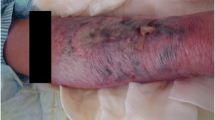
Abstract
In this review, we aimed to comprehensively summarize current literature on pathophysiology, relevance, diagnosis and treatment of fluid accumulation in patients with sepsis/septic shock. Fluid accumulation syndrome (FAS) is defined as fluid accumulation (any degree, expressed as percentage from baseline body weight) with new onset organ-failure. Over the years, many studies have described the negative impact of FAS on clinically relevant outcomes. While the relationship between FAS and ICU outcomes is well described, uncertainty exists regarding its diagnosis, monitoring and treatment. A stepwise approach is suggested to prevent and treat FAS in patients with septic shock, including minimizing fluid intake (e.g., by limiting intravenous fluid administration and employing de-escalation whenever possible), limiting sodium and chloride administration, and maximizing fluid output (e.g., with diuretics, or renal replacement therapy). Current literature implies the need for a multi-tier, multi-modal approach to de-resuscitation, combining a restrictive fluid management regime with a standardized early active de-resuscitation, maintenance fluid reduction (avoiding fluid creep) and potentially using physical measures such as compression stockings.
Trial registration: Not applicable.
Graphical Abstract

Similar content being viewed by others
Introduction
Fluids are widely used in critically ill patients to restore hemodynamic stability and tissue perfusion [1]. Fluid accumulation (FA) is very common in critical illness and occurs in at least 20% of the ICU population, particularly in patients with increased capillary leak due sepsis and septic shock [2, 3]. Many studies have previously described the negative impact of fluid accumulation syndrome (FAS) on clinically relevant outcomes. Several observational trials [4,5,6,7,8,9], as well as one meta-analysis (of mainly observational trials) [10], suggest that FAS is associated with increased mortality in critically ill patients. However, current RCTs on the topic have not found a mortality benefit with restrictive fluid management [11, 12] and protocolized de-resuscitation [13]. While FAS is a well described entity in critical care, with a serious impact on patient outcomes, its monitoring, prevention and treatment is less well described, with much uncertainty. In this review, we aimed to comprehensively summarize current literature on pathophysiology, relevance, diagnosis and treatment of fluid accumulation in patients with sepsis/septic shock in line with the ROSE framework (with the resuscitation—optimization—stabilization and evacuation phases) [14, 15].
What is FAS?
Fluid accumulation may be defined and calculated by dividing the cumulative fluid balance by the baseline body weight. Please see Fig. 1 for a critical appraisal of this definition. FAS is defined as any degree of fluid accumulation (expressed as a percentage) with new onset organ failure (which may be described by a sequential organ failure assessment (SOFA) organ sub-score equal to or greater than 3) that may be due to FA, [16]. Most organ systems, including the lungs, heart, and gastrointestinal tract, are negatively affected by FAS (see Fig. 2 for an overview) [16].
Potential adverse consequences of fluid accumulation. Adapted with permission from Malbrain et al. according to the Open Access CC BY License 4.0 [15,16,17]. Effects mentioned are related to the setting of sepsis, capillary leak and fluid accumulation. I.e. the numbness refers to the presence of peripheral edema and anasarca that may cause skin conduction disturbances, compression of nerves, reduced blood flow and reduced mobility. Additionally, severe and prolonged fluid imbalances can lead to a range of health issues and complications, including electrolyte imbalances, which may indirectly affect the body's ability to respond to stress, including the production of cortisol by the adrenal glands. APP: abdominal perfusion pressure (MAP minus IAP), RSB: rapid shallow breathing, HCS: hepatic congestion, GRV: gastro-esophageal reflux, CARS: cardiac-renal syndrome, AKI: acute kidney injury, JVP: jugular venous pressure, HJR: hepato-jugular reflux
In sepsis and septic shock, a cascade of circulatory effects, such as peripheral vasodilatation, myocardial depression, and increased metabolism, lead to an imbalance between systemic oxygen delivery and oxygen demand, resulting in global tissue hypoxia or shock [18, 19]. Sepsis and septic shock are not always associated with a volume depleted state, but rather it is the microcirculatory alterations together with vasodilatation and potentially cardiac dysfunction (myocardial depression) that lead to a reduction in stressed volume and cardiac output [20]. Stressed volume refers to the portion of circulating volume that actively contributes to tissue perfusion by being in direct contact with the vessel walls and thus exerting pressure against the walls [21,22,23]. Thus, the idea of fluid resuscitation in patients with sepsis is to increase the stressed volume and mean systemic filling pressure (Pmsf), thereby increasing cardiac preload via an increased gradient for venous return [the difference between Pmsf and central venous pressure (CVP)] [20]. In an international observational study it was shown that on average around fifty percent of ICU patients showed a response to a fluid bolus [24]. Importantly, while the ANDROMEDA-Shock study revealed that initially more than 50% of their study population was fluid responsive, this proportion decreased substantially during the intervention period [25, 26]. In clinical practise however, a large multi-centre cohort study demonstrated that a significant percentage of actually fluid unresponsive patients receive IV fluids on the ICU (approximately 50%) [24].
A profound inflammatory state, such as sepsis and septic shock, results in the activation of inflammatory mediators that initiate and perpetuate the degradation of endothelial glycocalyx, causing capillary leak [27]. The latter also decreases colloid oncotic pressure and impacts fluid haemostasis. Recent data suggest that IV fluid administration may promote this effect [27], leading to a vicious cycle through amplification of endothelial dysfunction.
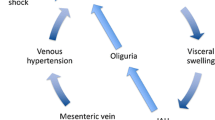
A further factor warranting consideration is organ congestion. The clinical impact of “venous congestion” in sepsis/septic shock is well described in the kidneys [28,29,30]. In a physiological state, both kidneys receive roughly a quarter of the cardiac output, whereas in a shocked state, such as the first phase of sepsis/septic shock (R and O of the ROSE model), this can decrease to 10% or less in order to divert blood flow away from the renal bed [31,32,33]. Urinary output/kidney function may decline as a result. However, in the stabilization and de-resuscitation phases of sepsis/septic shock, (phases S and E of the ROSE model) urinary output may still remain low despite the initial shock state having improved. However, in these stages, “renal venous congestion”, may cause impaired urinary output and kidney function i.e., because of FAS, intra-abdominal hypertension, abdominal compartment syndrome and/or right heart failure. This impaired renal venous outflow triggered kidney injury is associated with mortality in the critically ill [28, 29]. Unfortunately, the still low urinary output/impaired kidney function may (wrongly) entice physicians to administer additional IV fluid with the aim of increasing urinary output, creating a vicious cycle.
How do I recognize and monitor FAS?
There are many modalities to diagnose and monitor FAS, but there is currently no gold standard [34]. Clinical examination may provide valuable clues for detection of fluid accumulation, such as peripheral (pitting or anasarc) edema, respiratory distress and (prolonged) circulatory failure without clear lung/cardiac pathology. These are rather non-specific and may not reflect intravascular fluid status and are hard to distinguish from other causes of organ failure. Focussed ultrasonography and echocardiography may provide further insights whether organ failure might be related to fluid accumulation (i.e., VExUS score, increased end-diastolic volumes, inferior vena cava collapsibility index). Chest radiographs have been one of the most commonly used (albeit non-specific) tests to evaluate hypervolemia. Radiographic signs of volume overload include dilated upper lobe vessels, cardiomegaly, interstitial edema, enlarged pulmonary artery, pleural effusions, alveolar edema, prominent superior vena cava, and Kerley B-lines [35]. Bedside ultrasonography can examine all these signs and is a useful diagnostic tool for assessing pulmonary congestion [35, 36]. Advanced cardiac monitoring tools that may be of use include transpulmonary thermodilution [e.g., with PICCO (Getinge, Sölna, Sweden) or Volumeview (Edwards Lifesciences, Irvine, California, USA)] that provides information on extravascular lung water and pulmonary vascular permeability index. Furthermore, bioelectrical impedance analysis (BIA) is a less well known, but fully non-invasive, and inexpensive monitor [37]. BIA is a conductive property-based method of detecting soft tissue hydration with a 2–3% measurement error [37,38,39]. It is considered an easy and sensitive method to assess total body water (TBW), extracellular water content (ECW), intracellular water content (ICW), the ECW/ICW ratio overhydration (OH), volume excess, body cell mass (BCM) and derived phase angle [40,41,42,43].. Further validation of this tool in clinical practice and specific patient populations is needed but initial studies in ICU patients show promising results [43, 44]. Depending on the clinical context and resources available, the authors suggest a combination of non-invasive parameters like cumulative fluid balance, clinical assessment, BIA-derived parameters and ultra-sound/echocardiography for initial assessment and monitoring of FA.
How can FAS be prevented?
Prevention of FAS is probably the best treatment, please see Table 1 for an overview on prophylactic measures. Currently proposed strategies are de-escalation/ minimization, fluid restriction and small fluid resuscitation by means of albumin [45].
De-escalation/minimization implies limiting fluid intake to avoid unnecessary intravenous fluid administration i.e. by only administrating IV fluids to patients who are hypovolemic, fluid responsive and show signs of shock with tissue hypoxia. Additionally, early administration of norepinephrine was shown to have a positive effect on cumulative fluid balance in a propensity-score matched analysis in 337 patients where patients were allocated either to the very early vasopressor group (< 1 h) or a delayed vasopressor group [48]. A recent study found that a bolus of fluid of the same volume has a greater hemodynamic effect and increase in mean systemic filling pressure at a high dose than at a low dose of norepinephrine during septic shock confirming a synergistic effect [49]. However, further research is needed.
Further measures are i.e., to switch medication from intravenous to oral (or nasogastric) where possible [14, 15] to minimize fluid creep (fluids given with medication and flushes), to use concentrated parenteral or enteral nutrition formulas, and to only give maintenance fluids that are required (see Table 2 for a definition of fluid types). Fluid creep and maintenance fluids are substantial contributors to overall fluid balance (> 60%), and thus a reduction in creep/maintenance fluid may considerably reduce total fluid input [50]. Fluid creep and maintenance fluids impose also a substantial sodium (and chloride) burden and may thereby perpetuate fluid retention [50]. Choosing a low salt maintenance fluid strategy resulted in 0.6 L less fluid accumulation in healthy volunteers within 48 h, and almost 1 L less in peri-operative patients [51,52,53]. Another important potential intervention is the use of more concentrated, high density nutritional formulations (2 kcal/mL). Nutrition accounts, on average, for approximately 25–33% of the total fluid intake in critically ill patients [50]. As current guidelines propose some form of enteral feeding (e.g., trophic) as early as possible [54], fluids administered with nutrition are a significant contributor to overall fluid balance. Thus, changing to a more concentrated enteral formula would allow for a substantial reduction in overall fluid intake [55]. These concepts warrant evaluation in high quality studies.
Another strategy proposed to prevent FAS is fluid restriction. Two large RCTs (CLASSIC, CLOVERS) failed to prove that restrictive fluid management regimens are superior to usual care in terms of mortality [12, 57]. The failure to change clinical outcomes may be explained by the substantial volume of fluids administered to patients before randomization (i.e., in the emergency or operating room) and the lack of minimizing fluid creep in the trial. Furthermore, as many pragmatic trials, the CLASSIC trial was not able to demonstrate a clear separation of total fluid volumes administered between the restrictive versus liberal groups. In addition, what has been defined as “restrictive” in one trial may have been “liberal” in another due to the lack of a current gold standard definition. In a recent meta-analysis of 13 RCTs, including almost 4000 patients, adverse events were similar in the liberal and restrictive fluid management groups [58]. However, the number of patients harmed by fluid restriction was similar to the number of patients it helped; potential benefit or harm cannot be excluded thus [58]. Similar results were found in a Bayesian analysis of the CLASSIC trial data [59].
Another measure to prevent FAS may lie in the administration of 20% albumin, which was shown to increase intravascular volume twofold [60]. In the clinical setting, the SWIPE, ALBIOS and even the SAFE trials demonstrated better fluid balances with the use of albumin [61,62,63], but without improving mortality [63].
How can we treat FAS?
De-resuscitation specifically refers to late goal-directed fluid removal together with a late conservative fluid management strategy (see Table 2 for definitions), that involves active fluid removal using diuretics and renal replacement therapy (RRT) with net ultrafiltration [45]. The goal in treating FAS should be to increase diuresis and/or fluid removal, preferably following a multi-modal, multi-tier approach, as illustrated in Table 3.
There is currently no established de-escalation or de-resuscitation strategy in the critically ill literature, and high quality RCTs are scarce. Several studies have demonstrated that a more progressive use of loop diuretics to achieve a greater volume of fluid removal in fluid overloaded patients (with or without AKI) is associated with improved outcomes [5, 64,65,66]. This is also true for critically ill patients that are still on vasopressor support [67]. Hence, the authors suggest that therapy with furosemide may be started independent of actual vasopressor doses once the criteria for de-resuscitation are met (i.e., the patient has FAS with venous congestion, is hemodynamically stable, fluid unresponsive, and shows no signs of tissue hypoperfusion). The dosing regimen for diuretic therapy should be based on pharmacodynamics and pharmacokinetic considerations, whereas the dose is dependent on the patient’s kidney function, previous exposure to the drug, and potential tolerance [68]. Doses may be titrated to achieve an output that is greater than the input [69]. If the response to furosemide therapy is limited, evidence from the heart failure population shows a combination therapy of diuretics may be considered using spironolactone, acetazolamide, or indapamide but this needs to be studied in mixed ICU poulations (Table 3) [70, 71]. Other studies showed the beneficial effect on fluid removal by using hyperoncotic 20% albumin preceding furosemide use [72], or the combination of PEEP levels set to counteract IAP, followed by 20% albumin and furosemide (PAL treatment) [45]. While there is conflicting data on the effect of PEEP levels on pulmonary edema. On the one hand, alveolar recruitment induced by PEEP may have effects on alveolar vessels. On the other hand, high PEEP may also be responsible of an increase in CVP, which represent the downstream pressure of the lymphatic drainage and may promote fluid accumulation. The PAL treatment as described herein was used in patients with increased IAP and the PEEP (in cmH2O) was set at the level of IAP (in mmHg) to counterbalance and neutralise the effects at the level of the diaphragm. This strategy also takes in to account the fact that on average the pressure transmission from the abdomen to thorax compartment is about 35–50%, since the conversion factor between cmH2O and mmHg is 1.36 (or thus 36%) [73]. However, the clinician should be aware of the interplay between IAP, PEEP and lymphatic drainage as well as heart–lung-abdomen interactions. The presence of mechanical ventilation with high PEEP reduces the lymph drainage further which together with the increase in IAP decreases the lymphatic pressure gradient in the splanchnic regions, thereby promoting fluid accumulation [74,75,76].
If a patient is already on RRT, mechanical fluid removal should be started if the patient meets the criteria for FAS. The total amount of fluid to be removed should be calculated based on the cumulative fluid balance and the current hemodynamic response. Once the criteria for de-resuscitation are met, active fluid removal by RRT should start, independent of the patient’s current vasopressor dose, as hypotension itself is not a criterion for hypovolaemia [67, 77]. Alternatively, intermittent haemodialysis may be used. Fluid removal is titrated to achieve a daily output that is greater than the input.
Transcapillary refill rate or plasma refill rate, which depends on the distribution of excess fluids (intravascular vs extravascular), should be considered for “dosing” of active de-resuscitation by means of diuretic therapy or RRT [78]. Loop diuretics mainly reduce circulating blood volume and thus reduce intravascular fluid overload. In a delayed fashion (triggered by osmotic shifts), fluids then translocate from tissues (e.g., the lungs, gastrointestinal (GI) tract) when the plasma refill rate is exceeded. RRT reduces both water and osmotically active molecules, thus the efficiency of water removal by RRT mainly depends on transcapillary refill rate [78]. A repaired and intact glycocalyx is required for fluid to remain intravascular [79, 80].
As an adjunct to fluid minimization and active de-resuscitation therapy, the application of leg compression bandages may be considered with the rationale of increasing the interstitial pressure and, therefore, reducing capillary leak, unless contraindications exist. Additionally, lymphatic drainage may be increased. This method was successful in patients with septic shock as well as liver transplant recipients [81, 82], however, firm evidence is still awaited. Importantly, contraindications for leg compressions, such as peripheral arterial disease, a history of peripheral bypass, and local skin or soft tissue conditions should be recognized [83].
Whether fluid de-resuscitation actually leads to improved critical care outcomes is currently uncertain. A recently published meta-analysis on de-resuscitation in patients with septic shock showed no difference in survival with the use of de-resuscitation measures [3]. The signal favours usual care over fluid de-resuscitation in this analysis [3]. However, in this investigation, only three out of five RCTs currently published on active de-resuscitation measures in patients with sepsis/septic shock achieved fluid separation between groups [3]. Silversides and colleagues evaluated in the RADAR-2 trial the feasibility of active fluid removal in the general ICU population and demonstrated a significant fluid separation in the intervention group [84]. However, the trial was not designed to assess patient-centred outcomes. The latter trial combined pharmacological and mechanical (RRT) measures to achieve de-resuscitation [84]. Recently, the POINTCARE-2 study, a stepped wedge cluster open-label randomized controlled trial, and the first published de-resuscitation study which was powered to assess clinical outcomes, revealed that a structured de-resuscitation protocol that combined a weight-driven fluid restriction, diuretics and ultrafiltration did not reduce 60-day mortality [13]. Of the pre-defined safety outcomes, only hypernatremia was more frequent in the structured de-resuscitation group [13]. A further large multi-centre study in the general ICU population investigating early goal-directed therapy with is currently recruiting and has already included more than 50% of the required patients [85]. Further high-quality studies on de-resuscitation measures evaluating patient-centred outcomes are highly warranted.
When shall I start and stop FAS treatment?
Fluid de-resuscitation should only start when the patient is fluid unresponsive, signs of tissue hypoperfusion are absent, and signs of FAS present. The main concern for fluid removal that is too early or too fast is hypovolemia and the subsequent hemodynamic instability and tissue hypoperfusion. There is currently no gold standard for the safety criteria of fluid de-resuscitation. Some potential criteria for when to start and when to stop FAS treatment are shown in Fig. 3.
The 4 phases conceptual ROSE model and deleterious effects of fluid accumulation syndrome. Adapted with permission from Malbrain et al. according to the Open Access CC BY Licence 4. 0 [15,16,17]. IAP: intra-abdominal pressure, BIA: bio-impedance analysis, COP: colloid oncotic pressure, ECW/ICW: extracellular/intracellular water, EVLWI: extra-vascular lung water index, GEDVI: global end-diastolic volume index, IVCCI: inferior vena cava collapsibility index, LVEDAI: left ventricular end-diastolic area index, MAP: mean arterial pressure, OCS: ocular compartment syndrome, PAOP: pulmonary artery occlusion pressure. PLR: passive leg raising, PPV: pulse pressure variation, PVPI: pulmonary vascular permeability index, RVEDVI: right ventricular end-diastolic volume index, RVR: renal vascular resistance, ScvO2: central venous oxygen saturation, SvO2: mixed venous oxygen saturation, SV: stroke volume, SVV: stroke volume variation
Conclusions
This comprehensive review underlines the importance of FAS in patients with sepsis/septic shock. There is currently a lack of international consensus on diagnosis and monitoring tools of FAS. Prevention of FAS is as important as treatment. Therefore, a differentiated individualized stepwise approach, including minimizing fluid intake (e.g., limiting IV fluids and de-escalation whenever possible) and maximizing fluid output (e.g., by diuretics or combination therapy or renal replacement therapy with net ultrafiltration) depending on the patient’s current phase of septic shock and fluid requirement is needed. Treatment of FAS is symptomatic as there are currently no viable treatment options for the underlying problem of capillary leakage/increased vascular permeability. This huge knowledge gap requires research and evidence for clinicians to be able to therapeutically target the underlying disease and pathophysiology. However, with no such therapeutic targets and targeted inventions available, the only option is symptomatic treatment. Symptomatic treatment is extremely complex and requires a personalized approach as we try to treat the right patient (depending on the underlying disease, i.e. sepsis/septic shock) at the right time (i.e., stages of the ROSE model) with the right intervention (fluids, preventive measures, de-resuscitation measures). Thus, fluid management strategies should not be “liberal” or “restrictive” but patient-centered and individualized.
Future studies should focus on the different triggers, targets and safety limits to initiate and stop de-resuscitation, as well as the potential side effects of de-resuscitation that is inappropriately early, late, too rapid, too long, too little, or too liberal. The impact of FAS and de-resuscitation on capillary leak and the integrity of the endothelial glycocalyx requires further investigation, as does the joint application of vasopressors with fluid therapy (indication, dose, duration, and how to balance with fluid therapy).
Availability of data and materials
Not applicable.
References
Messmer AS, Zingg C, Müller M, Gerber JL, Schefold JC, Pfortmueller CA. Fluid overload and mortality in adult critical care patients-a systematic review and meta-analysis of observational studies. Crit Care Med. 2020;48(12):1862–70.
Messmer AS, Moser M, Zuercher P, Schefold JC, Müller M, Pfortmueller CA. Fluid overload phenotypes in critical illness-a machine learning approach. J Clin Med. 2022;11(2):336.
Messmer AS, Dill T, Müller M, Pfortmueller CA. Active fluid de-resuscitation in critically ill patients with septic shock: a systematic review and meta-analysis. Eur J Intern Med. 2023;109:89–96.
Chao W-C, Tseng C-H, Chien Y-C, Sheu C-C, Tsai M-J, Fang W-F, et al. Association of day 4 cumulative fluid balance with mortality in critically ill patients with influenza: a multicenter retrospective cohort study in Taiwan. PLoS ONE. 2018;13(1): e0190952.
Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6(5):966–73.
Han MJ, Park KH, Shin J-H, Kim SH. Influence of daily fluid balance prior to continuous renal replacement therapy on outcomes in critically ill patients. J Korean Med Sci. 2016;31(8):1337–44.
Neyra JA, Li X, Canepa-Escaro F, Adams-Huet B, Toto RD, Yee J, et al. Cumulative fluid balance and mortality in septic patients with or without acute kidney injury and chronic kidney disease. Crit Care Med. 2016;44(10):1891–900.
Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12(3):R74.
Teixeira C, Garzotto F, Piccinni P, Brienza N, Iannuzzi M, Gramaticopolo S, et al. Fluid balance and urine volume are independent predictors of mortality in acute kidney injury. Crit Care. 2013;17(1):R14.
Messmer AS, Zingg C, Müller M, Gerber JL, Schefold JC, Pfortmueller CA. Fluid overload and mortality in adult critical care patients—a systematic review and meta-analysis of observational studies. Crit Care Med. 2020. https://doi.org/10.1097/CCM.0000000000004617.
Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, DeBoisblanc B, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–75.
Meyhoff TS, Hjortrup PB, Wetterslev J, Sivapalan P, Laake JH, Cronhjort M, et al. Restriction of intravenous fluid in ICU patients with septic shock. N Engl J Med. 2022;386(26):2459–70.
Bollaert PE, Monnier A, Schneider F, Argaud L, Badie J, Charpentier C, et al. Fluid balance control in critically ill patients: results from POINCARE-2 stepped wedge cluster-randomized trial. Crit Care. 2023;27(1):66.
Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46(5):361–80.
Malbrain M, Van Regenmortel N, Saugel B, De Tavernier B, Van Gaal PJ, Joannes-Boyau O, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care. 2018;8(1):66.
Malbrain M, Martin G, Ostermann M. Everything you need to know about deresuscitation. Intensive Care Med. 2022;48(12):1781–6.
Malbrain M, Langer T, Annane D, Gattinoni L, Elbers P, Hahn RG, et al. Intravenous fluid therapy in the perioperative and critical care setting: executive summary of the International Fluid Academy (IFA). Ann Intensive Care. 2020;10(1):64.
Beal AL, Cerra FB. Multiple organ failure syndrome in the 1990s: systemic inflammatory response and organ dysfunction. JAMA. 1994;271(3):226–33.
Annane D, Bellissant E, Cavaillon J-M. Septic shock. Lancet. 2005;365(9453):63–78.
Marik P, Bellomo R. A rational approach to fluid therapy in sepsis. Br J Anaesth. 2016;116(3):339–49.
Funk DJ, Jacobsohn E, Kumar A. The role of venous return in critical illness and shock-part I: physiology. Crit Care Med. 2013;41(1):255–62.
Persichini R, Lai C, Teboul JL, Adda I, Guérin L, Monnet X. Venous return and mean systemic filling pressure: physiology and clinical applications. Crit Care. 2022;26(1):150.
Magder S. Volume and its relationship to cardiac output and venous return. Crit Care. 2016;20(1):271.
Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, et al. Fluid challenges in intensive care: the FENICE study: a global inception cohort study. Intensive Care Med. 2015;41(9):1529–37.
Hernández G, Ospina-Tascón GA, Damiani LP, Estenssoro E, Dubin A, Hurtado J, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA. 2019;321(7):654–64.
Kattan E, Ospina-Tascón GA, Teboul JL, Castro R, Cecconi M, Ferri G, et al. Systematic assessment of fluid responsiveness during early septic shock resuscitation: secondary analysis of the ANDROMEDA-SHOCK trial. Crit Care. 2020;24(1):23.
Hippensteel JA, Uchimido R, Tyler PD, Burke RC, Han X, Zhang F, et al. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit Care. 2019;23(1):259.
Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 2016;12(10):610–23.
Bielecka-Dabrowa A, Godoy B, Schefold JC, Koziolek M, Banach M, von Haehling S. Decompensated heart failure and renal failure: what is the current evidence? Curr Heart Fail Rep. 2018;15(4):224–38.
Verbrugge FH, Dupont M, Steels P, Grieten L, Malbrain M, Tang WH, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol. 2013;62(6):485–95.
Prowle JR, Molan MP, Hornsey E, Bellomo R. Measurement of renal blood flow by phase-contrast magnetic resonance imaging during septic acute kidney injury: a pilot investigation*. Crit Care Med. 2012;40(6):1768–76.
Tristani FE, Cohn JN. Studies in clinical shock and hypotension. VII. Renal hemodynamics before and during treatment. Circulation. 1970;42(5):839–51.
Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2(2):1303–53.
Malbrain MLNG, Wilkinson J, Malbrain L, Nasa P, Wong A. Fluid accumulation and deresuscitation. In: Malbrain ML, Wong A, Nasa P, Ghosh S, editors. Rational use of intravenous fluids in critically ill patients, vol. 1. Cham: Springer; 2024.
Claure-Del Granado R, Mehta RL. Fluid overload in the ICU: evaluation and management. BMC Nephrol. 2016;17(1):109.
Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, et al. “Ultrasound comet-tail images”: a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest. 2005;127(5):1690–5.
Piccoli A. Patterns of bioelectrical impedance vector analysis: learning from electrocardiography and forgetting electric circuit models. Nutrition. 2002;18(6):520–1.
Johnson HL, Virk SP, Mayclin P, Barbieri T. Predicting total body water and extracellular fluid volumes from bioelectrical measurements of the human body. J Am Coll Nutr. 1992;11(5):539–47.
Cleymaet R, Scheinok T, Maes H, Stas A, Malbrain L, De Laet I, et al. Prognostic value of bioelectrical impedance analysis for assessment of fluid overload in ICU patients: a pilot study. Anaesthesiol Intensive Ther. 2021;53(1):10–7.
Thomas BJ, Ward LC, Cornish BH. Bioimpedance spectrometry in the determination of body water compartments: accuracy and clinical significance. Appl Radiat Isot. 1998;49(5–6):447–55.
Dabrowski W, Kotlinska-Hasiec E, Jaroszynski A, Zadora P, Pilat J, Rzecki Z, et al. Intra-abdominal pressure correlates with extracellular water content. PLoS ONE. 2015;10(4): e0122193.
Dabrowski W, Kotlinska-Hasiec E, Schneditz D, Zaluska W, Rzecki Z, De Keulenaer B, et al. Continuous veno-venous hemofiltration to adjust fluid volume excess in septic shock patients reduces intra-abdominal pressure. Clin Nephrol. 2014;82(1):41–50.
Cleymaet R, D’Hondt M, Scheinok T, Malbrain L, De Laet I, Schoonheydt K, et al. Comparison of bioelectrical impedance analysis (BIA)-derived parameters in healthy volunteers and critically ill patients. Life. 2023;14(1):27.
Moonen H, Van Zanten ARH. Bioelectric impedance analysis for body composition measurement and other potential clinical applications in critical illness. Curr Opin Crit Care. 2021;27(4):344–53.
Cordemans C, De Laet I, Van Regenmortel N, Schoonheydt K, Dits H, Martin G, et al. Aiming for a negative fluid balance in patients with acute lung injury and increased intra-abdominal pressure: a pilot study looking at the effects of PAL-treatment. Ann Intensive Care. 2012;2(Suppl 1):S15.
Finfer S, Bellomo R, McEvoy S, Lo SK, Myburgh J, Neal B, et al. Effect of baseline serum albumin concentration on outcome of resuscitation with albumin or saline in patients in intensive care units: analysis of data from the saline versus albumin fluid evaluation (SAFE) study. BMJ. 2006;333(7577):1044.
Wiedermann CJ. Phases of fluid management and the roles of human albumin solution in perioperative and critically ill patients. Curr Med Res Opin. 2020;36(12):1961–73.
Ospina-Tascón GA, Hernandez G, Alvarez I, Calderón-Tapia LE, Manzano-Nunez R, Sánchez-Ortiz AI, et al. Effects of very early start of norepinephrine in patients with septic shock: a propensity score-based analysis. Crit Care. 2020;24(1):52.
Adda I, Lai C, Teboul JL, Guerin L, Gavelli F, Monnet X. Norepinephrine potentiates the efficacy of volume expansion on mean systemic pressure in septic shock. Crit Care. 2021;25(1):302.
Van Regenmortel N, Verbrugghe W, Roelant E, Van den Wyngaert T, Jorens PG. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: a retrospective study in a tertiary mixed ICU population. Intensive Care Med. 2018;44(4):409–17.
Van Regenmortel N, De Weerdt T, Van Craenenbroeck AH, Roelant E, Verbrugghe W, Dams K, et al. Effect of isotonic versus hypotonic maintenance fluid therapy on urine output, fluid balance, and electrolyte homeostasis: a crossover study in fasting adult volunteers. Br J Anaesth. 2017;118(6):892–900.
Van Regenmortel N, Hendrickx S, Roelant E, Baar I, Dams K, Van Vlimmeren K, et al. 154 compared to 54 mmol per liter of sodium in intravenous maintenance fluid therapy for adult patients undergoing major thoracic surgery (TOPMAST): a single-center randomized controlled double-blind trial. Intensive Care Med. 2019;45(10):1422–32.
Van Regenmortel N, Langer T, De Weerdt T, Roelant E, Malbrain M, Van den Wyngaert T, et al. Effect of sodium administration on fluid balance and sodium balance in health and the perioperative setting: extended summary with additional insights from the MIHMoSA and TOPMAST studies. J Crit Care. 2022;67:157–65.
Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36(1):49–64.
Oh H, Seo W. Alterations in fluid, electrolytes and other serum chemistry values and their relations with enteral tube feeding in acute brain infarction patients. J Clin Nurs. 2007;16(2):298–307.
Aya HD, Rhodes A, Chis Ster I, Fletcher N, Grounds RM, Cecconi M. Hemodynamic effect of different doses of fluids for a fluid challenge: a quasi-randomized controlled study. Crit Care Med. 2017;45(2):e161–8.
National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury Clinical Trials Network, Shapiro NI, Douglas IS, Brower RG, et al. Early restrictive or liberal fluid management for sepsis-induced hypotension. N Engl J Med. 2023;388(6):499–510.
Sivapalan P, Ellekjaer KL, Jessen MK, Meyhoff TS, Cronhjort M, Hjortrup PB, et al. Lower vs higher fluid volumes in adult patients with sepsis: an updated systematic review with meta-analysis and trial sequential analysis. Chest. 2023;164(4):892–912.
Sivapalan P, Meyhoff TS, Hjortrup PB, Lange T, Kaas-Hansen BS, Kjaer MN, et al. Restrictive versus standard IV fluid therapy in adult ICU patients with septic shock-Bayesian analyses of the CLASSIC trial. Acta Anaesthesiol Scand. 2024;68(2):236–46.
Zdolsek M, Hahn RG. Kinetics of 5% and 20% albumin: a controlled crossover trial in volunteers. Acta Anaesthesiol Scand. 2022;66(7):847–58.
Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370(15):1412–21.
Mårtensson J, Bihari S, Bannard-Smith J, Glassford NJ, Lloyd-Donald P, Cioccari L, et al. Small volume resuscitation with 20% albumin in intensive care: physiological effects: the SWIPE randomised clinical trial. Intensive Care Med. 2018;44(11):1797–806.
Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–56.
Uchino S, Doig GS, Bellomo R, Morimatsu H, Morgera S, Schetz M, et al. Diuretics and mortality in acute renal failure*. Crit Care Med. 2004;32(8):1669–77.
Cantarovich F, Rangoonwala B, Lorenz H, Verho M, Esnault VL, High-Dose Flurosemide in Acute Renal Failure Study Group. High-dose furosemide for established ARF: a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Am J Kidney Dis. 2004;44(3):402–9.
Mehta RL, Pascual MT, Soroko S, Chertow GM, PICARD Study Group. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288(20):2547–53.
Shen Y, Zhang W, Shen Y. Early diuretic use and mortality in critically ill patients with vasopressor support: a propensity score-matching analysis. Crit Care. 2019;23(1):9.
Brater DC. Diuretic therapy. N Engl J Med. 1998;339(6):387–95.
Swissmedic. https://www.swissmedic.ch/swissmedic/en/home/services/medicinal-product-information.html. Accessed 12 Apr 2023
Verbrugge FH. Editor’s choice-diuretic resistance in acute heart failure. Eur Heart J Acute Cardiovasc Care. 2018;7(4):379–89.
Mullens W, Dauw J, Martens P, Verbrugge FH, Nijst P, Meekers E, et al. Acetazolamide in acute decompensated heart failure with volume overload. N Engl J Med. 2022;387(13):1185–95.
Martin GS, Moss M, Wheeler AP, Mealer M, Morris JA, Bernard GR. A randomized, controlled trial of furosemide with or without albumin in hypoproteinemic patients with acute lung injury. Crit Care Med. 2005;33(8):1681–7.
Malbrain ML, Roberts DJ, Sugrue M, De Keulenaer BL, Ivatury R, Pelosi P, et al. The polycompartment syndrome: a concise state-of-the-art review. Anaesthesiol Intensive Ther. 2014;46(5):433–50.
Regli A, Pelosi P, Malbrain M. Ventilation in patients with intra-abdominal hypertension: what every critical care physician needs to know. Ann Intensive Care. 2019;9(1):52.
de Carvalho EB, Battaglini D, Robba C, Malbrain M, Pelosi P, Rocco PRM, et al. Fluid management strategies and their interaction with mechanical ventilation: from experimental studies to clinical practice. Intensive Care Med Exp. 2023;11(1):44.
Malbrain ML, Pelosi P, De Laet I, Lattuada M, Hedenstierna G. Lymphatic drainage between thorax and abdomen: please take good care of this well-performing machinery. Acta Clin Belg Suppl. 2007;62(1):152–61.
Berger D, Takala J. Hypotension and hypovolemia during hemodialysis: is the usual suspect innocent? Crit Care. 2016;20(1):140.
Mitsides N, Pietribiasi M, Waniewski J, Brenchley P, Mitra S. Transcapillary refilling rate and its determinants during haemodialysis with standard and high ultrafiltration rates. Am J Nephrol. 2019;50(2):133–43.
Kundra P, Goswami S. Endothelial glycocalyx: role in body fluid homeostasis and fluid management. Indian J Anaesth. 2019;63(1):6–14.
Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012;108(3):384–94.
Mathews S, James S, Anderson JD, Merchant M, Benenati S, Henry S, et al. Effect of elastic bandage wraps on leg edema in patients before and after liver transplant. Prog Transplant. 2015;25(4):302–31.
Dargent A, Large A, Soudry-Faure A, Doise J-M, Abdulmalak C, Jonval L, et al. Corporeal Compression at the Onset of Septic shock (COCOONs): a compression method to reduce fluid balance of septic shock patients. Sci Rep. 2019;9(1):11566.
Lim CS, Davies AH. Graduated compression stockings. CMAJ Can Med Assoc J. 2014;186(10):E391–8.
Silversides JA, McMullan R, Emerson LM, Bradbury I, Bannard-Smith J, Szakmany T, et al. Feasibility of conservative fluid administration and deresuscitation compared with usual care in critical illness: the Role of Active Deresuscitation After Resuscitation-2 (RADAR-2) randomised clinical trial. Intensive Care Med. 2022;48(2):190–200.
Wichmann S, Lange T, Perner A, Gluud C, Itenov TS, Berthelsen RE, et al. Furosemide versus placebo for fluid overload in intensive care patients—the randomised GODIF trial second version: statistical analysis plan. Acta Anaesthesiol Scand. 2024;68(1):130–6.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
CAP and MLNGM performed the literature search and selected eligible trials. CAP drafted the manuscript, with all other authors co-drafting and revising the manuscript for important intellectual content. All authors approved the final version of the manuscript and agreed to submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors approved the final manuscript.
Competing interests
The authors declare no competing interests. CAPs report grants from Orion Pharma, Abbott Nutrition International, B. Braun Medical AG, CSEM AG, Edwards Lifesciences Services GmbH, Kenta Biotech Ltd, Maquet Critical Care AB, Omnicare Clinical Research AG, Nestle, Pierre Fabre Pharma AG, Pfizer, Bard Medica S.A., Abbott AG, Anandic Medical Systems, Pan Gas AG Healthcare, Bracco, Hamilton Medical AG, Fresenius Kabi, Getinge Group Maquet AG, Dräger AG, Teleflex Medical GmbH, Glaxo Smith Kline, Merck Sharp and Dohme AG, Eli Lilly and Company, Baxter, Boehringer-Ingelheim, Aseptuva, Astellas, Astra Zeneca, CSL Behring, Novartis, Covidien, and Nycomed outside the submitted work. The money was paid into departmental funds; no personal financial gain applied. NVR received unrestricted educational grants (paid to institution) by Baxter Heatlhcare and speaker’s fees from Baxter and Nestlé Healthcare. He resided in advisory boards organized by Baxter Healthcare. MLNGM is co-founder, past-President and current Treasurer of WSACS (The Abdominal Compartment Society, http://www.wsacs.org). He is member of the medical advisory Board of Pulsion Medical Systems (part of Getinge group), Serenno Medical, Potrero Medical, Sentinel Medical and Baxter. He consults for BBraun, Becton Dickinson, ConvaTec, Maltron, Spiegelberg, Medtronic, MedCaptain, and Holtech Medical, and received speaker's fees from PeerVoice and Nestlé. He holds stock options for Serenno and Potrero. He is co-founder and President of the International Fluid Academy (IFA). The IFA (http://www.fluidacademy.org ) is integrated within the not-for-profit charitable organization iMERiT, International Medical Education and Research Initiative, under Belgian law.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pfortmueller, C.A., Dabrowski, W., Wise, R. et al. Fluid accumulation syndrome in sepsis and septic shock: pathophysiology, relevance and treatment—a comprehensive review. Ann. Intensive Care 14, 115 (2024). https://doi.org/10.1186/s13613-024-01336-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-024-01336-9