Abstract
Background
Sigh breaths may impact outcomes in acute hypoxemic respiratory failure (AHRF) during assisted mechanical ventilation. We investigated whether sigh breaths may impact mortality in predefined subgroups of patients enrolled in the PROTECTION multicenter clinical trial according to: 1.the physiological response in oxygenation to Sigh (responders versus non-responders) and 2.the set levels of positive end-expiratory pressure (PEEP) (High vs. Low-PEEP). If mortality differed between Sigh and No Sigh, we explored physiological daily differences at 7-days.
Results
Patients were randomized to pressure support ventilation (PSV) with Sigh (Sigh group) versus PSV with no sigh (No Sigh group). (1) Sighs were not associated with differences in 28-day mortality in responders to baseline sigh-test. Contrarily-in non-responders-56 patients were randomized to Sigh (55%) and 28-day mortality was lower with sighs (17%vs.36%, log-rank p = 0.031). (2) In patients with PEEP > 8cmH2O no difference in mortality was observed with sighs. With Low-PEEP, 54 patients were randomized to Sigh (48%). Mortality at 28-day was reduced in patients randomised to sighs (13%vs.31%, log-rank p = 0.021). These findings were robust to multivariable adjustments. Tidal volume, respiratory rate and ventilatory ratio decreased with Sigh as compared with No Sigh at 7-days. Ventilatory ratio was associated with mortality and successful extubation in both non-responders and Low-PEEP.
Conclusions
Addition of Sigh to PSV could reduce mortality in AHRF non-responder to Sigh and exposed to Low-PEEP. Results in non-responders were not expected. Findings in the low PEEP group may indicate that insufficient PEEP was used or that Low PEEP may be used with Sigh. Sigh may reduce mortality by decreasing physiologic dead space and ventilation intensity and/or optimizing ventilation/perfusion mismatch.
Clinical Trial Registration: ClinicalTrials.gov; Identifier: NCT03201263.
Similar content being viewed by others
Background
Occasional spontaneous deep breathing—known as sigh—is a physiological feature in healthy subjects during spontaneous ventilation. The first physiological characterization of sighs in healthy subjects dates back more than 100 years ago [1]. During normal breathing, sighs seem to play a key role in the prevention of atelectasis [2,3,4] and experimental data suggest that sighs improve the secretion of active alveolar surfactant [5].
The use of sighs during controlled mechanical ventilation was proposed to improve respiratory mechanics and gas exchange in patients undergoing surgical procedures [6] or in the presence of respiratory failure (ARDS) [7]. A renewed interest on the use of sigh during passive ventilation was recently reported in the setting of trauma patients at risk of developing ARDS for its potential benefit on outcome [8].
Sigh was also implemented during spontaneous breathing. In pressure support ventilation (PSV) – one among the most used modes of assisted mechanical ventilation [9]—Sighs improved respiratory mechanics and oxygenation, while decreasing lung heterogeneity, respiratory drive and effort [10, 11]. In 2021, the PROTECTION trial explored the feasibility of the application of sighs in PSV with acute hypoxemic respiratory failure (AHRF) or ARDS. Sigh was proved feasible and safe in this population but no differences on outcomes were reported between PSV with or without sigh breathing [12]. However, increasing awareness is emerging on the importance of phenotyping patients that may benefit the most from a therapeutic intervention based on clinical, laboratory, imaging or physiological criteria [13,14,15]. This may allow to reduce sample heterogeneity, leading to heterogeneity of treatment effects. Further, this may optimize the population enrichment of targeted subjects that are most likely to positively respond to a specific treatment in terms of hard outcomes.
In this secondary analysis of the PROTECTION trial we aimed at exploring the role of sigh breathing during PSV in specific predefined physiological subgroups of patients on outcomes.
These analyses may serve as preliminary, exploratory and hypotheses generating to understand whether the use of Sigh may be a ventilatory option based on the physiological response in oxygenation to Sigh and in regard to the set levels of PEEP. We based our analyses on a physiological rationale.
We started from the hypothesis that sigh breathing may be beneficial on outcome in the presence of oxygenation response during the sigh test (responders)—which was defined by SpO2/FiO2 criteria > 1%—as compared with No Sigh. Therefore, we explored differences on outcome between Sigh and No Sigh treatment (primary outcome).
Subsequently, we hypothesized that patients exposed to low levels of PEEP (PEEP ≤ 8cmH2O—PEEP = 8cmH2O defines two size balanced subgroups in the PROTECTION trial and seemed clinically reasonable [9]—Low PEEP group) may show a lower mortality rate by adding Sigh as compared with No Sigh (primary outcome).
In the presence of mortality differences between Sigh and No Sigh, we explored daily differences in physiological parameters between the 2 randomized groups, and whether physiological parameters were associated with outcomes (secondary outcomes).
Methods
Patients, study design and setting
These are prespecified secondary analyses of an international, multicenter, randomized clinical trial (NCT03201263) [12] aimed at exploring predefined physiological subgroups of patients potentially responsive to sighs in terms of outcomes. Further, we investigated whether differences in respiratory physiology might have a role as underpinning mechanisms of outcomes differences by using sigh.
The PROTECTION trial included 20 centers from 8 countries between December 2017 to May 2019 through a call of the Pleural Pressure Working Group (PLUG) of the European Society of Intensive Care Medicine (ESICM) who endorsed and partially funded the trial.
The PROTECTION trial included patients with acute hypoxemic respiratory failure (AHRF) (PaO2/FiO2 ≤ 300 with a PEEP of 5 cmH2O) who were mechanically ventilated between 24 h and 7 days and who were switched from mechanical ventilation to pressure support ventilation between 4 and 24 h. Furthermore, at the enrolment, the Richmond Agitation-Sedation Scale was −2 to 0 [16].
Further details about study design, population, exclusion criteria and methods were previously described [17].
Sigh test, randomization, interventions and spontaneous breathing trial
All enrolled patients underwent a responsivity test to Sigh. Specifically, patients were exposed for 30 min to Sigh (i.e. 30 cmH2O for 3-s insufflation one each minute) starting with a FiO2 tailored to target a SpO2 between 90 and 96%. After the Sigh test, patients were defined as Sigh responders versus Sigh non-responders whether SpO2/FiO2 improved by > 1%.
After completion of the Sigh test, patients were randomized to PSV with Sigh (Sigh group) or to PSV with no sigh (No Sigh group).
PSV setting after randomization targeted a Vt 6–8 mL/kg of predicted body weight, respiratory rate (RR) 20–35 breaths/minute, while clinical PEEP and FiO2 were unchanged.
In the Sigh group, Sigh was promptly added as a pressure control breath at total end-inspiratory
Pressure of 30 cmH2O for 3 s delivered once per minute. Ventilators were switched to biphasic synchronized positive airway pressure mode (also known as synchronized intermittent mandatory ventilation combining pressure control and PSV) with the lower pressure level set at clinical PEEP and the higher pressure level set at 30 cmH2O with a 3-s inspiratory time. Sigh settings were left unchanged until switch to controlled ventilation, day 28, death, or performance of a successful spontaneous breathing trial. In the No Sigh group, after randomization, PSV was set to obtain the same targets as above with clinical PEEP and the FiO2 selected during the prerandomization sigh test. Subsequent changes in PSV in both groups, were considered at least every 8 h to reach the randomization target of Vt and RR, while PEEP and FiO2 were adjusted to maintain SpO2 90–96%. In both groups, switch to protective controlled ventilation was considered when in the presence of specific predefined criteria. Patients switched to controlled ventilation were reassessed at least every 8 h and switched back to the Sigh or No Sigh group as soon as predefined criteria for improvement were met [17].
A spontaneous breathing trial (SBT) was considered if SpO2 ≥ 90% on FiO2 ≤ 0.4 and PEEP ≤ 5 cmH2O with no agitation and unstable hemodynamics. In the sigh group, the attending physician withdrew sigh, waited 60 min, confirmed the above-mentioned criteria, and performed the SBT. If criteria were no longer met, sigh was reintroduced and this procedure was repeated after at least 8 h. The SBT lasted at least 60 min with a combination of PEEP of 0 to 5 cmH2O and PSV level of 0 to 5 cmH2O. Criteria for success vs failure of the SBT were predefined by study protocol [17]. After successful completion of the SBT, patients were promptly extubated or, in the presence of tracheostomy, mechanical ventilation was discontinued. After SBT failure, patients were switched back to the Sigh or No Sigh group, and criteria for SBT were checked again after at least 6 h. After extubation, reintubation was performed if at least one of the criteria predefined by the study protocol was present [17].
Comprehensive information on randomization, interventions and SBT was previously described [17]. The complete study protocol is included in the Supplemental material.
Predefined physiological subgroups
Responders versus non-responders were defined based on the 30-min Sigh test based on oxygenation criteria, and—as previously explained—patients were defined as Sigh responders versus Sigh non-responders whether SpO2/FiO2 improved by > 1%.
The specific cut-off used to define High versus Low PEEP group was decided based on statistical reasons (i.e. to obtain balanced samples between the 2 predefined subgroups) and on baseline oxygenation criteria (i.e. patients with mild hypoxemia, average 200 < PaO2/FiO2 ≤ 300).
Measurements and study outcomes
After enrolment and at randomization, data on demographics, past and recent medical history, systemic severity, lung injury risk factors, ventilation clinical settings and etiology of AHRF were collected. Furthermore, daily physiological measurements were collected during the first 7 days after randomization. Study outcomes including 28-day mortality and successful extubation with more than 48 h free from reintubation at 28-day and data on sigh feasibility were explored.
Statistical analysis
Continuous data were described with median and quartiles (Q1–Q3). Categorical data were reported as count (proportion). Descriptive statistics were used to characterize the study population. A two-tailed p-value below 0.05 was considered statistically significant. Differences between the randomized groups (Sigh versus No Sigh groups) are reported by Mann–Whitney Wilcoxon-test and by Chi-square or Fisher’s exact test, as appropriate.
Differences in 28-day mortality and successful extubation with more than 48 h free from reintubation at 28-day were evaluated by survival curves using the Kaplan–Meier approach with log-rank p-value and competing risk non-parametric method with Fine & Gray p-value, respectively. The association of the study intervention (Sigh versus No Sigh) with 28-day mortality and successful extubation was investigated by using multivariable Cox-proportional and Fine & Gray models using mortality as a competitive event, respectively. The number of covariates used to adjust the multivariable model for the explored outcomes were decided based on the explored outcome of the sample size of to avoid overfitting. The specific covariates for multivariable adjustment were decided based on clinical meaning and their known association with outcomes including:
-
Age;
-
Patient past medical history – that was described by the presence of any comorbidities among the following ones (Chronic cardiovascular disease, Chronic pulmonary disease, Diabetes, Chronic renal disease, Cancer); and
-
Patients current clinical illness severity by using SOFA score.
Results of the multivariate models were reported as β coefficient, Hazard Ratio (HR) with 95% CI (95% CI).
Daily differences up to 7 days since randomization in physiological variables between the study interventions (Sigh versus No Sigh) in the investigated physiological subgroups were assessed by using generalized estimating equation models account for repeated measures for subjects. Association between average physiological parameters within 7-d and study outcomes were performed by using Cox-proportional (i.e. 28-day mortality) and Fine & Gray models using mortality as a competitive event (i.e. successful extubation with more than 48 h free from reintubation at 28-day). Differences in ventilatory ratio between survivors and non-survivors were assessed by using Mann–Whitney U-test. Statistical analyses were performed with SAS 9.4 TS Levek 1M7 (2020 SAS Institute Inc., Cary, NC, USA) and R Studio 2002.07.1 (2009-2002Rstudio PBC).
Further details on methods are reported in the Supplemental material.
Results
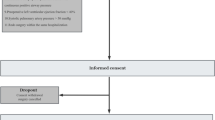
We explored differences in 28-day mortality and successful extubation in Sigh versus No Sigh treatments based on the oxygenation responsive to the baseline sigh test (responders versus non-responders) and to the exposure to different levels of PEEP (High versus Low PEEP). Patients included in the current analyses are reported in Fig. 1. Outcomes differed, in the 1) Oxygenation non-responder group (Fig. 2A, B, Supplemental Fig. 1A,B); and in the 2) Low PEEP group (Fig. 2C, D, Supplemental Fig. 1C,D). Therefore, we investigated physiological differences between Sigh versus No Sigh treatments in these 2 specific subgroups of patients.
Death (A) and successful extubation (B) at 28-day follow-up in patients stratified by Sigh versus No Sigh in the Non-responders subgroup. Death (C) and successful extubation (D) at 28-day follow-up in patients stratified by Sigh versus No Sigh in the Low PEEP subgroup. (p) = probability. Number of patients at risk by groups are reported below each panel timeline
Baseline characteristics
Non-responders
Patients included in the Protection Trial fulfilling the criteria of oxygenation non-responder group were 102 out of 258 (40%). Fifty-six patients were randomized to Sigh (55%), while 46 to No Sigh (45%). Baseline characteristics of non-responders stratified by the randomization to Sigh were reported in Table 1. Only comorbidities differed between the study groups and were lower in the Sigh arm.
Low PEEP group
Patients included in the Protection Trial fulfilling the criteria of Low PEEP (PEEP levels ≤ 8cmH2O—median PEEP level of the original study) were 113 out of 258 (44%). Fifty-four patients were randomized to Sigh (48%), while 59 to No Sigh (52%). Baseline characteristics of Low PEEP patients stratified by the randomization to sigh were reported in Table 2. No differences were reported between the two arms.
Clinical outcomes
Non-responders
We evaluated differences in outcomes between the study arms. In the Sigh treatment, 28-day mortality was lower, proportion of patients successfully extubated was higher and duration of ventilator free days was longer as compared with No Sigh treatment (Supplemental Table 1).
We explored differences in mortality by time-to-event analysis between the study arms. We observed that mortality over 28-day follow-up was significantly lower in the Sigh versus No Sigh arm (log-rank p = 0.031) (Fig. 2A, Supplemental Fig. 1A). After adjusting the multivariate model for clinically meaningful variables (i.e.age, comorbidities and SOFA score) the use of Sigh was consistently associated with a decreased mortality (HR 0.40; 95% CI 0.17–0.92; p = 0.030) (Table 3).
As a second clinical outcome, we investigated differences in the proportion of patients successfully extubated by competing risk analyses. We observed that the proportion of patients with a successful extubation at 28-day follow-up was higher in the Sigh versus No Sigh arm (Fine & Gray p = 0.024) (Fig. 2B, Supplemental Fig. 1B). After adjusting the model for clinically meaningful variables the use of Sigh was consistently associated with an increased successful extubation (HR 1.78; 95% CI 1.08–2.93; p = 0.024) (Table 3).
Of note, Sigh was not associated with differences in 28-day mortality (16% vs. 13%, p = 0.575) and successful extubation (81% vs. 85%, p = 0.6017) in patients with positive response to baseline sigh test (Fig. 2A, B).
Low PEEP group
We evaluated differences in outcomes between the study arms. In the Sigh arm, 28-day mortality was lower, and proportion of patients successfully extubated was higher in survivors, while duration of ventilator free days did not differ as compared with No Sigh treatment (Supplemental Table 2). We explored differences in mortality by time-to-event analyses between the study arms. We observed that mortality over 28-day follow-up was significantly lower in the Sigh versus No Sigh arm (log-rank p = 0.021) (Fig. 2C, Supplemental Fig. 1C). After adjusting the multivariate model for clinically meaningful variables the use of sigh was consistently associated with a decreased mortality (HR 0.26; 95% CI 0.10–0.68; p = 0.005) (Table 4).
As a second clinical outcome, we investigated differences in the proportion of patients successfully extubated by competing risk analyses. We observed that the proportion of patients successfully extubated over 28-day follow-up trended higher in the Sigh arm as compared with the No Sigh (Fine & Gray p = 0.061) (Fig. 2D, Supplemental Fig. 1D). After adjusting the model for clinically meaningful variables the use of sigh was associated with an increased successful extubation (HR 1.75; 95% CI 1.15–2.66; p = 0.010) (Table 4).
Of note, Sigh treatment was not associated with differences in 28-day mortality (19% versus 13%, p = 0.339) and successful extubation (79% vs. 84%, p = 0.8367) in patients with clinical PEEP > 8cmH2O (Fig. 2C, D).
Exploratory differences in the proportion of 28-day mortality and successful extubation by competing risk analyses between predefined physiological subgroups exposed or not exposed to SIGH are reported in Supplemental Table 3.
Ventilatory parameters at 7 days
Non-responders
We explored longitudinal physiological daily differences between arms up to 7 days since randomization. Ventilator settings did not differ between the groups (i.e.PSV, PEEP and FiO2). While oxygenation did not change between the groups, PaCO2 trended to lower levels in the Sigh arm (Supplemental Fig. 2). Despite minute ventilation did not significantly decrease in the Sigh arm (Supplemental Fig. 2), Vt/PBW trended to lower levels while RR decreased significantly in the Sigh arm (Fig. 3A, B). Therefore, we explored differences in proxies of pulmonary dead space between the 2 groups. We observed that both standardized minute ventilation (Supplemental Fig. 2) and ventilatory ratio were significantly lower in the Sigh versus No Sigh arm (Fig. 3C).
Vt/PBW (A), RR (B) and Ventilatory ratio (C) differences over 7-day follow up since randomization between Sigh versus No Sigh in the Non-responders group. Vt/PBW (D), RR (E) and Ventilatory ratio (F) differences over 7-day follow up since randomization between Sigh versus No Sigh in the Low PEEP group. PBW predicted body weight, PEEP positive end-expiratory pressure, RR respiratory rate, SE standard error, VT tidal volume
Low PEEP group
We explored longitudinal physiological daily differences between arms up to 7 days since randomization. Ventilator settings did not differ between the groups (i.e.PSV, PEEP and FiO2) and so did not gas exchange. Interestingly, minute ventilation decreased significantly in the Sigh treatment (Supplemental Fig. 3). This was led by both a decrease in Vt/PBW and lower respiratory rate as compared with the Sigh treatment (Fig. 3D, E). We explored differences in proxies of pulmonary dead space between the 2 groups. We observed that both standardized minute ventilation (Supplemental Fig. 3) and ventilatory ratio (Fig. 3F) were significantly lower in the Sigh versus No Sigh arm.
Comprehensive data about differences over 7-day follow-up about ventilatory and physiological parameters between Sigh versus No Sigh in both physiological subgroups are reported in the Supplemental material.
Association between ventilatory parameters and outcomes
Non-responders
While different ventilatory parameters during 7-day follow-up were correlated with successful extubation, only RR and ventilatory ratio were both positively correlated with 28-day mortality (Table 5, Supplemental Fig. 4).
Low PEEP group
While different ventilatory parameters during 7-day follow-up were correlated with successful extubation, only respiratory rate, ventilatory ratio and standardized minute ventilation were both positively correlated with 28-day mortality (Table 5, Supplemental Fig. 4).
Discussion
In this secondary analysis of the PROTECTION randomized controlled trial -assessing the feasibility of sigh during pressure support ventilation- we aimed at reducing patient heterogeneity by investigating the role of sigh breathing in different predefined physiological subgroups of patients. This was based on 1. the oxygenation response to a 30-min sigh test before randomization, and 2. different levels of set PEEP.
The main findings of our investigation include the following ones:
-
sigh was not associated with differences in 28-day mortality in responders and in patients with clinical set PEEP > 8cmH2O; surprisingly, 28-d mortality was significantly lower in the Sigh versus No Sigh arm in non-responders, and in patients exposed to Low PEEP levels; this was further confirmed after adjustment in multivariable models;
-
in non-responders, successful extubation was significantly higher in the Sigh versus No Sigh arm, and similarly, a trend was observed in the Low PEEP group; this was confirmed in both subgroups after adjustment in multivariable models;
-
daily Vt/PBW and respiratory rate levels were lower in the Sigh versus No Sigh arm up to 7-day follow-up in both subgroups;
-
pulmonary dead space and ventilation-perfusion mismatch -estimated by using ventilatory ratio- was lower in the Sigh versus No Sigh arm at 7-day follow-up in both subgroups;
-
ventilatory ratio was the only parameter associated with both 28-day mortality and successful extubation in both predefined physiological subgroups of patients (i.e. Non-responders and Low PEEP groups).
In these prespecified secondary analyses of the PROTECTION trial we observed that the use of sigh was associated with favourable outcomes. So far, the only RCT exploring differences on outcome using sigh during controlled mechanical ventilation—the SiVent study—suggested a promising beneficial role on outcome in the treatment arm with SIGH [8]. In our analysis, we confirmed the positive association with the use of sigh during PSV and a better outcome in 2 different predefined physiological subgroups. This was confirmed after multivariable adjustment with robust clinical variables known to have an impact on outcome in patients with respiratory failure undergoing mechanical ventilation: age [18]; patient past clinical history (i.e. comorbidities) [19]; and clinical illness severity estimated by the severity of organ failures [20]. Although these findings are exploratory and preliminary, they question whether a periodic brief recruitment manoeuvre during assisted mechanical ventilation may contribute somehow to the optimization of the pulmonary function and consequently may influence outcomes. The recent ESICM guidelines suggestions against routine use of brief high-pressure RM to reduce mortality in patients of ARDS consider a RM ≥ 35 cmH2O for less than a minute. In our setting, a RM pressure of 30 cmH2O for a duration of only 3 s may probably and unlikely result in complications, including barotrauma and hemodynamic instability [21].
We further evaluated daily differences in the levels of ventilatory variables over time (7 days after randomization) between Sigh versus No Sigh arm to infer on mechanisms that may support differences in major outcomes between predefined physiological subgroups.
The physiological benefit of sigh during controlled mechanical ventilation in ARDS is widely recognized [7, 22, 23]. During spontaneous breathing sigh promotes variability of tidal and minute volume ventilation in healthy infants [24]. Tidal volume variability is suggested to improve patient-ventilator asynchronies [25], which is associated with better outcomes [26]. Furthermore, in patients with respiratory failure a low tidal volume variability seems to be associated with the presence of dyspnoea as compared to healthy subjects [27]. Sigh was described to improve both respiratory mechanics—by increasing EELI and consequently the respiratory system compliance—and gas exchange during PSV [10]. Furthermore, sigh makes the regional distribution of the tidal ventilation more homogeneous [11]. In our analyses we observed a decrease in pulmonary dead space in the Sigh arm in both physiological subgroups. This was observed by a lower standardized minute ventilation and a lower ventilatory ratio. Further, this was achieved by decreasing PaCO2 levels over time in the Sigh versus No Sigh arm—although not significantly—and by both a decrease in Vt/PBW and respiratory rate. The beneficial role of sigh on decreasing the pulmonary dead space may suggest a potential contribution in the decrease of death in our population [28]. Both increasing levels of standardized minute ventilation [9, 29] and ventilatory ratio [30] are associated with worse outcomes in patients with ARDS. Furthermore, the decrease in wasted ventilation may suggest an improved homogeneous regional ventilation leading to a better optimization of the ventilation perfusion matching [11] which is associated with a better outcome [31]. These findings are potentially of high clinical relevance during the ventilatory management. The decision on setting sigh during pressure support ventilation may not be driven only by an improvement of oxygenation—as it was performed in the PROTECTION original trial. Sigh may be set during PSV by assessing the response on the decrease in physiologic deadspace—that can be easily estimated at bedside by using standardized minute ventilation or ventilatory ratio. We may speculate that patients exposed to Sigh may show a better outcome in the presence of a decreased ventilatory ratio as compared with No Sigh. This is in line with the superior role of CO2 clearing—as compared to oxygenation improvement—in predicting a lower mortality rate in ARDS patients as a consequent effect of lung recruitment after prone-positioning [32]. Of note, in both our physiological subgroups, ventilatory ratio was the only variable positively associated with both 28-day mortality and successful extubation.
Another key physiological finding is the enhancement of protective ventilation by Sigh treatment. This may contribute to the beneficial role of SIGH on outcome by decreasing the intensity of ventilation [29]. The decrease of tidal volume was recently suggested to protect the lung from the patient self-inflicted lung injury [33, 34]. Furthermore, the lower respiratory rate seems to reduce the risk of lung injury [35] and may have an independent contribution on outcome in patients with ARDS [36].
Taking all these findings together, we may question on the reasons why sigh is beneficial when the patients are non-responders to the Sigh test as compared with responders. In non-responders to the 30-min Sigh test, Sigh does not seem to provide a significant benefit on oxygenation either during the Sigh test or after randomization at 7 days, suggesting that the main mechanism of Sigh breathing may not be immediate lung recruitment during PSV. The beneficial role of Sigh after randomization may be explained by a decrease of physiologic dead-space and therefore of ventilation intensity (i.e. tidal volume and respiratory rate). However, it is also possible that the repeated sighs allow lung volume to remain stable instead of gradually decreasing over hours without sighs, which may not be captured by the immediate response to the Sight test. Patients exposed to Low PEEP as compared with High PEEP may also experience a much better maintenance of lung volume over time. In patients exposed to Low PEEP, two conclusions may be inferred: 1. insufficient PEEP was used, as mortality was higher as compared with the High PEEP group; or 2. low PEEP should be used with intermittent sighs. Interestingly, even in this setting, the use of Sigh after randomization in the Low PEEP group does not seem to play a relevant role on optimizing oxygenation. It may also act by decreasing physiologic dead-space and ventilation intensity.
Strengths of the study include the secondary analysis on prespecified subgroups from a RCT. We have daily granular information on physiological parameters in all patients. The physiological findings in the 2 different subgroups are similar suggesting that differences on outcomes driven by Sigh treatment may include an optimization in ventilation perfusion matching, as seen by VR modulation. This analysis has also some limitations. We cannot infer on causal-effect interpretation. However, we adjusted our analyses for major predictors of clinical outcomes. As the sample size is limited, our results are exploratory and hypothesis generating and need further investigation. However, this is the only RCT performed in patients with AHRF exploring the role of Sigh treatment and investigating its role on the heterogeneity of treatment response in predefined physiological subgroups.
Conclusions
In conclusions, sigh breathing during PSV was independently associated with better outcomes as compared with No Sigh ventilation in specific physiological subgroups of patients with AHRF. The findings in non-responders were not expected and require further exploration. The findings in the low PEEP group may indicate that insufficient PEEP was used or that low PEEP should be used with intermittent sighs [37].
Sigh treatment showed lower Vt/PBW and respiratory rate despite similar/lower CO2 levels leading to better ventilation/perfusion mismatch as compared with No Sigh. This was independently associated with major outcomes. Responsivity to brief recruitment manoeuvres during PSV may be investigated by the decrease of wasted ventilation (i.e. decreased pulmonary dead space)—that can be easily evaluated at bedside. This may introduce the concept of dead space responder as compared with oxygenation responder to Sigh.
These exploratory findings may help to identify distinct physiological subgroups of AHRF undergoing PSV who may benefit of Sigh breathing.
Availability of data and materials
Data are available upon reasonable request to the Corresponding Author.
Abbreviations
- AHRF:
-
Acute hypoxemic respiratory failure
- ARDS:
-
Acute respiratory distress syndrome
- ARF:
-
Acute respiratory failure
- BMI:
-
Body mass index
- COPD:
-
Chronic obstructive pulmonary disease
- ESICM:
-
European Society of Intensive Care Medicine
- FiO2 :
-
Inspiratory oxygen fraction
- ICU:
-
Intensive care unit
- LOS:
-
Length of stay
- Mve:
-
Expiratory minute ventilation
- PaCO2 :
-
Arterial carbon dioxide partial pressure
- PaO2 :
-
Arterial oxygen partial pressure
- PEEP:
-
Positive end-expiratory pressure
- pH:
-
Negative logarithm of hydrogen concentration
- PLUG:
-
Pleural Pressure Working Group
- PSV:
-
Pressure support ventilation
- P0.1:
-
Occlusion pressure at 100 ms
- Q:
-
Quartile
- RASS:
-
Richmond Agitation-Sedation Scale
- RM:
-
Recruitment maneuver
- RR:
-
Respiratory rate
- SAPS:
-
Simplified Acute Physiology Score
- SBT:
-
Spontaneous breathing trial
- SOFA:
-
Sequential organ failure assessement
- SpO2 :
-
Peripheral oxygen saturation
- stMve:
-
Standardized expiratory minute ventilation
- TRALI:
-
Transfusion related acute lung injury
- VR:
-
Ventilatory ratio
- Vt:
-
Tidal volume
References
Haldane JS, Meakins JC, Priestley JG. The effects of shallow breathing. J Physiol. 1919;52(6):433–53. https://doi.org/10.1113/jphysiol.1919.sp001842.
Reynolds LB. Characteristics of an inspiration-augmenting reflex in anesthetized cats. J Appl Physiol. 1962;17:683–8. https://doi.org/10.1152/jappl.1962.17.4.683.
Bendixen HH, Smith GM, Mead J. Pattern of ventilation in young adults. J Appl Physiol. 1964;19:195–8. https://doi.org/10.1152/jappl.1964.19.2.195.
Bartlett D. Origin and regulation of spontaneous deep breaths. Respir Physiol. 1971;12(2):230–8. https://doi.org/10.1016/0034-5687(71)90055-7.
Massaro GD, Massaro D. Morphologic evidence that large inflations of the lung stimulate secretion of surfactant. Am Rev Respir Dis. 1983;127(2):235–6. https://doi.org/10.1164/arrd.1983.127.2.235.
Bendixen HH, Hedley-Whyte J, Laver MB. Impaired oxygenation in surgical patients during general anesthesia with controlled ventilation. a concept of atelectasis. N Engl J Med. 1963;269:991–6. https://doi.org/10.1056/NEJM196311072691901.
Foti G, Cereda M, Sparacino ME, De Marchi L, Villa F, Pesenti A. Effects of periodic lung recruitment maneuvers on gas exchange and respiratory mechanics in mechanically ventilated acute respiratory distress syndrome (ARDS) patients. Intensive Care Med. 2000;26(5):501–7. https://doi.org/10.1007/s001340051196.
Albert RK, Jurkovich GJ, Connett J, Helgeson ES, Keniston A, Voelker H, Lindberg S, Proper JL, Bochicchio G, Stein DM, Cain C, Tesoriero R, Brown CVR, Davis J, Napolitano L, Carver T, Cipolle M, Cardenas L, Minei J, Nirula R, Doucet J, Miller PR, Johnson J, Inaba K, Kao L. Sigh ventilation in patients with trauma: the SiVent randomized clinical trial. JAMA. 2023;330(20):1982–90. https://doi.org/10.1001/jama.2023.21739.
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A, LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. https://doi.org/10.1001/jama.2016.0291.
Patroniti N, Foti G, Cortinovis B, Maggioni E, Bigatello LM, Cereda M, Pesenti A. Sigh improves gas exchange and lung volume in patients with acute respiratory distress syndrome undergoing pressure support ventilation. Anesthesiology. 2002;96(4):788–94. https://doi.org/10.1097/00000542-200204000-00004.
Mauri T, Eronia N, Abbruzzese C, Marcolin R, Coppadoro A, Spadaro S, Patroniti N, Bellani G, Pesenti A. Effects of Sigh on regional lung strain and ventilation heterogeneity in acute respiratory failure patients undergoing assisted mechanical ventilation. Crit Care Med. 2015;43(9):1823–31. https://doi.org/10.1097/CCM.0000000000001083.
Mauri T, Foti G, Fornari C, Grasselli G, Pinciroli R, Lovisari F, Tubiolo D, Volta CA, Spadaro S, Rona R, Rondelli E, Navalesi P, Garofalo E, Knafelj R, Gorjup V, Colombo R, Cortegiani A, Zhou JX, D’Andrea R, Calamai I, Vidal González Á, Roca O, Grieco DL, Jovaisa T, Bampalis D, Becher T, Battaglini D, Ge H, Luz M, Constantin JM, Ranieri M, Guerin C, Mancebo J, Pelosi P, Fumagalli R, Brochard L, Pesenti A, PROTECTION Trial Collaborators. Sigh in patients with acute hypoxemic respiratory failure and ARDS: the PROTECTION pilot randomized clinical trial. Chest. 2021;159(4):1426–36. https://doi.org/10.1016/j.chest.2020.10.079.
Shah FA, Meyer NJ, Angus DC, Awdish R, Azoulay É, Calfee CS, Clermont G, Gordon AC, Kwizera A, Leligdowicz A, Marshall JC, Mikacenic C, Sinha P, Venkatesh B, Wong HR, Zampieri FG, Yende S. A research agenda for precision medicine in sepsis and acute respiratory distress syndrome: an official american thoracic society research statement. Am J Respir Crit Care Med. 2021;204(8):891–901. https://doi.org/10.1164/rccm.202108-1908ST.
Hochberg CH, Sahetya SK. Laying the groundwork for physiology-guided precision medicine in the critically Ill. NEJM Evid. 2023;2(5):EVIDe2300051. https://doi.org/10.1056/EVIDe2300051.
Rezoagli E, Xin Y, Signori D, Sun W, Gerard S, Delucchi KL, Magliocca A, Vitale G, Giacomini M, Mussoni L, Montomoli J, Subert M, Ponti A, Spadaro S, Poli G, Casola F, Herrmann J, Foti G, Calfee CS, Laffey J, Bellani G, Cereda M, CT-COVID19 Multicenter Study Group. Phenotyping COVID-19 respiratory failure in spontaneously breathing patients with AI on lung CT-scan. Crit Care. 2024;28(1):263. https://doi.org/10.1186/s13054-024-05046-3.
Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–44. https://doi.org/10.1164/rccm.2107138.
Mauri T, Foti G, Fornari C, Constantin JM, Guerin C, Pelosi P, Ranieri M, Conti S, Tubiolo D, Rondelli E, Lovisari F, Fossali T, Spadaro S, Grieco DL, Navalesi P, Calamai I, Becher T, Roca O, Wang YM, Knafelj R, Cortegiani A, Mancebo J, Brochard L, Pesenti A, Protection Study Group. Pressure support ventilation + sigh in acute hypoxemic respiratory failure patients: study protocol for a pilot randomized controlled trial, the PROTECTION trial. Trials. 2018;19(1):460. https://doi.org/10.1186/s13063-018-2828-8.
Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguía C, Nightingale P, Arroliga AC, Tobin MJ, Mechanical Ventilation International Study Group. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287(3):345–55. https://doi.org/10.1001/jama.287.3.345.
Rezoagli E, McNicholas BA, Madotto F, Pham T, Bellani G, Laffey JG, LUNG SAFE Investigators, the ESICM Trials Group. Presence of comorbidities alters management and worsens outcome of patients with acute respiratory distress syndrome: insights from the LUNG SAFE study. Ann Intensive Care. 2022;12(1):42. https://doi.org/10.1186/s13613-022-01015-7.
Vincent JL, Akça S, De Mendonça A, Haji-Michael P, Sprung C, Moreno R, Antonelli M, Suter PM, SOFA Working Group. Sequntial organ failure assessment. The epidemiology of acute respiratory failure in critically ill patients(*). Chest. 2002;121(5):1602–9. https://doi.org/10.1378/chest.121.5.1602.
Grasselli G, Calfee CS, Camporota L, Poole D, Amato MBP, Antonelli M, Arabi YM, Baroncelli F, Beitler JR, Bellani G, Bellingan G, Blackwood B, Bos LDJ, Brochard L, Brodie D, Burns KEA, Combes A, D’Arrigo S, De Backer D, Demoule A, Einav S, Fan E, Ferguson ND, Frat JP, Gattinoni L, Guérin C, Herridge MS, Hodgson C, Hough CL, Jaber S, Juffermans NP, Karagiannidis C, Kesecioglu J, Kwizera A, Laffey JG, Mancebo J, Matthay MA, McAuley DF, Mercat A, Meyer NJ, Moss M, Munshi L, Myatra SN, Ng Gong M, Papazian L, Patel BK, Pellegrini M, Perner A, Pesenti A, Piquilloud L, Qiu H, Ranieri MV, Riviello E, Slutsky AS, Stapleton RD, Summers C, Thompson TB, Valente Barbas CS, Villar J, Ware LB, Weiss B, Zampieri FG, Azoulay E, Cecconi M, European Society of Intensive Care Medicine Taskforce on ARDS. ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023;49(7):727–59. https://doi.org/10.1007/s00134-023-07050-7.
Pelosi P, Cadringher P, Bottino N, Panigada M, Carrieri F, Riva E, Lissoni A, Gattinoni L. Sigh in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;159(3):872–80. https://doi.org/10.1164/ajrccm.159.3.9802090.
Pelosi P, Bottino N, Chiumello D, Caironi P, Panigada M, Gamberoni C, Colombo G, Bigatello LM, Gattinoni L. Sigh in supine and prone position during acute respiratory distress syndrome. Am J Respir Crit Care Med. 2003;167(4):521–7. https://doi.org/10.1164/rccm.200203-198OC.
Baldwin DN, Suki B, Pillow JJ, Roiha HL, Minocchieri S, Frey U. Effect of sighs on breathing memory and dynamics in healthy infants. J Appl Physiol (1985). 2004;97(5):1830–9. https://doi.org/10.1152/japplphysiol.00298.2004.
Spieth PM, Güldner A, Huhle R, Beda A, Bluth T, Schreiter D, Ragaller M, Gottschlich B, Kiss T, Jaber S, Pelosi P, Koch T, de Abreu MG. Short-term effects of noisy pressure support ventilation in patients with acute hypoxemic respiratory failure. Crit Care. 2013;17(5):R261. https://doi.org/10.1186/cc13091.
Blanch L, Villagra A, Sales B, Montanya J, Lucangelo U, Luján M, García-Esquirol O, Chacón E, Estruga A, Oliva JC, Hernández-Abadia A, Albaiceta GM, Fernández-Mondejar E, Fernández R, Lopez-Aguilar J, Villar J, Murias G, Kacmarek RM. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med. 2015;41(4):633–41. https://doi.org/10.1007/s00134-015-3692-6.
Brack T, Jubran A, Tobin MJ. Dyspnea and decreased variability of breathing in patients with restrictive lung disease. Am J Respir Crit Care Med. 2002;165(9):1260–4. https://doi.org/10.1164/rccm.2201018.
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346(17):1281–6. https://doi.org/10.1056/NEJMoa012835.
Rezoagli E, Laffey JG, Bellani G. Monitoring lung injury severity and ventilation intensity during mechanical ventilation. Semin Respir Crit Care Med. 2022;43(3):346–68.
Sinha P, Calfee CS, Beitler JR, Soni N, Ho K, Matthay MA, Kallet RH. physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2019;199(3):333–41. https://doi.org/10.1164/rccm.201804-0692OC.
Spinelli E, Kircher M, Stender B, Ottaviani I, Basile MC, Marongiu I, Colussi G, Grasselli G, Pesenti A, Mauri T. Unmatched ventilation and perfusion measured by electrical impedance tomography predicts the outcome of ARDS. Crit Care. 2021;25(1):192. https://doi.org/10.1186/s13054-021-03615-4.
Gattinoni L, Vagginelli F, Carlesso E, Taccone P, Conte V, Chiumello D, Valenza F, Caironi P, Pesenti A, Prone-Supine Study Group. Decrease in PaCO2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit Care Med. 2003;31(12):2727–33. https://doi.org/10.1097/01.CCM.0000098032.34052.F9.
Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195(4):438–42. https://doi.org/10.1164/rccm.201605-1081CP.
Mascheroni D, Kolobow T, Fumagalli R, Moretti MP, Chen V, Buckhold D. Acute respiratory failure following pharmacologically induced hyperventilation: an experimental animal study. Intensive Care Med. 1988;15(1):8–14. https://doi.org/10.1007/BF00255628.
Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit Care Med. 2012;40(5):1578–85. https://doi.org/10.1097/CCM.0b013e3182451c40.
Costa ELV, Slutsky AS, Brochard LJ, Brower R, Serpa-Neto A, Cavalcanti AB, Mercat A, Meade M, Morais CCA, Goligher E, Carvalho CRR, Amato MBP. Ventilatory variables and mechanical power in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021;204(3):303–11. https://doi.org/10.1164/rccm.202009-3467OC.
Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, Brochard L, Richard JC, Lamontagne F, Bhatnagar N, Stewart TE, Guyatt G. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303(9):865–73. https://doi.org/10.1001/jama.2010.218.
Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8.
Acknowledgements
None.
Pleural Pressure Working Group (PLUG) collaborators
Riccardo Colombo MD (Department of Anesthesiology and Intensive Care, ASST Fatebenefratelli Sacco, Milan, Italy), Andrea Cortegiani MD (Section of Anesthesia, Analgesia, Intensive Care and Emergency, Department of Surgical, Oncological and Oral Science, Policlinico Paolo Giaccone, University of Palermo, Palermo, Italy), Jian-Xin Zhou MD (Department of Critical Care Medicine, Beijing Tiantan Hospital, Capital Medical University, Beijing, China), Rocco D’Andrea MD (Department of Anesthesiology, Intensive Care and Transplants, University Hospital St. Orsola-Malpighi, Bologna, Italy), Italo Calamai MD (Anesthesia and Intensive Care Unit AUSL Toscana Centro, Ospedale San Giuseppe, Empoli, Italy), Ánxela Vidal González MD (the Hospital Universitario Fundación Jiménez Díaz de Madrid, Madrid, Spain), Oriol Roca MD (Critical Care Department, Vall d’Hebron University Hospital, Vall d’Hebron Research Institute, Universitat Autònoma de Barcelona, Barcelona, Spain; the Ciber Enfermedades Respiratorias (CibeRes), Instituto de Salud Carlos III, Madrid, Spain), Domenico Luca Grieco MD (the Department of Anesthesiology and Intensive Care Medicine, Catholic University of the Sacred Heart, IRCCS Fondazione Policlinico A. Gemelli, Rome, Italy), Tomas Jovaisa MD (the Critical Care Service, Anaes- thetics Division, Barking Havering and Redbridge University Hospitals NHS Trust, London, United Kingdom), Dimitrios Bampalis MD (the Intensive Care Unit, Larissa General Hospital, Larissa, Greece), Tobias Becher MD (the Klinik für Anästhesiologie und Operative Intensivmedizin, Universitätsklinikum Schleswig-Holstein, Campus Kiel, Kiel, Germany), Denise Battaglini MD (Department of Surgical Sciences and Integrated Diagnostics, University of Genoa, Genoa, Italy; Anesthesia and Intensive Care, San Martino Policlinico Hospital, IRCCS for Oncology and Neurosciences, Genoa, Italy), Huiqing Ge MD (Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, Chin), Mariana Luz MD (Intensive Care Department, Hospital da Mulher, Salvador, Bahia, Brazil; the Intensive Care Department, Hospital Universitário Professor Edgard Santos, Universidade Federal da Bahia, Salvador, Bahia, Brazil), Jean-Michel Constantin MD (Sorbonne University, GRC 29, AP-HP, DMU DREAM, Department of Anesthesiology and Critical Care, Pitié-Salpêtrière Hospital, Paris, France), Marco Ranieri MD (Department of Anesthesiology, Intensive Care and Transplants, University Hospital St. Orsola-Malpighi, Bologna, Italy), Claude Guerin MD (Médecine Intensive-Réanimation Groupement Hospitalier Edouard Herriot, Université de Lyon Faculté de Médecine Lyon-Est, Lyon, France), Jordi Mancebo MD (the Servei de Medicina Intensiva, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona (UAB), Barcelona, Spain), Paolo Pelosi MD (Department of Surgical Sciences and Integrated Diagnostics, University of Genoa, Genoa, Italy; Anesthesia and Intensive Care, San Martino Policli- nico Hospital, IRCCS for Oncology and Neurosciences, Genoa, Italy)
Funding
The study was supported, in part, by the ESICM Clinical Research Award 2016 (TM) and, in part, by Italian Ministry of Health—Current research IRCCS, Rome, Italy (AP, GG, TM).
Author information
Authors and Affiliations
Consortia
Contributions
E.R. conceived the study, analyzed and interpreted data, wrote the manuscript. C.F. analyzed data, reviewed and edited the manuscript; R.F., G.G., C.A.V., P.N., R.K., L.B. and A.P. interpreted data and reviewed and edited the manuscript; T.M. and G.F. conceived the study, interpreted data, and reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study was conducted according to the principles of the Declaration of Helsinki, and it was approved by the Ethics Committee of the Coordinating Center Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico of Milan, Italy (ref. 318/2017). The institutional review boards of all participating centers approved the trial. Informed consent was obtained for each patient according to local regulations.
Consent for publication
Not applicable.
Competing interests
E.R. received fees from Draeger Medical, BURKE&BURKE and PALL outside of the present work. G.G. received funding from Fischer&Paykel, MSD, Pfizer, and received fees from Getinge, Draeger Medical, Cook, MundiPharma, Fischer&Paykel, Pfizer outside of the present work. P.N. research lab received grants/research equipment from Draeger, Intersurgical SPA, and Gilead. P.N. receives royalties from Intersurgical SPA for the Helmet Next invention, and received speaking fees from Getinge, Mindray, Intersurgical SPA, Gilead, GSK and Draeger outside of the present work. T.M. received personal fees from Fisher & Paykel, Dräger, and Mindray outside of the present work. G.F. received fees from Draeger Medical, DIMAR and Siaretron outside of the present work. The other authors have no conflict of interest to declare. The sponsors had no role in the design of the study; the collection, analysis, and interpretation of data; the writing of the manuscript; or the decision to submit it.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rezoagli, E., Fornari, C., Fumagalli, R. et al. Heterogeneous impact of Sighs on mortality in patients with acute hypoxemic respiratory failure: insights from the PROTECTION study. Ann. Intensive Care 14, 153 (2024). https://doi.org/10.1186/s13613-024-01385-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-024-01385-0