Abstract
Background
In the last decade, Ibrutinib has become the standard of care in the treatment of several lymphoproliferative diseases such as chronic lymphocytic leukemia (CLL) and several non-Hodgkin lymphoma. Beyond Bruton tyrosine kinase inhibition, Ibrutinib shows broad immunomodulatory effects that may promote the occurrence of infectious complications, including opportunistic infections. The infectious burden has been shown to vary by disease status, neutropenia, and prior therapy but data focusing on severe infections requiring intensive care unit (ICU) admission remain scarce. We sought to investigate features and outcomes of severe infections in a multicenter cohort of 69 patients receiving ibrutinib admitted to 10 French intensive care units (ICU) from 1 January 2015 to 31 December 2020.
Results
Median time from ibrutinib initiation was 6.6 [3–18] months. Invasive fungal infections (IFI) accounted for 19% (n = 13/69) of severe infections, including 9 (69%; n = 9/13) invasive aspergillosis, 3 (23%; n = 3/13) Pneumocystis pneumonia, and 1 (8%; n = 1/13) cryptococcosis. Most common organ injury was acute respiratory failure (ARF) (71%; n = 49/69) and 41% (n = 28/69) of patients required mechanical ventilation. Twenty (29%; n = 20/69) patients died in the ICU while day-90 mortality reached 55% (n = 35/64). In comparison with survivors, decedents displayed more severe organ dysfunctions (SOFA 7 [5–11] vs. 4 [3–7], p = 0.004) and were more likely to undergo mechanical ventilation (68% vs. 31%, p = 0.010). Sixty-three ibrutinib-treated patients were matched based on age and underlying malignancy with 63 controls receiving conventional chemotherapy from an historic cohort. Despite a higher median number of prior chemotherapy lines (2 [1–2] vs. 0 [0–2]; p < 0.001) and higher rates of fungal [21% vs. 8%, p = 0.001] and viral [17% vs. 5%, p = 0.027] infections in patients receiving ibrutinib, ICU (27% vs. 38%, p = 0.254) and day-90 mortality (52% vs. 48%, p = 0.785) were similar between the two groups.
Conclusion
In ibrutinib-treated patients, severe infections requiring ICU admission were associated with a dismal prognosis, mostly impacted by initial organ failures. Opportunistic agents should be systematically screened by ICU clinicians in this immunocompromised population.
Similar content being viewed by others
Background
Recent advancements in our understanding of carcinogenesis of hematological malignancies have paved the way for the development of targeted therapies, aiming to address the limited efficacy of conventional chemotherapy and reduce its associated toxicity. In the last decade, the emergence of targeted drugs against B-cell receptor (BCR) has reshaped the standard of care of B-cell malignancies with durable responses reported even in patients with refractory disease [1,2,3,4,5].
Ibrutinib, an irreversible inhibitor of Bruton tyrosine kinase (BTK), has become a key molecule in the treatment of chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), Waldenström macroglobulinemia (WM), diffuse large B-cell lymphoma (DLBCL), and follicular lymphoma [6,7,8,9,10,11,12].
BTK is a non-receptor kinase that plays a critical role in the BCR transduction pathway, driving B lymphocytes activation, differentiation, proliferation, and survival [13, 14]. Beyond BTK blockade, ibrutinib also shows broad immunomodulatory effects that have been shown to be associated with infectious complications, including opportunistic infections [15,16,17]. Disease status, prior chemotherapy and neutropenia have been shown to influence the incidence of severe infections [12, 18], which is one of the main reasons for ibrutinib discontinuation [19].
Because the use of targeted therapies is expanding, the need for critical care management of toxicities related to these new drugs is likely to grow. However, data focusing on most severe infections leading to organ failure remain scarce and generally extrapolated from randomized control trials [16, 20].
Accordingly, we sought to investigate features and outcomes of severe infections requiring intensive care unit (ICU) admission in a multicenter cohort of patients receiving ibrutinib for a lymphoproliferative disease, with a special focus on opportunistic infections.
Methods
This study was approved by the institutional review board of the French Intensive Care Society. Patient’s consent was waived for this retrospective study.
Patients
All consecutive patients receiving ibrutinib for a hematological malignancy admitted to 10 French ICU departments affiliated to the Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique (GRRR-OH [21]) from 1 January 2015 to 31 December 2020 were screened for inclusion. Only patients admitted for severe infections were included in the study analysis. In case of multiple ICU admissions, only the first stay was considered.
Data collection
Patient’s demographics, underlying disease, treatments exposure, clinical outcomes, and microbiological analysis were collected from individual medical records by three independent investigators. Primary endpoint of this study was ICU mortality, defined as death from any cause within ICU stay. Ibrutinib discontinuation and disease status evolution were also recorded.
Definitions
Organ injuries were reported using Early Warning System definitions (oxygen requirement, low blood-pressure, altered mental status) and biological definitions used in the SOFA score [22, 23].
All types of severe infections requiring ICU admission and occurring any time from ibrutinib initiation until 3 months after its discontinuation were considered. Severe infections were identified by reviewing patient medical record, laboratory data, imaging studies and histopathological or cytology results when available. For cases with microbiological and/or radiological findings suggestive of infection, we further reviewed the clinical chart to confirm the presence of associated symptoms to exclude colonization and ascertain clinical outcome.
The source of infection was classified according to clinical and microbiological criteria following the Center for Disease Control guidelines [24]. In patients with multiple infections, only the main infectious episode leading to ICU admission was considered for the analysis. Invasive fungal infections (IFI) were defined according to the 2020 Revision and Update of the Consensus Definition of Invasive Fungal Disease from the EORTC/MSGERC Consensus Group [25]. Neutropenia was defined by a neutrophil count < 500/mm3.
Additionally, infectious features and outcomes were compared between 63 ibrutinib-treated patients and controls receiving conventional chemotherapies from an historic cohort reported by the same research network (TRIAL-OH [21]).
Statistical analysis
Quantitative variables were described as median with interquartile range (IQR) and compared using Wilcoxon’s test. Qualitative variables were described as count and percentages and compared using Fisher’s exact test. ICU survivors and decedents were compared using univariate analysis. Due to small sample size and various underlying diseases, multivariate analysis was not relevant. Second, ibrutinib-treated patients and controls were matched using a 1:1 algorithm based on age and underlying malignancy. Ibrutinib-treated patients for WM and graft-versus-host disease were excluded from the matched analysis because these diseases were not represented in TRIAL-OH study [21]. Survival functions were computed using Kaplan–Meier’s estimates on right-censored data and group comparison was performed using univariate analysis.
All tests were two-sided, and a p-value of less than 0.05 was considered significant. Statistical analyses were carried out using R version 4.2.3 (http://www.R-project.org) with the following packages: Matchit, Survival and survminer.
Results
Patient characteristics
Among 92 critically ill patients receiving ibrutinib admitted to the ICU within the study period, 75% (n = 69/92) were admitted for a severe infection and were included in our cohort (Fig. 1). Other adverse events included hematological complications such as tumor lysis syndrome or disease progression (n = 16), cardiovascular diseases (n = 3), and bleedings (n = 4).
Description of the overall study population is detailed in Table 1. Median age at ICU admission was 73 [IQR 66–78] years and 64% (n = 44/69) were male. The most common underlying malignancy was CLL (59%; n = 41/69). Other hemopathies included DLBCL (13%; n = 9/69), MCL (15%; n = 10/69), Waldenström macroglobulinemia (7%; n = 5/69), marginal zone lymphoma (4%; n = 3/69), and graft-versus-host disease (1%; n = 1/69). This last patient had undergone allogenic stem cell transplantation over three years before admission to the ICU.
Median time from ibrutinib initiation to ICU admission was 6.6 months [IQR 2.6–17.5]. Median number of prior therapy lines was 2 [1, 2], including 29 (42%; n = 29/69) patients treated by other chemotherapy within the last 6 months, and 7 (10%; n = 7/69) patients receiving ibrutinib as first line therapy. Prior therapy lines included anti-CD20 therapy, fludarabine, and alemtuzumab in 54 (78%; n = 54/69), 24 (35%; n = 24/69) and 2 (3%; n = 2/69) patients respectively. Most patients (67%; n = 46/69) received ibrutinib as monotherapy. In patients on combination therapy (33%; n = 23/69), concurrent treatments included anti-CD20 monoclonal antibodies in 48% (n = 11/23) and corticosteroids in 39% (n = 9/23). Additional infectious risk factors included diabetes in 19% (n = 13/69), hypogammaglobulinemia in 23% (n = 16/69), neutropenia in 10% (n = 7/69), and HIV infection in 1% (n = 1/69) of patients. Anti-Pneumocystis and anti-viral prophylaxis were administered to 58% (n = 40/69) and 65% (n = 45/69) of patients respectively.
Types of infection
Among 69 infectious episodes leading to ICU admission, the most frequent were bacterial infections (65%; n = 45/69 episodes). Main bacterial infection sources were lungs (40%; n = 18/45) and urinary tract (16%; n = 7/45), while 6 (13%) catheter-related infections were detected.
Regarding viral infections, 11 (16%) episodes were recorded. Severe acute coronavirus 2 (SARS-CoV-2) infections accounted for 54% (n = 6/11) of viral infections. Other viral episodes included cytomegalovirus infections in 3 (27%; n = 3/11) patients, herpes simplex virus reactivation and disseminated adenovirus infection in 1 patient each.
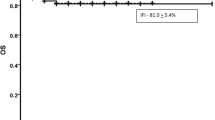
Overall, 13 (19%) fungal episodes were reported in the whole cohort (Table 2), including 11 (84%; n = 11/13) patients with CLL, 3 (23%; n = 3/13) patients receiving concomitant corticosteroids therapy and 1 (8%; n = 1/13) allogeneic hematopoietic stem-cell recipient. The proportion of IFI was not significantly higher in CLL patients in comparison with other hematological malignancies (27% vs. 11%, p = 0.105). Additional risk factors included neutropenia in 8% (n = 1/13), hypogammaglobulinemia in 15% (n = 2/13) and diabetes in 23% (n = 3/13). The most frequent fungal infection was proven or probable invasive aspergillosis (69%; n = 9/13). Other fungal episodes included Pneumocystis jirovecii pneumonia in 3 (23%; n = 3/13) patients and Cryptococcus neoformans infection in 1 (8%; n = 1/13) patient.
The spectrum of infections was similar between patients receiving ibrutinib as single-drug therapy and patients on combination regimen, including 21% (n = 10/46) and 13% (n = 3/23) of fungal episodes respectively (p = 0.422).
Overall, 81 infections were reported among the 69 patients, including 54 bacterial infections (67%; n = 54/81), 14 viral infections (17%; n = 14/81) and 13 fungal infections (16%; n = 13/81). Twelve (17%; n = 12/69) patients displayed polymicrobial infections, including 8 (12%; n = 8/69) patients with IFI associated with bacterial (75%; n = 6/8) or viral coinfections (25%; n = 2/8).
ICU admission
At ICU admission, most patients (68%; n = 47/69) displayed multi-organ failure and median SOFA score was 5 [IQR 3–8]. Most common organ injury at admission was acute respiratory failure (ARF) (71%; n = 49/69), including all patients with IFI. Overall, invasive mechanical ventilation (IMV) was required in 41% (n = 28/69) of patients. The median time from ICU admission to IMV was inferior to one day [IQR 0–1]. At ICU admission, shock, and acute kidney injury (AKI) were reported in 49% (n = 34/69) and 33% (n = 23/69) patients respectively. Renal replacement therapy (RRT) was initiated in 15% (n = 10/69) patients throughout the ICU stay. Median time from hospital to ICU admission was 2 [IQR 1–13] days.
Outcomes and predictive factors of ICU mortality
Median ICU and hospital lengths of stay were 5 [2–11] and 21 [IQR 7–43] days respectively. Twenty (29%; n = 20/69) patients died in the ICU. End-of-life decisions preceded ICU deaths in 65% (n = 13/20). Comparison of baseline characteristics between ICU survivors and decedents is summarized in Table 1. There was no difference regarding baseline characteristics, underlying malignancy, and infection type. Of note, the proportion of polymicrobial infections was similar between ICU survivors and decedents (20 vs. 16%; p = 0.715). Organ dysfunctions at ICU admission were more severe in non-survivors (SOFA 7 [IQR 5–11] vs. 4 [IQR 3–7], p = 0.004. In comparison with survivors, decedents were more likely to display ARF at ICU admission (95% vs. 61%, p = 0.005) and to require IMV (68% vs. 31%, p = 0.010).
Follow up data after ICU discharge was missing for 5 patients. Overall, hospital mortality reached 50% (n = 32/64) and day-90 mortality 55% (n = 35/64), affecting 34% (n = 15/44) of ICU survivors. Ibrutinib was discontinued during the ICU stay in 57 patients (83%; n = 57/69), including all patients with IFI. Among survivors in whom ibrutinib treatment had been discontinued through ICU stay, discontinuation was permanent in 65% (n = 24/37) and only temporary in 35% (n = 13/37). After a median follow up of 5 [IQR 1–18] months, 61% (n = 27/44) of ICU survivors displayed B-cell malignancy progression or recurrence. Individual patient clinical trajectories from ibrutinib introduction to loss of follow up or death are displayed in Fig. 2.
Swimmer’s plot of patients admitted to the ICU for severe infections, from ibrutinib introduction to loss to follow up or death. Patients are classified by underlying disease group and final diagnosis during ICU stay. The straight line represents the median time from ibrutinib introduction to ICU admission
Comparison between ibrutinib-treated patients and controls receiving conventional chemotherapy
Severe infections features and outcomes were compared between 63 ibrutinib-treated patients and 63 controls receiving conventional chemotherapy from an historic cohort (TRIAL-OH (21)), matched on age and underlying malignancy (Table 3). Median number of prior chemotherapy lines was higher in ibrutinib-treated patients in comparison with controls (2 [IQR 1–2] vs. 0 [0–2] p = 0.001). Other baseline characteristics were similar between the 2 groups.
Regarding infection type, ibrutinib-treated patients were more likely to display IFI (21% [n = 13/63] vs. 8% [n = 5/63], p = 0.001) or viral episodes (17% [n = 11/63] vs. 5% [n = 3/63], p = 0.027) while infections in patients receiving conventional chemotherapy were more frequently bacterial (87% [n = 55/63] vs. 62% [n = 39/63], p = 0.039).
Median SOFA score at ICU admission was similar between the 2 groups but ibrutinib-treated patients were more likely to display acute liver injury (19% [n = 12/63] vs. 0% [n = 0/63], p < 0.001) and impaired consciousness (43% [n = 27/63] vs. 19% [n = 12/63], p = 0.004). There was no difference regarding ARF incidence, but control patients were more likely to undergo IMV (62% [n = 39/63] vs. 41% [n = 26/63], p = 0.032).
However, ibrutinib-treated patients and controls receiving conventional chemotherapies displayed similar ICU (27% vs. 38%, p = 0.254) and day-90 (52% vs. 48%, p = 0.785) mortality rates (Fig. 3).
Discussion
This is the first series focusing on severe infectious episodes requiring ICU admission in patients receiving ibrutinib in a real-life setting. Beyond the predominance of bacterial infections, our results underline the risk of opportunistic infections within the first year of treatment. Overall, severe infections requiring ICU admission are associated with a dismal prognosis, with over 50% of mortality at day 90, mostly impacted by initial organ dysfunctions.
In our cohort, infectious features corroborated recent studies not restricted to critically ill patients [16, 20]. The predominance of bacterial episodes, which accounted for nearly 60% of severe infections, was previously reported in studies focusing on ibrutinib-associated adverse events [18]. This finding may be promoted by ibrutinib off-target effects including innate immunity impairment (involving macrophages, neutrophils [26,27,28] and natural killer cells [29] function, and modulation of T-cell activation through Interleukin 2 Receptor Kinase (ITK) inhibition [30]. In contrast, minimal off-target effects among other members of the TEC tyrosine kinase family are reported with more specific second-generation BTK inhibitor such as acalabrutinib [30]. Further studies are warranted to determine infections incidence and severity associated with these new molecules [31, 32].
A specific concern regarding IFI in patients treated with targeted therapies including ibrutinib has previously been pointed out [12, 17, 20, 33]. In the present series, one out of five infection was an IFI. IFI proportion even reached 27% in patients admitted for acute respiratory failure, suggesting that opportunistic agents should be systematically screened by ICU clinicians (serum galactomannan, ß-D-Glucan, PCR, etc.…) in this setting. As expected, the spectrum of fungal episodes included a majority of pulmonary aspergillosis, three Pneumocystis pneumonia and one pulmonary cryptococcosis [17, 18, 33, 34]. Aspergillus susceptibility in ibrutinib-treated patients may rely on the critical role of BTK activation in macrophages for TNFα response, neutrophil recruitment and functions including reactive oxygen species production, and A.fumigatus clearance in the respiratory airways [26, 27, 35]. In contrast with previous reports, we report only one case of extra-pulmonary fungal dissemination in the present cohort [33, 36]. Interestingly, almost all fungal episodes were diagnosed in CLL patients with additional risk factors, including diabetes, prior chemotherapies, concomitant corticosteroids and/or anti-CD20 monoclonal antibodies. IFI proportion tended to be higher in CLL patients in comparison with other hematological malignancies in line with the data reported by Gold et al. [37].
Main limitations of this study include its retrospective design and numerous confounding factors, particularly regarding immunosuppression. Ibrutinib-related infectious burden probably partly reflects the impact of previous chemotherapies and uncontrolled malignancy status at the beginning of the targeted-drug treatment [38]. In the present cohort, the short time from ibrutinib introduction to severe infection requiring ICU admission, consistent with previous studies, supports this assumption. Infections incidence has previously been shown to be higher in the first year of ibrutinib exposure and to progressively decrease over time [2, 18, 37]. Moreover, in vitro studies have identified a partial recovery of both humoral immune function [39] and T Cell Receptor (TCR) repertoire diversity via ITK inhibition [30, 40, 41]. In line with these findings, the matched comparison between ibrutinib-treated patients and controls receiving conventional chemotherapies should not be interpretated as an attempt to differentiate molecules-related infectious adverse events. Thus, higher proportion of fungal infections may simply reflect the higher number of prior lines of chemotherapy in patients receiving ibrutinib. Given the discrepancies between the two cohorts regarding inclusion periods, baseline characteristics, organ injuries at ICU admission and IMV requirement, outcomes must be compared cautiously.
Furthermore, increased proportion of viral episodes in ibrutinib-treated patients in the present study may be due to the emergence of Severe Acute Respiratory Virus Coronavirus 2 (SARS-CoV-2) in 2020, which accounted for over half of viral infections. Several studies have shown a severe prognosis among CLL patients diagnosed with SARS CoV2 infection [42]. In a multicentre retrospective study including 198 patients with symptomatic SARS CoV-2 infection, the authors reported a mortality reaching 33% [43]. The impact of ibrutinib treatment on the course of SARS-CoV-2 infection is still uncertain, and ibrutinib discontinuation in SARS-CoV-2-infected patients remains controversial [42, 44, 45]. In our cohort only one in five SARS CoV-2 infected patients died in the ICU. However, hence the dismal prognosis among CLL patients, evaluation of ibrutinib treatment impact on the course of Coronavirus Induce Disease 2019 (COVID 19) in these patients must be taken carefully.
In this study, severe infections in critically ill patients receiving ibrutinib were associated with an unexpectedly high day-90 mortality above 50%. However, outcomes comparison with studies in which most of severe infections do not require ICU admission would be inappropriate. Organ failures at ICU admission were the major determinant of mortality, suggesting that ICU transfer should be considered as soon as these high-risk patients show any sign of organ injury. Last, severe infection conveyed the need for ibrutinib discontinuation in a high proportion of cases, but further studies are warranted to evaluate its impact on long-term outcomes.
Conclusion
In patients receiving ibrutinib for hematological diseases, severe infections requiring ICU admission are associated with a dismal prognosis, mostly impacted by initial organ failures. Due to the high proportion of fungal infections, opportunistic agents should be systematically screened by ICU clinicians in this immunocompromised population, especially in the setting of acute respiratory failure.
Availability of data and materials
The datasets used and analyzed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- BCR:
-
B cell receptor
- BTK:
-
Bruton tyrosine kinase
- CLL:
-
Chronic lymphocytic leukemia
- MCL:
-
Mantel cell lymphoma
- WM:
-
Waldenström macroglobulinemia
- DLBCL:
-
Diffuse large B cell lymphoma
- ICU:
-
Intensive care unit
- IFI:
-
Invasive fungal infection
- IQR:
-
Interquartile range
- HIV:
-
Human immunodeficiency virus
- ARF:
-
Acute respiratory failure
- IMV:
-
Invasive mechanical ventilation
- AKI:
-
Acute kidney infection
- RRT:
-
Renal replacement therapy
- ITK:
-
Interleukin 2 receptor kinase
- TCR:
-
T cell receptor
References
Jain N, O’Brien S. Targeted therapies for CLL: practical issues with the changing treatment paradigm. Blood Rev. 2016;30(3):233–44.
Munir T, Brown JR, O’Brien S, Barrientos JC, Barr PM, Reddy NM, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353–63.
Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–28.
Dimopoulos MA, Trotman J, Tedeschi A, Matous JV, Macdonald D, Tam C, et al. Ibrutinib for patients with rituximab-refractory Waldenström’s macroglobulinaemia (iNNOVATE): an open-label substudy of an international, multicentre, phase 3 trial. Lancet Oncol. 2017;18(2):241–50.
Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–23.
Hallek M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol. 2021;96(12):1679–705.
Gertz MA. Waldenström macroglobulinemia: 2021 update on diagnosis, risk stratification, and management. Am J Hematol. 2021;96(2):258–69.
Kumar A, Eyre TA, Lewis KL, Thompson MC, Cheah CY. New directions for mantle cell lymphoma in 2022. Am Soc Clin Oncol Educ Book. 2022;42:614–28.
Eichhorst B, Robak T, Montserrat E, Ghia P, Niemann CU, Kater AP, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(1):23–33.
Barr PM, Owen C, Robak T, Tedeschi A, Bairey O, Burger JA, et al. Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022;6(11):3440–50.
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–37.
Chamilos G, Lionakis MS, Kontoyiannis DP. Call for action: invasive fungal infections associated with Ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways. Clin Infect Dis. 2018;66(1):140–8.
Corneth OBJ, Klein Wolterink RGJ, Hendriks RW. BTK signaling in B cell differentiation and autoimmunity. Curr Top Microbiol Immunol. 2016;393:67–105.
McDonald C, Xanthopoulos C, Kostareli E. The role of Bruton’s tyrosine kinase in the immune system and disease. Immunology. 2021;164(4):722–36.
Stephens DM, Byrd JC. How I manage ibrutinib intolerance and complications in patients with chronic lymphocytic leukemia. Blood. 2019;133(12):1298–307.
Tillman BF, Pauff JM, Satyanarayana G, Talbott M, Warner JL. Systematic review of infectious events with the Bruton tyrosine kinase inhibitor ibrutinib in the treatment of hematologic malignancies. Eur J Haematol. 2018;100(4):325–34.
Rogers KA, Mousa L, Zhao Q, Bhat SA, Byrd JC, El Boghdadly Z, et al. Incidence of opportunistic infections during ibrutinib treatment for B-cell malignancies. Leukemia. 2019;33(10):2527–30.
Varughese T, Taur Y, Cohen N, Palomba ML, Seo SK, Hohl TM, et al. Serious infections in patients receiving Ibrutinib for treatment of lymphoid cancer. Clin Infect Dis. 2018;67(5):687–92.
Maddocks KJ, Ruppert AS, Lozanski G, Heerema NA, Zhao W, Abruzzo L, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1(1):80–7.
O’Brien S, Hillmen P, Coutre S, Barr PM, Fraser G, Tedeschi A, et al. Safety analysis of four randomized controlled studies of Ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma or mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2018;18(10):648-57.e15.
Azoulay E, Mokart D, Pène F, Lambert J, Kouatchet A, Mayaux J, et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium–a groupe de recherche respiratoire en réanimation onco-hématologique study. J Clin Oncol. 2013;31(22):2810–8.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Almutary A, Althunayyan S, Alenazi K, Alqahtani A, Alotaibi B, Ahmed M, et al. National early warning score (NEWS) as prognostic triage tool for septic patients. Infect Drug Resist. 2020;13:3843–5.
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16(3):128–40.
Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis. 2020;71(6):1367–76.
Bercusson A, Colley T, Shah A, Warris A, Armstrong-James D. Ibrutinib blocks Btk-dependent NF-ĸB and NFAT responses in human macrophages during Aspergillus fumigatus phagocytosis. Blood. 2018;132(18):1985–8.
Blez D, Blaize M, Soussain C, Boissonnas A, Meghraoui-Kheddar A, Menezes N, et al. Ibrutinib induces multiple functional defects in the neutrophil response against Aspergillus fumigatus. Haematologica. 2020;105(2):478–89.
Mangla A, Khare A, Vineeth V, Panday NN, Mukhopadhyay A, Ravindran B, et al. Pleiotropic consequences of Bruton tyrosine kinase deficiency in myeloid lineages lead to poor inflammatory responses. Blood. 2004;104(4):1191–7.
Kohrt HE, Sagiv-Barfi I, Rafiq S, Herman SEM, Butchar JP, Cheney C, et al. Ibrutinib antagonizes rituximab-dependent NK cell-mediated cytotoxicity. Blood. 2014;123(12):1957–60.
Shirley M. Bruton tyrosine kinase inhibitors in B-cell malignancies: their use and differential features. Target Oncol. 2022;17(1):69–84.
Byrd JC, Hillmen P, Ghia P, Kater AP, Chanan-Khan A, Furman RR, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39(31):3441–52.
Tam CS, Opat S, D’Sa S, Jurczak W, Lee HP, Cull G, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood. 2020;136(18):2038–50.
Ghez D, Calleja A, Protin C, Baron M, Ledoux MP, Damaj G, et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood. 2018;131(17):1955–9.
Woyach JA. Ibrutinib and Aspergillus: a Btk-targeted risk. Blood. 2018;132(18):1869–70.
Herbst S, Shah A, Mazon Moya M, Marzola V, Jensen B, Reed A, et al. Phagocytosis-dependent activation of a TLR9-BTK-calcineurin-NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus. EMBO Mol Med. 2015;7(3):240–58.
Lionakis MS, Dunleavy K, Roschewski M, Widemann BC, Butman JA, Schmitz R, et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31(6):833-843.e5.
Gold JAW, Tolu SS, Chiller T, Benedict K, Jackson BR. Incidence of invasive fungal infections in patients initiating ibrutinib and other small molecule kinase inhibitors-United States, July 2016–June 2019. Clin Infect Dis. 2022;75(2):334–7.
Stefania Infante M, Fernández-Cruz A, Núñez L, Carpio C, Jiménez-Ubieto A, López-Jiménez J, et al. Severe infections in patients with lymphoproliferative diseases treated with new targeted drugs: a multicentric real-world study. Cancer Med. 2021;10(21):7629–40.
Sun C, Tian X, Lee YS, Gunti S, Lipsky A, Herman SEM, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood. 2015;126(19):2213–9.
Solman IG, Blum LK, Burger JA, Kipps TJ, Dean JP, James DF, et al. Impact of long-term ibrutinib treatment on circulating immune cells in previously untreated chronic lymphocytic leukemia. Leuk Res. 2021;102:106520.
Yin Q, Sivina M, Robins H, Yusko E, Vignali M, O’Brien S, et al. Ibrutinib therapy increases T cell repertoire diversity in patients with chronic lymphocytic leukemia. J Immunol. 2017;198(4):1740–7.
Merli M, Ferrarini I, Merli F, Busca A, Mina R, Falini B, et al. SARS-CoV-2 infection in patients with chronic lymphocytic leukemia: the Italian hematology alliance on COVID-19 cohort. Hematol Oncol. 2023;41(1):128–38.
Mato AR, Roeker LE, Lamanna N, Allan JN, Leslie L, Pagel JM, et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134–43.
McGee MC, August A, Huang W. BTK/ITK dual inhibitors: modulating immunopathology and lymphopenia for COVID-19 therapy. J Leukoc Biol. 2021;109(1):49–53.
Coutre SE, Barnett C, Osiyemi O, Hoda D, Ramgopal M, Fort AC, et al. Ibrutinib for hospitalized adults with severe coronavirus disease 2019 infection: results of the randomized, double-blind, placebo-controlled iNSPIRE study. Open Forum Infect Dis. 2022;9(5):104.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
LB collected and interpretated patient data, drafted and edited the manuscript. AL collected and analyzed patient data and was a major contributor in writing the manuscript. VL and AJ performed statistical analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the institutional review board of the French Intensive Care Society. Patient’s consent was waived for this retrospective study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baucher, L., Lemiale, V., Joseph, A. et al. Severe infections requiring intensive care unit admission in patients receiving ibrutinib for hematological malignancies: a groupe de recherche respiratoire en réanimation onco-hématologique (GRRR-OH) study. Ann. Intensive Care 13, 123 (2023). https://doi.org/10.1186/s13613-023-01219-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-023-01219-5