Abstract
Background
Homonymous visual field defects represent the most frequent type of visual field loss after stroke, affecting nearly 30% of individuals with unilateral post-chiasmal brain damage. This review aimed to gather the available evidence on the biomechanical changes to visual field loss following stroke.
Methods
A systematic review was conducted inclusive of randomised controlled trials, cohort studies, before-after studies and case-controlled studies. Studies including adult and paediatric participants that investigated eye, head, or body movements in post-stroke visual field loss during visual exploration tasks were included. Search terms included a range of MESH terms as well as alternative terms relating to stroke, visual field loss, hemianopia, visual functions and scanning behaviour. Articles were selected by two authors independently. Data were extracted by one author and verified by a second. All included articles were assessed for risk of bias using checklists appropriate to the study design.
Results
Thirty-six articles (1123 participants) were included in the overall review (Kappa 0.863) and categorised into simulated or true visual field loss (typically hemianopia). Seven studies identified the biomechanical alterations to simulated hemianopia compared to normal performance. Twenty-nine studies detailed eye, head and body movement parameters in true hemianopia. Hemianopic participants and healthy adults with simulated hemianopia differed significantly from controls in various fixation and saccade parameters as indicated by increased number and duration of fixations, number and duration of saccades and scan path length with shorter mean saccadic amplitude. Under simulated hemianopia, participants were consistently biased towards the sighted visual field while gaze behaviour in true hemianopia was biased in the direction of the blind hemifield.
Conclusions
There is considerable evidence on the altered eye movements that occur in true hemianopia and in healthy adults with simulated hemianopia. Successful performance in naturalistic tasks of visual exploration appears to be related to compensatory mechanisms of visual exploratory behaviour, namely, an increase in the amplitude and peak velocity of saccades, widening horizontally the distribution of eye movements, and a shift of the overall distribution of saccades into the blind field. This review highlights the lack of studies reporting head and other body movement parameters in hemianopia. Further studies with robust methodology and large sample sizes involving participants with post-stroke visual field loss are needed.
Systematic review registration
PROSPERO CRD42020194403
Similar content being viewed by others
Background
Stroke affects approximately 100,000 persons per annum in the UK [1]. As stroke is more common with older age, and the UK population is one of many countries with an ageing population, stroke is likely to be an ongoing health concern. A common problem post-stoke is visual impairment, with an estimated 65% of stroke survivors having visual impairment in the immediate aftermath of stroke [2]. The large prevalence of sight loss post-stroke imposes significant costs on public funds, private expenditure and health: an estimated £28.1 billion in 2013 in the UK [3].
Stroke-associated visual impairment can include impairment to central vision and peripheral vision (visual field), eye movement disorders, reading difficulties and visual perception disorders including visual neglect [2, 4]. Visual field defect (VFD) encompasses hemianopia, quadrantanopia, temporal crescent defect and scotoma, among others, with the most common defect being homonymous hemianopia (HH). HH involves vision loss on the same side of the visual field in both eyes and is associated with a worse prognosis for successful rehabilitation [5, 6], especially when combined with visual neglect [7]. Approximately 30% of stroke survivors have this visual field loss acutely while approximately 8–10% of stroke survivors have a permanent HH [8].
HH seriously impacts functional ability and quality of life following stroke [9]. For example, it causes an increased risk of falling, impaired ability to read, poor mood and higher levels of institutionalisation [10, 11]. Moreover, HH impacts participation in post-stroke rehabilitation and may result in poor long-term recovery, leading to loss of independence, social isolation and depression [12].
Individuals with VFD cannot process images in the same way as those with a full visual field [13]. Those with normal visual function use their peripheral field of vision as a guidance system and cue for generating eye movements to look towards objects of interest. To attain the highest quality of visual information, one must position the area of interest on the fovea [14,15,16]. Gaze scanning can be accomplished through eye, head and body movements, with the choice of movement depending on the demands of the activity and the environment. For example, when switching visual attention from one target to a closely adjacent target, the task usually requires only small eye movements. Conversely, when crossing the street, a task that involves acquiring information over a large area, individuals prefer to make a head movement to increase the scan area in a short period. This occurs because, in an outdoor environment, about 85% of naturally occurring human saccades have magnitudes of ≤ 15° [17]. Head movements also serve to recentre the head on the torso and serve as a reference frame for body movements, important for walking and maintaining consistent heading direction [18].
Individuals with normal or corrected-to-normal vision always move their eyes during the acquisition of visual information, even when attempting to maintain a steady gaze on a single point. Rapid gaze shifts known as saccades typically occur 2–3 times per second, bringing a new portion of the visual scene on to the fovea. In between these movements, the eyes essentially stop scanning about the scene, holding the central foveal vision in place so that the visual system can take in detailed information about what is being looked at [16, 19, 20]. Impaired peripheral vision (as occurs in hemianopia), will affect the visual feedback system that guides and cues eye movements into the affected visual field [21, 22]. Those with HH receive no visual cues from peripheral vision as to when to scan or how far to scan into the blind hemifield (BHF) and spend most of their time looking towards their BHF when viewing simple patterns in order to bring more of the visual scene into their seeing hemifield (SHF). They demonstrate numerous refixations (additional eye movements) and inaccurate saccades which result in impaired scanning, longer search times (trying to find objects) and the failure to detect relevant objects [23,24,25,26].
Training programmes exist to improve these eye movements—visual scanning or visual search strategies [27]. Research on the impact of HH on eye movements plus research on improving eye movements through visual scanning training is largely based on computerised tasks with participants seated in head-fixed positions [28]. While more recent research includes studies with free head movement, there has been limited investigation regarding the impact of hemianopia on eye movements when individuals are walking around with free head and trunk movements [29].
Why this review is important
In real-life settings, some stroke survivors with HH spontaneously adapt to their visual field loss through effective compensatory eye and head movements and, within weeks of their stroke onset, can read easily, negotiate familiar and unfamiliar environments and appear to have little detriment to their everyday activities. Others appear to be more affected by visual field loss and continue using ineffective scanning strategies. This causes difficulties when carrying out daily activities such as reading, driving and locating objects around them [30]. It is not fully understood why some individuals adapt at a different rate to others using compensatory eye and head movements. Those who adapt well have a noticeable improvement in activities of daily living over those who do not. An important consideration for understanding the scanning performance is the extent to which individuals with these visual field defects might adopt patterns of eye and head movements that assist them to compensate to their visual impairment. The review will outline these compensatory strategies and provide indications as to whether some of these scanning patterns could be trained in rehabilitation programmes with the aim of improving visual search performance in this population.
To our knowledge, no systematic review has attempted to collate the available evidence on biomechanical alterations in stroke survivors with HH.
Aim
To provide a comprehensive systematic overview of the biomechanical alterations to post-stroke VFD to identify which movement parameters are the most relevant, commonly used or have specific clinical relevance. This will guide current practice and aid in the design of future research into this subject area. In this review, the term biomechanics refers to changes in eye, head or body movements in response to the visual field loss.
Objectives
The primary objective was to determine how eye, head and body movements are affected by the visual field loss that occurs following a stroke compared to healthy controls and participants with simulated VFD.
The secondary objectives were to determine how biomechanical factors are affected by extent and side of visual field loss.
Methods
This systematic review aimed to bring together the biomechanical evidence relating to eye, head and body movements in stroke-related VFD. The review was observed and reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Supplementary Figure 1) [31]. A detailed protocol was developed prior to the review and registered with PROSPERO (international prospective register of systematic reviews; CRD42020194403) [32].
Inclusion criteria for considering studies for this review
Types of studies
The following types of studies were included in the review: randomised controlled trials, controlled trials, prospective cohort studies, before-after studies, case-controlled studies and case series. Articles that discussed other visual impairments alongside VFD were included if visual field loss was discussed separately. Interventional studies for visual field loss were included provided that they formally recorded eye, head or body movements. Studies that only recorded behavioural outcomes (for example, reading) to assess the effectiveness of an intervention were excluded. Case reports and letters were excluded due to the risk of bias associated with these types of reports. All languages were included and translations were obtained when necessary.
Participants
We included studies of adult participants of all ages and children diagnosed with post-stroke VFD. Studies which included mixed populations were included if over 50% of the participants had a diagnosis of stroke and data were available for this subgroup. Studies which included participants with HH and other VFD were included if over 50% of the participants had hemianopia and data were available for this subgroup. Studies that included participants with both HH and neglect were also included if HH data could be extracted separately to that of HH with neglect or neglect only.
Target condition
The target condition was visual field loss of any severity which occurred acutely following a stroke event, and simulated HH.
Study tasks
Studies that investigated the compensatory eye, head or body movements to post-stroke VFD or simulated HH during visual exploration tasks were included. Studies that investigated practice-related changes in movement strategy were also included provided that eye, head or body movements were quantitatively measured using kinematic equipment (e.g. eye trackers).
Comparator(s)/control
Eye, head or body movements in individuals with VFD were compared to other groups (i.e. individuals with simulated HH and normally sighted participants).
Outcome measures
Eye, head or body movement parameters expressed in quantitative data during different task types: Percentage change or difference in measurement from baseline to primary endpoint or before/after intervention for; number of fixations, refixations, saccades, saccade amplitude, proportion of saccades made into the BHF and SHF, head turn, shoulder movement, etc.
Information sources and search strategy
We used systematic search strategies to search key electronic databases. The following electronic databases were searched: PsycINFO (1887 to 2020), Scopus (1823 to 2020), MEDLINE (1948 to 2020), CINAHL (1937 to 2020). No language restrictions were applied. We also hand-searched the reference lists of all included studies for relevant papers. Search terms are detailed in Table 1.
Data management
References from all searches were uploaded into EndNote (X9, Clarivate Analytics, USA) bibliographic software. Duplicates were removed using the Endnote deduplication tool.
Selection process
The titles and abstracts identified from the search were independently screened by one reviewer (AE) and at least 10% were double checked by a second author (AC) using the pre-stated inclusion criteria. A secondary review of the full papers was then undertaken independently by two reviewers (AE reviewed all studies along with either KD, RL, NT, or FR) and a Cohen’s Coefficient of Agreement [33] (Kappa score) calculated to quantify inter-reviewer agreement. Any disagreements over inclusion of studies were discussed and resolved by discussion between all reviewers.
Data extraction process
A pre-designed data extraction form was used to gather information on study design, aims, sample size, numbers recruited and analysed, intervention and outcome measures related to eye/head/body movement. Data was extracted and documented by one reviewer (AE) and verified by a second (either KD, RL, LH, NT or FR). Data was reported by numbers, means, standard deviation (SD) and standard error of mean (SEM). Of note, measurement data are provided in the tables where available and p values when no hard data is reported in the primary studies.
Quality assessment
Two reviewers (AE reviewed all studies along with either KD, RL, NT or FR) independently reviewed the quality of the studies included in this review using the following two checklists: (1) For the quality assessment of randomised control trials, the CONSORT (Consolidated Standards of Reporting Trials) statement was used. The CONSORT statement covers 25 items within the following domains; title/abstract, introduction, methods, results, discussion and other information [34].
(2) The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement was used to assess the quality of cohort, before-after and case-control studies. The STROBE statement covers 22 items from introduction, methods, results and discussion [35]. A study was considered of good quality if it scored 75% or greater on the relevant checklist.
Results
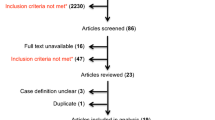
Figure 1 illustrates the flowchart for this review. Thirty-six articles (1123 participants) published in English were included and the overall Kappa score for inter-rater agreement was 0.863 indicating substantial agreement. Due to the heterogeneity across the included studies with respect to reporting of outcomes as well as recruitment and selection of participants, a meta-analysis of studies could not be undertaken. A narrative summary of the data is presented in relation to included studies to highlight how visual field loss impacted on eye, head or body movements across the included studies. Results are split into simulated HH and true VFD and then discussed by type of movement analysis (eye/head/body movement). Data analysis was conducted by one reviewer (AE) and double checked by another two reviewers (FR, LH).
Quality assessment
A total of 36 articles were included in this review paper and the risk of bias was assessed for each (Supplementary Tables 1, 2). Overall, no article scored 100% for quality assessment in this section. Twenty-nine of the 36 articles scored between 76 and 90% of the checklist items assessed and were deemed to have good quality. Seven studies scored between 59 and 71% on the relevant quality checklists. All articles were included in this review.
Simulated hemianopia
Seven papers recruited healthy adult participants and simulated HH (n = 269 summed sample size). None of the included studies recruited children in their sample. These included the following study types; one randomised cross-over trial (n = 24), three cohort studies (n = 147), one case-control study (n = 34) and two before-and-after studies (n = 58). HH was simulated in these studies using a gaze-contingent visual display paradigm. When simulating HH, a window, with the same properties as the background, continuously and completely blanked part of the screen with reference to the gaze position. The blanked area could either be left or right of fixation in order to simulate left- or right-sided HH respectively. Table 2 summarises the key data extracted from the simulated HH studies.
None of these simulation studies assessed either head or body movement; therefore, this section will focus solely on eye movements. Eye movements were recorded in three studies using a pupil and dual Purkinje image video eye-tracker [39,40,41]. In two studies, eye movements were recorded using pupil-corneal-reflection method [37, 38]. Two studies recorded eye movements with infrared reflection oculography [36, 42].
Eye movement parameters
Number, duration of fixations and percentage of fixation repetitions
The most commonly reported eye movement measurements included number of fixations, duration of fixations and percentage of fixation repetitions. These eye movement parameters were measured in six of the seven studies (n = 203). During visual tasks with simulated HH (visual exploration, line bisection), participants showed significantly more and longer fixations and refixations when compared with their normal performance [37, 39,40,41]. However, eye movement recordings during tasks that require complex cognitive processing (e.g. visual mental imagery of complex pictures) revealed that participants made significantly fewer fixations compared to normal viewing of stimuli [36]. Participants with simulated HH also changed their oculomotor search strategy in response to increasing search difficulty as demonstrated by a significantly decreased fixation duration for detailed tasks (to memorise the details of each picture) and recollection tasks (to look for relationships between items) compared to easy tasks (to look at the whole picture) [36].
Efficient spontaneous oculomotor changes to simulated HH occurred after 15 min of visual exploration practice [40]. Improvements in visual exploration mirrored changes of the respective oculomotor measures as participants showed a significant decrease in the number and duration of fixations. Zangemeister et al. [42] also demonstrated that training of parafoveal eccentric viewing for 20 min helped the oculomotor change of participants with simulated HH significantly. Fixation durations decreased significantly immediately after training and 14 days later compared to pre-training.
Saccade amplitude and scan path length
Saccadic amplitude (i.e. the angular distance travelled by the eye between two fixation points) and scan path (i.e. the sum of all saccadic amplitudes) eye movement parameters were measured in six studies (n = 235). Scan paths were significantly longer across various visual search tasks with simulated HH [39,40,41]. The amplitude of saccades, however, varied significantly and was task dependent. Eye movement recording during viewing and subsequent visual imagery of complex pictures revealed that saccade amplitudes were significantly lower in visual imagery scan paths compared to the normal viewing condition [36]. During visual exploration, however, participants did not show the expected decrease in return sweep and exploration saccadic amplitude [39]. In a study of visual search for an emotional face among neutral faces, no significant differences in saccadic amplitude were found between the saccades made into the SHF and BHF in the unmodified condition. However, in the masked conditions (blank/grey background), dot (black and white dots representing the spatial location of the faces) and filtered (the low spatial frequency version of the face), saccades were shorter when made towards the SHF compared with the BHF [37].
Practice-related changes in saccadic amplitude and scan path length under simulated HH were found. After visual exploration practice, participants showed a significant decrease in scan path length [40]. Participants undergoing training of parafoveal eccentric fixation also showed a significant increase in saccadic amplitude immediately after training and 14 days later compared to pre-training [42].
Proportion of saccades into the blind hemifield
As a measure of directional bias, three studies (n = 167) examined the mean proportion of all saccades executed towards the BHF. In various visual tasks (object naming, detection and search) [37, 38], participants with simulated HH were consistently biased towards the visible part of their visual field. Participants preferred to saccade first, and more often, into the SHF as opposed to the BHF, irrespective of task difficulty. The bias to preferentially search the sighted field persisted even in easy search. Liman et al recorded eye movements during viewing and subsequent visual imagery of complex pictures [36]; under masked conditions, the proportion of all saccades made into the BHF was significantly smaller in the blank, dot and filtered conditions compared with the unmodified condition . With practice, most participants made saccades that went further into the BHF and earlier in the search process, specifically under conditions where little information about the target location would be gained by inspecting the SHF. Contrary to the search required for a full-view environment, participants made significantly larger saccades towards to their BHF during line bisection than into their SHF when compared to normal viewing conditions [41]. In participants with left HH (LHH), the amplitude of leftward saccades was significantly higher than that of rightward saccades. In participants with right HH (RHH), the amplitude of rightward saccades was significantly higher than that of leftward saccades.
Global/local ratio
Global/local ratio (g/l) is a measure to distinguish between global versus local viewing strategies (saccades smaller than 1.0° were taken as local and those larger than 1.1° were taken as global). The g/l ratio was measured in one study (n = 20) that examined the impact of simulated HH on scan path eye movements during visual mental imagery [36]. It was reported to be significantly lower with simulated HH when compared to eye movements during regular viewing of stimuli.
Significant effects of tasks and picture content on fixation g/l ratio were also found. Participants demonstrated a significantly increased median g/l ratio for abstract and realistic picture content. The highest g/l ratio was detected for abstract picture content and for a detailed viewing task. The task effect in the “detailed” condition elicited a more global scanning of the whole image. This effect was stronger in abstract than in realistic or search pictures reflecting in part the visual content of the inspected image.
Ratio of overshoot one-step/undershoot stair-step saccades to the blind hemifield
The ratio of overshoot one-step/undershoot stair-step saccades to the BHF was recorded in one study (n = 16) as a measure of efficient adaptation [42]. The ratio was significantly higher 14 days after training of parafoveal eccentric viewing as compared to pre-training. This is an indicator of efficacy in improving gaze by use of saccades of larger amplitudes towards the BHF.
Head movements
None of the simulated hemianopia studies measured head movements.
Shoulder movements
None of the simulated hemianopia studies measured shoulder movements.
Summary of key findings from simulated hemianopia studies
Simulated HH induced an inefficient and unsystematic oculomotor scanpath for exploring and processing visual information during visual exploration tasks, as indicated by the increase in number and duration of fixations as well as in scan path length. Participants preferred to saccade first, and more often, into the SHF as opposed to the BHF to a similar extent across various difficulty levels. During tasks that required complex cognitive processing and under masked conditions, saccade amplitudes were significantly lower compared to normal viewing and the proportion of all saccades made into the BHF was significantly smaller.
After visual exploration training, however, participants developed a more efficient oculomotor response to the visual-sensory loss which improved their visual exploration performance. Participants showed a significant decrease in the number and duration of fixations and scan path length. Over time, participants scanned further into their BHF than into their SHF and made significantly larger saccades towards the side of space corresponding to their BHF.
True hemianopia
Twenty papers recruited adult participants with VFD (n = 397 summed sample size) and healthy controls (n = 284). Nine further studies recruited only participants with VFD (n = 173). None of the included studies recruited children in their sample. Only two studies included participants with both HH and visual neglect but reported results separately for these groups. Together these papers included the following study types; two randomised control trials (n = 50), five before-after studies (n = 153), 18 case-control studies (n = 632), three cohort studies (n = 45) and one case series (n = 6). Eye movements were recorded in 27 studies (n = 846) and eight studies (n = 221) recorded head or body movements. Only two studies (n = 100) recorded eye, head and shoulder movements. Table 3 summarises the key data extracted from the real VFD studies.
Eye movements were recorded using an infrared eye tracking in 16 studies [26, 43, 44, 46, 47, 49,50,51,52, 54, 55, 59,60,61,62, 64]. In two studies, subjects’ eye movements were recorded using a scleral search coil system [56, 57]. Other methods used to record eye movements were as follows: infrared reflection oculography [48, 67], electro-oculography [53], pupil-corneal-reflection method [23, 68], digital, head-worn video camera [63, 66] and the P scan system [25, 58]. In all the included studies, gaze parameters were measured while head movements were unrestricted.
Head movements were recorded in eight studies by means of a head-mounted binocular infrared video pupil tracker [44, 51, 55], a high-resolution accelerometer [63], digital head-worn video camera [65, 66], a low-torque potentiometer [67] or a remote infrared system [45]. Shoulder movements were recorded in two studies by means of a video camera [51, 66].
Eye movement parameters
Number, duration of fixations and percentage of fixation repetitions
The most commonly reported eye movement parameters included number of fixations, duration of fixations and percentage of fixation repetitions, measured in 17 studies (n = 524). Participants in these studies were tested in various visual tasks, from those requiring mostly simple visual scanning (e.g. dot counting) to more cognitively demanding visual search tasks (e.g. comparative visual search task).
In comparison to normal controls, the scanning pattern of participants with HH was characterised by significantly higher numbers of fixations, a higher proportion of fixations towards the BHF and a higher proportion of refixations [23, 25, 57].
Different studies showed different findings regarding mean fixation durations. Eye movement recording during the inspection of a stimulus pattern showed that mean fixation durations were longer in the impaired groups compared to controls [68]. However, compensatory behaviour of participants with HH while they assembled models and viewing naturalistic pictures was characterised by slightly shorter fixation durations than controls. Fixation durations did not differ significantly between the SHF and BHF while participants assembled wooden models [26]. Participants made significantly more fixations in the area corresponding to their respective BHF compared to controls while viewing filtered images [25].
In the naturalistic setting of a driving simulation, no significant difference was found regarding the duration of fixations between the healthy controls and participants with HH [44].
When participants with HH were divided into two subgroups by the median of their task performance into “high performance” and a “low performance” groups, participants in the high performance group showed no statistically significant differences from the normal controls regarding fixation number, duration of fixations and proportion of refixations [44, 50, 59].
Practice-related improvement in scanning efficiency was reported in five studies (n = 169) [23, 49, 58, 60, 62]. The number of fixations and refixations significantly reduced after treatment by at least 20% as compared to pre-treatment values. After explorative saccade training [62], the number of fixations during natural scene exploration increased significantly towards the BHF (follow-up/predifference, 238%). However, the proportion of fixations made into the BHF did not change as a result of visual search training [58].
Number and the duration of saccades
The number and duration of saccades were measured in five studies (n = 111). In a driving simulation experiment and visual search tasks, no statistically significant differences were found between participants with HH and healthy controls concerning the number and duration of saccades [26, 44, 57]. Passamonti et al. studied oculomotor responses during a visual search task before and after visual (control) or audio-visual (experimental) training [60]. Before training, duration of saccades was significantly longer in participants with HH compared to controls. After training, saccadic duration markedly reduced by about 40% in participants with HH. Keller et al. also reported that audio-visual stimulation training (AVT) significantly increased the number of saccades in participants with HH compared to visual stimulation training (VT) [53]. Participants in the AVT group nearly doubled the number of saccades into their BHF while participants in the VT group increased the number of saccades by only about 11%.
Amplitude, peak velocity of saccades and scan path length
The amplitude of saccades was measured in 14 studies (n = 497) [23, 25, 26, 44, 47, 48, 50, 53, 54, 57, 59, 60, 64, 68] while the peak velocity of saccades was measured in two studies (n = 49) [26, 44]. Scan path length (the sum of all saccadic amplitudes) was measured in four studies (n = 124) [25, 50, 59, 60].
Compared to normal controls, visual exploration by participants with HH was mostly characterised by significantly smaller mean saccadic amplitude. In contrast, analysing the gaze patterns of participants with HH and visually intact controls while they assembled wooden models [26] and during memory recall in an imagery task, a non-imagery task (verbal fluency) and a visually guided task [47], participants performed a roughly similar number of saccades of similar amplitude compared to normal controls.
Recording eye movements in a driving simulation experiment [44], participants with HH had significantly lower peak velocity of saccades compared to normal controls.
Participants with HH whose performance on the relative search task was rated as high showed no statistically significant differences from the normal controls regarding the amplitude, peak velocity of saccades and scan path length. The low performance group showed significantly smaller amplitude, lower velocity and longer scanpaths than either the control or high performance groups [44, 50, 59].
Saccadic training which took different forms across different studies [23, 53, 54, 60] demonstrated increased saccadic amplitude and reduced length of scan path compared to pre-training. Scan path length significantly reduced after audio-visual training by about 50% compared to pre-training.
Percentage of saccades made towards the blind hemifield
Four studies (n = 92) measured the proportion of saccades made towards the BHF [25, 56,57,58]. Recording eye movements of participants with acute and chronic HH while they performed an exploratory visual search task, Machner et al. found that the number of saccades did not differ significantly between the blind and the intact hemifield [56, 57]. However, Pambakian et al. examined the scanpaths of participants with HH while viewing naturalistic pictures in their original and also spatially filtered forms and participants made significantly higher proportion of saccades towards their BHF compared to the SHF for both filtered and unfiltered images [25]. This variation supports the view that the contralesional bias towards the BHF reflects a compensatory eye movement strategy which may be due to an attentional shift developed over time.
After visual search training, participants with HH made a higher proportion of saccades in the direction of the target, i.e. participants made more contralesional (57%) than ipsilesional saccades (23%) when the target was in hemianopic hemispace. After training, participants were able to locate targets within a larger area of their blindfield within a single saccade [58].
Gaze eccentricity on the blind and seeing sides
Two studies (n = 72) measured gaze eccentricity (i.e. the average gaze position from the straight-ahead position) on the blind and seeing hemifields [43, 59]. In a driving simulator experiment, Alberti et al. calculated gaze and pedestrian eccentricities with respect to the car heading direction for all pedestrians that appeared at approximately 14° on the blind side. It was reported that only 40% of scans to reach the pedestrian on the BHF were made within 1 s of the pedestrian appearing compared with approximately 70% on the SHF [43]. Papageorgiou et al. also examined gaze patterns applied by participants with HH under virtual reality conditions in a dynamic collision avoidance task. Low-performing participants with HH exhibited lower mean gaze eccentricity than controls and showed a higher proportion of gaze eccentricity to the BHF at lower traffic densities [59]. High-performing participants with HH, however, had higher mean gaze eccentricity than controls and higher proportion of gaze eccentricity to the BHF at 50% density compared to 75% density [59].
Horizontal gaze activity
Two studies (n = 60) investigated the horizontal gaze exploration ability of participants with HH by assessing the horizontal standard deviation of the pupil (measured on the x-axis) [51, 52]. Kasneci et al. recorded eye movements of participants with HH while they collected products placed on two supermarket shelves. No statistically significant difference was found regarding the horizontal gaze activity between participants with HH and age-matched controls [52]. Similarly, a study that assessed the on-road driving performance of HH participants with simultaneous eye and head tracking found no statistically significant difference in the horizontal gaze activity between participants with HH and healthy controls [51].
Glance proportion and glance frequency
Two studies (n = 60) measured the proportion of gazes towards a defined area of interest during a specific time interval and glance frequency. In a supermarket search task, no significant difference was found between the participant subgroups regarding the proportion of glances beyond the 30° visual field [52]. In a study to assess on-road driving performance, participants with HH who passed the driving test glanced more towards the BHF than participants who failed [51].
Mean amplitude and velocity of eye-head gaze saccades
One study (n = 22) measured the amplitude and velocity of eye-head gaze saccades (saccades accompanied by head movements) [63]. According to their adaptive state of reading, the better adapted hemianopic participants showed significantly increased amplitudes of eye-head gaze saccades and corresponding velocities were faster with increasing target frequencies.
No significant differences were reported between saccades directed either into the SHF or into the BHF. Less adapted participants, however, showed significantly lower amplitude and velocity of eye-head saccades directed into the BHF than saccades that were aimed into the SHF.
Other less reported eye movement parameters
Mean deviation of saccade from horizontal and amplitude of first saccade
One study recorded the mean amplitude of all measured saccades and amplitude of first saccade (n = 16). This study examined the scan paths of participants with HH while viewing naturalistic pictures in their original and also spatially filtered forms [25]. The mean angle of deviation for participants with HH was significantly lower compared to the control group when viewing filtered and unfiltered images. The mean amplitude of the first saccade was significantly larger for participants with HH than controls for both filtered and unfiltered images.
Spatial consistency between gaze positions and spatial distribution of the gaze throughout the task
One study (n = 19) recorded the gaze position and its spatial distribution during memory recall of French towns in an imagery task, a non-imagery task (verbal fluency) and a visually guided task in participants with left or right HH [47]. Gaze was constantly shifted across all tasks with respect to their body midline, contralesionally for all participants with HH without neglect and ipsilesionally for the two participants with HH with neglect. For each participant, horizontal shift was statistically significant when compared to healthy controls who systematically positioned their gaze at their body midline when they started their mental imagery.
Mean number of gaze shifts
One study (n = 60) recorded the mean number of gaze shifts (i.e. gaze transitions between left and right hemifield) in participants with HH under virtual reality conditions in a dynamic collision avoidance task [59]. The subgroup of participants who adapted successfully to their VFD showed increased number of gaze shifts at 50% and 70% traffic density conditions compared to healthy controls. Participants who failed to adapt exhibited lower number of gaze shifts at 50% and 70% traffic density conditions than controls.
Progressive and regressive fixations
One study (n = 8) recorded the proportion of progressive and regressive fixations in participants with HH and healthy controls while they assembled wooden models [26]. Progressive fixations were defined as fixations upon a location in the 10 s prior to a pickup from that location, while regressive fixations were defined as fixations to a location within 10 s after a pickup from that location, with at least one fixation to a different location since the pickup. Participants with HH exhibited a significantly higher proportion of progressive fixations than controls. Regressive fixations were very rare [26].
Number of progressive and regressive saccades
One study (n = 24) recorded the number of progressive (left-to-right eye movement between fixations) and regressive (right-to-left eye movements) saccades. Passamonti et al. recorded oculomotor responses of participants with HH during a visual search task before and after AVT. Before training, HH participants had significantly higher number of progressive and regressive saccades compared to controls. After AVT, participants with RHH made fewer number of progressive saccades and fewer mean number of regressions compared to pre-training. Participants with LHH made slightly fewer progressive and regressive saccades compared to pre-training [60].
Landing accuracy of saccades and fixation stability after landing
Landing accuracy of saccades and fixation stability were measured in one study (n = 47) aimed to detect potential spontaneous adaptive mechanisms in participants with HH during saccadic and fixation tasks [61]. Landing accuracy was decreased in participants with HH, indicated by significantly more hypometric (single-step saccades in which the eye undershoots the target) and hypermetric saccades (saccades that overshoot the target) to the BHF compared with the SHF. Fixation after landing in participants with HH was less stable on the BHF compared with the SHF.
Head movements
Head movements were recorded in eight studies (n = 221) [44, 45, 51, 55, 63, 65,66,67]. Examining the head movement parameters during various visual tasks (e.g. visual search during driving and tracking flying basketballs), no statistically significant differences regarding the number, the amplitude, the duration and the peak velocity of head movements were reported between participants with HH and healthy controls [44, 55]. Bowers et al. [45] examined the effects of HH on head scanning behaviours using a driving simulator. Drivers with HH were found to make a greater proportion of head scans overall to the blind side when compared to normal control drivers. However, amplitudes of scans were smaller for HH than for control drivers. For participants with HH, BHF scans were not larger than SHF scans. Similarly, head scanning behaviour of participants with HH during on-road driving was characterised by increased head movements in the direction of the BHF, particularly for those with a left-sided defect [51]. On average, 59% of head movements were made into the BHF compared with the SHF, with large (+ 30%) and small (+ 60%) head movements made into the BHF significantly greater than into the SHF [66].
In a study that examined eye-head coordination in HH, participants seemingly simplified search and fixation strategies by minimising or eliminating head movements and relying solely on eye movements instead with significantly greater delays in head movements towards the BHF [67]. Similarly, Schoepf et al. [63] examined the influence of target predictability on the distribution of coordinated eye-head gaze saccades in participants with HH. The head contribution to the gaze shift was often reduced with significantly longer delays in head movements towards the BHF.
For rehabilitation purposes, Turton et al. [65] assessed the acceptability of search training for people with visual field loss after stroke and reported increased searching on the blind side, from head movement, following the intervention.
The variation in the degree of head movement employed as a compensatory strategy by participants with HH across studies could be attributed to how well they adapt to their visual field loss; better adapted patients seem to use more head movements towards their BHF compared to patients who fail to adapt.
Shoulder movements
Shoulder movements were recorded in two studies (n = 80) [51, 66]. Scanning behaviour of participants with HH during on-road driving [66] was characterised by a statistically significant increase in shoulder movement compared to controls. Participants with HH who failed the driving assessment displayed significantly less shoulder movements than control subjects and participants with HH who passed the driving test [51].
Summary of key findings regarding movement parameters in true hemianopia
The gaze behaviour of participants with HH differs from that of visually intact observers when performing simple laboratory as well as naturalistic tasks. Participants with HH showed significantly increased number of fixations, proportion of fixations to the BHF, scan path length and repetition of fixations. Visual exploration by participants with HH was mostly characterised by a significantly increased number and duration of saccades and scan path length with shorter mean saccadic amplitude and lower peak velocity of saccades. In contrast to the scanning behaviour of participants under simulated HH, participants with HH made significantly more hypometric and hypermetric saccades to the BHF than saccades made into the SHF. Participants with HH also displayed significantly lower amplitude and velocity of eye-head saccades directed into the BHF than saccades that were aimed into the SHF.
The absence of a compensatory gaze bias towards the BHF or of compensatory saccadic search strategies by participants with post-stroke HH was evident during simple, naturalistic tasks (e.g. assembling wooden models). This may reflect the static nature of the task environment which eliminated the need for participants to perform new visual searches and lent strength to the hypothesis that participants with HH might indeed place greater reliance on spatial memory when performing simple, real-world tasks. Similarly, there was no direction-specific bias in participants with acute HH during an exploratory visual search task, i.e. saccades directed towards the BHF did not differ concerning frequency and amplitude from those directed towards the SHF. This suggests that probably due to their acute stage of stroke a compensation has not taken place yet. No significant differences between participants with HH and healthy controls regarding the number, the amplitude, the duration and the peak velocity of head movements were found during various visual tasks. Participants’ head scanning behaviour during on-road driving, however, was characterised by increased head and shoulder movements in the direction of the BHF compared to healthy controls.
Several groups have trained participants with HH in visual search tasks and have concluded that they adopt compensatory eye and head movement strategies with training. Training confers several advantages on the observer. By making more saccades towards their blind side, they bring ever increasing areas into their seeing side, which they examine with numerous rapid fixations.
Discussion
The aim of this systematic review was to gather and summarise the available evidence on biomechanical alterations to post-stroke VFD. In simulated HH, healthy adults temporarily deprived of information from half of their visual field tended to preferentially move their eyes towards their sighted field of vision, especially during tasks that required complex cognitive processing [37, 38]. Nonetheless, the bias to preferentially search the SHF persisted, even in easy search, and participants continued to direct eye movements into the SHF even when these eye movements gained them very little new information and impeded search performance. Training or repeated exposure to a simulated VFD led to the development of a more efficient visual search strategy. Participants’ fixations moved progressively deeper into the BHF, and this shift was seen in the easy search condition, and to a lesser extent, in the hard condition [38]. Saccades into the BHF were strongly associated with improved search performance.
The scanning behaviour of participants with true HH was mostly characterised by a significantly increased number and duration of fixations, duration of saccades and scan path length with shorter mean saccadic amplitude and lower peak velocity of saccades [25, 26, 56, 57, 59]. This abnormal scanning behaviour was associated with impaired visual exploration, longer visual search times, target omissions and longer, unsystematic scanpaths. Driving was also considered to be problematic for participants with HH. The majority of on-road studies and simulator experiments highlighted poor steering control, incorrect lane position and difficulty in gap judgment [51, 59, 66]. The gaze pattern of drivers with HH was also characterised by increased numbers of fixations, longer search times, longer saccadic amplitudes and more head movements, particularly towards moving objects of interest on their BHF.
In contrast to the shifting bias observed in simulated HH, participants with post-stroke HH tended to spend more time overall looking to the side associated with the deficit during free viewing tasks. These compensatory strategies of biasing gaze in the direction of the BHF were found to be most evident when participants with HH were in dynamic and unpredictable environments [66], where they could not rely on spatial memory to locate salient objects. The differences found between the simulated HH and the true HH may be attributed to several reasons. First, it may be that because of the limited time duration of simulated HH participants did not develop a consistent search strategy. Second, participants with simulated HH are normally aware that their deficit will end with the experiment, whereas participants with HH would be more motivated and have more time to adapt to a long-term deficit. Third, there may be a particular effect of brain damage over and above the visual deficit that is responsible for the specific eye movement pattern. Tant et al. [64] stated that the visual deficit is the main but not the only factor that contributes to the abnormal oculomotor behaviour seen in post-stroke HH. Fourth, participants with HH move more to their BHF because they possess some residual visual abilities in their affected visual field that guides their search more effectively while healthy participants with simulated HH do not have any residual visual information in their blind side. This hemifield bias could also be related to the fact that eye movements in studies involving participants with HH were largely recorded in the chronic stage [57]. Machner et al. [56] recorded eye movements while participants with HH in an acute stage searched for targets among distractors. Both participants with true and simulated HH displayed no direction-specific bias, i.e. saccades directed towards the BHF did not differ in frequency or amplitude from those directed towards the SHF suggesting that compensation had not yet occurred. Some participants discussed in the true hemianopia studies had visual field defects other than hemianopia, for example quadrantanopia and bitemporal visual field loss. However, in the simulated HH group, only the complete right or left hemianopia condition was simulated, and this might account for some differences found in the scanning behaviour across studies. Finally, some individuals with HH are not aware of their visual field loss (especially when combined with spatial neglect) and cannot use voluntary, cognitive control to guide their blind side scanning or have multiple visual impairments that can exacerbate the impact of their hemianopia.
Several limitations to these conclusions should be acknowledged. One critical consideration is that most of the included studies in this review recruited a small number of participants with post-stroke VFD in their study populations (n < 30 in 72% of the included studies). Some studies in this review had a mixture of HH aetiologies and did not focus on a specific stroke survivor population. Few studies explored the importance of head movements in compensatory visual search behaviour of participants with HH. Furthermore, the evidence from these studies is inconsistent and it is not clear why some studies demonstrated that successful task performance is related to an increased number of head movements into the BHF while other studies found no difference. The literature on biomechanical changes to post-stroke VFD varies considerably. Significant heterogeneity exists among the included studies with respect to the selection of biomechanical outcome measures. We recommend that researchers should focus on reporting a small, consistent set of key measures of gaze. Research is warranted for the development of a core outcome set of measurements and it is apparent that some gaze parameters (e.g. proportion of saccades made into the BHF, amplitude of saccades and velocity of movements) appear to have more clinical significance than others (e.g. global/local ratio).
The extent to which conclusions drawn from any experiment using simulated HH can be applied to individuals with visual field deficits may be limited. The method employed to simulate HH works by completely removing all the visual information from one half of the display screen in a gaze-contingent manner. This method of “hard edge” hemianopia is only an approximation of what happens in post-stroke VFD and is not entirely consistent with the effects of damage to postgeniculate visual pathways. The loss of vision in the contralateral visual field is sometimes accompanied by residual visual capacity, known as blind sight [69]. Furthermore, the majority of included studies recorded eye and head movements in stroke survivors with HH in the chronic stage and little is known about search behaviour during exploratory visual search tasks within acute phase, in the first few weeks following a stroke.
Findings from this comprehensive review will underpin future clinical research. The review highlights that numerous studies have explored the eye and head movements and scanning behaviour of participants with HH in well-controlled laboratory-based settings but that there has been only limited investigation into what happens under real-world conditions. Future research should aim to establish the extent to which people with HH use effective scanning strategies in real-world situations, whether they are able to adapt their scanning strategies in response to differing task demands, and whether scanning training can be generalised to everyday mobility tasks. The review outlines an extensive list of eye, head and body movement parameters that have been used in the scientific literature to examine scanning behaviour in individuals with HH.
Findings from this review have important implications for clinical practice. Some stroke survivors appear to spontaneously adopt some compensatory strategies [68], and those who do not can be trained to do so. One effective strategy to compensate for a lateralised field deficit is to fixate and saccade as far into the BHF as possible to maximise the proportion of the search area that falls into the SHF [25, 38, 58, 62]. Based on self-report, encouraging stroke survivors with HH to utilise such strategies in real life improves general functioning [58]. Real-life improvements were demonstrated by Bahnemann et al. [44], who compared participants with HH with high and low hazard detection rates in a simulated driving task on a number of eye and head movement measures. Successful performance appeared to be related to compensatory mechanisms of visual exploratory behaviour, namely, an increase in the amplitude and peak velocity of saccades, widening horizontally the distribution of eye movements and a shift of the overall distribution of saccades into the BHF. Similarly, large eye and head movements directed towards the BHF improved search for specific items in a supermarket [52] and collision avoidance [51, 59, 66].
The benefits of rehabilitation for individuals with HH are often perceived as offering only marginal gains. However, we propose that practitioners should be directed to promote active rehabilitation programmes to improve scanning/search performance for individuals with HH. These could employ simple yet effective, user-friendly techniques that can be practised in people’s own homes, causing minimal disruption to their daily lives.
Conclusion
This systematic review provides a substantial amount of evidence about the inefficient oculomotor scanning behaviour of individuals with HH and healthy participants with simulated HH. Under simulated HH, participants were consistently biased towards the visible part of their visual field whereas the gaze behaviour of participants with HH was biased in the direction of the blind side. With practice, participants with true and simulated HH developed compensatory mechanisms of visual exploratory behaviour, namely, an increase in the amplitude and peak velocity of saccades, widening horizontally the distribution of eye movements and a shift of the overall distribution of fixations and saccades into the blind side. This evidence can be used to underpin the further development, refinement and implementation of visual rehabilitation programmes for hemianopia.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Abbreviations
- AS:
-
Anti-saccades
- AV:
-
Audio-visual exploration training
- AVM:
-
Arteriovenous malformation
- BHF:
-
Blind hemifield
- CEMs:
-
Compensatory eye movements
- CFD:
-
Cumulative fixation duration
- CINAHL:
-
Cumulative Index to Nursing and Allied Health Literature
- CVS:
-
Comparative visual search
- d/s:
-
Degree per second
- d:
-
Difference
- DC:
-
Dot counting
- EL:
-
Latencies of eye movement
- EST:
-
Explorative saccade training
- FEMs:
-
Fixational eye movements
- FS:
-
Fixation stability
- FT:
-
Flicker training
- GF:
-
Glance frequency
- GPP:
-
Glance proportion in percentage
- HGA:
-
Horizontal gaze activity
- HGD:
-
Horizontal gaze distribution
- HH:
-
Homonymous hemianopia
- HHf:
-
Homonymous hemianopia who failed
- HHp:
-
Homonymous hemianopia who passed
- HL:
-
Latencies of head movement
- HP:
-
High performance
- IQR:
-
Interquartile range
- LHH:
-
Left homonymous hemianopia
- LP:
-
Low performance
- M:
-
Mean
- MEDLINE:
-
Medical Literature Analysis and Retrieval System Online
- NV:
-
Normal vision
- Pf:
-
Participants who failed
- Pp:
-
Participants who passed
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PsycINFO:
-
Psychological Information Database
- RHH:
-
Right homonymous hemianopia
- SD:
-
Standard deviation
- SEM:
-
Standard error of the mean
- SHF:
-
Sighted hemifield
- sHH:
-
Simulated homonymous hemianopia
- VF:
-
Visual field
- VFD:
-
Visual field defect
- VT:
-
Visual exploratory training
References
Stroke Association. UK. State of the nation stroke statistics-May 2020. https://www.stroke.org.uk/resources/state-nation-stroke-statistics. last accessed 12/11/2020
Rowe FJ, Hepworth LR, Howard C, Hanna KL, Cheyne CP, Currie J. High incidence and prevalence of visual problems after acute stroke: an epidemiology study with implications for service delivery. Plos One. 2019;14(3):e0213035. https://doi.org/10.1371/journal.pone.0213035.
Pezzullo L, Streatfeild J, Simkiss P, Shickle D. The economic impact of sight loss and blindness in the UK adult population. BMC Health Serv Res. 2018;18(1):63. https://doi.org/10.1186/s12913-018-2836-0.
Hepworth LR, Rowe FJ, Walker MF, Rockliffe J, Noonan C, Howard C, et al. Post-stroke visual impairment: a systematic literature review of types and recovery of visual conditions. Ophth Res An Int J. 2016;5(1):1-43. https://doi.org/10.9734/OR/2016/21767.
Han L, Law-Gibson D, Reding MJS. Key neurological impairments influence function-related group outcomes after stroke. Stroke. 2002;33(7):1920–4. https://doi.org/10.1161/01.STR.0000019792.59599.CC.
Patel AT, Duncan PW, Lai S-M, Studenski SJ. The relation between impairments and functional outcomes post stroke. Arch Phys Med Rehab. 2000;81(10):1357–63. https://doi.org/10.1053/apmr.2000.9397.
Cassidy TP, Bruce DW, Lewis S, Gray CS. The association of visual field deficits and visuo-spatial neglect in acute right-hemisphere stroke patients. Age Ageing. 1999;28(3):257–60. https://doi.org/10.1093/ageing/28.3.257.
Zhang X, Kedar S, Lynn MJ, Newman NJ, Biousse VJ. Homonymous hemianopia in stroke. J Neuro Ophth. 2006;26(3):180–3. https://doi.org/10.1097/01.wno.0000235587.41040.39.
Jones SA, Shinton RA. Improving outcome in stroke patients with visual problems. Age Ageing. 2006;35(6):560–5. https://doi.org/10.1093/ageing/afl074.
Granger CV, Divan N, Fiedler RC. Functional assessment scales. A study of persons after traumatic brain injury. Arch Phys Med Rehab. 1995;74(2):107–13.
Ramrattan RS, Wolfs RC, Panda-Jonas S, Jonas JB, Bakker D, Pols HA, Hofman A, de Jong PT. Prevalence and causes of visual field loss in the elderly and associations with impairment in daily functioning: the Rotterdam Study. Arch Ophth. 2001;119(12):1788–94. https://doi.org/10.1001/archopht.119.12.1788.
MacIntosh CJ. Review articles-Stroke re-visited: visual problems following stroke and their effect on rehabilitation. Br Orth J. 2003;60:10–4.
Hepworth LR, Rowe FJ. Visual impairment following stroke-the impact on quality of life: a systematic review. Ophth Res. An Int J. 2016;5(2):1–15. https://doi.org/10.9734/OR/2016/23272.
Williams DR. Aliasing in human foveal vision. Vision research. 1985;25(2):195–205. https://doi.org/10.1016/0042-6989(85)90113-0.
Atchison DA, Smith G. Optics of the human eye. Oxford: Butterworth-Heinemann; 2000.
Kowler E. Eye movements: the past 25 years. Vision Res. 2011;51(13):1457–83. https://doi.org/10.1016/j.visres.2010.12.014.
Bahill AT, Adler D, Stark L. Most naturally occurring human saccades have magnitudes of 15 degrees or less. Invest Ophthalmol Vis Sci. 1975;14(6):468–9.
Hollands MA, Patla AE, Vickers JN. “Look where you’re going!”: gaze behaviour associated with maintaining and changing the direction of locomotion. Exp Brain Res. 2002;143(2):221–30. https://doi.org/10.1007/s00221-001-0983-7.
Martinez-Conde S, Macknik SL, Hubel DH. The role of fixational eye movements in visual perception. Nat Rev Neurosci. 2004;5(3):229–40. https://doi.org/10.1038/nrn1348.
Rucci M, Poletti M. Control and functions of fixational eye movements. Annu Rev Vis Sci. 2015;1:499–518, 1. https://doi.org/10.1146/annurev-vision-082114-035742.
Niehorster DC, Peli E, Haun A, Li L. Influence of hemianopic visual field loss on visual motor control. Plos One. 2013;8(2):e56615. https://doi.org/10.1371/journal.pone.0056615.
Leff AP, Scott SK, Crewes H, Hodgson TL, Cowey A, Howard D, Wise RJS. Impaired reading in patients with right hemianopia. Ann Neurol. 2000;47:171–8, 2. https://doi.org/10.1002/1531-8249(200002)47:2<171::AID-ANA6>3.0.CO;2-P.
Zihl J. Visual scanning behavior in patients with homonymous hemianopia. Neuropsychol. 1995;33(3):287–303. https://doi.org/10.1016/0028-3932(94)00119-A.
Mena-Garcia L, Maldonado-Lopez MJ, Fernandez I, Coco-Martin MB, Finat-Saez J, Martinez-Jimenez JL, Pastor-Jimeno JC, Arenillas JF. Visual processing speed in hemianopia patients secondary to acquired brain injury: a new assessment methodology. J NeuroEngineering Rehabil. 2020;17(1):12. https://doi.org/10.1186/s12984-020-0650-5.
Pambakian ALM, Wooding DS, Patel N, Morland AB, Kennard C, Mannan SK. Scanning the visual world: a study of patients with homonymous hemianopia. J Neurol Neurosurg Psych. 2000;69:751–9, 6. https://doi.org/10.1136/jnnp.69.6.751.
Martin T, Riley ME, Kelly KN, Hayhoe M, Huxlin KR. Visually-guided behavior of homonymous hemianopes in a naturalistic task. Vision Res. 2007;47(28):3434–46. https://doi.org/10.1016/j.visres.2007.09.021.
Rowe FJ, Conroy EJ, Bedson E, Cwiklinski E, Drummond A, Garcia-Finana M, et al. A pilot randomized controlled trial comparing effectiveness of prism glasses, visual search training and standard care in hemianopia. Acta Neurol Scand. 2017;136(4):310–21. https://doi.org/10.1111/ane.12725.
Aimola L, Lane AR, Smith DT, Kerkhoff G, Ford GA, Schenk T. Efficacy and feasibility of home-based training for individuals with homonymous visual field defects. Neurorehab Neural Repair. 2014;28(3):207–18. https://doi.org/10.1177/1545968313503219.
Hollands KL, Pelton TA, van der Veen S, Alharbi S, Hollands MA. A novel and simple test of gait adaptability predicts gold standard measures of functional mobility in stroke survivors. Gait Posture. 2016;43:170–5. https://doi.org/10.1016/j.gaitpost.2015.09.018.
Howard C, Rowe FJ. Adaptation to poststroke visual field loss: a systematic review. Brain Behav. 2018;8(8):e01041. https://doi.org/10.1002/brb3.1041.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Plos Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. https://doi.org/10.1016/j.jclinepi.2009.06.006.
Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20(1):37–46. https://doi.org/10.1177/001316446002000104.
Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340(1):c869. https://doi.org/10.1136/bmj.c869.
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. Epidemiology. 2007;18(6):800–4. https://doi.org/10.1097/EDE.0b013e3181577654.
Liman TG, Zangemeister WH. Scanpath eye movements during visual mental imagery in a simulated hemianopia paradigm. J Eye Movement Res. 2012;5(1). https://doi.org/10.16910/jemr.5.1.2.
Nowakowska A, Clarke ADF, Sahraie A, Hunt AR. Inefficient search strategies in simulated hemianopia. J Exp Psychol. 2016;42(11):1858–72.
Nowakowska A, Clarke ADF, Sahraie A, Hunt AR. Practice-related changes in eye movement strategy in healthy adults with simulated hemianopia. Neuropsychol. 2019;128:232–40. https://doi.org/10.1016/j.neuropsychologia.2018.01.020.
Schuett S, Kentridge RW, Zihl J, Heywood CA. Are hemianopic reading and visual exploration impairments visually elicited? New insights from eye movements in simulated hemianopia. Neuropsychol. 2009;47(3):733–46. https://doi.org/10.1016/j.neuropsychologia.2008.12.004.
Schuett S, Kentridge RW, Zihl J, Heywood CA. Adaptation of eye-movements to simulated hemianopia in reading and visual exploration: transfer or specificity? Neuropsychol. 2009;47(7):1712–20. https://doi.org/10.1016/j.neuropsychologia.2009.02.010.
Schuett S, Kentridge RW, Zihl J, Heywood CA. Is the origin of the hemianopic line bisection error purely visual? Evidence from eye movements in simulated hemianopia. Vis Res. 2009;49(13):1668–80. https://doi.org/10.1016/j.visres.2009.04.004.
Zangemeister WH, Utz P. An increase in a virtual hemianopic field defect enhances the efficiency of secondary adaptive gaze strategies. Cahiers Psychol Cog. 2002;21(2-3):281–303.
Alberti CF, Goldstein RB, Peli E, Bowers AR. Driving with Hemianopia v: do individuals with hemianopia spontaneously adapt their gaze scanning to differing hazard detection demands? Trans Vis Sci Tech. 2017;6(5):11. https://doi.org/10.1167/tvst.6.5.11.
Bahnemann M, Hamel J, De Beukelaer S, Ohl S, Kehrer S, Audebert H, et al. Compensatory eye and head movements of patients with homonymous hemianopia in the naturalistic setting of a driving simulation. J Neurol. 2015;262(2):316–25. https://doi.org/10.1007/s00415-014-7554-x.
Bowers AR, Ananyev E, Mandel AJ, Goldstein RB, Peli E. Driving with hemianopia: IV. Head scanning and detection at intersections in a simulator. Invest Ophthalmol Vis Sci. 2014;55(3):1540–8. https://doi.org/10.1167/iovs.13-12748.
Cazzoli D, Hopfner S, Preisig B, Zito G, Vanbellingen T, Jäger M, Nef T, Mosimann U, Bohlhalter S, Müri RM, Nyffeler T. The influence of naturalistic, directionally non-specific motion on the spatial deployment of visual attention in right-hemispheric stroke. Neuropsychol. 2016;92:181–9. https://doi.org/10.1016/j.neuropsychologia.2016.04.017.
Fourtassi M, Rode G, Tilikete C, Pisella L. Spontaneous ocular positioning during visual imagery in patients with hemianopia and/or hemineglect. Neuropsychol. 2016;86:141–52. https://doi.org/10.1016/j.neuropsychologia.2016.04.024.
Gbadamosi J, Zangemeister WH. Visual imagery in hemianopic patients. J Cog Neurosci. 2001;13(7):855–66. https://doi.org/10.1162/089892901753165782.
Grasso PA, Làdavas E, Bertini C. Compensatory recovery after multisensory stimulation in hemianopic patients: behavioral and neurophysiological components. Front Syst Neurosci. 2016;10:45.
Hardiess G, Papageorgiou E, Schiefer U, Mallot HA. Functional compensation of visual field deficits in hemianopic patients under the influence of different task demands. Vis Res. 2010;50(12):1158–72. https://doi.org/10.1016/j.visres.2010.04.004.
Kasneci E, Sippel K, Aehling K, Heister M, Rosenstiel W, Schiefer U, Papageorgiou E. Driving with binocular visual field loss? A study on a supervised on-road parcours with simultaneous eye and head tracking. Plos One. 2014;9(2):e87470. https://doi.org/10.1371/journal.pone.0087470.
Kasneci E, Sippel K, Heister M, Aehling K, Rosenstiel W, Schiefer U, Papageorgiou E. Homonymous visual field loss and its impact on visual exploration: a supermarket study. Trans Vis Sci Tech. 2014;3(6):2. https://doi.org/10.1167/tvst.3.6.2.
Keller I, Lefin-Rank G. Improvement of visual search after audiovisual exploration training in hemianopic patients. Neurorehab Neural Rep. 2010;24(7):666–73. https://doi.org/10.1177/1545968310372774.
Levy-Bencheton D, Pelisson D, Prost M, Jacquin-Courtois S, Salemme R, Pisella L, et al. The effects of short-lasting anti-saccade training in homonymous hemianopia with and without saccadic adaptation. Front Behav Neurosci. 2015;9:332. https://doi.org/10.3389/fnbeh.2015.00332.
Iorizzo DB, Riley ME, Hayhoe M, Huxlin KR. Differential impact of partial cortical blindness on gaze strategies when sitting and walking - an immersive virtual reality study. Vis Res. 2011;51(10):1173–84. https://doi.org/10.1016/j.visres.2011.03.006.
Machner B, Sprenger A, Kömpf D, Sander T, Heide W, Kimmig H, Helmchen C. Visual search disorders beyond pure sensory failure in patients with acute homonymous visual field defects. Neuropsychol. 2009;47(13):2704–11. https://doi.org/10.1016/j.neuropsychologia.2009.05.016.
Machner B, Sprenger A, Sander T, Heide W, Kimmig H, Helmchen C, Kömpf D. Visual search disorders in acute and chronic homonymous hemianopia: lesion effects and adaptive strategies. Ann NY Acad Sci. 2009;1164:419–26, 1. https://doi.org/10.1111/j.1749-6632.2009.03769.x.
Mannan SK, Pambakian ALM, Kennard C. Compensatory strategies following visual search training in patients with homonymous hemianopia: an eye movement study. J Neurol. 2010;257(11):1812–21. https://doi.org/10.1007/s00415-010-5615-3.
Papageorgiou E, Hardiess G, Mallot HA, Schiefer U. Gaze patterns predicting successful collision avoidance in patients with homonymous visual field defects. Vis Res. 2012;65:25–37. https://doi.org/10.1016/j.visres.2012.06.004.
Passamonti C, Bertini C, Làdavas E. Audio-visual stimulation improves oculomotor patterns in patients with hemianopia. Neuropsychol. 2009;47(2):546–55. https://doi.org/10.1016/j.neuropsychologia.2008.10.008.
Reinhard JI, Damm I, Ivanov IV, Trauzettel-Klosinski S. Eye movements during saccadic and fixation tasks in patients with homonymous hemianopia. J Neuro-Ophthalmol. 2014;34(4):354–61. https://doi.org/10.1097/WNO.0000000000000146.
Roth T, Sokolov AN, Messias A, Roth P, Weller M, Trauzettel-Klosinski S. Comparing explorative saccade and flicker training in hemianopia: a randomized controlled study. Neurol. 2009;72(4):324–31. https://doi.org/10.1212/01.wnl.0000341276.65721.f2.
Schoepf D, Zangemeister WH. Target predictability influences the distribution of coordinated eye- head gaze saccades in patients with homonymous hemianopia. Neurol Res. 1996;18(5):425–39. https://doi.org/10.1080/01616412.1996.11740447.
Tant MLM, Cornelissen FW, Kooijman AC, Brouwer WH. Hemianopic visual field defects elicit hemianopic scanning. Vis Res. 2002;42(10):1339–48. https://doi.org/10.1016/S0042-6989(02)00044-5.
Turton AJ, Angilley J, Longley V, Clatworthy P, Gilchrist ID. Search training for people with visual field loss after stroke: a cohort study. Br J Occ Ther. 2018;81(5):255–65. https://doi.org/10.1177/0308022617743481.
Wood JM, McGwin G Jr, Elgin J, Vaphiades MS, Braswell RA, DeCarlo DK, et al. Hemianopic and quadrantanopic field loss, eye and head movements, and driving. Invest Ophthalmol Vis Sci. 2011;52(3):1220–5. https://doi.org/10.1167/iovs.10-6296.
Zangemeister WH, Meienberg O, Stark L, Hoyt WF. Eye-head coordination in homonymous hemianopia. J Neurol. 1982;226(4):243–54. https://doi.org/10.1007/BF00313397.
Zihl J. Oculomotor scanning performance in subjects with homonymous visual field disorders. Vis Imp Res. 1999;1(1):23–31. https://doi.org/10.1076/vimr.1.1.23.4450.
Weiskrantz L. Blindsight: a case study and implications. Oxford: Oxford University Press; 1986.
Acknowledgements
Not applicable.
Funding
The review was funded by the University of Liverpool. The funder did not influence the conduct or reporting of this review.
Author information
Authors and Affiliations
Contributions
All authors drafted this review and contributed to the development of the selection criteria, the risk of bias assessment strategy and data extraction criteria. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was not required for this study.
Consent for publication
All authors consent to publication for this manuscript.
Competing interests
The authors report no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Elfeky, A., D’Août, K., Lawson, R. et al. Biomechanical adaptation to post-stroke visual field loss: a systematic review. Syst Rev 10, 84 (2021). https://doi.org/10.1186/s13643-021-01634-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-021-01634-4