Abstract
Background
Benign prostate hyperplasia (BPH) is the most common urological problem in elderly males. Recent studies have reported polymorphism in various metabolic genes in BPH. However, their association with the susceptibility of BPH is still inconsistent. Here, we systematically reviewed and performed a meta-analysis of CYP17, VDR, and ACE genes to determine their precise association with the risk of BPH.
Methods
A comprehensive literature search for published studies on candidate gene associations involving vitamin D receptor (VDR), angiotensin-converting enzyme (ACE), and CYP17 genes with the risk of BPH was done up to April 2020 in PubMed, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), and Google Scholar databases. Fixed/random effects models were used to estimate the odd’s ratio (OR) and 95% confidence intervals (CIs). Begg’s funnel plot was used to assess the potential for publication bias.
Results
We found a total of 23 studies containing 3461 cases and 3833 controls for these gene polymorphisms. A significant association of ACE gene polymorphism was observed under the recessive (II vs. ID + DD) model for BPH susceptibility compared to control subjects (overall OR = 1.67, 95% CI = 1.03–2.73). Similar trends were observed for ACE gene polymorphism in Caucasian (OR = 6.18, 95% CI = 1.38–27.68) and Asian (OR = 1.42, 95% CI = 0.99–2.03) populations under study. No significant association was observed in VDR and CYP17 gene polymorphisms in any dominant or recessive models.
Conclusion
Significant OR demonstrated the implication of ACE gene polymorphism in the proliferation of prostate tissue, which in turn is associated with BPH susceptibility. However, prospective studies at large scale and sample size are needed to confirm the current findings.
Similar content being viewed by others
Introduction
Benign prostatic hyperplasia (BPH) is one of the most common diseases in lower urinary tract symptoms (LUTS), in middle-aged and elderly men. It is a non-malignant enlargement of the prostate which can cause urinary dysfunction and may affect the quality of life of patients [1]. Being a progressive disease, it results in more severe LUTS, making the life of patients more difficult and after the age of 80 years; various treatments are given to patients according to their symptoms. BPH is a multifactorial and complex disease. Roles of ageing, heritability, ethnicity, and family history have been demonstrated in BPH development [2]. Still, its aetiology remains unclear. However, recent literature demonstrates the role of gene polymorphisms, metabolic changes, and inflammation among ageing males in BPH [3].
Androgens are required for normal growth and development of the prostate gland and even reported in the maintenance of BPH [4]. The CYP17 gene codes for the cytochrome P450c17α enzyme, which plays a crucial role in the synthesis of testosterone from its precursor cholesterol. The CYP17 gene, in its 5′-untranslated region, harbours a nucleotide substitution (rs743572) of ‘T’ (A1 allele) to ‘C’ (A2 allele) resulting in higher levels of androgens. This change in nucleotide results in a new Sp-1 site (CCACC box) at 34 bp upstream of the translation site and downstream of the transcription site, which in turn acts as an additional promoter elevating CYP17 transcription [5]. Several studies have investigated the association between CYP17 rs743572 polymorphism and BPH susceptibility, but still there is no clarity.
Another metabolic factor, angiotensin-converting enzyme (ACE), plays a significant role in “classical” renin–angiotensin–aldosterone system (RAAS) through which the proliferation of cellular elements in the prostate is regulated [6]. One of the well-known polymorphisms in the ACE gene is the insertion/deletion polymorphism, which is 287 bp long and results in three genotypes (II, ID, and DD). These genotypes have been shown to be associated with ACE activity and levels in plasma and tissues. The primary transcript of the gene is differentially spliced, and different versions of the mature mRNAs are translated to synthesize various isoforms of the enzyme; of them, one isoform is adequately expressed in the testis. This polymorphism of the ACE gene has been associated with an increased risk for many diseases [7, 8]. High levels of ACE are associated with BPH [9]. The hyperactivity of local tissue RAAS is considered involved in the pathophysiology of BPH [10]. Considering the suggested involvement of RAAS in BPH, the ACE insertion/deletion (I/D) polymorphism could have implications in the pathophysiology of BPH.
Vitamin D and its analogues possess antiproliferative and differentiation effects on prostate cells in both in vivo and in vitro [11, 12]. The antineoplastic actions of vitamin D appear to be mediated primarily through the vitamin D receptor (VDR), which is a member of the steroid/thyroid hormone receptor superfamily. Low vitamin D level is an independent risk factor for BPH [13]. More than 11 single nucleotide polymorphisms (SNPs) have been reported in VDR gene’s coding and promoter [14]. Several studies have been done investigating their role in the progression of the BPH [15,16,17,18]. Still, there is no consensus between VDR polymorphism and the risk of BPH.
Therefore, we performed the present meta-analysis based on the multivariate method to evaluate the possible role of CYP17, VDR, and ACE gene polymorphisms towards the risk of BPH.
Methods
Literature search
This systematic literature review was performed using the guidelines of the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses) [19] and Cochrane Handbook for Systematic Reviews of Intervention. An electronic search of PubMed, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), and Google Scholar databases was performed for English language papers published up to 31 April 2020. The following key terms were used: ‘Candidate Association studies OR ‘cytochrome P450c17alpha’ OR ‘Angiotensin Converting Enzyme’, OR ‘Vitamin D Receptor’ OR ‘Gene Polymorphisms’ AND ‘Benign Prostate Hyperplasia’. Additionally, the reference list of retrieved studies, review articles, and previous meta-analyses were manually searched for collecting more relevant studies often missed while performing the electronic search. During the literature search, all candidate gene association studies involving VDR, ACE, and CYP17 polymorphisms on the risk of benign prostate hyperplasia compared to the control group were included, whereas duplicates, case reports, and case series were excluded.
Selection criteria
Case–control candidate gene association studies reporting genetic polymorphisms of VDR, ACE, and CYP17 polymorphisms with the risk of BPH were included. Two authors independently and in duplicate screened titles, abstracts, and full texts determined eligibility, abstracted data, and assessed the credibility of pooled associations. Meta-analyses were performed for genetic variants assessed in more than two studies.
Data extraction
Two investigators independently extracted the data. The following data were extracted from each study: first author’s name, published year, ethnicity, country, number of cases and controls, mean age, genotyping method, source of control population, either hospital- or population-based, and frequency distribution of selected polymorphisms. Hardy–Weinberg equilibrium (HWE) was calculated for the allelic frequency distribution. Ethnicities were categorized as Asian and Caucasian populations.
Quality assessment
The Newcastle–Ottawa Scale (NOS) [20] was used for assessing the quality of the included studies based on three components: selection, comparability, and ascertainment of outcome. Scores ranged from 01 to 09. Two authors independently assessed the quality of the included studies. Discrepancies over quality scores were resolved by discussion among all authors and a subsequent consensus was reached.
Risk of bias assessment
The risk of bias was assessed with Newland Ottawa Scale, and publication bias was assessed using Begg’s and Egger’s funnel plot analysis.
Statistical analysis
Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to investigate the relationship between ACE, Cyp17, and ACE gene polymorphisms and the risk of BPH using fixed (Mantel–Haenszel method) [21] or random effects (Dersimonian and Laird method) models [22]. Heterogeneity between the studies was compared by using Cochran’s-Q statistic and I2 metric [23, 24]. The I2 metric was used to describe the degree of heterogeneity between the included studies, where 0–25% indicated no observed heterogeneity and larger values showed increasing heterogeneity, with 25–50% regarded as low, 50–75% as moderate, and 75–100% as high. Heterogeneity between studies was adjusted by subgroup analysis, HWE status, and meta-regression by quality score of the included studies.
One-way sensitivity analyses were performed to assess the stability of the results, namely, a single study in which the meta-analysis was deleted each time to reflect the influence of the individual dataset on the pooled OR. Funnel plots and Egger’s linear regression test were used to obtain the potential publication bias [25, 26]. The presence of selection bias in control participants was evaluated by calculating HWE, and genotypic frequencies of the control subjects were compared by using the chi-square test. Stratified analysis based on ethnicity (Asian versus Caucasian) was performed. To ensure the reliability and accuracy of the results, two investigators entered data into the software and reached a consensus. All statistical analyses were performed using STATA 13.0 software. P value <0.05 was considered statistically significant.
Results
Literature search
The initial search yielded 310 records from PubMed, Embase, Scopus, Web of Science Cochrane Central Register of Controlled Trials (CENTRAL), and Google Scholar databases. Of them, 281 were excluded after the review of the title/abstract, leaving 29 potential studies for full-text information review. Finally, 23 studies met the inclusion criteria and were included in this study (Fig. 1).
Characteristics of eligible studies
The main characteristics of the included studies are presented in Table 1. The publication years of the studies included in our analysis ranged from 2000 to 2020. The sample size in each study ranged from 20 to 588. Total 23 case–control studies, 11 for CYP17 [2, 5, 16, 27,28,29,30,31,32,33,34], 10 for VDR [15,16,17, 34,35,36,37,38,39,40], and 4 studies for ACE I/D [41,42,43,44] polymorphisms were included in our meta-analysis (Table 1). Studies were carried out in two major ethnic populations; 13 studies were in Asian while 10 studies were in the Caucasian population. All studies in this meta-analysis had controls in HWE. The quality scores of all included studies were moderately high. Out of 23 studies, 21 studies were hospital-based, 02 studies were population-based, and 01 study had a mixed source of controls. Table 1 gives a summary of the characteristics and methodological quality of all included studies.
Association between CYP17 (rs743572) gene polymorphism and BPH susceptibility
A non-significant relationship between CYP17 (rs743572) gene polymorphism and risk of BPH was observed under dominant model (OR = 0.96, 95% CI = 0.87–1.06) and recessive model (OR = 0.82, 95% CI = 0.60–1.13), respectively (Table 2, Supplementary Fig. S1A). Upon conducting the subgroup analysis based on the ethnicity of the study population, no significant association was observed based on the Asian population under the dominant model (OR = 0.96, 95% CI = 0.83–1.11) and recessive model (OR = 0.77, 95% CI = 0.43–1.36); also, on the Caucasian population, no significant association was observed under dominant (OR = 0.96, 95% CI = 0.83–1.12) as well as recessive model (OR = 0.93, 95% CI = 0.74–1.17), respectively (Table 2). Overall, no evidence of heterogeneity was observed (I2 = 0.00%, P = 0.67).
Association between ACE I/D gene polymorphisms and risk of BPH
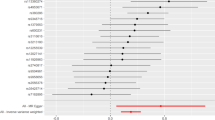
A significant association between angiotensin-converting enzyme (ACE) insertion/deletion (I/D) polymorphism and risk of BPH was observed under the recessive (II vs. ID + DD) model (OR = 1.67, 95% CI = 1.03–2.73), but not in the dominant (II + ID vs. DD) model (OR = 1.0, 95% CI = 0.75–1.35) (Table 2, Fig. 2). Upon conducting the subgroup analysis based on the ethnicity of the study population, a significant association was also observed based in the Asian population under the recessive model (OR = 1.42, 95% CI = 0.99–2.03) as well as the Caucasian population (OR = 6.18, 95% CI = 1.38–27.68), respectively. However, no significant association was observed under the dominant model (OR = 0.98, 95% CI = 0.76–1.35) in Asian as well as in Caucasian (OR = 1.56, 95% CI = 0.38–6.40) (Table 2, Fig. 2). Overall, slight non-significant evidence of heterogeneity was observed (I2 = 28.5%, P = 0.24).
Association between vitamin D receptor (VDR) gene polymorphisms and risk of BPH
Four polymorphisms (Taq-I, Bsm-I, Apa-I, and Fok-I) of the VDR gene with risk of BPH from seven case–control studies were identified. Seven case–control studies focused on Taq-I, six case–control studies focused on Fok-I, and five case–control studies focused on Bsm-I and Apa-I gene polymorphisms of VDR. The pooled analyses indicated that these four polymorphisms might not be associated with the risk of BPH.
All four VDR polymorphisms were not associated with the risk of BPH under the dominant model: Taq-I: OR 1.16, 95% CI (0.95–1.42) for TT + Tt vs. tt; Bsm-I: OR 1.05, 95% CI (0.80–1.39) for BB + Bb vs. bb; Apa-I: OR 1.07, 95% CI (0.77–1.51) for AA +Aa vs. aa; Fok-I: OR 0.90, 95% CI (0.78–1.04) for FF + Ff vs. ff and recessive model (Taq-I: OR 1.36, 95% CI (0.91–2.02) for tt vs. TT; Bsm-I: OR 1.34, 95% CI (0.90–1.99) for bb vs. BB; Apa-I: OR 1.23, 95% CI (0.57–2.64) for aa vs. AA; Fok-I: OR 0.76, 95% CI (0.54–1.07) for ff vs. FF) (Table 2, Supplementary Figs. S3A–S6A). Subgroup analysis according to ethnicity also did not confirm any risk of association for all these four polymorphisms and the risk of BPH in both Asian as well as Caucasian populations. No heterogeneity for all these four polymorphisms was observed.
Publication bias
Begg’s funnel plot and the Egger test were performed to assess the publication bias arising from the literature for all three genes under study. No obvious asymmetry was observed in any genetic model in any of the genes according to the visual assessment of the funnel plot (Supplementary Figs. S1B, S2B, S3B–S6B). In addition, there was no statistical evidence of publication bias among studies using Egger’s regression test.
Sensitivity analyses
Furthermore, we performed sensitivity analyses to assess the influence of each individual study of every gene on the pooled ORs by sequential omission of individual included studies. However, the corresponding pooled ORs were not significantly altered by removing any individual study except for allelic models of all three genes (Supplementary Figs. S1C, S2C, S3C–S6C). Therefore, the sensitivity analysis confirmed that the results of this meta-analysis were statistically reliable and robust.
Meta-regression analysis
Meta-regression analysis based on the quality scores for the relationship between all three gene polymorphisms and the risk of BPH did not confirm any deviation of the findings (Supplementary Fig. S1D).
Discussion
BPH affects the quality of life of patients including both young to old men. Its aetiology has been described by multiple hypotheses of the involvement of several genetic and metabolic factors. Polymorphism in several genes has been linked to the high susceptibility of BPH. For example, SNPs in CYP17, CYP19, VDR, and SRD5A2 [34] and in chemokine genes CCR2 (rs1799864) and CCL5 (rs2107538) [45] genes have been reported in BPH. Individuals with metabolic syndrome (MetS) or its individual components—including central obesity, hyperinsulinemia, insulin resistance, and dyslipidemia, are more prone to develop BPH and LUTS [46,47,48]. However, the molecular and stromal mechanisms involved in the pathogenesis of BPH have not yet been fully elucidated. Genetic polymorphisms in vital genes of metabolic pathways associated with BPH impact the phenotype and its severity. Therefore, in the present study, we systematically reviewed and analyzed genetic variations in important genes towards the susceptibility of BPH.
Our systematic review and meta-analysis evaluated the significance of three highly frequent polymorphisms and their association with the risk of BPH by pooling 11 studies for CYP17 (cases = 2078, controls = 2110), 10 studies for VDR (cases = 1539, controls = 1915), and 4 studies for ACE (cases = 364, controls = 388) across two different ethnicities (Asians and Caucasian) (Table 1). The findings from our analysis reveal that genetic polymorphism in the ACE gene was significantly associated with the risk of BPH when compared with control subjects, whereas the polymorphism located in VDR and CYP17 genes failed to do so (Table 2, Fig. 2).
The hydroxylase enzyme encoded by the CYP17 gene regulates steroid hormone synthesis and may play a crucial role in the etiology of hormone-related cancers such as prostate cancer and breast cancer. There is no consensus on the effect of genetic polymorphisms of these genes on BPH susceptibility. For example, the A1 allele with a gene dosage effect and -34T>C polymorphisms of CYP17 have been associated with an increased risk of BPH and its clinical progression, while no positive association was found in Orientals [49]. Similarly, the VDR gene polymorphism was not found significantly associated with BPH in Asians and Caucasians [18], whereas a significant association was demonstrated by two variants (Taq-I and Bsm-I) in Asians [15] and the other two variants (ApaI and BsmI) in Lebanese men [16]. However, by pooling these and similar shortlisted reports, in the present analysis, no significant association was observed with the BPH susceptibility. Even after omitting one of these studies (either CYP17 or VDR), we observed that the overall effect size did not change significantly in the leave-one-out analysis ordered by both heterogeneity and effect size. Similar results were observed in the analysis of VDR polymorphism.
However, in the case of analysis of ACE gene polymorphism for BPH susceptibility, we found a significant association between them. The ACE gene is involved in the hyperactivity of local RAAS in the prostate and has to be demonstrated to be involved in the pathogenesis of BPH. The insertion/deletion polymorphism of the ACE gene is directly related to ACE plasma levels [50]. This variation has also previously been found to be associated with LUTS or surgery for LUTS in study populations of Mexico and India [43, 45]. Thus, such reports prove the robustness of the current study.
Publication bias and heterogeneity could in turn distort the results of the meta-analysis. However, the publication bias was not detected in Begg’s funnel plots for per-allele models or their combinations for all three genes. In addition to bias, heterogeneity was also not found in the current study, which could in turn distort the results of the meta-analysis. These all further strengthen our results.
Conclusion
We found a significant association of ACE gene and negative association of CYP17 and VDR gene polymorphisms with the risk of BPH, which patients with ACE polymorphism (recessive) are more susceptible to BPH onset. Although relatively few studies on ACE polymorphism than VDR/CYP17 genes were analysed in the present study, large studies with prospective data and large sample size should be conducted.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Madersbacher S, Sampson N, Culig Z. Pathophysiology of benign prostatic hyperplasia and benign prostatic enlargement: a mini-review. Gerontology. 2019;65(5):458–64.
Abdullah RG, Altaee AH, Joodi MR. Association of -34 T > C CYP17 gene polymorphism with benign prostatic hyperplasia in Babylon Province. Iraq J Glob Pharma Technol. 2018;10(10):154–5.
Fan G-R, Yang E-G, Gao S-Z, Wang Z-P. Genetic polymorphisms in benign prostatic hyperplasia: progress in studies. Zhonghua Nan Ke Xue. 2019;25(10):934–8.
Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation. 2011;82(4–5):184–99.
Kumar V, Banerjee BD, Datta SK, Yadav CS, Singh S, Ahmed RS, et al. Association of CYP1A1, CYP1B1 and CYP17 gene polymorphisms and organochlorine pesticides with benign prostatic hyperplasia. Chemosphere. 2014;108:40–5.
Singh Y, Gupta G, Sharma R, Matta Y, Mishra A, Pinto TDJA, et al. Embarking effect of ACE2-angiotensin 1-7/Mas receptor axis in benign prostate hyperplasia. Crit Rev Eukaryot Gene Expr. 2018;28(2):115–24.
Pinheiro DS, Santos RS, Jardim PCBV, Silva EG, Reis AAS, Pedrino GR, et al. The combination of ACE I/D and ACE2 G8790A polymorphisms revels susceptibility to hypertension: a genetic association study in Brazilian patients. PLoS One. 2019;14(8):e0221248.
Gumus E. The powerful association of angiotensin-converting enzyme insertion/deletion polymorphism and idiopathic recurrent pregnancy loss. Ginekol Pol. 2018;89(10):573–6.
Nassis L, Frauman AG, Ohishi M, Zhuo J, Casley DJ, Johnston CI, et al. Localization of angiotensin-converting enzyme in the human prostate: pathological expression in benign prostatic hyperplasia. J Pathol. 2001;195(5):571–9.
Dinh DT, Frauman AG, Somers GR, Ohishi M, Zhou J, Casley DJ, et al. Evidence for activation of the renin-angiotensin system in the human prostate: increased angiotensin II and reduced AT(1) receptor expression in benign prostatic hyperplasia. J Pathol. 2002;196(2):213–9.
Ingles SA, Coetzee GA, Ross RK, Henderson BE, Kolonel LN, Crocitto L, et al. Association of prostate cancer with vitamin D receptor haplotypes in African-Americans. Cancer Res. 1998;58(8):1620–3.
Peehl DM, Skowronski RJ, Leung GK, Wong ST, Stamey TA, Feldman D. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res. 1994;54(3):805–10.
Hammarsten J, Damber JE, Johnell O, Knutson T, Ljunggren O, Ohlsson C, et al. A low vitamin D level is an independent risk factor for the development of benign prostatic hyperplasia. Urology. 2006;PD 02.03(68):5.
Zajícková K, Krepelová A, Zofková I. A single nucleotide polymorphism under the reverse primer binding site may lead to BsmI mis-genotyping in the vitamin D receptor gene. J Bone Miner Res. 2003;18(10):1754–7.
Manchanda PK, Konwar R, Nayak VL, Singh V, Bid HK. Association of genetic variants of the vitamin D receptor (VDR) gene (Fok-I, Taq-I and Bsm-I) with susceptibility of benign prostatic hyperplasia in a North Indian population. Asian Pac J Cancer Prev. 2010;11(4):1005–8.
El Ezzi AA, Zaidan WR, El-Saidi MA, Al-Ahmadieh N, Mortenson JB, Kuddus RH. Association of benign prostate hyperplasia with polymorphisms in VDR, CYP17, and SRD5A2 genes among Lebanese men. Asian Pac J Cancer Prev. 2014;15(3):1255–62.
Ruan L, Zhu J, Pan C, Hua X, Yuan D, Li Z, et al. Association between single nucleotide polymorphism of vitamin D receptor gene FokI polymorphism and clinical progress of benign prostatic hyperplasia. ScientificWorldJournal. 2015;2015:235895.
Zeng X-T, Yao Q-S, Weng H, Li S, Huang J-Y, Wang X-H. Meta-analysis of vitamin D receptor gene polymorphisms and benign prostatic hyperplasia risk. Mol Biol Rep. 2014;41(10):6713–7.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48.
DerSimonian R. Meta-analysis in the design and monitoring of clinical trials. Stat Med. 1996;15(12):1237–48 discussion 1249-1252.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Habuchi T, Liqing Z, Suzuki T, Sasaki R, Tsuchiya N, Tachiki H, et al. Increased risk of prostate cancer and benign prostatic hyperplasia associated with a CYP17 gene polymorphism with a gene dosage effect. Cancer Res. 2000;60(20):5710–3.
Madigan MP, Gao Y-T, Deng J, Pfeiffer RM, Chang B-L, Zheng S, et al. CYP17 polymorphisms in relation to risks of prostate cancer and benign prostatic hyperplasia: a population-based study in China. Int J Cancer. 2003;107(2):271–5.
Tigli H, Yazici H, Dalay N. Cyp17 genetic polymorphism in prostate cancer and benign prostatic hyperplasia. Res Commun Mol Pathol Pharmacol. 2003;113–114:307–14.
Gunes S, Bagci H, Sarikaya S, Bilen CY, Kara N. Prostate-specific antigen and 17-hydroxylase polymorphic genotypes in patients with prostate cancer and benign prostatic hyperplasia. DNA Cell Biol. 2007;26(12):873–8.
Sobti RC, Gupta L, Singh SK, Seth A, Kaur P, Thakur H. Role of hormonal genes and risk of prostate cancer: gene-gene interactions in a North Indian population. Cancer Genet Cytogenet. 2008;185(2):78–85.
Antognelli C, Mezzasoma L, Mearini E, Talesa VN. Glyoxalase 1-419C>A variant is associated with oxidative stress: implications in prostate cancer progression. PLoS One. 2013;8(9):e74014.
Ananthan V, Presanna BS, Dolia PB, Ramadesikan VK, Sumathy S, Priya CS. Association of CYP 17 gene polymorphism with benign prostatic hyperplasia. Sch J App Med Sci. 2016;4(7E):2630–5.
Zhang L-L, Song Y, He L-L, Chen G-Q, Fu J-C, Liu L, et al. Associations of SRD5A2/CYP17/CYP19/VDR gene polymorphisms with the development and clinical progression of benign prostatic hyperplasia: a case-control study in northern Chinese population. Int J Clin Exp Pathol. 2017;10(8):8660–76.
Bousema JT, Bussemakers MJ, van Houwelingen KP, Debruyne FM, Verbeek AL, de La Rosette JJ, et al. Polymorphisms in the vitamin D receptor gene and the androgen receptor gene and the risk of benign prostatic hyperplasia. Eur Urol. 2000;37(2):234–8.
Habuchi T, Suzuki T, Sasaki R, Wang L, Sato K, Satoh S, et al. Association of vitamin D receptor gene polymorphism with prostate cancer and benign prostatic hyperplasia in a Japanese population. Cancer Res. 2000;60(2):305–8.
Hamasaki T, Inatomi H, Katoh T, Ikuyama T, Matsumoto T. Significance of vitamin D receptor gene polymorphism for risk and disease severity of prostate cancer and benign prostatic hyperplasia in Japanese. Urol Int. 2002;68(4):226–31.
Chaimuangraj S, Thammachoti R, Ongphiphadhanakul B, Thammavit W. Lack of association of VDR polymorphisms with Thai prostate cancer as compared with benign prostate hyperplasia and controls. Asian Pac J Cancer Prev. 2006;7(1):136–9.
Huang S-P, Huang C-Y, Wu W-J, Pu Y-S, Chen J, Chen Y-Y, et al. Association of vitamin D receptor FokI polymorphism with prostate cancer risk, clinicopathological features and recurrence of prostate specific antigen after radical prostatectomy. Int J Cancer. 2006;119(8):1902–7.
Nunes SBR, de Matos Oliveira F, Neves AF, Araujo GR, Marangoni K, Goulart LR, et al. Association of vitamin D receptor variants with clinical parameters in prostate cancer. Springerplus. 2016;5:364.
El Ezzi AA, Clawson JM, El-Saidi MA, Zaidan WR, Kovash A, Orellana J, et al. Association of angiotensin I converting enzyme insertion/287 bp deletion polymorphisms and proliferative prostatic diseases among Lebanese men. Prostate Cancer. 2020;2020:5959134.
Bid HK, Manchanda PK, Konwar R, Hanif K, Nayak VL, Singh V. Does angiotensin-converting enzyme polymorphism have association with symptomatic benign prostatic hyperplasia? Indian J Urol. 2010;26(4):497–501.
Shubbar HA, Umran MA. Ace gene polymorphism insertion deletion association with prostate cancer. Int J Pharm Qual Assur. 2018;9(2):187–9.
Sierra Díaz E, Sánchez Corona J, Rosales Gómez RC, Gutierrez Rubio SA, Vázquez Camacho JG, Solano Moreno H, et al. Angiotensin-converting enzyme insertion/deletion and angiotensin type 1 receptor A1166C polymorphisms as genetic risk factors in benign prostatic hyperplasia and prostate cancer. J Renin Angiotensin Aldosterone Syst. 2009;10(4):241–6.
Pang Y, Li H, Gong Y, Jing S, Peng C, Liu W, et al. Association of CCL2, CCR2 and CCL5 genetic polymorphisms with the development and progression of benign prostatic hyperplasia. Oncol Rep. 2019;41(4):2491–501.
Sajjaboontawee N, Supasitthumrong T, Tunvirachaisakul C, Nantachai K, Snabboon T, Reiche EMV, et al. Lower thiol, glutathione, and glutathione peroxidase levels in prostate cancer: a meta-analysis study. Aging Male. 2020:23(5):1533–44.
Hashim NA, Al-Ali ZAJR, Syhood AA. Association between metabolic syndrome (MetS) and benign prostatic hyperplasia (BPH) in Amara city. Iraq Eurasia J Biosci. 2020;14:93–8.
Grzesiak K, Rył A, Ratajczak W, Stachowska E, Rotter I, Słojewski M, et al. Influence of metabolic syndrome on the relationship between fatty acids and the selected parameters in men with benign prostatic hyperplasia. Aging (Albany NY). 2019;11(5):1524–36.
Weng H, Fang C, Geng P-L, Jin Y-H, Zeng X-T, Wang X-H. Role of CYP17 rs743572 polymorphism in benign prostatic hyperplasia: a multivariate integrated analysis. Front Physiol. 2019;10:774.
Hasanzad M, Samzadeh M, Jamaldini SH, Haghdoost AA, Afshari M, Ziaei SAM. Association of angiotensin I converting enzyme polymorphism as genetic risk factor in benign prostatic hyperplasia and prostate cancer. Genet Test Mol Biomarkers. 2012;16(7):770–4.
Acknowledgements
Not applicable.
Funding
This meta-analysis was funded by the National Natural Science Foundation of China, grant number 81602914.
Author information
Authors and Affiliations
Contributions
LL and HNS designed the complete study. LL and PL performed the literature search and data extraction and assessed the quality of the included articles. XL and XX prepared the figures. LL, PL, LL, and AKS drafted the manuscript. All authors reviewed and revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable; this analysis is based on published aggregate data and does not require ethical approval or informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Figure S1.
Meta-analysis of included studies reporting on CYP17 polymorphism in BPH susceptibility compared with controls. Figure S2. Meta-analysis of included studies reporting on ACE gene polymorphism in BPH susceptibility compared with controls. Figure S3. Meta-analysis of included studies reporting on VDR Taq1 polymorphism in BPH susceptibility compared with controls. Figure S4. Meta-analysis of included studies reporting on VDR bsm1 polymorphism in BPH susceptibility compared with controls. Figure S5. Meta-analysis of included studies reporting on VDR APa1 polymorphism in BPH susceptibility compared with controls. Figure S6. Meta-analysis of included studies reporting on VDR Fok1 polymorphism in BPH susceptibility compared with controls.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, L., Li, P., Liu, X. et al. Systematic review and meta-analysis of candidate gene association studies of benign prostate hyperplasia. Syst Rev 11, 60 (2022). https://doi.org/10.1186/s13643-022-01914-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-022-01914-7