Abstract
Background
The sedative effect of intraoperative sedation in elderly surgery exerts critical influence on the prognosis. Comparison on the safety and efficacy between dexmedetomidine and midazolam in many clinical randomized controlled trials (RCTs) was inconsistent and suspicious. We aim to comprehensively evaluate the safety and efficacy between dexmedetomidine and midazolam for intraoperative sedation in the elderly via meta-analysis and systematic reviews.
Methods
RCTs regarding to the comparison of sedative effects and safety between dexmedetomidine and midazolam in elderly patients (aged ≥ 60 years) will be comprehensively searched from 2000 October to 2022 May through 4 English databases and 4 Chinese databases. After extraction in duplicate, the systematic review and meta-analysis will be performed on the primary outcomes (hemodynamic changes, sedative effect, cognitive function) and secondary outcomes (analgesic effect, surgical characteristics, complications, or adverse reactions) for assessing the two therapy methods using Review Manager software (Version 5.3). Sensitivity analysis will be conducted to evaluate the heterogeneity of the results; funnel plot and Egger’s trial will be performed to analyze publication bias of the included studies, and trial sequential analysis will be applied to assess the robustness and reliability of preliminary meta-analysis results. Finally, rating quality of evidence and strength of recommendations on the meta results will be summarized by Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach.
Discussion
This systematic review and meta-analysis will evaluate the safety and efficacy between dexmedetomidine and midazolam for intraoperative sedation in the elderly; it will give an insight on the application of dexmedetomidine and midazolam and will provide evidence-based reference for clinical decision-making.
Systematic review registration
PROSPERO CRD42021221897
Similar content being viewed by others
Background
In clinical practice, elderly population (aged ≥ 60 years) suffers from increased risk of diseases due to their reduced physical function and weakened immune function, and they are increasingly dependent on surgical treatment [1, 2]. Older people have surgery at a higher rate than other age groups, making up 15% of the total population [3]. Despite significant advances in surgery and anesthesia, anesthetics can still affect the outcome of disease treatment. Therefore, the better and more rational anesthetic medication in elderly surgery attracts increasing concern and interest.
Intraoperative sedation, often used to improve patient comfort, also regarded as surgical anesthesia [4, 5]. In order to reduce the occurrence of postoperative complications, safe and effective anesthetics are issues that need be considered in clinic. Dexmedetomidine is a highly selective α2-adrenergic receptor agonist; its sedation and analgesic effects are similar to opioids [6,7,8,9]. Dexmedetomidine provides a unique “conscious sedation” in which the patient appears to be sleeping and easily aroused, and the sedation and breathing patterns it provides are consistent with natural sleep [10, 11]. Dexmedetomidine is an important option for sedation in intensive care [12]. Dexmedetomidine applied in anesthesia can prolong the duration of sensory and motor block and reduce the cumulative analgesic dosage for 24 h after surgery [13]. Midazolam, belongs to midazolam benzodiazepines, represses the excitatory response combined with γ-aminobutyric acid receptors [14]. It is widely used for hypnotic, anticonvulsant, sedation, anxiolytic, and amnesia [15]. Midazolam has a high affinity for benzodiazepine receptors in the central nervous system, with data showing twice the affinity of diazepam [16]. Midazolam prevents autonomic, hormonal, and circulatory adverse effects without causing nausea and vomiting [17, 18]. Thus, midazolam and dexmedetomidine were the subjects of study among numerous anesthetics. However, studies have shown that sudden cessation of dexmedetomidine can lead to clonidin-like withdrawal syndromes, including tension, headache, and agitation [19]. The specific antagonist atipamezole can easily reverse the situation [20]. It is reported that dexmedetomidine application may result in intraoperative comorbidities including uncontrollable hypotension, severe bradycardia, and asystole [21, 22]. Studies have also shown that midazolam exerts fast anesthetic effect within 5 min after intramuscular injection, but it may cause loss of airway reflex, respiratory depression and even apnea during anesthesia, etc. [23].
The elderly population is at risk of serious complications during the operation and needs special care [24]. It is necessary to compare the safety of effective sedatives for the elderly. No reliable and consistent conclusions have been reached regarding dexmedetomidine and midazolam for sedation in elderly patients. Only a few meta-analyses related to anesthesia in children have confirmed that dexmedetomidine is safer and more effective than midazolam in sedation and reducing postoperative complications [25,26,27]. However, there still lacks comparison on the safety and efficacy between dexmedetomidine and midazolam in elderly surgery due to significant heterogeneities between the clinical RCTs, resulting in inconsistent and controversial conclusions. Some studies also reported that there is no significant difference in the sedation between dexmedetomidine and midazolam [28,29,30]; thus, the conclusions of these researches have been questioned to a certain extent.
This study is designed to compare the safety and sedative effects between dexmedetomidine and midazolam in elderly patients with surgery. In order to minimize the heterogeneity and bias, we will select RCTs addressing the safety and sedative effects of dexmedetomidine and midazolam in elderly patients. Systematic reviews and meta-analysis represent an appropriate design to elucidate the clinical usage and efficacy of dexmedetomidine and midazolam in previous RCTs, providing clinicians and policymakers with an overall assessment of the evidence on the safety and sedative effects between dexmedetomidine and midazolam, which is necessary for both practitioners and elderly patients.
Methods
Study design and objectives
The program will be designed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocol (PRISMA-P) [31]. It has been registered in the PROSPERO database (CRD42021221897). It aims to provide a systematic review on the effect between dexmedetomidine and midazolam in elderly patients. Additionally, meta-analysis of RCTs will be performed in order to assess the safety and efficacy of dexmedetomidine and midazolam on primary and secondary outcomes of RCTs.
Eligibility criteria
Types of participants
Patients with surgery age more than 60 or older and receive anesthetics intervention will be included, whose ASA status is I-III. Regardless of sex, region, race, and the type of surgery, as detailed in Table 1, the MESH term of population will include “The elderly,” “The old man,” “The old patient,” “The elderly patients,” “The aged” and “Intraoperative sedation,” “Sedation,” “Surgery sedation,” “Surgical sedation,” “Anesthesia and analgesia,” “Anesthesia sedation,” “Intraoperative anesthesia,” “Surgery anesthesia,” and “Surgical anesthesia.”
Types of intervention in the experimental group and control group
Patients in the experimental group and control group received dexmedetomidine or midazolam, respectively, with or without the same conventional treatment other anesthetics. The MESH term of interventions included “Dexmedetomidine,” “Dexmedetomidine Hydrochloride” and “Hydrochloride Dexmedetomidine” in the experimental group, and “Midazolam,” “Benzodiazepine,” “Benzodiazepine,” and “Benzodiazepinones, devazepide” in the control group (Table 1).
Type of included indicators or outcomes
The primary results will contain hemodynamic changes (mean arterial pressure (MAP), heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), saturation of pulse oxygen (SPO2)), sedative effect (observer assessment of alertness/sedation scale (OAA/S), bispectral index (BIS), Richmond Agitation-Sedation Scale (RASS)) and cognitive function (mini-mental state examination (MMSE), postoperative cognitive dysfunction (POCD)). The secondary results will include analgesic effect (Visual Analogue Scale/Score (VAS)), surgical characteristics (operation time, the volume of intraoperative blood loss, and extubation time), complications, or adverse reactions (hypotension, agitation, postoperative nausea and vomiting, bradycardia, respiratory depression, delirium, and shivering).
Search strategy
Four English databases (PubMed, Embase, Web of Science, Cochrane Library) and four Chinese databases (Chinese Biomedical Literature Database, Chinese Science and Technology Journals VIP Database, China National Knowledge Infrastructure (CNKI), Wan-fang Database) will be searched for literatures published between October 2001 and May 2022. The search strategy will be conducted according to the patients, interventions, comparisons, outcomes, and study design (PICOS) components (Table 1). The complete search strategy will use a combination of PICOS framework (P+I+C+O+S or P+I+C). Only clinical randomized controlled trials (RCTs) published in English or Chinese will be included. Search strategy in PubMed is shown in Table 2. Any differences can be resolved through discussion with other reviewers (ZW, DBH).
Study selection
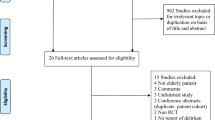
Two independent reviewers (HCX, LZJ) will screen relevant trials by examining titles and abstracts of the identified studies. Any studies that do not conform to the PICOS framework will be excluded. Full articles will be obtained and checked for extracted details (LYX, LDL). Any reason for extraction will be recorded. Possible divergency will be resolved by discussion or a third reviewer (DBH). The selection process will be presented in a PRISMA flow diagram (Fig. 1).
Data extraction and exclusion criteria
We will extract each qualified data. Data will be extracted including year of publication, author’s name, study type, randomized methodology, and basic characteristics of participants (ASA classification, mean age, sample size, type of surgery, body mass index/weight, duration of anesthesia, duration of surgery, medication use, and outcomes) (Tables 3 and 4).
Nonhuman trials, non-peer-reviewed publications, retrospective observational studies, prospective observational studies, review article, case reports, cohort studies, and letters to the editor will be excluded. Any other factors that do not meet with the inclusion criteria will also be excluded as well.
Risk-of-bias assessment
The updated ROB2 tool according to the instructions will be used for risk-of-bias assessment (https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials). The criteria for risk of bias will be assessed according to randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Quality assessments will be performed by two independent authors, and the results will be rated as “high risk,” “some concerns,” or “unclear” risk of bias. Discussions will be held by all authors to resolve any disagreements.
Statistical analysis
The effect size will be pooled using the Review Manager software tool (RevMan, v.5.3; The Cochrane Collaboration). Fixed-effect model will be used for the pool with low heterogeneity, and random-effects model will be used for the pool with high heterogeneity. Mean deviation (MD) or standard mean difference (SMD) and 95% confidence intervals (CI) will be utilized for continuous data, and relative risk (RR) with 95% CI will be calculated for dichotomous data. Subgroup analysis and sensitivity analysis will be also used to investigate potential sources of heterogeneity.
Sensitivity analysis and subgroup analysis
When it occurs relatively high heterogeneity of the results, sensitivity analysis will be performed to explore the source of high heterogeneity in order to enhance the credibility of the results. Any possibility (methodological quality, heterogeneity, studies quality, surgery duration, number of samples etc.) resulted in high heterogeneity will be considered for subgroup analysis.
Potential publication and reporting bias analysis
After preliminary meta-analysis, potential publication bias will be assessed visually using funnel plots, and Egger’s regression test and Begg’s test will be utilized to detect the funnel plot asymmetry.
Trial sequential analysis (TSA)
For further illustrating and confirming the reliability of final results, trial sequential analysis will be performed on the analyzed outcomes via TSA software (version 0.9.5.10 Beta; Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark), aiming to rule out the possibility of false positives and confirm firm evidence of final results.
Rating quality of evidence and strength of recommendations
Finally, we will conduct evaluation on the quality of evidence regarding to the main positive outcomes using the GRADEprofiler 3.6.1 evaluation tool. Briefly, the quality of the evidence will be divided into four levels: high, medium, low, and very low, and strength of recommendations will be divided into strong recommendation and weak recommendation. RCT trials will be regarded as high quality of evidence, and then, the evidence quality level of the outcomes will be assessed from the five downgrading factors of research limitations, inconsistency, indirectness, imprecision, and publication bias.
Discussion
Secure and effective anesthetics effectively reduce the occurrence of postoperative complications and improve perioperative cognitive dysfunction or intraoperative awareness, providing clinicians with better surgical procedures [32]. However, appropriate anesthetic for elderly patients with surgery is still a serious challenge for clinicians [33].
Up to date, there is not enough evidence to determine a better anesthesia plan for elderly patients [34].
Various drugs including ketamine, propofol, benzodiazepines, fentanyl, etomidate, and dexmedetomidine exert intraoperative sedation. Midazolam and dexmedetomidine is both applied for anesthesia and sedation in the elderly [35]. However, studies have indicated that midazolam led to cognitive impairment and postoperative or delirium in the elderly [36]. Although dexmedetomidine does not directly damage the respiratory system and cause apnea, it has been shown to cause hypoxia and hypercapnia [36]; it can also lead to hemodynamic effects such as hypertension, hypotension, and bradycardia, whereas, in some clinical studies, their conclusions are inconsistent and suspicious due to their heterogeneity in research methods [37, 38]. Up to date, it still lacks comprehensive acknowledgement on the safety and efficacy of dexmedetomidine and midazolam.
In conclusion, systematic evaluation on the efficacy and safety of dexmedetomidine and midazolam for intraoperative sedation in elderly patients can provide the anesthesiologist with insight to the appropriate anesthetic choice, aiming to reducing the potential postoperative complications of elderly patients [34]. This protocol maximizes the extraction of relevant information with a clear and structured procedure; however, we also acknowledge several limitations existing in this systematic review and meta-analysis. The assessment methods differ in different included studies may result in medium or high heterogeneity. Some of the included studies may be of low quality. Moreover, only studies published in English and Chinese will be included. Therefore, we will continue to pay attention to update our conclusions in future.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- RCTs:
-
Randomized controlled trials
- RASS:
-
Richmond Agitation-Sedation Scale
- MMSE:
-
Mini-mental state examination
- PICOS:
-
Patients, interventions, controls, outcomes, and study design
- BIS:
-
Bispectral index
- VAS:
-
Visual Analogue Scale/Score
- HR:
-
Heart rate
- ASA:
-
American Society of Anesthesiologists
- SMD:
-
Standard mean difference
- MAP:
-
Mean arterial pressure
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- OAA/S:
-
Observer assessment of alertness/sedation scale
- POCD:
-
Postoperative cognitive dysfunction
- SPO2:
-
Saturation of pulse oxygen
- OP:
-
Odds ratio
- 95% CI:
-
95% confidence intervals
- TSA:
-
Trial sequential analysis
References
Jaul E, Barron J. Age-related diseases and clinical and public health implications for the 85 years old and over population. Front Public Health. 2017;5. https://doi.org/10.3389/fpubh.2017.00335.
Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123(3):958–65. https://doi.org/10.1172/JCI64096.
Aucoin S, McIsaac DI. Emergency general surgery in older adults: a review. Anesthesiol Clin. 2019;37(3):493–505. https://doi.org/10.1016/j.anclin.2019.04.008.
Mei B, Meng G, Xu G, Cheng X, Chen S, Zhang Y, et al. Intraoperative sedation with dexmedetomidine is superior to propofol for elderly patients undergoing hip arthroplasty: a prospective randomized controlled study. Clin J Pain. 2018;34(9):811–7. https://doi.org/10.1097/ajp.0000000000000605.
Chui J, Alimiri R, Parrent A, Craen RA. The effects of intraoperative sedation on surgical outcomes of deep brain stimulation surgery. Can J Neurol Sci. 2018;45(2):168–75. https://doi.org/10.1017/cjn.2017.269.
Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med. 2004;32(6):1322–6. https://doi.org/10.1097/01.ccm.0000128579.84228.2a.
Taniguchi T, Kurita A, Kobayashi K, Yamamoto K, Inaba H. Dose- and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats. J Anesth. 2008;22(3):221–8. https://doi.org/10.1007/s00540-008-0611-9.
Herr DL, Sum-Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. 2003;17(5):576–84. https://doi.org/10.1016/s1053-0770(03)00200-3.
Aydin C, Bagcivan I, Gursoy S, Altun A, Topcu O, Koyuncu A. Altered spontaneous contractions of the ileum by anesthetic agents in rats exposed to peritonitis. World J Gastroenterol. 2009;15(13):1620–4. https://doi.org/10.3748/wjg.15.1620.
Afonso J, Reis F. Dexmedetomidine: current role in anesthesia and intensive care. Braz J Anesthesiol. 2012;62(1):118–33. https://doi.org/10.1016/s0034-7094(12)70110-1.
Romera Ortega MA, Chamorro Jambrina C, Lipperheide Vallhonrat I, Fernández Simón I. Indications of dexmedetomidine in the current sedoanalgesia tendencies in critical patients. Med Int. 2014;38(1):41–8. https://doi.org/10.1016/j.medin.2013.03.008.
Keating GM. Dexmedetomidine: a review of its use for sedation in the intensive care setting. Drugs. 2015;75(10):1119–30. https://doi.org/10.1007/s40265-015-0419-5.
Vorobeichik L, Brull R, Abdallah FW. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. 2017;118(2):167–81. https://doi.org/10.1093/bja/aew411.
Ng E, Taddio A, Ohlsson A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochr Database Syst Rev. 2017;1(1):Cd002052. https://doi.org/10.1002/14651858.CD002052.pub3.
Kupietzky A, Houpt MI. Midazolam: a review of its use for conscious sedation of children. Pediatr Dent. 1993;15(4):237–41.
Reves JG, Fragen RJ, Vinik HR, Greenblatt DJ. Midazolam: pharmacology and uses. Anesthesiology. 1985;62(3):310–24.
Kanto J. Benzodiazepines as oral premedicants. Br J Anaesth. 1981;53(11):1179–88. https://doi.org/10.1093/bja/53.11.1179.
Clarke RSJ. New drugs--boon or bane? Premedication and intravenous induction agents. Can Anaesthetists' Soc J. 1983;30(2):166.
Naaz S, Ozair E. Dexmedetomidine in current anaesthesia practice- a review. J Clin Diagn Res. 2014;8(10):GE01-4. https://doi.org/10.7860/JCDR/2014/9624.4946.
Scheinin H, Aantaa R, Anttila M, Hakola P, Helminen A, Karhuvaara S. Reversal of the sedative and sympatholytic effects of dexmedetomidine with a specific alpha2-adrenoceptor antagonist atipamezole: a pharmacodynamic and kinetic study in healthy volunteers. Anesthesiology. 1998;89(3):574–84. https://doi.org/10.1097/00000542-199809000-00005.
Gerlach AT, Murphy CV. Dexmedetomidine-associated bradycardia progressing to pulseless electrical activity: case report and review of the literature. Pharmacother J Human Pharmacol Drug Ther. 2009;29(12):1492. https://doi.org/10.1592/phco.29.12.1492.
Aikaterini A, Ioannis D, Dimitrios G, Konstantinos S, Vasilios G, George P. Bradycardia leading to asystole following dexmedetomidine infusion during cataract surgery: dexmedetomidine-induced asystole for cataract surgery. Case Rep Anesthesiol. 2018;2018:2896032. https://doi.org/10.1155/2018/2896032.
Taylor JW, Simon KB. Possible intramuscular midazolam-associated cardiorespiratory arrest and death. DICP. 1990;24(7-8):695–7. https://doi.org/10.1177/106002809002400707.
Mézière A, Paillaud E, Plaud B. Anesthesia in the elderly. Presse Medicale (Paris, France : 1983). 2013;42(2):197–201. https://doi.org/10.1016/j.lpm.2012.07.040.
Pasin L, Febres D, Testa V, Frati E, Borghi G, Landoni G, et al. Dexmedetomidine vs midazolam as preanesthetic medication in children: a meta-analysis of randomized controlled trials. Pediatr Anesth. 2015;25(5):468–76. https://doi.org/10.1111/pan.12587.
Sun Y, Lu Y, Huang Y, Jiang H. Is dexmedetomidine superior to midazolam as a premedication in children? A meta-analysis of randomized controlled trials. Pediatr Anesth. 2014;24(8):863–74. https://doi.org/10.1111/pan.12391.
Lang B, Zhang L, Zhang W, Lin Y, Fu Y, Chen S. A comparative evaluation of dexmedetomidine and midazolam in pediatric sedation: a meta-analysis of randomized controlled trials with trial sequential analysis. CNS Neurosci Therapeut. 2020;26(8):862–75. https://doi.org/10.1111/cns.13377.
Sathyamoorthy M, Hamilton TB, Wilson G, Talluri R, Fawad L, Adamiak B, et al. Pre-medication before dental procedures: a randomized controlled study comparing intranasal dexmedetomidine with oral midazolam. Acta Anaesthesiol Scand. 2019;63(9):1162–8. https://doi.org/10.1111/aas.13425.
Abdel-Ghaffar HS, Kamal SM, El Sherif FA, Mohamed SA. Comparison of nebulised dexmedetomidine, ketamine, or midazolam for premedication in preschool children undergoing bone marrow biopsy. Br J Anaesth. 2018;121(2):445–52. https://doi.org/10.1016/j.bja.2018.03.039.
Sajid B, Mohamed T, Jumaila M. A comparison of oral dexmedetomidine and oral midazolam as premedicants in children. J Anaesthesiol Clin Pharmacol. 2019;35(1):36–40. https://doi.org/10.4103/joacp.JOACP_20_18.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. https://doi.org/10.1186/2046-4053-4-1.
Gray LD, Morris C. The principles and conduct of anaesthesia for emergency surgery. Anaesthesia. 2013;68(s1):14–29. https://doi.org/10.1111/anae.12057.
Kruijt Spanjer MR, Bakker NA, Absalom AR. Pharmacology in the elderly and newer anaesthesia drugs. Best Pract Res Clin Anaesthesiol. 2011;25(3):355–65. https://doi.org/10.1016/j.bpa.2011.06.002.
Lim B-G, Lee I-O. Anesthetic management of geriatric patients. Korean J Anesthesiol. 2020;73(1):8–29. https://doi.org/10.4097/kja.19391.
Barends CR, Absalom A, van Minnen B, Vissink A, Visser A. Dexmedetomidine versus midazolam in procedural sedation. A systematic review of efficacy and safety. PLoS One. 2017;12(1):e0169525. https://doi.org/10.1371/journal.pone.0169525.
Griebling Tomas L. Re: American Geriatrics Society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Urol. 2019;202(3):438. https://doi.org/10.1097/JU.0000000000000409.
White SM, Foss NB, Griffiths R. Anaesthetic aspects in the treatment of fragility fracture patients. Injury. 2018;49(8):1403–8. https://doi.org/10.1016/j.injury.2018.06.027.
White SM, Altermatt F, Barry J, Ben-David B, Coburn M, Coluzzi F, et al. International fragility fracture network Delphi consensus statement on the principles of anaesthesia for patients with hip fracture. Anaesthesia. 2018;73(7):863–74. https://doi.org/10.1111/anae.14225.
Funding
This work was supported by the special foundation of Guangzhou Key Laboratory (No. 202002010004), Science and Technology Planning Project of Guangdong Province (No. 2017B030314166), Guangdong Provincial Hospital of Chinese Medicine Science and Technology Research Program (No. YN2019MJ11), and Secondary Development Project of Guangdong Provincial Pharmaceutical Chinese medicine (No. 20170410).
Author information
Authors and Affiliations
Contributions
DBH and ZW are contributed to the conception of the study, this manuscript was drafted by HCX and LZJ, and it was revised by LYX. The search strategy was developed by all authors and will be conducted by HCX, and LZJ, LYX, and LDL will extract data from included studies and assess the risk of bias. HCX and HMH will conduct and finish the data synthesis. DBH and ZW will arbitrate in cases of disagreement and ensure no errors occur during the study. All authors have approved the publication of the protocol. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, C., Li, Z., Long, Y. et al. A comprehensive evaluation between dexmedetomidine and midazolam for intraoperative sedation in the elderly: protocol for a systematic review and meta-analysis of randomized controlled trials. Syst Rev 11, 278 (2022). https://doi.org/10.1186/s13643-022-02144-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-022-02144-7