Abstract
Background
The extent of the consequences of an episode of severe acute kidney injury (AKI) on long-term outcome of critically ill patients remain debated. We conducted a prospective follow-up of patients included in a large multicenter clinical trial of renal replacement therapy (RRT) initiation strategy during severe AKI (the Artificial Kidney Initiation in Kidney Injury, AKIKI) to investigate long-term survival, renal outcome and health related quality of life (HRQOL). We also assessed the influence of RRT initiation strategy on these outcomes.
Results
Follow-up of patients extended from 60 days to a median of 3.35 years [interquartile range (IQR), 1.89 to 4.09] after the end of initial study. Of the 619 patients included in the AKIKI trial, 316 survived after 60 days. The overall survival rate at 3 years from inclusion was 39.4% (95% CI 35.4 to 43.4). A total of 46 patients (on the 175 with available data on long-term kidney function) experienced worsening of renal function (WRF) at the time of follow-up [overall incidence of 26%, cumulative incidence at 4 years: 20.6% (CI 95% 13.0 to 28.3)]. Fifteen patients required chronic dialysis (5% of patients who survived after day 90). Among the 226 long-term survivors, 80 (35%) answered the EQ-5D questionnaire. The median index value reported was 0.67 (IQR 0.40 to 1.00) indicating a noticeable alteration of quality of life. Initiation strategy for RRT had no effect on any long-term outcome.
Conclusion
Severe AKI in critically ill patients was associated with a high proportion of death within the first 2 months but less so during long-term follow-up. A quarter of long-term survivors experienced a WRF and suffered from a noticeable impairment of quality of life. Renal replacement therapy initiation strategy was not associated with mortality outcome.
Graphical Abstract

Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is frequent among critically ill patients and associates with increased morbidity and mortality [1] First considered as a totally reversible syndrome, AKI is now recognized as a risk factor for chronic kidney disease (CKD) [2, 3]. This risk persists even after the normalization of serum creatinine (SCr) level following the acute episode [4, 5]. Patients requiring dialysis for CKD have an altered health related quality of life (HRQOL) and renal replacement therapy (RRT) represents a major cost item for health care systems [6, 8]. In addition, AKI may also impact long-term survival [4, 9, 10]. The majority of studies evaluating long-term outcome after AKI were retrospective and were based on administrative databases and not on actual patient data for a large part [1, 11]. In addition, patient population was markedly heterogeneous in terms of baseline characteristics and of AKI severity [12]. These considerations explain why prospective long-term follow-up of critically ill patients with severe AKI has been considered as a research priority [13, 14]. We took advantage of our large multicenter randomized controlled trial (RCT) on renal replacement therapy initiation strategy for severe AKI (stage 3 of KDIGO classification [15]) in critically ill patients [16] to conduct a long-term follow-up study. The aim of the present study was to investigate long-term survival, renal outcomes and health related quality of life (HRQOL). We hypothesized that some critically ill patients with severe AKI will have poor long-term outcomes. We also aimed to investigate the influence of the RRT initiation strategy on these outcomes because recent large randomized controlled trials on this subject did not assess long-term outcomes.
Methods
Study design and patients
The AKIKI trial was an institutionally sponsored, unblinded, prospective, multicenter, open-label, two-group RCT conducted in 31 intensive care units in France from September 2013 through January 2016. Design and methods of the trial have been previously published [17]. Patients with severe AKI, defined by KDIGO stage 3 classification, compatible with the diagnosis of acute tubular necrosis in a context of ischemic or toxic aggression and receiving invasive mechanical ventilation and/or catecholamine infusion (see inclusion criteria in supplementary appendix) were randomly allocated to one of the two following RRT initiation strategies: an early strategy where RRT was initiated within 6 h after AKI KDIGO 3 status was documented; a delayed strategy where RRT was initiated if one or more of the following events occurred: serum potassium concentration greater than 6 mmol/L (or greater than 5.5 mmol/L that persisted despite well-conducted medical treatment), a pH below 7.15 in the context of pure metabolic or mixed acidosis, an acute overload pulmonary edema generating severe hypoxemia, a serum urea concentration higher than 40 mmol /L, oliguria or anuria for more than 72 h.
The duration of the initial follow-up for each patient was 60 days from randomization. For the present study, we extended this follow-up from day 60 and prospectively assessed survival, renal outcomes and HRQOL. Five patients only over a total of 619 in the original study [16] were lost to follow-up. However, we retrieved long-term vital status for 3 through administrative data.
Informed consent was obtained after oral and written delivery of information. Patients who were reached by phone during the long-term follow-up were asked to verbally confirm their consent. The protocol was approved by the ethics committee of the French Society of Intensive Care Medicine and by the appropriate French legal authority (Comité de Protection des Personnes d’Ile de France VI) for all participating centers. Patients or their surrogates were informed that they could decline to participate at any time.
Data collection
Clinical and biological data were collected each day from randomization in the trial until ICU discharge or until 60 days after randomization in the initial study. For the present study and in order to adequately evaluate kidney function course, we estimated glomerular filtration rate (eGFR) using the Cockcroft formula [18]. We defined CKD by an eGFR < 60 ml/min [19] (Additional file 1: Table S1). We identified all patients who survived 60 days after randomization. We tried to reach all surviving patients (or their surrogate) by phone. During telephone interview, we collected the value for last known Scr concentration, the need for chronic RRT and we assessed HRQOL using the EuroQol Group 5 dimensions tool (EQ-5D-5L) [20]. We first ruled-out any acute event before considering Scr concentration. This allowed exclusion of the influence of a new AKI episode on this value. We also retrieved when available, the nearest non-emergency department SCr concentration obtained 7 to 365 days prior to index hospitalization in order to refine baseline kidney function previously collected in AKIKI. The interviewers were blinded to original study treatment allocation. We attempted to reach the general practitioner (GP) of each surviving patient by phone or email. When neither the patient, surrogate nor GP was available, we requested a certificate of birth from the birthplace townhall (as allowed by French law) that indicates the date in case of death. To avoid missing data on the need for chronic RRT (hemodialysis, peritoneal dialysis or renal transplantation), we queried the French National REIN registry (hosted by French Agency of Biomedicine) which aggregates all patients receiving chronic RRT in France [21].
Long-term outcomes
-
Survival
Overall survival was assessed at the time of the long-term follow-up (up to 7 years after the ICU stay).
-
Renal outcomes
Because CKD is defined by the persistence of kidney disease for a period of more than 90 days [19], all renal outcomes were assessed beyond this point. They included worsening renal function (WRF) which was defined differently according to CKD status at baseline.
-
For non-CKD patients at baseline, WRF was defined by the occurrence of CKD (progression from stage 1 or 2 of KDIGO CKD nomenclature to a stage 3, 4 or 5) [15].
-
For CKD patients at baseline, WRF was defined by the progression from a stage 3 to a stage 4 or 5.
We also assessed the number of patient dependent on chronic dialysis and/or who benefited from kidney transplantation.
-
HRQOL
Health-related quality of life was assessed with the EQ-5D questionnaire [22] which consists in evaluation of mobility, self-care, usual activities, pain and anxiety and derives an index value from 0 to 1 where 0 is a health state equivalent to death and 1 the best imaginable state. A negative score indicates a state considered “worse than death” (Additional file 1: Figure S2).
Statistical analyses
Qualitative variables were compared using a Chi-squared test or a Fisher’s exact test as appropriate, unless otherwise stated. Continuous variables were compared using a Student’s t-test or a Wilcoxon test as appropriate. Where data were missing, we reported the number of available observations and made no assumptions about missing values. We present the results for the overall sample and according to randomization groups (early versus delayed RRT initiation strategy).
Survival curves were generated from randomization to long-term follow-up according to the Kaplan–Meier method. The log-rank test was used to compare the 2 groups survival curves.
Univariate and multivariate analysis with Cox model were used to identify risk factors associated with long-term mortality. Variables with p < 0.1 in the univariate analysis as well as those that are clinically important like severity, age and some comorbidities were included in the model. Schoenfeld residuals are presented in the supplementary appendix (Additional file 1: Figure S3) to check the proportional hazards assumption. We analyzed risk factors associated with the occurrence of WRF. In this context, we tested variables which have been reported to be potentially associated with worse renal outcomes (CKD, CCF, diabetes, age, hypertension, severity scores) [23]. We also assessed a potential association between RRT initiation strategy and WRF. Since WRF and death are competing events, the use of separate Kaplan–Meier curve and proportional hazard (PH) Cox model (“cause-specific analysis”) for each type of event is inappropriate, because censoring mechanism could not be considered uninformative. In order to appreciate the occurrence of WRF, we calculated the cumulative incidence function (CIF) of each event type, overall and by each factor of interest. The CIF curves represent the cumulative probability of failure from a specific cause over time. The analysis used PH Fine and Gray model which extends the PH Cox model to the presence of competing events. The effect of each factor on each type of event in competition was estimated using the sub-hazards ratio (SHR) and its 95% confidence interval. Interpretation of SHR is similar to that of the Cox model (cause specific) hazard ratio.
We also drew an alluvial diagram showing movement of patients between eGFR categories (see KDIGO CKD nomenclature in Supplementary appendix) between baseline (before severe AKI episode) and long-term follow-up.
All tests were two-sided at a confidence level of 5%. Statistical analyses were conducted using R v.4.1.0 and Prism v 8.4.2. Flowchart was provided with whimsical tool.
Results
Study patients
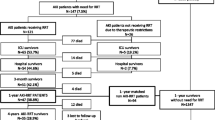
Of the 619 patients included in the AKIKI trial [16], 316 (51%) survived for at least 60 days after randomization (Fig. 1). Fifty (16%) surviving patients had CKD at baseline. The Kaplan–Meier estimate of median follow-up after the AKI episode was 3.35 years (95% CI 2.72 to 3.16; IQR: 1.21 to 4.06). Baseline characteristics of patients are depicted in Table 1.
Long-term outcomes
-
Survival
Overall survival rate was 259/619 patients (41.8% (95% CI, 38 to 45.8)) and 244/619 (39.4% (95% CI 35.4 to 43.4)), at two and three years from inclusion, respectively. At the end of long-term follow-up, 226 patients were still alive accounting for an overall survival rate after day 60 of 226/316 (71.5%) and for a total of 226/619 (36.5%) of patients initially enrolled. Figure 2A shows the overall long-term survival for the 619 patients included in AKIKI trial. Survival did not differ according to the RRT initiation strategy allocated by randomization (Fig. 2B).
Results of the univariate analysis with all screened variables are presented in supplementary appendix (Additional file 1: Table S2). Multivariate Cox models are summarized in Table 2. The only baseline variable that was statistically significant predictive of long-term mortality was age (HR 1.02, 95%CI 1.01 to 1.03).
-
Renal outcomes
There were no missing data for dialysis dependency and kidney transplantation. Values for SCr concentration beyond day 90 were available in 175 (63%) of 280 patients who survived after day 90. The median time between inclusion and latest SCr concentration assessment was 2.78 years (CI95% 1.58 to 3.14).
The cumulative incidence of WRF was 15/175 (8.5% (CI95% 4.0–12.7)), and 36/175 (20.6% (CI 95% 13.0–28.3)) at three and four years after the severe AKI episode, respectively (Fig. 3A). At the end of follow-up, 46/175 patients (26.2%) presented WRF. Among these patients, 7 had CKD at baseline and eventually progressed from stage 3 to a higher stage of CKD, whereas 39 had normal renal function at baseline. The occurrence of WRF did not differ according to RRT initiation strategy (Fig. 3B). In the univariate Fine and Gray analysis, no variable was associated with the occurrence of WRF (Table 3).
The evolution of eGFR between baseline and long-term follow-up of surviving patients is presented in Fig. 4.
Among the 280 patients who survived after day 90, six remained dialysis-dependent after their ICU stay. Fifteen patients eventually needed chronic dialysis (5%), after a median of 2.3 months (0 to 42) (after day 90). No patient received a kidney transplant.
-
HRQOL
Among long-term survivors, 80/226 (35%) answered the EQ-5D questionnaire. The median index value was 0.67 (IQR 0.40 to 1.00). The violin plot of index values is presented in supplementary appendix (Additional file 1: Figure S1). The values for the 5 dimensions of the questionnaire are presented in the supplementary appendix (Additional file 1: Figure S2).
Inclusion of deceased patients in the calculation yielded a median index of 0.39 (IQR 0.20 to 0.81) (Table 4). The RRT initiation strategy had no effect on results of HRQOL (Table 4).
Probability of survival. Panel A shows Kaplan–Meier curves of the probability of survival for all study participants from randomization to end of extended follow-up. Panel B shows Kaplan–Meier curves of the probability of survival from randomization to end of extended follow-up, according to RRT initiation strategy group
Cumulative incidence of WRF and death. Panel A shows cumulative incidence of WRF from D90 to end of extended follow-up for all study participants Panel B shows cumulative incidence of WRF and death from D90 to end of extended follow-up according to RRT initiation strategy group. WRF Worsening renal function (Defined for non-CKD patients at baseline by the occurrence of CKD and for CKD patients at baseline by the progression from a stage 3 to a stage 4 or 5)
Alluvial diagram illustrating CKD stages evolution according to eGFR from baseline to end of follow-up. Each black bar represents an eGFR KDIGO stage at baseline (on the left of the diagram) and at the end of long-term follow-up (on the right of diagram). eGFR KDIGO stage: higher stages indicate more severe renal disease (see Additional file 1: Table S1 of the supplementary appendix).The height of the bars represents the proportion of patients. Alluvial diagram shows the evolution of the kidney function from baseline to long-term follow-up. The height of each stream represents the proportion of patient. Numbers and proportions of patients are presented next to each stage. Among these patients, 7 had CKD at baseline and eventually progressed from stage 3 to a higher stage of CKD, whereas 39 had normal renal function at baseline. Some patients had apparent eGFR improvement. This point is discussed in the text
Discussion
In this prospective study, 619 critically ill patients with severe AKI were followed for a median time of 3.35 years and for a maximum of 7.39 years. As previously reported, D60 mortality was 49% (95% IC 43 to 54.2) [16]. Death eventually occurred in 28.5% of patients who had survived until day 60 in the initial study. Then, overall survival at 3 years from inclusion was 39.4% (95% CI 35.4 to 43.4). During long-term follow-up, WRF developed in more than a quarter of patients. The occurrence of WRF was noticeable in the first months of follow-up and increased substantially 3 years after the severe AKI episode. However, the proportion of patients needing chronic dialysis was small (5%) and no patient received a kidney transplant. The alluvial diagram (Fig. 4) shows that end stage renal disease (ESRD) was the final path in all patients with CKD at baseline who suffered from worsening of their renal function after the episode of AKI. Then, such patients should be followed very carefully since worsening of renal function can be dramatically rapid. The HRQOL was noticeably impaired in this population. Finally, long-term survival, kidney function and quality of life did not significantly differ according to the RRT initiation strategy.
As could be anticipated, mortality was high in the first 60 days (303/619, 49%) [16] but less so during long-term follow-up. The RENAL trial reported comparable figures (long-term survival of 38% with most deaths occurring in the first 3 months [24]). These observations are consistent with the hypothesis that comorbidities become the most impacting entity on life expectancy after recovery from an episode of AKI [25]. However, the RENAL study included only patients who received RRT, excluding patients with severe AKI who died or recovered before initiation of RRT, whereas ours concerns all patients with severe AKI allocated to an early or delayed RRT initiation strategy (which allowed a substantial proportion of patients to escape RRT).
A high proportion (more than one-quarter) of patients experienced WRF during follow-up in our study. These figures differ from those reported in a study on cardiac surgery patients [26]. Indeed, in that study only 5.7% of patients who experienced AKI and survived developed CKD during follow-up (that study did not provide a median time of follow-up). However, that study comprised a population with different stages (KDIGO 1 to 3) of reversible AKI, whereas ours included only patients with AKI KDIGO stage 3. A recent prospective study (ASSESS-AKI) [27] found consistent results with our study but included only 97 patients with AKI stage 3 on a total of 769 patients (561 had stage 1 AKI and 111 stage 2). Our study focused on a large (n = 619) and homogeneous population of AKI stage 3. Indeed, the risk of developing long-term renal consequences varies probably with the severity of renal insult as well as with the global severity of patients. The small proportion of patients who eventually needed chronic dialysis (5%) in our study is, however, comparable to most studies which provide a follow-up of more than 3 years [28–30]. However, another study reported a higher proportion of chronic dialysis (25%) after an episode of AKI in a population of critically ill patients [31]. This difference might be explained by a high proportion (28%) of patients with CKD before the index hospitalization admission in that study. In contrast, only 19% of patients had preexisting CKD in our study.
A noticeable caveat of all studies, including ours, reporting renal function outcome stems from the fact eGFR may be overestimated by Cockcroft formula at the end of ICU-stay in patients because of muscular loss [32]. This may be responsible for some of our patients moving up into a lower stage of CKD in our study (Fig. 4).
Another important issue is related to the influence of the RRT initiation strategy during AKI. Since three recent large multicenter studies did not show any difference of short-term (60 to 90 days) outcome according to RRT initiation strategy, delaying RRT initiation in patients with severe AKI and no life-threatening complication will probably become standard of care [33]. In such conditions, it is important to evaluate long-term outcomes and particularly whether the initiation strategy affects any of these outcomes. This issue seems controversial at present. Indeed, the follow-up of patients included in the ELAIN trial [34] for one year was the first study to report mortality data at a distance of an initial episode of AKI, according to RRT initiation strategy. Findings indicated that an early initiation of RRT during AKI was associated with a considerably lower mortality rate after 90 days in the early strategy group as compared with the delayed one (19% additional versus 39% in the delayed group, p 0.005). In that study, the time difference of RRT initiation between the two strategies was less than 24 h. Authors do not offer explanation for the finding that such small difference produced very important effects on long- term outcomes. Several features of the initial ELAIN study differ with ours. ELAIN was a single-center study that included mainly post-cardiac surgery patients with a high proportion of fluid overload and worsening pulmonary edema [35], whereas the present one was a multicenter study that included patients with various medical conditions and a high proportion with sepsis or septic shock. In addition, ELAIN study reported a noticeable difference in short-term (90 days) survival between patients allocated to an early RRT strategy (39%) and those allocated to a delayed strategy (55%, p < 0.03), whereas the 3 above-mentioned large multicenter RCTs (accounting for a total of 4034 patients) as well as a large (1879 patients) individual patient-data meta-analysis did not report difference in mortality at D60 or 90 according to RRT initiation strategy.
In fact, the risk of renal sequelae might be higher with an early strategy RRT initiation strategy during AKI for various reasons [33]. Indeed, delayed renal function recovery was observed with such strategy in both AKIKI [16] and STARRT-AKI study [36]. These findings might suggest an association between an early RRT strategy and a risk for CKD [37]. Our present study was unable to confirm this hypothesis. In summary, the discrepancy concerning long-term outcome between the ELAIN study and ours is unexplained at present and underlines the importance of gathering data from more studies before a definite conclusion can be drawn.
Multiple evidence suggests that AKI portends long-term risks although not reported in this study (cardiovascular events, sepsis, fracture risk, CKD and chronic dialysis) making a HRQOL assessment interesting to analyze [3, 38, 39]. In our study, HRQL was noticeably impaired among survivors of AKI with a score comparable to what was found in a systematic review published in 2016 [40]. The initial RRT strategy had no effect on the quality of life.
The main strength of the present study is the extended follow-up of more than 3 years, with some patients followed for more than 7 years and the provision of long-term follow-up according to initial RRT timing strategy in critically ill patients. Results suggest that delaying RRT for critically ill patients in the absence of life-threatening condition does not affect long-term outcomes, in addition to be safe on a short-term basis. The long duration of follow-up allowed to identify definitive WRF. Indeed, a previous study demonstrated that it takes time (up to one year) in order for patients to reach their highest eGFR after an episode of AKI [28], leading to overestimation of WRF if follow-up is too short.
Our study suffers from some limitations. First, a substantial proportion of data on kidney function and HRQOL was not available at the time of follow-up leading to missing data. Since data were missed completely at random [41] (patients and families contact information missing at admission), bias in data collection is unlikely. However, missing data may have affected the precision of our results; therefore, caution is advised in drawing inferences from these findings. This issue is common to all studies assessing long-term renal follow-up [42] and the proportion of missing data in our study is comparable to others [24, 40]. Second, we did not perform a population-based study with non-AKI matched cohort. Then, we do not provide hazard ratio for risk of death, WRF and HRQOL impairment. Third, we did not assess urinary sediment or proteinuria although it is an important information for CKD staging in KDIGO guidelines [15, 43]. Nevertheless, studies reporting these data are often flawed by the non-separation of preexisting proteinuria and hematuria from newly discovered cases after admission [44]. Since our study included more than a quarter of patients with diabetes, it is likely that it would have suffered the same pitfall.
Conclusion
The present study documents long-term outcomes of critically ill patients with severe AKI accurately. Although death rate markedly abates after the first 2 months, death toll remains noticeable and a substantial proportion of survivors progress to CKD and have HRQOL impairment more than 3 years after. These outcomes did not differ according to RRT initiation strategy.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14:607–25.
Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887-899.
Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66.
Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE, Perkins RM. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012;81:477–85.
Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76:893–9.
Li PK-T, Chan GC-K, Chen J, Chen H-C, Cheng Y-L, Fan SL-S, et al. Tackling dialysis burden around the world: a global challenge. KDD. Karger Publishers; 2021;7:167–75.
All-Cause Costs Increase Exponentially with Increased Chronic Kidney Disease Stage. AJMC. https://www.ajmc.com/view/all-cause-costs-increase-exponentially-with-increased-chronic-kidney-disease-stage-article. Accessed 28 Jul 2021.
Elshahat S, Cockwell P, Maxwell AP, Griffin M, O’Brien T, O’Neill C. The impact of chronic kidney disease on developed countries from a health economics perspective: a systematic scoping review. PLoS ONE. 2020;15:e0230512.
Lafrance J-P, Miller DR. Acute kidney injury associates with increased long-term mortality. JASN Am Soc Nephrol. 2010;21:345–52.
Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–53.
James MT, Bhatt M, Pannu N, Tonelli M. Long-term outcomes of acute kidney injury and strategies for improved care. Nat Rev Nephrol. 2020;16:193–205.
Ikizler TA, Parikh CR, Himmelfarb J, Chinchilli VM, Liu KD, Coca SG, et al. A prospective cohort study of acute kidney injury and kidney outcomes, cardiovascular events, and death. Kidney Int. 2021;99:456–65.
Pickkers P, Ostermann M, Joannidis M, Zarbock A, Hoste E, Bellomo R, et al. The intensive care medicine agenda on acute kidney injury. Intensive Care Med. 2017;43:1198–209.
Pickkers P, Darmon M, Hoste E, Joannidis M, Legrand M, Ostermann M, et al. Acute kidney injury in the critically ill: an updated review on pathophysiology and management. Intensive Care Med. 2021;47:835–50.
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-184.
Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375:122–33.
Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Tubach F, Pons B, et al. Comparison of two strategies for initiating renal replacement therapy in the intensive care unit: study protocol for a randomized controlled trial (AKIKI). Trials. 2015;16:170.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat Rev Nephrol. 2017;13:241–57.
Devlin NJ, Brooks R. EQ-5D and the EuroQol group: past, present and future. Appl Health Econ Health Policy. 2017;15:127–37.
Couchoud C, Stengel B, Landais P, Aldigier J-C, de Cornelissen F, Dabot C, et al. The renal epidemiology and information network (REIN): a new registry for end-stage renal disease in France. Nephrol Dial Transplant. 2006;21:411–8.
EuroQol Group. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208.
Jonsson AJ, Lund SH, Eriksen BO, Palsson R, Indridason OS. Incidence of and risk factors of chronic kidney disease: results of a nationwide study in Iceland. Clin Kidney J. 2022;15:1290–9.
Gallagher M, Cass A, Bellomo R, Finfer S, Gattas D, Lee J, et al. Long-term survival and dialysis dependency following acute kidney injury in intensive care: extended follow-up of a randomized controlled trial. PLoS Med. 2014;11: e1001601.
Fortrie G, Stads S, Aarnoudse A-JH, Zietse R, Betjes MG. Long-term sequelae of severe acute kidney injury in the critically ill patient without comorbidity: a retrospective cohort study. PLoS ONE. 2015;10:e0121482.
Legouis D, Galichon P, Bataille A, Chevret S, Provenchère S, Boutten A, et al. Rapid occurrence of chronic kidney disease in patients experiencing reversible acute kidney injury after cardiac surgery. Anesthesiology. 2017;126:39–46.
Hsu C, Chinchilli VM, Coca S, Devarajan P, Ghahramani N, Go AS, et al. Post-acute kidney injury proteinuria and subsequent kidney disease progression: the assessment, serial evaluation, and subsequent sequelae in acute kidney injury (ASSESS-AKI) Study. JAMA Intern Med. 2020;180:402–10.
Brito GA, Balbi AL, Abrão JMG, Ponce D. Long-term outcome of patients followed by nephrologists after an acute tubular necrosis episode. Int J Nephrol. 2012;2012:e361528.
Macedo E, Zanetta DMT, Abdulkader RCRM. Long-term follow-up of patients after acute kidney injury: patterns of renal functional recovery. PLoS ONE. 2012;7:e36388.
Riffaut N, Moranne O, Hertig A, Hannedouche T, Couchoud C. Outcomes of acute kidney injury depend on initial clinical features: a national French cohort study. Nephrol Dial Transplant. 2018;33:2218–27.
Wald R, Shariff SZ, Adhikari NKJ, Bagshaw SM, Burns KEA, Friedrich JO, et al. The association between renal replacement therapy modality and long-term outcomes among critically ill adults with acute kidney injury: a retrospective cohort study*. Crit Care Med. 2014;42:868–77.
Schortgen F. Defining renal recovery: pitfalls to be avoided. Intensive Care Med. 2015;41:1993–5.
Gaudry S, Palevsky PM, Dreyfuss D. Extracorporeal kidney-replacement therapy for acute kidney injury. New Engl J Med. 2022;386:964–75.
Meersch M, Küllmar M, Schmidt C, Gerss J, Weinhage T, Margraf A, et al. Long-term clinical outcomes after early initiation of rrt in critically ill patients with AKI. J Am Soc Nephrol. 2018;29:1011–9.
Dreyfuss D, Hajage D, Gaudry S. Why ELAIN and AKIKI should not be compared: resolving discordant studies. Am J Kidney Dis. 2017;69:864.
STARRT-AKI Investigators, Canadian Critical Care Trials Group, Australian and New Zealand Intensive Care Society Clinical Trials Group, United Kingdom Critical Care Research Group, Canadian Nephrology Trials Network, Irish Critical Care Trials Group, et al. Timing of Initiation of Renal-Replacement Therapy in Acute Kidney Injury. N Engl J Med. 2020. 383:240–51.
Benichou N, Gaudry S, Dreyfuss D. The artificial kidney induces acute kidney injury: yes. Intensive Care Med. 2019. https://doi.org/10.1007/s00134-019-05891-9.
Wang W-J, Chao C-T, Huang Y-C, Wang C-Y, Chang C-H, Huang T-M, et al. The impact of acute kidney injury with temporary dialysis on the risk of fracture. J Bone Miner Res. 2014;29:676–84.
Wu V-C, Wu P-C, Wu C-H, Huang T-M, Chang C-H, Tsai P-R, et al. The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc. 2014;3:e000933.
Villeneuve P-M, Clark EG, Sikora L, Sood MM, Bagshaw SM. Health-related quality-of-life among survivors of acute kidney injury in the intensive care unit: a systematic review. Intensive Care Med. 2016;42:137–46.
Bhaskaran K, Smeeth L. What is the difference between missing completely at random and missing at random? Int J Epidemiol. 2014;43:1336–9.
Herbert RD, Kasza J, Bø K. Analysis of randomised trials with long-term follow-up. BMC Med Res Methodol. 2018;18:48.
Wong CS, Pierce CB, Cole SR, Warady BA, Mak RHK, Benador NM, et al. Association of proteinuria with race, cause of chronic kidney disease, and glomerular filtration rate in the chronic kidney disease in children study. Clin J Am Soc Nephrol. 2009;4:812–9.
Han SS, Ahn SY, Ryu J, Baek SH, Chin HJ, Na KY, et al. Proteinuria and hematuria are associated with acute kidney injury and mortality in critically ill patients: a retrospective observational study. BMC Nephrol. 2014;15:93.
Acknowledgements
We thank Aline Dechanet for the administrative management of the project. We thank Marouane Boubaya and David Hajage for their valuable advice on statistical analysis
Funding
The AKIKI trial was supported by a grant from the Programme Hospitalier de Recherche Clinique National, 2012 (AOM12456), funded by the French Ministry of Health. The extended follow-up Severe Acute kidney injury Long-Term Outcomes (SALTO) had no financial support.
Author information
Authors and Affiliations
Contributions
This is an investigator-initiated multicenter study led by Stephane Gaudry and the members of the steering committee (Didier Dreyfuss, Stephane Gaudry, David Hajage Laurent Martin Lefevre, Jean Damien Ricard, Frederique Schortgen, Florence Tubach). All the investigators mentioned as co-authors gathered the data. The statistical analysis was performed by Khalil Chaïbi. The paper was written by Khalil Chaïbi, Stéphane Gaudry, and Didier Dreyfuss. The paper was submitted to all the co-authors who made substantial contributions and agreed to submit to Annals of Intensive Care. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol was approved by the ethics committee of the French Society of Intensive Care Medicine and by the appropriate French legal authority (Comité de Protection des Personnes d’Ile de France VI) for all participating centers. Patients or their surrogates were informed that they could decline to participate at any time.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Current Chronic kidney disease Nomenclature used by KDIGO. Table S2. Univariate analysis of mortality predictors. Figure S1. Violin plot HRQL. Figure S2. EQ5D five dimensions of health in SALTO according to RRT strategy group. Figure S3. Schoenfeld residuals for proportional hazards assumption.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chaïbi, K., Ehooman, F., Pons, B. et al. Long-term outcomes after severe acute kidney injury in critically ill patients: the SALTO study. Ann. Intensive Care 13, 18 (2023). https://doi.org/10.1186/s13613-023-01108-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-023-01108-x