Abstract
Background
Multiple randomized controlled studies have compared numerous antibiotic regimens, including new, recently commercialized antibiotics in the treatment of nosocomial pneumonia (NP). The objective of this Bayesian network meta-analysis (NMA) was to compare the efficacy and the safety of different antibiotic treatments for NP.
Methods
We conducted a systematic search of PubMed, Medline, Web of Science, EMBASE and the Cochrane Library databases from 2000 through 2021. The study selection included studies comparing antibiotics targeting Gram-negative bacilli in the setting of NP. The primary endpoint was 28 day mortality. Secondary outcomes were clinical cure, microbiological cure and adverse events.
Results
Sixteen studies encompassing 4993 patients were included in this analysis comparing 13 antibiotic regimens. The level of evidence for mortality comparisons ranged from very low to moderate. No significant difference in 28 day mortality was found among all beta-lactam regimens. Only the combination of meropenem plus aerosolized colistin was associated with a significant decrease of mortality compared to using intravenous colistin alone (OR = 0.43; 95% credible interval [0.17–0.94]), based on the results of the smallest trial included. The clinical failure rate of ceftazidime was higher than meropenem with (OR = 1.97; 95% CrI [1.19–3.45]) or without aerosolized colistin (OR = 1.40; 95% CrI [1.00–2.01]), imipemen/cilastatin/relebactam (OR = 1.74; 95% CrI [1.03–2.90]) and ceftazidime/avibactam (OR = 1.48; 95% CrI [1.02–2.20]). For microbiological cure, no substantial difference between regimens was found, but ceftolozane/tazobactam had the highest probability of being superior to comparators. In safety analyses, there was no significant difference between treatments for the occurrence of adverse events, but acute kidney failure was more common in patients receiving intravenous colistin.
Conclusions
This network meta-analysis suggests that most antibiotic regimens, including new combinations and cefiderocol, have similar efficacy and safety in treating susceptible Gram-negative bacilli in NP. Further studies are necessary for NP caused by multidrug-resistant bacteria.
Registration PROSPERO CRD42021226603
Similar content being viewed by others
Introduction
Over the two last decades, bacterial multidrug resistance (MDR) has emerged and spread widely all around the world. The burden of this issue was estimated to be around five million deaths associated with bacterial resistance in 2019 [1]. The World Health Organization emphasized that it represents one of the biggest threats to global health by putting the achievements of modern medicine at risk [2]. Among emerging MDR bacteria, Gram-negative bacilli (GNB) are at forefront of concerns [3]. Consequently, rates of infections due to third-generation cephalosporin-resistant Enterobacterales (3GCRE) have dramatically increased in most countries [4, 5].
New antibiotics have recently been developed to offer alternatives in the treatment of infections due to MDR or extensively drug resistant (XDR) GNB. However, the emergence of metallo-beta-lactamase and class D beta-lactamase producing bacteria has made antimicrobial treatment challenging despite the development of these new antibiotics.
Nosocomial pneumonia represents the second most frequent healthcare-associated infection [6], of which GNB are leading pathogens [7]. Nosocomial pneumonia (NP) includes hospitalized-acquired pneumonia (HAP) and ventilator-acquired pneumonia (VAP) which carry high attributable costs as well as high morbidity and mortality [8].
Four new antibiotics including three combinations of beta-lactam/beta-lactamase inhibitor (BLBLI)—namely ceftolozane/tazobactam, ceftazidime/avibactam, imipemen/cilastatin/relebactam—and cefiderocol have been assessed in separate randomized controlled trials (RCTs) and then commercialized in the treatment of nosocomial pneumonia [9,10,11,12]. Most of these RCTs demonstrated a non-inferiority versus comparator (meropenem or piperacillin/tazobactam), but no study compared theses different molecules face-to-face. In this Bayesian network meta-analysis (NMA), we aimed to assess the efficacy and safety of antibiotics targeting GNB used in the treatment of nosocomial pneumonia and compare them, in order to determine the best available treatment.
Material and methods
This study was a systematic review with Bayesian NMA performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations statements [13]. The protocol was registered in the PROSPERO database prior to study initiation (CRD42021226603).
Search strategy
Two authors (DLP, DC) conducted a systematic search of PubMed, Medline, Web of Science, EMBASE and the Cochrane Library databases from January 1st, 2000, to December 31st 2020 for studies comparing antibiotics in the treatment of NP, including VAP, with available data on outcomes in each group of treatment. We initially used a broad search strategy by using MeSH search terms detailed in the Additional file 6.
Among records identified, we restricted the search for articles written in English dealing with human adults. We excluded studies which were performed on animals or specific populations (pregnant women, children). We also excluded studies focusing on community-acquired pneumonia or with mixed infection types. In the remaining articles, we sought studies which reported mortality data for each group of antibiotic treatment. Particular attention was paid to the risk of duplicate reports, and whenever identified, duplicate studies were excluded.
Eligibility criteria and study selection
First, we included RCTs, clinical trials, observational comparative studies that compared outcomes in patients who had received different antibiotics targeting GNB in confirmed nosocomial pneumonia. Inclusion and exclusion criteria are fully detailed in the Additional file 6. These studies had to provide 28 day mortality rates (or if not available, in-hospital or crude mortality rates with follow-up exceeding 14 day) for each treatment group. We did not include preprints or non-peer-reviewed works.
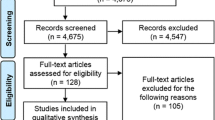
The articles were first screened by two authors (DLP, DC) independently based on title and abstract. Selected articles were assessed by full-text reviewing and studies fulfilling the predetermined inclusion criteria were included. In addition, reference lists of relevant articles were screened using the snowballing method. All disagreement over study inclusion led to discussion in order to find a consensus or, if necessary, were solved by the adjudication of a third author (DD). We excluded articles reporting subgroup analyses data that overlap with other studies. Participants, study design and comparisons of all included studies are described in Table 1.
Assessment of risk of bias
Risk of bias (RoB) for studies included in the NMA was independently assessed by two authors (DLP, DC), and a third author (DD) was solicited to solve disagreements.
Outcomes definition
The primary outcome was all causes 28 day mortality. Clinical cure, microbiological cure and the occurrence of adverse effects were assessed as secondary outcomes. Clinical cure was defined as resolution or substantial improvement of baseline symptoms/signs and the absence of additional antibiotic treatment at the end of treatment or test-of-cure visit. Microbiological cure was achieved when baseline Gram-negative pathogen(s) were eradicated or presumed to be eradicated on clinical culture specimen at the end of treatment of test-of-cure visit.
Statistical analysis
In this study, a Bayesian network meta-analysis with an unconstrained random effect model was performed. Gibbs sampler in Bayesian hierarchical model with binomial prior distribution was used to assess the consistency of estimates. Odds Ratio (ORs) with their 95% credible interval (95% CrI) was used to summarize to treatment effect. Convergence of relative treatment effects, baseline effect, and heterogeneity parameter was tested using the Brooks-Gelman-Rubin statistics. A node-splitting model was performed for all loops of the network to detect the inconsistency between direct and indirect comparisons [14].
A probabilistic analysis was also realized and summarized using the surface under the cumulative ranking curve (SUCRA), and an overall ranking based on the probability that a treatment was the most effective for the outcome of interest. A subgroup analysis was performed according to risk of bias.
Furthermore, network meta-regressions were realized to assess different treatment effect: age, kidney failure, adjunctive use of amikacin, non-fermenting GNB, severity at randomization and study design.
All statistical analysis were performed using R software version 4.0.3 (R Foundation for Statistical Computing). These analyses required the use of the following R packages: “bnma”, “rjags”, and “ggplot2”.
Results
Characteristics of included trials
A total of 5113 citations were initially identified, of which 4540 articles were screened. After screening for exclusion criteria, 114 studies were full text reviewed. Only RCTs met inclusion criteria, and all observational studies were excluded. The process of inclusion and exclusion is detailed in the PRISMA diagram (Fig. 1). Accordingly, 16 RCTs of 13 antibiotic regimens were included in the final analysis for the present NMA [9,10,11,12, 15,16,17,18,19,20,21,22,23,24,25,26].
References and characteristics of trials finally included are summarized in Table 1. A total of 4993 participants were included for the primary endpoint across 16 trials. Baseline characteristics were reported for 4568 patients of which 3215 (70.4%) were men, mean age was 59.5 years. VAP was found in 3121 (68.2%) patients enrolled in 15 of the 16 RCTs. Kidney failure, defined by a creatinine clearance < 60 ml/min, was reported in 451 patients. A bacterial pneumonia was documented in 2738 patients among 15 RCTs. Main pathogens identified were K. pneumonia (n = 745), P. aeruginosa (n = 651), E. coli (n = 361) and A. baumannii (n = 288). Clinical outcomes are compiled in Table 2.
Risk of bias assessment and certainty of evidence
Thirteen trials provided a detailed and adequate randomization process. Two studies had a major deviation from the intended intervention [19, 26]. Only one trial suffered from concerns about missing data [16]. Nine trials were open-label studies and five were evaluated by investigator in charge of included patients, which were at high risk of bias in measurement of the outcome. The risk of bias was low, intermediate and high in six, three and seven trials respectively (Fig. 2). The certainty of evidence evaluating the head-to-head comparisons between different antibiotic regimens was assessed using the GRADE approach (Additional file 1: Table S1).
Network
The visualization of network geometry of comparisons in all RCTs is shown in the Fig. 3. This network contained two closed loops consisting of three nodes and eight direct comparisons, which assessed a total of 13 antibiotic regimens.
Primary outcome: 28-day mortality
Twelve of the 16 RCTs reported precisely 28-day mortality as an endpoint. Two trials mentioned in-hospital mortality [16, 24] and two other trials reported crude mortality rates [23, 26]. Meropenem plus an adjunctive aerosolized colistin was associated with a significant decrease of mortality compared to colistin only (OR = 0.43; 95% CrI [0.17–0.94]). There were no significant differences for mortality among all others comparisons between antibiotic regimens. The results are summarized in Additional file 2: Fig. S1. None of the covariates improved the fit of the model in meta-regression analyses and therefore explained variation in treatment effects. Comparisons of antibiotic regimens using piperacillin/tazobactam as comparator are represented in Fig. 4.
The inconsistency of the model was tested on the direct comparison loops with a non-significant p-value (p = 0.96) allowing whole network estimates. The ranking of the probability for being the most effective treatment in terms of mortality is represented in Table 3. Meropenem plus aerosolized colistin had the highest likelihood of being top ranked (SUCRA = 92.0%), followed by imipenem/cilastatin (SUCRA = 72.2%) and imipenem/cilastatin/relebactam (SUCRA = 68.9%), while ciprofloxacin had the lowest (SUCRA = 24.0%). Assessment of transitivity is detailed in the Additional file 6. We found no concern about the transitivity across the comparisons. In addition, a subgroup analysis according to the risk of bias was not possible because no loop was present.
Secondary outcomes
Clinical cure
Data on clinical cure were available in all 16 included RCTs. Meropenem with (OR 1.97; 95% CrI [1.19–3.45]) or without aerosolized colistin (OR 1.40; 95% CrI [1.00–2.01]), imipemen/cilastatin/relebactam (OR 1.74; 95% CrI [1.03–2.90]) and ceftazidime/avibactam (OR 1.48; 95% CrI [1.02–2.20]) were associated with higher clinical cure rates compared to ceftazidime. Clinical cure was also higher in patients treated by meropenem plus aerosolized colistin in comparison with intravenous colistin alone (OR 1.48; 95% CrI [1.06–2.23]). There were no significant differences for clinical cure across the comparisons between other regimens (Additional file 3: Fig. S2). The inconsistency of the model was tested on the direct comparison loops with a non-significant p-value (p = 0.16) allowing whole network estimates. Meropenem plus aerosolized colistin (SUCRA 86.5%) and imipemen/cilastatin/relebactam (SUCRA 80.7%) were the two treatments with the highest probability of being superior to comparators, while ceftazidime had the lowest probability of being the most effective therapy (SUCRA 4.0%) (Table 3).
Microbiological cure
Microbiological cure was reported in 15 trials. In comparison with ceftazidime, ceftolozane/tazobactam (OR 1.62; 95% CrI [1.16–2.31]), intravenous colistin (OR 1.52; 95%CrI [1.01–2.35]) and meropenem (OR 1.41; 95% CrI [1.05–1.94]) were associated with increased microbiological cure. Patients treated by ceftolozane/tazobactam had higher microbiological cure than those treated by piperacillin/tazobactam (OR 1.42; 95% CrI [1.07–2.01]) or ceftazidime/avibactam (OR 1.33; 95% CrI [1.07–1.68]). All other comparisons between antibiotic regimens were not significant (Additional file 4: Fig. S3). The inconsistency of the model was tested on the direct comparison loops with a non-significant p-value (p = 0.06) allowing whole network estimates. Ceftolozane/tazobactam was the treatment with the highest probability of being superior to comparators (SUCRA 89.6%), while ceftazidime had the lowest (SUCRA 9.9%) (Table 3).
Adverse events
Adverse events were reported in 15 trials. There were no significant differences between treatments in the occurrence of at least one adverse event among trials. Meropenem had the highest probability of being the treatment with the fewest adverse events (SUCRA = 74.5%), while ciprofloxacin had the lowest probability (SUCRA = 4.8%) (Table 3). Information regarding the severe drug-related adverse events and drug-related discontinuation were reported in 10 and 8 trials, respectively. Additional analysis on these events were not allowed by NMA because the corresponding networks did not include any closed loop. Types of adverse events are specified in the Additional file 5: Table S2. Acute kidney injury (AKI) was reported in 13 of the 16 RCTs, and the greatest proportion of AKI occurred in patients treated by intravenous colistin (17%, N = 25/150). Data about epilepsy were available in 6 trials, of which imipenem/cilastatin had the most important seizure rate (4%, N = 10/263). Abnormal hepatic function and Clostridium difficile infections were reported in five and four studies respectively, and were uncommon (0.9, 0.8%).
Discussion
This NMA including 16 trials evaluated the efficacy and the safety of 13 antibiotic regimens for the treatment of NP in approximately 5000 hospitalized patients. For 28 day mortality, we found no substantial differences between regimens. To our knowledge, this Bayesian NMA is the first to compare the efficacy of antibiotics targeting GNB in the setting of NP. In the field of nosocomial pneumonia, almost all RCTs are non-inferiority trials, using piperacillin or carbapenem as comparator. It is therefore unlikely that new antibiotics will be compared with each other in further large randomized trials. Based on direct and indirect comparisons, our work underlines important findings.
First, there were no significant differences between new antibiotics in terms of efficacy or safety. Importantly, the lack of difference among the antibiotic regimens does not imply that they are equal. In these non-inferiority trials, new antibiotics were started as empirical treatment of nosocomial pneumonia and pursued even if an antimicrobial de-escalation was possible. Current guidelines highly recommend the use of new BLBLI or cefiderocol in MDR/XDR infections after antibiotic stewardship considerations [27]. A major issue of this NMA is that analyses were unable to compare these new antibiotics in their real-life use in cases of bacterial resistance, considering intrinsic differences in spectrum or antimicrobial activity.
Interestingly, the only significant difference in mortality analysis was found in favor of using aerosolized colistin associated to meropenem over intravenous colistin. This result relies on a small trial which included 30 patients with VAP caused by MDR K. pneumoniae in each arm. This finding is consistent with previous trial, in which IV colistin alone showed a trend towards higher in-hospital mortality compared to a combination of IV/AS colistin in the treatment of MDRGNB in ICU [28]. Two observational studies and one NMA evaluating the adjunction of AS colistin found a benefit in clinical cure and microbiological eradication in the treatment of VAP [29,30,31]. The inferiority of IV colistin in the setting of pneumonia could be explained by its poor distribution into lung parenchyma (improved with inhaled use) and its nephrotoxicity [32]. Another hypothesis could be that this finding, derived from a small-sized RCT may indicate inconsistency within the network analysis. Indeed, most registration trials included hundreds of participants and targeted non-inferiority compared to a comparator, while this RCT (conducted in a MDR setting) is the only trial included in this NMA with significant differences in mortality rates between the two arms. This could lead to an overestimation of this result.
Microbiological data is a key issue and the source of heterogeneity among the included trials. Only one study was based on proven bacterial documentation at randomization [24]. To obtain culture-documentation is one of the challenges in nosocomial pneumonia, but the studies in this NMA reported high rates of isolated pathogens. For example, in RESTORE-IMI 2 and APEKS-NP trials, pneumonias were culture-documented in more than 80% of cases. However, real-life data showed that pathogens are identified on culture only in 50% of these patients [37]. This emphasizes the discrepancies between registration trials and clinical practice attributable to selection bias, introducing heterogeneity to the analyses.
This meta-analysis compiled data on various regimen of antibiotics targeting GNB. Across the last two decades, antibiotic’s resistance has raised dramatically, especially in Enterobacterales. For example, 3GCRE have emerged and represents one third of infections in some ICUs [33]. As a consequence, empirical treatment of NP relies on broad-spectrum antibiotics. This may explain why ceftazidime was associated with the lowest probability of clinical and microbiological cure despite its excellent activity against P. aeruginosa, including in the setting of respiratory infections [34]. Furthermore, it has been well-established that ceftazidime is inferior to comparators as empiric treatment in febrile neutropenic patients [35, 36]. On the other hand, our findings do not advocate for using the empirical antibiotic with the largest spectrum regimen available. Indeed, most regimens compared in this NMA had no significant differences between them in terms of mortality, clinical or microbiological cure.
The tolerability of antibiotics is another key element in the rationale of prescription. Because most antibiotics had similar clinical outcomes, the occurrence of adverse effects is even more important. Intravenous colistin is associated with important nephrotoxicity [37]. The two trials using intravenous colistin included on our NMA confirm this trend with 17% rates of acute renal failure [19, 24]. Another important issue is the neurologic tolerance to antibiotics, especially beta-lactams, colistin and quinolones. The occurrence of seizures were sparsely recorded in trials, but as expected, imipemen/cilastatin was the treatment with the highest risk of seizures (4%) [17]. Surprisingly, no epilepsy was reported among the 266 patients treated by imipenem/cilastatin/relebactam [11]. This could be related to the relatively low dose of imipenem (2 g/d) administered, which may limit the risk of seizures [38]. This suggests that antibiotics are well tolerated in the setting of nosocomial pneumonia, with the exception of intravenous colistin.
This study has limitations. First, among the 16 RCTs included in this NMA, only six (38%) were considered at low risk of bias. The network of these six trials did not contain a closed loop, which prevented us from performing sensitivity analyses to estimate difference of treatment effects in studies with low risk of bias. Second, this NMA did not integrate antibiotics dosages or treatment durations in the analyses. However, trials have demonstrated that a short treatment is non-inferior to longer antibiotic courses in nosocomial pneumonia [39,40,41]. Moreover, standard doses of antibiotics in lung infections are often sufficient, except for critically ills patients [42]. This is why these two variables not taken into account would probably not have had an impact on our analyses. Third, the network of this NMA contained more indirect than direct comparisons. In addition, heterogeneity among studies and the presence of outliers might have influenced the SUCRA analyses, potentially leading to instability in the findings related to efficacy and safety. Fourth, subgroup analyses according to the severity or to the bacteria would be clinically relevant but cannot be performed because of the lack of closed loop in dedicated networks. To address these points, an individual personalized data NMA should be performed. Fifth, registration trials in this meta-analysis excluded MDR/XDR pathogens. As a consequence, the findings of this meta-analysis may not be generalizable to difficult-to-treat resistant GNB infections in which antibiotic stewardship remains pivotal. All the limitations mentioned above lead to conditional findings.
Conclusions
This NMA provides data suggesting that most of beta-lactams regimen had similar outcomes in terms of efficacy and safety, including new BLBLI, in the treatment of NP. Considering the very low to moderate certainty of evidence for the comparisons assessed in this meta-analysis, further studies are needed, especially in the field of multidrug-resistant Gram-negative bacterial pneumonia.
Availability of data and materials
All data in this study are available from the corresponding author (david.luque.paz@chu-rennes.fr) upon reasonable request.
Abbreviations
- MDR:
-
Multidrug resistance
- GNB:
-
Gram-regative bacilli
- 3GCRE:
-
Third-generation cephalosporin-resistant Enterobacterales
- MDRGNB:
-
Multidrug resistant Gram-negative bacteria
- XDRGNB:
-
Extensively drug resistant Gram-negative bacteria
- NP:
-
Nosocomial pneumonia
- HAP:
-
Hospitalized-acquired pneumonia
- VAP:
-
Ventilator-acquired pneumonia
- BLBLI:
-
Beta-lactam/beta-lactamase inhibitor
- RCT:
-
Randomized controlled trials
- NMA:
-
Network meta-analysis
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RoB:
-
Risk of bias
- OR:
-
Odds Ratio
- 95% CrI:
-
95% Credible interval
- SUCRA:
-
Surface Under the Cumulative Ranking Curve
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- AKI:
-
Acute kidney injury
References
Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–55.
Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–27.
Mancuso G, Midiri A, Gerace E, Biondo C. Bacterial antibiotic resistance: the most critical pathogens. Pathogens. 2021;10(10):1310.
Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European economic area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56–66.
Bush K, Bradford PA. Epidemiology of β-lactamase-producing pathogens. Clin Microbiol Rev. 2020;33(2):e00047-e119.
Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European respiratory society (ERS), European society of intensive care medicine (ESICM), European society of clinical microbiology and infectious diseases (ESCMID) and asociación latinoamericana del tórax (ALAT). Eur Respir J. 2017;50(3):1700582.
Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362(19):1804–13.
Melsen WG, Rovers MM, Groenwold RHH, Bergmans DCJJ, Camus C, Bauer TT, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13(8):665–71.
Torres A, Zhong N, Pachl J, Timsit JF, Kollef M, Chen Z, et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis. 2018;18(3):285–95.
Wunderink RG, Matsunaga Y, Ariyasu M, Clevenbergh P, Echols R, Kaye KS, et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2020. https://doi.org/10.1016/S1473-3099(20)30731-3.
Titov I, Wunderink RG, Roquilly A, Rodríguez Gonzalez D, David-Wang A, Boucher HW, et al. A randomized, double-blind, multicenter trial comparing efficacy and safety of imipenem/cilastatin/relebactam versus piperacillin/tazobactam in adults with hospital-acquired or ventilator-associated bacterial pneumonia (RESTORE-IMI 2 Study). Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa803.
Kollef MH, Nováček M, Kivistik Ü, Réa-Neto Á, Shime N, Martin-Loeches I, et al. Ceftolozane–tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): a randomised, controlled, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2019;19(12):1299–311.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84.
Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7–8):932–44.
Zanetti G, Bally F, Greub G, Garbino J, Kinge T, Lew D, et al. Cefepime versus imipenem-cilastatin for treatment of nosocomial pneumonia in intensive care unit patients: a multicenter, evaluator-blind, prospective, randomized study. AAC. 2003;47(11):3442–7.
Schmitt DV, Leitner E, Welte T, Lode H. Piperacillin/tazobactam vs imipenem/cilastatin in the treatment of nosocomial pneumonia—a double blind prospective multicentre study. Infection. 2006;34(3):127–34.
Chastre J, Wunderink R, Prokocimer P, Lee M, Kaniga K, Friedland I. Efficacy and safety of intravenous infusion of doripenem versus imipenem in ventilator-associated pneumonia: a multicenter, randomized study*. Crit Care Med. 2008;36(4):1089–96.
Kollef MH, Chastre J, Clavel M, Restrepo MI, Michiels B, Kaniga K, et al. A randomized trial of 7-day doripenem versus 10-day imipenem-cilastatin for ventilator-associated pneumonia. Crit Care. 2012;16(6):R218.
Cisneros JM, Rosso-Fernández CM, Roca-Oporto C, De Pascale G, Jiménez-Jorge S, et al. Colistin versus meropenem in the empirical treatment of ventilator-associated pneumonia (Magic Bullet study): an investigator-driven, open-label, randomized, noninferiority controlled trial. Crit Care. 2019;23(1):383.
Réa-Neto Á, Niederman M, Margareth Lobo S, Schroeder E, Lee M, Kaniga K, et al. Efficacy and safety of doripenem versus piperacillin/tazobactam in nosocomial pneumonia: a randomized, open-label, multicenter study. Curr Med Res Opin. 2008;24(7):2113–26.
Yamamoto Y, Izumikawa K, Nakamura S, Imamura Y, Miyazaki T, Kakeya H, et al. Prospective randomized comparison study of piperacillin/tazobactam and meropenem for healthcare-associated pneumonia in Japan. J Infect Chemother. 2013;19(2):291–8.
Lerma FA. Efficacy of meropenem as monotherapy in the treatment of ventilator-associated pneumonia. J Chemother. 2001;13(1):70–81.
Torres A. Treatment of severe nosocomial pneumonia: a prospective randomised comparison of intravenous ciprofloxacin with imipenem/cilastatin. Thorax. 2000;55(12):1033–9.
Abdelsalam MFA, Abdalla MS, El-Abhar HSED. Prospective, comparative clinical study between high-dose colistin monotherapy and colistin–meropenem combination therapy for treatment of hospital-acquired pneumonia and ventilator-associated pneumonia caused by multidrug-resistant Klebsiella pneumoniae. J Glob Antimicrob Resist. 2018;15:127–35.
Álvarez-Lerma F, Insausti-Ordeñana J, Jordá-Marcos R, Maraví-Poma E, Torres-Martí A, Nava J, et al. Efficacy and tolerability of piperacillin/tazobactam versus ceftazidime in association with amikacin for treating nosocomial pneumonia in intensive care patients: a prospective randomized multicenter trial. Intensive Care Med. 2001;27(3):493–502.
Joshi M, Metzler M, McCarthy M, Olvey S, Kassira W, Cooper A. Comparison of piperacillin/tazobactam and imipenem/cilastatin, both in combination with tobramycin, administered every 6h for treatment of nosocomial pneumonia. Respir Med. 2006;100(9):1554–65.
Paul M, Carrara E, Retamar P, Tängdén T, Bitterman R, Bonomo RA, et al. European society of clinical microbiology and infectious diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect. 2022;28(4):521–47.
Abdellatif S, Trifi A, Daly F, Mahjoub K, Nasri R, Ben LS. Efficacy and toxicity of aerosolised colistin in ventilator-associated pneumonia: a prospective, randomised trial. Ann Intensive Care. 2016;6(1):26.
Doshi NM, Cook CH, Mount KL, Stawicki SP, Frazee EN, Personett HA, et al. Adjunctive aerosolized colistin for multi-drug resistant gram-negative pneumonia in the critically ill: a retrospective study. BMC Anesthesiol. 2013;13(1):45.
Tumbarello M, De Pascale G, Trecarichi EM, De Martino S, Bello G, Maviglia R, et al. Effect of aerosolized colistin as adjunctive treatment on the outcomes of microbiologically documented ventilator-associated pneumonia caused by colistin-only susceptible gram-negative bacteria. Chest. 2013;144(6):1768–75.
Xu F, He LL, Che LQ, Li W, Ying SM, Chen ZH, et al. Aerosolized antibiotics for ventilator-associated pneumonia: a pairwise and Bayesian network meta-analysis. Crit Care. 2018;22(1):301.
Michalopoulos AS, Falagas ME. Colistin: recent data on pharmacodynamics properties and clinical efficacy in critically ill patients. Ann Intensive Care. 2011;2(1):30.
Réseau REA-Raisin, France. Surveillance des infections nosocomiales en réanimation adulte, résultats, résultats 2015. 2017; https://www.santepubliquefrance.fr/content/download/182996/2308612?version=1
Choi SH, Koh Y. Ceftazidime for respiratory infections. Expert Opin Pharmacother. 2012;13(14):2097–109.
Glasmacher A, Von Lilienfeld-Toal M, Schulte S, Hahn C, Schmidt-Wolf IGH, Prentice A. An evidence-based evaluation of important aspects of empirical antibiotic therapy in febrile neutropenic patients. Clin Microbiol Infect. 2005;11:17–23.
Roberts JA, Webb SAR, Lipman J. Cefepime versus ceftazidime: considerations for empirical use in critically ill patients. Int J Antimicrob Agents. 2007;29(2):117–28.
Falagas ME, Kyriakidou M, Voulgaris GL, Vokos F, Politi S, Kechagias KS. Clinical use of intravenous polymyxin B for the treatment of patients with multidrug-resistant gram-negative bacterial infections: an evaluation of the current evidence. J Glob Antimicrob Resist. 2021;24:342–59.
Cannon JP, Lee TA, Clark NM, Setlak P, Grim SA. The risk of seizures among the carbapenems: a meta-analysis. J Antimicrob Chemother. 2014;69(8):2043–55.
Chastre J, Wolff M, Fagon JY, Chevret S, Thomas F, Wermert D, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588–98.
Bouglé A, Tuffet S, Federici L, Leone M, Monsel A, Dessalle T, et al. Comparison of 8 versus 15 days of antibiotic therapy for pseudomonas aeruginosa ventilator-associated pneumonia in adults: a randomized, controlled, open-label trial. Intensive Care Med. 2022;48(7):841–9.
Zaragoza R, Vidal-Cortés P, Aguilar G, Borges M, Diaz E, Ferrer R, et al. Update of the treatment of nosocomial pneumonia in the ICU. Crit Care. 2020;24(1):383.
Veiga RP, Paiva JA. Pharmacokinetics–pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit Care. 2018;22(1):233.
Acknowledgements
None.
Funding
This work was not funded. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
DavLP and DC had full access to all of the data in the study and takes responsibility for the integrity of the data. DD and JR did the analysis. DavLP, DD and JR co-wrote the original draft. DamLP, BB, AK and PT critically reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have nothing to disclose and no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Appendix including PRISMA checklist.
Additional file 2: Table S1.
Quality of evidence for primary endpoint using GRADEframework. Table S2. Summary of adverse events reported in trials included in the meta-analysis.
Additional file 3: Figure S1.
Rank-heat plot of 28-day mortality of interventions in the treatment ofnosocomial pneumonia.
Additional file 4: Figure S2.
Rank-heat plot of clinical cure of interventions in the treatment ofnosocomial pneumonia.
Additional file 5: Figure S3.
Rank-heat plot of microbiological cure of interventions in the treatmentof nosocomial pneumonia.
Additional file 6: Figure S4.
Rank-heat plot of adverse events of interventions in the treatment ofnosocomial pneumonia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luque Paz, D., Chean, D., Tattevin, P. et al. Efficacy and safety of antibiotics targeting Gram-negative bacteria in nosocomial pneumonia: a systematic review and Bayesian network meta-analysis. Ann. Intensive Care 14, 66 (2024). https://doi.org/10.1186/s13613-024-01291-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-024-01291-5