM, et al. Mass azithromycin distribution for hyperendemic trachoma following a cluster-randomized trial: a continuation study of randomly reassigned subclusters (TANA II). PLoS Med. 2018;15(8):1–17.
Mulugeta A, Gebregergs GB, Asfaw S, Yemane D, Mitiku M, Meresa B, et al. Coverage, social mobilization and challenges of mass Zithromax administration campaign in south and south east zones of Tigray, northern Ethiopia: a cross sectional study. PLoS Negl Trop Dis. 2018;12(2):e0006288. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5854420/pdf/pntd.0006288.pdf.
Ebert CD, Astale T, Sata E, Zerihun M, Nute AW, Stewart AEP, et al. Population coverage and factors associated with participation following a mass drug administration of azithromycin for trachoma elimination in Amhara, Ethiopia. PLoS One. 2019;24(4):493–501. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6850572/pdf/TMI-24-493.pdf.
Oldenburg CE, Arzika AM, Amza A, Gebre T, Kalua K, Mrango Z, et al. Mass azithromycin distribution to prevent childhood mortality: a pooled analysis of cluster-randomized trials. Am J Trop Med Hyg. 2019;100(3):691–5. Available from: https://www.ajtmh.org/downloadpdf/journals/tpmd/100/3/article-p691.pdf.
Habtamu E, Wondie T, Aweke S, Tadesse Z, Zerihun M, Gashaw B, et al. Oral doxycycline for the prevention of postoperative trachomatous trichiasis in Ethiopia: a randomised, double-blind, placebo-controlled trial. Lancet Glob Heal. 2018;6(5):e579–92. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5912946/pdf/main.pdf.
Adafrie Y, Redae G, Zenebe D, Adhena G. Uptake of trachoma trichiasis surgery and associated factors among trichiasis-diagnosed clients in southern Tigray, Ethiopia. Clin Ophthalmol. 2021;15:1939–48.
Habtamu E, Wondie T, Gobezie W, Tadesse Z, Gashaw B, Gebeyehu A, et al. Effect of repeated epilation for minor trachomatous trichiasis on lash burden, phenotype and surgical management willingness: a cohort study. PLoS Negl Trop Dis. 2020;14(12):e0008882. https://doi.org/10.1371/journal.pntd.0008882.
Asfaw MA, Zerdo Z, Churko C, Seife F, Yihune M, Chisha Y, et al. Preventive chemotherapy coverage against soil-transmitted helminth infection among school age children: implications from coverage validation survey in Ethiopia, 2019. PLoS One. 2020;15:1–11. https://doi.org/10.1371/journal.pone.0235281.
Eshetu T, Aemero M, Zeleke AJ. Efficacy of a single dose versus a multiple dose regimen of mebendazole against hookworm infections among school children: a randomized open-label trial. BMC Infect Dis. 2020;20(1):376. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7251741/pdf/12879_2020_Article_5097.pdf.
Shumbej T, Menu S, Girum T, Bekele F, Gebru T, Worku M, et al. Impact of annual preventive mass chemotherapy for soil-transmitted helminths among primary school children in an endemic area of Gurage zone: a prospective cross-sectional study. Res Rep Trop Med. 2019;10:109. Available from: https://www.dovepress.com/getfile.php?fileID=50991.
Chisha Y, Zerdo Z, Asnakew M, Churko C, Yihune M, Teshome A, et al. Praziquantel treatment coverage among school age children against schistosomiasis and associated factors in Ethiopia: a cross-sectional survey, 2019. BMC Infect Dis. 2020;20(1):872. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7682081/pdf/12879_2020_Article_5519.pdf.
Tesfie A, Getnet G, Abere A, Yihenew G, Belete Y, Kassa M, et al. Praziquantel is an effective drug for the treatment of Schistosoma mansoni infection among school-aged children in northwest Ethiopia. Trop Med Health. 2020;48(28):28. Available from: https://tropmedhealth.biomedcentral.com/track/pdf/10.1186/s41182-020-00204-z.pdf.
Levecke B, Vlaminck J, Andriamaro L, Ame S, Belizario V, Degarege A, et al. Evaluation of the therapeutic efficacy of praziquantel against schistosomes in seven countries with ongoing large-scale deworming programs. Int J Parasitol Drugs Drug Resist. 2020;14:183–7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7595844/pdf/main.pdf.
Ayode D, McBride CM, De Heer HD, Watanabe E, Gebreyesus T, Tora A, et al. A qualitative study exploring barriers related to use of footwear in rural highland ethiopia: implications for neglected tropical disease control. PLoS Negl Trop Dis. 2013;7(4):e2199. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3636134/pdf/pntd.0002199.pdf.
Teshome A, Asfaw MA, Churko C, Yihune M, Chisha Y, Getachew B, et al. Coverage validation survey for lymphatic filariasis treatment in Itang Special District of Gambella Regional State of Ethiopia: a cross-sectional study. Infect Drug Resist. 2021;14:1537–43. Available from: https://www.dovepress.com/getfile.php?fileID=68744.
Negussie H, Molla M, Ngari M, Berkley JA, Kivaya E, Njuguna P, et al. Lymphoedema management to prevent acute dermatolymphangioadenitis in podoconiosis in northern Ethiopia (GoLBeT): a pragmatic randomised controlled trial. Lancet Glob Heal. 2018;6(7):e795-803. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6562300/pdf/main.pdf.
Deribe K, Kebede B, Tamiru M, Mengistu B, Kebede F, Martindale S, et al. Integrated morbidity management for lymphatic filariasis and podoconiosis, Ethiopia. Bull World Heal Organ. 2017;95(9):652–6. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5578380/pdf/BLT.16.189399.pdf.
Tsegay G, Wubie M, Degu G, Tamiru A, Cooper M, Davey G. Barriers to access and re-attendance for treatment of podoconiosis: a qualitative study in northern Ethiopia. Int Heal. 2015;7(4):285–92. Available from: https://watermark.silverchair.com/ihu085.pdf?token=AQECAHi208BE49Ooan9kkhW_Ercy7Dm3ZL_9Cf3qfKAc485ysgAAAs0wggLJBgkqhkiG9w0BBwagggK6MIICtgIBADCCAq8GCSqGSIb3DQEHATAeBglghkgBZQMEAS4wEQQM_0sqGLsls5E5DOViAgEQgIICgCPFDROlM220jo5SLzi3g_W-L2Kt_XQGsPV8nP-AMgvcKSYk.
Kelemework A, Tora A, Amberbir T, Agedew G, Asmamaw A, Deribe K, et al. “Why should I worry, since I have healthy feet?” A qualitative study exploring barriers to use of footwear among rural community members in northern Ethiopia. Eur J Hum Genet. 2016;6(3):e010354. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4809094/pdf/bmjopen-2015-010354.pdf.
Shahvisi A, Meskele E, Davey G. A human right to shoes? Establishing rights and duties in the prevention and treatment of podoconiosis. Heal Hum Rights. 2018;20(1):53–65. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6039730/pdf/hhr-20-053.pdf.
van ’t Noordende AT, Aycheh MW, Schippers A. The impact of leprosy, podoconiosis and lymphatic filariasis on family quality of life: a qualitative study in northwest Ethiopia. PLoS Negl Trop Dis. 2020;14(3):e0008173. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7083326/pdf/pntd.0008173.pdf.
Phillips C, Samuel A, Tiruneh G, Deribe K, Davey G. The impact of acute adenolymphangitis in podoconiosis on caregivers: a case study in Wayu Tuka woreda, Oromia, Western Ethiopia. ‘Ifshe was healthy, I would be free. PLoS Negl Trop Dis. 2018;12(1):e0006126. Available from: https://storage.googleapis.com/plos-corpus-prod/10.1371/journal.pntd.0007487/2/pntd.0007487.pdf?X-Goog-Algorithm=GOOG4-RSA-SHA256&X-Goog-Credential=wombat-sa%40plos-prod.iam.gserviceaccount.com%2F20210611%2Fauto%2Fstorage%2Fgoog4_request&X-Goog-Date=20210.
van‘t Noordende AT, Aycheh MW, Tadesse T, Hagens T, Haverkort E, Schippers AP. A family-based intervention for prevention and self-management of disabilities due to leprosy, podoconiosis and lymphatic filariasis in Ethiopia: a proof of concept study. PLoS Negl Trop Dis. 2016;10(3):e0004531. Available from: https://storage.googleapis.com/plos-corpus-prod/10.1371/journal.pntd.0009167/2/pntd.0009167.pdf?X-Goog-Algorithm=GOOG4-RSA-SHA256&X-Goog-Credential=wombat-sa%40plos-prod.iam.gserviceaccount.com%2F20210611%2Fauto%2Fstorage%2Fgoog4_request&X-Goog-Date=20210.
Wharton-Smith A, Rassi C, Batisso E, Ortu G, King R, Endriyas M, et al. Gender-related factors affecting health seeking for neglected tropical diseases: findings from a qualitative study in Ethiopia. PLoS Negl Trop Dis. 2019;13(12):e0007840.
Tekola F, Bull S, Farsides B, Newport MJ, Adeyemo A, Rotimi CN, et al. Impact of social stigma on the process of obtaining informed consent for genetic research on podoconiosis: a qualitative study. BMC Med Ethics. 2009;10(1):1–10.
Hounsome N, Kinfe M, Semrau M, Ali O, Tesfaye A, Mengiste A, et al. Economic assessment of a community-based care package for people with lower limb disorder caused by lymphatic filariasis, podoconiosis and leprosy in Ethiopia. Trans R Soc Trop Med Hyg. 2020;114(12):1021–34. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7738652/pdf/traa111.pdf.
Tora A, Mengiste A, Davey G, Semrau M. Community involvement in the care of persons affected by podoconiosis—a lesson for other skin. Trop Med Infect Dis. 2017;55(Suppl 1):45–54. Available from: https://res.mdpi.com/d_attachment/tropicalmed/tropicalmed-03-00087/article_deploy/tropicalmed-03-00087.pdf.
Engdawork K, Davey G, Ayode D, McBride CM, Tadele G. A cross-sectional survey to assess the risk factors associated with stigmatizing attitudes towards patients with podoconiosis among rural youth in southern Ethiopia. Trans R Soc Trop Med Hyg. 2020. Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02201675/full.
Kebede B, Martindale S, Mengistu B, Kebede B, Mengiste A, H/Kiros F, et al. Lymphoedema management in podoconiosis. PLoS Negl Trop Dis. 2018;6(9):e962. Available from: https://storage.googleapis.com/plos-corpus-prod/10.1371/journal.pntd.0006491/2/pntd.0006491.pdf?X-Goog-Algorithm=GOOG4-RSA-SHA256&X-Goog-Credential=wombat-sa%40plos-prod.iam.gserviceaccount.com%2F20210611%2Fauto%2Fstorage%2Fgoog4_request&X-Goog-Date=20210.
Churko C, Yihune M, Teshome A, Chisha Y, Getachew B, Sleshi M, et al. Ivermectin treatment coverage validation in two onchocerciasis endemic districts in Ethiopia: a community-based cross-sectional study, 2019. J Multidiscip Healthc. 2021;14:137–44. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7822083/pdf/jmdh-14-137.pdf.
Samuel A, Belay T, Yehalaw D, Taha M, Zemene E, Zeynudin A. Impact of six years community directed treatment with ivermectin in the control of onchocerciasis, Western Ethiopia. PLoS ONE. 2016;11(3):e0141029. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4778937/pdf/pone.0141029.pdf.
Gebrezgabiher G, Mekonnen Z, Yewhalaw D, Hailu A. Status of parasitological indicators and morbidity burden of onchocerciasis after years of successive implementation of mass distribution of ivermectin in selected communities of Yeki and Asosa districts, Ethiopia. BMC Public Health. 2020;20(1):1233. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7425055/pdf/12889_2020_Article_9344.pdf.
Enbiale W, Ayalew A, Gebrehiwot T, Mulu Y, Azage M, Zachariah R, et al. Does mass drug administration for community-based scabies control works? The experience in Ethiopia. Dermatol Res Pr. 2020;14(6.1):78s–85s. Available from: https://jidc.org/index.php/journal/article/download/32614801/2275.
Surur AS, Fekadu A, Makonnen E, Hailu A. Challenges and opportunities for drug discovery in developing countries: the example of cutaneous leishmaniasis. ACS Med Chem Lett. 2020;11(11):2058–62.
Coulborn RM, Gebrehiwot TG, Schneider M, Gerstl S, Adera C, Herrero M, et al. Barriers to access to visceral leishmaniasis diagnosis and care among seasonal mobile workers in western Tigray, northern Ethiopia: a qualitative study. PLoS Negl Trop Dis. 2018;12(11):e0006778. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6224040/pdf/pntd.0006778.pdf.
Eyayu T, Zeleke AJ, Worku L. Current status and future prospects of protein vaccine candidates against Schistosoma mansoni infection. J Parasitol Res. 2020;11:e00176. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7475110/pdf/main.pdf.
Engels D, Zhou XN. Neglected tropical diseases: an effective global response to local poverty-related disease priorities. Infect Dis Poverty. 2020;9(10):1–9.
WHO. Ending the neglect to attain the sustainable development goals – a road map for neglected tropical diseases 2021–2030. Geneva: World Health Organization; 2020. p. 55. Available from: https://www.who.int/news-room/events/detail/2021/01/28/default-calendar/eliminating-ntds-together-towards-2030-formal-launch-of-the-new-road-map-for-neglected-tropical-diseases. Accessed on 7 July 2020.
Acknowledgements
This work was supported by the Wellcome Trust (Grant ID 221576/Z/20/Z). We would also like to thank the CDT-Africa, Addis Ababa University, University of Sussex and National Institute of Health Research (NIHR)-Wellcome Global Health Research Partnership, UK, for the financial support. AF receives support from the NIHR-Wellcome Trust to the Unit for Health Evidence and Policy (UHEP) Ethiopia (Grant ID 221576/Z/20/Z), and NIHR Global Health Research Unit on Neglected Tropical Diseases-Phase 1 (G2153) & Phase 2 (G3417). CH receives support from the National Institute of Health and Care Research through the NIHR Global Health Research Group on Homelessness and Mental Health in Africa (NIHR134325) and the SPARK project (NIHR200842) using UK aid from the UK Government. The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. CH receives support from the Wellcome Trust through grants 222154/Z20/Z and 223615/Z/21/Z.
Funding
This work was financially supported by Wellcome Trust, UK (Grant ID 221576/Z/20/Z). The funder has no role on the technical work and interpretation of the finding which is the responsibility of authors.
Author information
Authors and Affiliations
Contributions
AS, TM, BF, EA, EG, and AF participated from inception to design of this scoping review. AS and EG involved on the studies selection and data extraction. AS, EA, and EG drafted the scoping review manuscript. All authors review the scoping review manuscript, incorporate their scientific and intellectual inputs, provided feedback, and approved for final submission of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable (NA).
Consent for publication
NA.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist.
Additional file 2.
Search strategy.
Additional file 3: Table 1.
Description of relevant evidence included in scoping review of neglected tropical diseases (NTDs) burden in Ethiopia (Additional file 3).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Semahegn, A., Manyazewal, T., Getachew, E. et al. Burden of neglected tropical diseases and access to medicine and diagnostics in Ethiopia: a scoping review. Syst Rev 12, 140 (2023). https://doi.org/10.1186/s13643-023-02302-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-023-02302-5