Abstract
Background
Waiting lists are an ongoing issue for publicly funded community and hospital-based health services. Parents and caregivers are instrumental supports in the health and well-being of young and school-aged children, yet little is known about the way they can be supported during waiting periods. Given mounting evidence about the value of early intervention in physical and mental health literature, and waits for some public health services extending past 12 months, it is both timely and warranted to explore interim interventions that may be applied in this period.
Methods
Intervention studies that have applied an educational programme, information, group-based support or individualised therapy to primary caregivers of children (heron referred to as parent-directed interventions), waiting for diagnostic assessment at any inpatient or outpatient health service and aged between 1 and 12 years of age, will be reviewed. These will include intervention studies of any type that have included more than 5 participants or participant groups and where a control or comparison group has been included. Abstract screening, full-text review, data extraction and risk of bias will be conducted by two reviewers. Relevant databases in health and education will be systematically searched using key words and Medical Subject Headings (MeSH) and grey literature will be explored. Databases will include PubMed, Ovid for MEDLINE and PsycINFO, EBSCO for the Cumulative Index of Nursing and Allied Health Literature (CINAHL), and the Education Resources Information Center (ERIC). Covidence© will be used to support abstract and full text screening, which will be completed by two main reviewers. Results will be tabulated, summarised and meta-analysed using a random-effects model, in any instance where concordant outcome measures have been applied. Results will be published and reported in line with PRISMA reporting guidelines.
Discussion
Given little is known about effective support for families when children are awaiting diagnostic assessment for any medical, developmental or behavioural condition, the authors will synthesise existing evidence about parent-directed interventions in this period. It is hoped that by understanding the existing evidence interventions that are proven to be effective will be adopted and intervention innovation can occur.
Systematic review registration
PROSPERO 2020 CRD42020159360
Similar content being viewed by others
Background
For decades, family-centred care (FCC) is a philosophy that has gained momentum in paediatric healthcare and research [1, 2]. Bronfenbrenner’s ecological systems theory [3] and the World Health Organization’s International Classification of Functioning [4] have long described the link between family environment and an individual’s well-being. As an approach, however, FCC does not receive universal support [5, 6]. Despite observed evidence gaps, studies suggest factors such as parent-child attachment [7, 8], caregiver mental health [9, 10], resource access or socioeconomic status [11, 12], and health literacy or knowledge of caregivers [13, 14] can positively or negatively impact child health outcomes. Such findings continue to support the need for ongoing consideration of family-centred approaches when addressing child well-being in theory or practice.
One area of health-related disparity that continues to be explored is child and family access to healthcare services [15, 16]. As a construct, access to services has a number of dimensions including approachability, availability and affordability [16], where obstacles to access can include service cost, transportation limitations, and waiting times. Even in developed countries with well-resourced health care systems, waiting times for services remain a barrier to prompt receipt of care [17,18,19,20].
Researchers, clinicians and policy makers have sought to understand and address waiting list issues across a range of healthcare settings [21,22,23,24]. Issues, such as duration and variation of waiting times, and corresponding delays in service access, or service inequity, exist across diagnostic and intervention services in both public and private sectors, and typically occur when healthcare resources are allocated irrationally. For example being based on historical tertiary hospital allocations, rather than being based on statistical data justifying appropriate resource distribution [24, 25]. Across the National Health Service in the UK, one of the oldest public health system still in existence today, waiting lists have continued to impact on public perceptions and service quality since just after its inception [24, 25]. Referrers, service users, providers, policy makers and healthcare systems worldwide continue to be challenged by waiting lists despite innovation in approaches aimed at their reduction or eradication [25,26,27].
In paediatric healthcare, increased demand for assessment of neurological, cardiac, developmental and allergy-related conditions, at a secondary or tertiary service level, has been documented in recent years [28,29,30,31]. This has placed further pressure on paediatric public health services, who subsequently utilise waiting lists in the absence of, or in addition to, other strategies to manage demand. Waiting for a child’s assessment or access to intervention has been identified as a time of elevated parental stress by some researchers, and a period of “missed opportunities” for timely intervention by others. This period has subsequently been recognized as having the potential to negatively impact on the well-being of children and parents or caregivers of the child awaiting services [32,33,34,35,36,37].
To counteract the actual and possible negative effects of paediatric waiting periods, clinicians and researchers have begun to describe strategies to support parents, other caregivers and families in the interim [32, 38,39,40]. These strategies are directed to parents of children, rather than children themselves, in order to reduce parental stress, or increase parental knowledge and competence on the path to improving child and parent well-being. There remains no systematic review to the authors’ knowledge that has explored the efficacy of parent-directed interventions whilst their child waits for diagnostic or assessment-related service access. Preliminary searches suggest that there is paucity of studies in this area, hence the importance of exploring the true status of evidence relating to family support interventions during this stage of healthcare. Moreover, initial scoping of the literature has revealed significant variation in strategies that have been applied, ranging from information sheets to group-based intervention programmes [38,39,40]. Thus, the aims of this systematic review are:
-
1.
To explore the nature of interventions implemented for or via parents or primary caregivers of children waiting for a diagnostic assessment
-
2.
To evaluate the efficacy of parent-directed interventions compared with controls, for improving family health and service access outcomes, whilst their child waits for diagnostic assessment
Methods
Methodology for this systematic review will be guided by the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA–P) [41] and the Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 [42]. Abstract screening, full-text review and data extraction will be conducted by two independent reviewers. Disagreements that cannot be resolved at each of these stages will be referred to a third reviewer. All reviewing personnel have previous research and clinical expertise across different disciplines of paediatric healthcare. The Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines (Additional file 1) have supported the development of this protocol, which will further guide all actions taken by reviewers.
Search strategy
Relevant databases for initial electronic searches were reviewed with library-based content experts and paediatric researchers with extensive systematic review publication experience. Moreover, the authors considered existing relevant literature and databases within which such studies were indexed. The following electronic databases were selected for comprehensive searching using subject or MeSH headings and keywords as relevant. Search terms and synonyms will be considered in relation to previously known papers of relevance and adapted to each database search as appropriate, within:
-
PubMed
-
MEDLINE (OvidSP)
-
PsycINFO (OvidSP)
-
CINAHL (EBSCO)
-
ERIC (EBSCO)
-
The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, latest issue)
Search terms will broadly address the concepts of referral or waiting, diagnosis or assessment, caregiving or parenting, childhood, and intervention or programme. We present the search strategy in Additional file 2. We will refine other search strategies according to other database key word trees and report them in the review.
To identify studies not otherwise indexed, we will search trial registries, repositories and reference lists of included articles, as well as grey literature that may be inclusive of brief reports, conference-related publications and masters and PhD theses/dissertations e.g. via ProQuest Dissertation & Theses (PQDT). We will contact experts in the field and authors of potentially relevant studies where required, which may lead to further study inclusions.
No publication year or language limits will be applied for this systematic review in the interests of comprehensiveness and broad applicability of results.
Eligibility criteria and study selection
Design inclusion
Eligible study designs will include randomised controlled studies (RCTs) and non-randomised controlled studies, which have been peer-reviewed. The minimum requirement will be 5 or more participants, with a comparison group applied within the study. Such methods are selected to reduce risk of bias and ensure study conclusions/outcomes are considered in the context of usual or alternative paediatric care pathways. On the authors’ initial assessment, inclusion of RCTs only would be likely to result in a very low inclusion yield and would exclude studies using other controlled methodologies relevant to the objectives of the review. Where published papers include the same participants in their sample, the study with the largest sample size will be analysed. Table 1 presents inclusion and exclusion criteria for studies, with further descriptions detailed below.
Details of inclusions and exclusions are provided in Table 1. In brief, we will include all studies that evaluate interventions directed at parents or primary caregivers of children below 13 years of age. Strategies that are part of the intervention could address goals relating to the parent or primary caregiver, the referred child, siblings or the broader family as a unit. Children must be referred to or currently be waiting for a health-related diagnostic service. The assessment for diagnosis may be related to any medical, developmental or psychiatric condition. Subgroup data will be sought directly from authors where divisions are unclear and where appropriate for analysis. If no response is received, the paper will be excluded from further analysis.
Study selection process
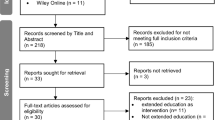
The first author will search all databases listed above. Retrieved articles will be stored in Endnote©. Duplicates will be removed via manual review and automatic functions contained within Endnote, and these numbers will be recorded. Remaining articles will then be uploaded into Covidence© with any additional duplicates removed as identified by this software. These yield numbers will be recorded and formulate the beginning of the PRISMA flow chart for later publication with the main review manuscript.
The first and second authors will independently screen all titles and abstracts identified from searches in Covidence©, filtering out those that do not meet the inclusion criteria. We will retrieve the full text of any papers identified as potentially meeting the criteria by at least one author. Two review authors will then independently screen full-text articles for inclusion or exclusion, with discrepancies resolved by discussion. When consensus cannot be reached, a third author will be consulted to reach a decision.
Full-text papers excluded from the review will be listed as excluded studies with reasons provided in a ‘Characteristics of excluded studies’ table. We will also provide citation details and any available information about ongoing studies and consider details of duplicate publications, so that each investigation (rather than each report) is the unit of interest in the review. We will report the screening and selection process in an adapted PRISMA flow chart [41].
Data extraction
Two review authors will extract data independently from included studies, using a custom designed electronic data extraction form in Microsoft Excel©. Any discrepancies in relation to data extraction will be resolved by discussion until consensus is reached or through consultation with a third reviewer if necessary. We will develop and pilot a data extraction form using the Cochrane guidance. This will be trialled on 3 papers, with relevant adjustments made, prior to its application in the extraction of data from all other papers.
Data to be extracted using categories detailed in Table 2.
In the case of missing data, the contact authors for the study will be contacted via email for further information.
All extracted data will be entered into the extraction form in Microsoft Excel by the first author and will be checked for accuracy against the data extraction sheets of a second reviewer working independently. Conflicts and inconsistencies will be resolved jointly or with support of a third reviewer where consensus is unable to be reached. Possible outcomes of interest to the authors are detailed in Table 3.
Adverse events
Serious adverse events are not expected in studies of interest to this systematic review. An increase in parenting stress or decrease in family quality of life following an intervention will be considered an adverse event for the purposes of analysis and discussion.
Data synthesis
We will decide whether to meta-analyse data based on whether the interventions in the included trials are similar enough in terms of participants, settings, intervention, comparison and outcome measures to ensure meaningful conclusions from a statistically pooled result. Where possible, we will standardise the data and generate pooled estimates, using the latest available version of Stata. For dichotomous data, we will use Stata to calculate odds ratios using inverse variance (IV) to produce a pooled estimate and explore random effects, with a 95% confidence interval. For continuous data, we will also use IV to explore the standardised mean difference, where pre- and post means and standard deviations are provided, again with a 95% confidence interval. We will visualise data for each outcome using forest plots and calculate heterogeneity. Forest plots will be created in Stata, with odds ratios or standard mean differences, weights and 95% confidence intervals. Using both visual inspection and the chi2 test for heterogeneity, further decisions will be made regarding meta-analyses. The I2 statistic will be used to quantify heterogeneity, with values of 50% or more representing levels that may preclude further meta-analysis. This will be considered within the context of the p value of the chi2 test, as well as the size and direction of the effects. If heterogeneity is high, we will conduct sensitivity analyses for methodological differences to explore the cause. We will present information about the impact of sensitivity analyses on heterogeneity and make a decision about presenting subgroup forest plots and conducting subgroup or overall meta-analyses on an individual outcome basis. We will conduct sensitivity analyses to assess heterogeneity and effect size comparing non-randomised and randomised studies and for randomised studies only. We will present randomised and non-randomised trials as subgroups in the same forest plot and only provide an overall effect size if heterogeneity is similar with and without non-randomised studies.
If we are unable to pool the data statistically using meta-analysis, we will provide clear reasons for this decision and will conduct a narrative synthesis of results. We will present the major outcomes and results, organised by intervention categories according to the major types and/or aims of the identified interventions. Depending on the assembled research, we may also explore the possibility of organising the data by the service category that the child is waiting for. Within the data categories, we will explore the main comparisons of the review:
-
Intervention versus a control group or usual care.
-
One form of intervention versus another.
Where studies compare more than one intervention, we will compare each separately to no intervention/control and with one another.
We will consider the following factors for subgroup analysis should this be possible:
-
1.
Participant characteristics: Gender of participants, group vs individual intervention focus, age of child, type of service not yet provided.
-
2.
Intervention: Study methodology used, type of intervention.
-
3.
Primary outcome type (see Table 2).
Where qualitative data is presented, key themes will be collated in this review.
Data quality and risk of bias
We will assess and report on the methodological risk of bias of included studies in accordance with the Cochrane Handbook [42], which includes recommendations around the explicit reporting of the following individual elements: random sequence generation, allocation sequence concealment, blinding (participants, personnel), blinding (outcome assessment), completeness of outcome data and selective outcome reporting. We will consider blinding separately for different outcomes where appropriate (for example, blinding may have the potential to differently affect subjective versus objective outcome measures). For non-randomised studies, we will also consider assignment of patients to treatment groups, the timing of intervention versus control groups, comparable baseline characteristics and loss to follow-up. We will judge each item as being at high, low or unclear risk of bias, and provide a quote from the study report and a justification for our judgement for each item in the risk of bias table.
In all cases, two authors will independently assess the risk of bias of included studies, with any disagreements resolved by discussion or referred to a third review in instances where consensus is unable to be reached. We will contact study authors for additional information about the included studies or for clarification of the study methods as required. We will incorporate the results of the risk of bias assessment into the review through standard tables, and systematic narrative description and commentary about each of the elements, leading to an overall assessment the risk of bias of each included study. As randomised and non-randomised studies will be included, it is likely that high levels of bias will be encountered and evaluated accordingly.
We will assess reporting bias qualitatively based on the characteristics of the included studies (e.g. if only small studies that indicate positive findings are identified for inclusion and if information that we obtain from contacting experts and authors or studies suggests that there are relevant unpublished studies).
If we identify sufficient studies for inclusion in the review, we will construct a funnel plot to investigate small study effects, which may indicate the presence of publication bias. We will formally test for funnel plot asymmetry, bearing in mind that there may be several reasons for funnel plot asymmetry when interpreting the results. We will conduct sensitivity analyses based on study quality.
Confidence in evidence and representation
We will prepare a 'Summary of findings' table to present the results of meta-analysis and/or narrative synthesis for the major comparisons of the review, for each of the primary outcomes listed in Table 2. We will provide a source and rationale for each assumed risk cited in the table(s) and will use the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria to rate the evidence based on the methods described in chapter 11 of the Cochrane Handbook [42]. If meta-analysis is not possible, we will present results in a narrative ‘Summary of findings’ table format.
Discussion
Despite initiatives to reduce waiting times across particular programmes, extended waiting periods that can last months or years continue to plague some public health assessment services. Perils relating to waiting lists and health service access issues continue to be reported as an area of concern in paediatric research. This systematic review aims to evaluate applied interventions that are directed at parents of children who are awaiting diagnostic assessments, when compared with controls.
The strengths of this review include the novel nature of the topic, its use of relevant systematic review guidelines such as PRISMA to support a standardised approach to the review’s methods, well-defined inclusion and exclusion criteria, and its breadth of inclusion relating to health services and settings where parent-directed interventions for children waiting for diagnostic assessment may occur. There will be possible challenges and potential limitations for the evidence synthesis, given variable interventions and outcomes that may be yielded in the search. Despite these challenges, this review aims to inform authors, and the wider community about the reported value of interventions currently provided to parents of children awaiting diagnostic assessment. The outcomes of this review will detail the range of interventions that are effective for improving family health and service-related outcomes whilst families wait for diagnostic assessment services for their child. This information will be useful for clinicians, service providers and policy makers, so that there may be consideration of implementation of effective waitlist interventions in existing services. It will also highlight any research gaps in this area, and authors will propose opportunities for innovation and future directions based on the evidence synthesised.
Availability of data and materials
Not applicable
Abbreviations
- CENTRAL:
-
Cochrane Central Register of Controlled Trials
- CINAHL:
-
Cumulative Index of Nursing and Allied Health Literature
- EBSCO:
-
Elton B. Stephens Co
- ERIC:
-
Education Resources Information Center
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- MEDLINE:
-
A bibliographic database of life sciences and biomedical information
- MeSH:
-
Medical Subject Headings
- OvidSP:
-
Research platform
- PQDT:
-
ProQuest Dissertation & Theses
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PRISMA-P:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses–Protocol extension
- PROSPERO:
-
International prospective register of systematic reviews
- PsycINFO:
-
Abstracting and indexing database covering behavioural sciences and mental health
- PubMed:
-
Biomedical and life sciences journal literature
- RCT:
-
Randomised control trial
References
Kuo DZ, Houtrow AJ, Arango P, Kuhlthau KA, Simmons JM, Neff JM. Family-centered care: current applications and future directions in pediatric health care. Matern Child Health J. 162012. p. 297–305.
Kuhlthau KA, Bloom S, Van Cleave J, Knapp AA, Romm D, Klatka K, et al. Evidence for family-centered care for children with special health care needs: a systematic review. Academic Pediatrics. 2011;11(2):136–43 e8.
Bronfenbrenner U. The ecology of human development: Harvard university press; 1979.
Organization WH. International classification of functioning, disability and health: children & youth version: ICF-CY2007 2007.
Shields L. Questioning family-centred care. Journal of clinical nursing. 2010;19(17-18):2629–38.
Coyne I, Hallström I, Söderbäck M. Reframing the focus from a family-centred to a child-centred care approach for children’s healthcare. J Child Health Care. 2016;20(4):494–502.
Barlow J, Schrader-McMillan A, Axford N, Wrigley Z, Sonthalia S, Wilkinson T, et al. Attachment and attachment-related outcomes in preschool children–a review of recent evidence. Child and Adolescent Mental Health. 2016;21(1):11–20.
Branjerdporn G, Meredith P, Strong J, Garcia J. Associations between maternal-foetal attachment and infant developmental outcomes: a systematic review. Maternal Child Health J. 2017;21(3):540–53.
Cousino MK, Hazen RA. Parenting stress among caregivers of children with chronic illness: a systematic review. J Pediatr Psychol. 2013;38(8):809–28.
van Santvoort F, Hosman CM, Janssens JM, van Doesum KT, Reupert A, van Loon LM. The impact of various parental mental disorders on children’s diagnoses: a systematic review. Clinical Child Family Psychol Review. 2015;18(4):281–99.
Villalonga-Olives E, Kawachi I. The dark side of social capital: a systematic review of the negative health effects of social capital. Social Science & Medicine. 2017;194:105–27.
Alvarez EC, Kawachi I, Romani JR. Family social capital and health–a systematic review and redirection. Sociol Health Illness. 2017;39(1):5–29.
Lee HY, Zhou AQ, Lee R, Dillon AL. Parents’ functional health literacy is associated with children’s health outcomes: implications for health practice, policy, and research. Children Youth Services Review. 2020;104801.
DeWalt DA, Hink A. Health literacy and child health outcomes: a systematic review of the literature. Pediatrics. 2009;124(Supplement 3):S265-S74.
Higgs G. A literature review of the use of GIS-based measures of access to health care services. Health Services and Outcomes Research Methodology. 2004;5(2):119–39.
Levesque J-F, Harris MF, Russell G. Patient-centred access to health care: conceptualising access at the interface of health systems and populations. Int J Equity Health. 2013;12(1):18.
Bachmann MO, Barron P. Why wait so long for child care? An analysis of waits, queues and work in a South African urban health centre. Tropical Doctor. 1997;27(1):34–8.
Grilli L, Feldman DE, Swaine B, Gosselin J, Champagne F, Pineault R. Wait times for paediatric rehabilitation. Healthcare Policy. 2007;2(3):e171.
Chen LE, Zamakhshary M, Foglia RP, Coplen DE, Langer JC. Impact of wait time on outcome for inguinal hernia repair in infants. Pediatric Surgery International. 2009;25(3):223–7.
Smith J, Kyle RG, Daniel B, Hubbard G. Patterns of referral and waiting times for specialist Child and Adolescent Mental Health Services. Child Adolescent Mental Health. 2018;23(1):41–9.
Duckett SJ. Private care and public waiting. Australian health review: a publication of the Australian Hospital Association. 2005;29(1):87-93.
Olivella P. Shifting public-health-sector waiting lists to the private sector. Eur J Political Econ. 2003;19(1):103–32.
Dangerfield B. System dynamics applications to European healthcare issues. Operational Research for Emergency Planning in Healthcare: Volume 2: Springer; 2016. p. 296–315.
Frankel S. The natural history of waiting lists--some wider explanations for an unnecessary problem. Health Trends. 1989;21(2):56–8.
Gebreiter F. Hospital accounting and the history of health-care rationing. http://dxdoiorg/101080/2155285120151086559. 2015.
Arnaboldi M, Lapsley I, Steccolini I. Performance management in the public sector: the ultimate challenge. Financial Accountability Management. 2015;31(1):1–22.
Siciliani L, Moran V, Borowitz M. Measuring and comparing health care waiting times in OECD countries. Health Policy. 2014;118(3):292–303.
Blanco-Lago R, Garcia-Ron A, Granizo-Martinez J, Ruibal J. Current situation of the demand for health care in neuropaediatrics. Characteristics of consultations and comparison with other paediatric specialties. Revista de Neurologia. 2014;59(9):392–8.
Diwakar L, Cummins C, Lilford R, Roberts T. Systematic review of pathways for the delivery of allergy services. BMJ Open. 2017;7(2):e012647.
Murugan SJ, Thomson J, Parsons JM, Dickinson DF, Blackburn ME, Gibbs JL. New outpatient referrals to a tertiary paediatric cardiac centre: evidence of increasing workload and evolving patterns of referral. Cardiol Young. 2005;15(1):43–6.
Wise MD, Little AA, Holliman JB, Wise PH, Wang CJ. Can state early intervention programs meet the increased demand of children suspected of having autism spectrum disorders? Journal of Developmental Behavioral Pediatrics. 2010;31(6):469–76.
Kratchman AR. The design, implementation, and formative evaluation of a program for waitlisted caregivers of individuals with developmental disabilities. New Jersey: Rutgers, The State University of New Jersey; 2011.
Bloch JR, Gardner M. Accessing a diagnosis for a child with an autism spectrum disorder: the burden is on the caregiver. Am J Nurse Practitioners. 2007;11(8):10–7.
Rodger S, Mandich A. Getting the run around: accessing services for children with developmental co-ordination disorder. Child Care Health Dev. 2005;31(4):449–57.
Anderson M, Elliott EJ, Zurynski YA. Australian families living with rare disease: experiences of diagnosis, health services use and needs for psychosocial support. Orphanet J Rare Dis. 2013;8:22.
Launay E, Morfouace M, Deneux-Tharaux C, Gras le-Guen C, Ravaud P, Chalumeau M. Quality of reporting of studies evaluating time to diagnosis: a systematic review in paediatrics. Arch Dis Child. 2014;99(3):244-250.
Gibbs V, Aldridge F, Sburlati E, Chandler F, Smith K, Cheng L. Missed opportunities: an investigation of pathways to autism diagnosis in Australia. Research in Autism Spectrum Disorders. 2019;57:55–62.
Connolly M, Gersch I. A support group for parents of children on a waiting list for an assessment for autism spectrum disorder. Educational Psychol Practice. 2013;29(3):293–308.
Hannan K, Hiscock H, Robert G, Sciberras E, McLean K, Quach J, et al. Sleep help@home. targeted behavioural interventions to improve sleep problems in children and reduce tertiary outpatient waitlists: a pilot randomised controlled trial. J Paediatr Child Health. 2018;54:5.
Bardsen T, Sorbye MH, Tronnes H, Greve G, Berg A. Parental anxiety related to referral of childhood heart murmur; an observational/interventional study. BMC Pediatr. 2015;15:193.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals Internal Med. 2009;151(4):W-65-W-94.
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Cochrane. 2019.
Acknowledgements
The authors would like to thank the support of the Department of Paediatrics at the University of Melbourne, the Developmental Disabilities and Rehabilitation Research Unit with the Murdoch Children’s Research Institute and the planning contribution of Jason Wozniak. The lead author would like to specifically acknowledge the contribution of the Department of Paediatrics’ Graduate Research Advisory Committee, and the contribution of the scholarship kindly donated by Sue and Leigh Clifford.
Additional resources
Not applicable
Funding
This project has been funding through the Australian Government Research Training Program Scholarship, The Sue and Leigh Clifford Family Scholarship and the Developmental Disabilities and Rehabilitation Research Top-up Scholarship. These funding bodies have financially supported the lead author to develop this protocol in collaboration with other authors, without having a direct role in its development.
Author information
Authors and Affiliations
Contributions
All authors CB, MM, TM and KW have contributed to this manuscript as well as the design and implementation of this protocol. All authors agreed on the approval for this manuscript to be submitted on finalisation. As the lead author and guarantor, CB will continue to coordinate systematic review activities and reporting. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
All authors agreed on approval for this manuscript to be submitted on finalisation.
Competing interests
Not applicable
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:.
Prisma P Checklist.
Additional file 2:.
Search Strategy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bernie, C., Mitchell, M., Williams, K. et al. Parent-directed intervention versus controls whilst their child waits for diagnostic assessment: a systematic review protocol. Syst Rev 10, 67 (2021). https://doi.org/10.1186/s13643-021-01615-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-021-01615-7