Abstract
Background
Bedside assessment of low levels of inspiratory effort, which are probably insufficient to prevent muscle atrophy, is challenging. The flow index, which is derived from the analysis of the inspiratory portion of the flow–time waveform, has been recently introduced as a non-invasive parameter to evaluate the inspiratory effort. The primary objective of the present study was to provide an external validation of the flow index to detect low inspiratory effort.
Methods
Datasets containing flow, airway pressure, and esophageal pressure (Pes)–time waveforms were obtained from a previously published study in 100 acute brain-injured patients undergoing pressure support ventilation. Waveforms data were analyzed offline. A low inspiratory effort was defined by one of the following criteria, work of breathing (WOB) less than 0.3 J/L, Pes–time product (PTPes) per minute less than 50 cmH2O•s/min, or inspiratory muscle pressure (Pmus) less than 5 cmH2O, adding “or occurrence of ineffective effort more than 10%” for all criteria. The flow index was calculated according to previously reported method. The association of flow index with Pes-derived parameters of effort was investigated. The diagnostic accuracy of the flow index to detect low effort was analyzed.
Results
Moderate correlations were found between flow index and WOB, Pmus, and PTPes per breath and per minute (Pearson’s correlation coefficients ranged from 0.546 to 0.634, P < 0.001). The incidence of low inspiratory effort was 62%, 51%, and 55% using the definition of WOB, PTPes per minute, and Pmus, respectively. The area under the receiver operating characteristic curve for flow index to diagnose low effort was 0.88, 0.81, and 0.88, for the three respective definition. By using the cutoff value of flow index less than 2.1, the diagnostic performance for the three definitions showed sensitivity of 0.95–0.96, specificity of 0.57–0.71, positive predictive value of 0.70–0.84, and negative predictive value of 0.90–0.93.
Conclusions
The flow index is associated with Pes-based inspiratory effort measurements. Flow index can be used as a valid instrument to screen low inspiratory effort with a high probability to exclude cases without the condition.
Similar content being viewed by others
Background
Pressure support ventilation (PSV) is one of the most widely used modes for mechanically ventilated patients [1]. The main advantage of PSV is to provide variable inspiratory flow to match the patient’s inspiratory effort, but so far there is no consensus on the adjustment of the optimal pressure support level [2]. Recent studies suggest that low levels of inspiratory effort due to over-assistance may adversely affect the respiratory system, probably leading to diaphragm atrophy and contractile dysfunction due to disuse [3, 4]. Bedside evaluation of potential injurious low effort is challenging. Indeed, the absence of signs related to respiratory workload (respiratory distress or recruitment of accessory respiratory muscles) is not sufficient: most over-assisted patients appear calm and comfortable [5, 6]. Therefore, early detection of low effort is essential for the appropriate management of patients receiving PSV.
Numerous instruments have been designed to assess inspiratory effort [7, 8]. Up to now, measurements based on esophageal pressure (Pes) are still being treated as the gold standard, including tidal swing of Pes (ΔPes), inspiratory muscle pressure (Pmus), Pes–time product (PTPes), and work of breathing (WOB) [9, 10]. However, these parameters are usually used for research purposes and not for routine clinical monitoring, mainly because they require relatively invasive procedures, special equipment, and high expertise with complex calculations. Recently, several non-invasive methods have been investigated, including airway occlusion pressure (P0.1) [11], the swing in airway pressure (Paw) generated by the patient’s respiratory effort against the occluded airway (ΔPOCC) [12], and pressure muscle index (PMI) [13], and the results show that these parameters can reliably assess inspiratory effort. In 2021, Albani and colleagues introduced a new parameter derived from the analysis of the inspiratory portion of the flow–time waveform, the flow index, which is independently correlated with inspiratory effort in patients receiving PSV [14]. Data from the same group of patients showed that the flow index could accurately identify high and low inspiratory effort [15]. The advantage of this monitoring method is that no airway manipulation is required, and continuous monitoring can be accommodated if automatic curve fitting is integrated into the ventilator design. However, the study was single-center and lacked additional evidence to verify its validity.
In the present study, we performed a secondary analysis of previously published data on brain-injured patients [16]. The primary aim was to provide external validation of the flow index to detect potential injurious low inspiratory effort. In addition, we specifically investigated whether the flow index could be used as a screening tool because of its continuous measurement characteristics.
Methods
This was a secondary analysis of data obtained from a previously published prospective observational cohort study (ClinicalTrials.gov: NCT03212482) [16]. Anonymous use of the data was approved by the Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University (KY 2017–028-02).
Data collection
More detailed information about the previous study can be found in the original publication [16]. The study enrolled 100 acute brain-injured patients undergoing mechanical ventilation (AVEA ventilator, CareFusion Co., USA) and Pes monitoring (SmartCath-G catheter, CareFusion Co., San Diego, CA, USA). The position of the esophageal balloon was confirmed by an occlusion test [17]. Flow, Paw, and Pes waveforms were recorded at 100 Hz for 15 min using the ventilator acquisition system (VOXP Research Data Collector 3.2, Applied Biosignals GmbH, Weener, Germany). The settings of the ventilator remained unchanged during the 15-min recording period. At the end of the recording, an arterial blood gas analysis was performed.
In the study unit, the ventilator mode was usually changed to PSV when all ventilator breaths were triggered by the patient during assist/control ventilation. Pressure support was set to obtain tidal volume (VT) of 6–8 ml/kg predicted body weight (PBW) with the respiratory rate (RR) lower than 30 breaths/min, and to maintain an arterial partial pressure of carbon dioxide of 35–40 mmHg (usually performed twice daily) as long as possible [16]. The trigger sensitivity was usually set as 1–2 L/min for the flow-trigger and 1.5–3 cmH2O for the pressure-trigger. The inspiratory-to-expiratory cycling was usually set as 25–30% of peak inspiratory flow. Inspired oxygen fraction (FiO2) and positive end-expiratory pressure (PEEP) were set according to the oxygenation condition of the patient. The first dataset undergoing PSV in each patient was selected for the present analysis. We chose five consecutive stable breaths without Pes artifacts, swallowing, and patient–ventilator asynchrony from the last 5 min in each 15-min dataset, and measurements were averaged. Data were offline analyzed by using a dedicated software (ICU-Lab 2.5 software package, KleisTEK, Bari, Italy).
Definitions and measurements
Measurements of respiratory mechanics variables were in accordance with previous recommendations [9, 10, 14]. The onset of inspiratory effort was defined as the point of negative deflection of Pes with a rapid change in slope [18]. The onset and the end of ventilator insufflation were identified as the first and the last positive value in the flow–time recording, respectively [10].
Inspiratory VT was integrated using a flow–time waveform. RR was calculated as 60 s divided by the time of the total breathing cycle (s) in each measured breath and averaged over the five selected breaths. The rapid shallow breathing index (RSBI) was calculated as the ratio between RR (breaths/min) and VT (L) [19]. Minute ventilation (MV) was also calculated.
The inspiratory ∆Pes was calculated as the difference in Pes between the onset of inspiratory effort and the maximal negative deflection during inspiration. Intrinsic PEEP (PEEPi) was measured as the Pes dropping from the onset of inspiratory effort to the onset of ventilator insufflation. Pmus was calculated as the maximal difference between the static recoil pressure of the chest wall (Pcw) and Pes during inspiration. A theoretical value of chest wall compliance, estimated as 4% of the predicted value of vital capacity [20], was used to construct Pcw.
The PTPes per breath (cmH2O•s) was measured as the area subtended by the Pes–time and Pcw–time curve from the onset of inspiratory effort to the end of ventilator insufflation [10]. PTPes per minute was calculated as the product of PTPes and RR, which was expressed as cmH2O•s/min. The inspiratory WOB was measured using the Campbell diagram and was expressed as joules/liter (J/L) [10, 21].
Original results of ineffective triggering were used in the present study [16]. The ineffective effort (IE) index was calculated as the percentage of ineffective triggers in total breaths in the entire 15-min dataset [22].
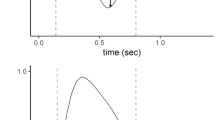
The flow index was calculated according to the method introduced by Albani and coworkers based on flow–time curve analysis during PSV [14]. The starting point on the flow–time waveform during inspiratory was defined as the flow increased less than 1% of the preceding measurement, and the ending point was identified as the flow decreased more than 10% of the former measurement (Fig. 1). Flow–time data between the starting and the ending point were fitted by using the equation:
Schematic show of flow index measurement. Flow–time waveform (grey circle, 100 Hz) for a single breath under pressure support ventilation is shown. The starting point of flow–time fitting was defined as the flow increased less than 1% of the preceding measurement, and the ending point was identified as the flow decreased more than 10% of the former measurement. The equation of fitting is also shown. The solid black line indicates the flow–time fitting curve (R2 = 0.997 and 0.994). The flow index was 1.3 and 3.1 in panel A and panel B, respectively
where Flow and Time were described as L/s and s, respectively. The parameter c was defined as the flow index [14].
Definition of low inspiratory effort
In the present study, the potential injurious low inspiratory effort was defined based on the three criteria introduced by previous studies, including (1) WOB less than 0.3 J/L [21]; (2) PTPes per minute less than 50 cmH2O•s/min [11]; and (3) Pmus less than 5 cmH2O [15], adding “or IE index more than 10%” for all criteria [23].
Statistical analysis
Categorical variables are expressed as counts and percentages, and continuous data are presented as median (25–75th percentile).
Pearson’s correlation was performed to evaluate the association of flow index with Pes-derived inspiratory effort assessment parameters, including Pmus, PTPes per breath, PTPes per minute, and WOB.
The incidence of low inspiratory effort was reported as percentage and 95% confidence interval (CI). The agreement of low effort identified by the three definitions was analyzed using the interclass correlation coefficient (ICC) with the two-way-random model. 95% CI of ICC was also calculated.
The primary endpoint of the present study was the validity of the flow index to detect potential injurious low inspiratory effort. The diagnostic accuracy of flow index and other non-invasive parameters (RSBI, and MV) was analyzed using the receiver operating characteristic curve (ROC), and the area under the curve (AUC) was calculated. A comparison of AUCs was performed using the DeLong test. The best cutoff value for the flow index to identify low effort was calculated using Youden’s index. For flow index, sensitivity, specificity, positive and negative predictive values (PPV and NPV), and accuracy with respective 95% CI were calculated using the best cutoff value derived from the present study and the value reported by Albani et al. [15].
Datasets with low inspiratory effort were identified according to the three definitions adopted in the present study. The association of low effort with pressure support level, type of brain injury, consciousness impairment as indicated by the Glasgow Coma Scale (GCS), and the use of analgesia and/or sedation was analyzed using a multivariate model with an enter logistic regression. Odds ratios (ORs) and 95% CIs were calculated for each factor.
The statistical analysis was conducted with SPSS 26.0 software. A P-value lower than 0.05 was regarded as statistically significant.
Results
Patients’ characteristics are shown in Table 1. At a median (25–75th percentile) pressure support of 7 (6–8) cmH2O, the RR and VT were 19 (16–22) breaths/min and 8.6 (7.4–9.8) ml/kg PBW, respectively. The three Pes-derived parameters used to define the low inspiratory effort were WOB of 0.20 (0.12–0.57) J/L, PTPes per minute of 72.6 (46.6–138.6) cmH2O•s/min, and Pmus of 5.6 (3.2–10.6) cmH2O. The median (25–75th percentile) of flow index was 1.7 (1.4–2.2), ranging from 1.0 to 4.7.
Moderate correlations were found between flow index and Pes-derived parameters for inspiratory effort evaluation, including Pmus, PTPes per breath and per minute, and WOB (Fig. 2, Pearson correlation coefficients ranged from 0.546 to 0.634, P < 0.001).
Correlation of flow index with inspiratory effort parameters derived from esophageal pressure. The flow index correlated significantly with the esophageal pressure–time product (PTPes) per breath and per minute, inspiratory muscle pressure (Pmus), and work of breathing (WOB). Pearson correlation coefficient (r) is shown
Incidence (95% CI) of low inspiratory effort was 62% (51.7–71.4%), 51% (40.9–61.1%), and 55% (44.8–64.9%) by the definition of WOB, PTPes per minute, and Pmus, respectively (Fig. 3). ICC (95% CI) of agreement among the three definitions was 0.923 (0.891–0.946).
Incidence of low inspiratory effort by different definitions. Data are shown as low inspiratory effort only defined by criteria of work of breathing (WOB), esophageal pressure–time product (PTPes) per minute, or inspiratory muscle pressure (Pmus), only defined by criteria of ineffective effort (IE) index, and defined by both effort parameters based on esophageal pressure and IE index criteria
Figure 4 shows the results of the ROC analysis for flow index and other non-invasive parameters to detect low effort. By each of the three definitions, the AUC of the flow index (0.81–0.88) was significantly higher than that of MV and RSBI (0.55–0.69) (Fig. 4). No significant differences were found in the AUCs of flow index among the three definitions (P > 0.05).
Receiver operating characteristic curve of non-invasive parameters for detecting low inspiratory effort. The area under the receiver operating characteristic curve (AUC) and 95% confidence interval (CI) are shown. By definition of work of breathing (A), the AUC of flow index was significantly higher than those of rapid shallow breathing index (RSBI) (P = 0.002) and minute ventilation (MV) (P < 0.001). By definition of esophageal pressure–time product per minute (B), the AUC of flow index was significantly higher than the other parameters (vs. RSBI: P = 0.002; vs. MV: P < 0.001). By definition of inspiratory muscle pressure (C), the AUC of flow index was significantly higher than the other two parameters (all P < 0.001)
The cutoff value of the flow index to detect low inspiratory effort was 2.1, 2.0, and 2.0 by the definition of WOB, PTPes per minute, and Pmus, respectively. Because we considered the sensitivity of the flow index to be the most important feature to avoid missing the detection of low effort, we set 2.1 as the cutoff value for all three definitions in diagnostic performance analysis. Although specificity was relatively low (0.57–0.71), high sensitivity (0.95–0.96) and NPV (0.90–0.93) were found (Table 2). The PPVs ranged from 0.70 to 0.84. By using a higher cutoff value (2.6) previous reported to detect low inspiratory effort [15], higher sensitivity and NPV (equals to 1.00), as well as lower specificity (0.33–0.42) and PPV (0.61–0.74), were found in our cohort (Table 3).
Multivariate logistic analysis showed that only the pressure support level was significantly associated with low inspiratory effort (OR ranged from 1.36 to 1.45, P < 0.05) (Table 4). The type of brain injury, GCS, and the use of analgesia and/or sedation did not enter the model of factors associated with low inspiratory effort.
Discussion
The present analyses of previously published data in mechanically ventilated brain-injured patients show that: (1) flow index, a novel parameter of inspiratory flow–time waveform fitting, is associated with Pes-derived inspiratory effort assessment parameters; (2) high sensitivity suggests that flow index can be used as a valid instrument to screen low inspiratory effort, and high NPV indicates a high probability of flow index to exclude cases without the condition of interest, while the relatively low specificity and PPV suggest that the flow index is less likely to rule in low effort when following values below the cutoff point; and (3) low inspiratory effort is not uncommon in brain-injured patients undergoing PSV, and the primary cause might have been over-assistance of pressure support.
The first difficulty encountered during the design of the present analysis was the definition of potential injurious low inspiratory effort. Although Pes-based parameters have been used as the golden standard for assessing inspiratory effort [7,8,9,10], there is no consensus on the criteria for low effort in critically ill patients undergoing mechanical ventilation. Therefore, we included the three most commonly used parameters, including WOB, PTPes per minute, and Pmus, to define the low effort. These criteria are all derived from Pes waveform analysis which was considered the gold diagnosis. In accordance with previous studies, the lower limits of these parameters in healthy subjects at rest were selected as the criteria for definition [11, 15, 23]. And our results showed a high agreement for low effort diagnosis among these three definitions (ICC 0.923, 95% CI 0.891–0.946).
In the present study, we also included the severity of ineffective triggering in the definition of potential injurious low effort as described by Pletsch-Assuncao and coworkers [23]. The main factors associated with ineffective triggering include low respiratory drive and effort, high trigger threshold loading (PEEPi), and insensitive trigger setting [24]. In our unit, a relatively sensitive trigger setting (1–2 L/min for flow-trigger and 1.5–3 cmH2O for pressure-trigger) is routinely employed. Because only breaths without patient–ventilator asynchrony were included for measurements of Pes-derived effort parameters and flow index, this resulted in a low measured PEEPi (median of 1.2 with 25–75th percentile of 0.8–2.1 cmH2O) in our assessments (Table 1). Notably, diagnosing low effort according to IE criteria alone was uncommon in our data, especially for the WOB definition (Fig. 3). However, further clinical outcome studies are needed to determine whether to incorporate ineffective triggering into the diagnosis of low inspiratory effort.
For bedside monitoring of inspiratory effort without additional invasive procedures, several Paw-based instruments have been developed, including P0.1 [11], ΔPOCC [12], and PMI [13]. Studies have shown that these parameters correlate with Pes-based effort assessing instruments and can reliably detect high and low inspiratory effort. However, these monitoring methods require airway manipulation and thus can only be performed intermittently, which may hinder their use as screening tools to detect low inspiratory effort. The newly introduced flow index, fitting the descending portion of inspiratory flow with time, represents the relationship of the patient’s inspiratory effort with ventilator flow insufflation after triggering [14]. It has been demonstrated by a single-center study that flow index correlates with inspiratory effort [14].
In Albani and coworkers’ study, pressure support was titrated to obtain low, intermediate, and high inspiratory effort, and they found the flow index was accurate to detect low inspiratory effort [15]. These results may be helpful for adjustment of pressure support to avoid over-assistance in a specific patient. In the present study, we were deliberately interested in the diagnostic performance of flow index as a screening tool to detect low inspiratory effort. ROC analysis showed that the flow index could accurately detect low effort (AUC 0.81–0.88, Fig. 4). Using less than 2.1 as the cutoff value, high sensitivity (0.95–0.96) indicated an excellent performance of the flow index as a screening tool (Table 2). Meanwhile, high NPV (0.90–0.93) indicated a high probability of excluding cases without low effort when the flow index was higher than or equal to 2.1. Similar diagnostic performances to detect low inspiratory effort were found in our cohort when using the previously reported cutoff value of 2.6 (Table 3) [15]. Additionally, we used datasets collected at clinical pressure support settings and did not perform adjustments. Therefore, our results may be more useful to screen low effort in the patient population undergoing PSV. However, it should be emphasized that lower specificity and PPV may indicate that the flow index is less likely to rule in low effort when values below the cutoff point are followed. Clinicians should use the flow index with caution when confirming the low inspiratory effort is the primary purpose.
Notably, the high inspiratory effort is also detrimental to mechanically ventilated patients. Vigorous inspiratory effort due to inadequate assistance may increase lung stress and strain, both global and regional, which is deemed as the major mechanism of patient self-inflicted lung injury [25]. Therefore, detecting high effort may be an important issue in preventing patient self-inflicted lung injury. However, our data do not allow us to describe whether the flow index can reliably detect high effort because of a relatively low incidence of high effort in the present cohort. Although Albani and coworkers demonstrated that the flow index is accurate in detecting high inspiratory effort during PSV [15], further analysis is required.
Unlike other studies on the general critically ill population [11,12,13,14,15], the present study analyzed brain-injured patients. The low inspiratory effort may have resulted from either low respiratory drive due to original brainstem impairment or over-assistance of mechanical ventilation, or inspiratory muscle dysfunction which is likely mainly due to over-assistance [7]. Because of the retrospective nature of the analysis, we did not have data on the prevalence and severity of brainstem damage in our cohort. Multivariate logistic analysis showed that low effort was only associated with pressure support level, but not with the type of brain injury, level of consciousness, and use of sedation/analgesia, which implied that the main reason for low effort might be over-assistance in our group of patients. However, this result should be interpreted with caution because a lot of data on brainstem impairment were not collected. Given the increased use of PSV in brain-injured patients [16, 26, 27], further studies are warranted in this population.
There are limitations in the present study. First, there is still the lack of a universally accepted definition of low inspiratory effort. We included the three most used Pes-derived criteria plus ineffective triggering. Our results showed a high agreement for the diagnosis of low effort among these criteria. Second, we did not analyze the high inspiratory effort in the present study, mainly because high effort rarely occurred in our cohort. Third, this was a retrospective analysis of previous data in brain-injured patients. Our results may not be applicable to other populations. Fourth, we did not measure other non-invasive inspiratory effort parameters, such as P0.1, ΔPOCC and PMI, because no formal airway occlusion was performed in the original research. Therefore, we cannot provide the comparison of flow index with these parameters in diagnostic performance for detecting low effort.
Conclusions
The flow index is associated with Pes-based inspiratory effort measurements. Flow index can be used as a valid instrument to screen low inspiratory effort with a high probability to exclude cases without the condition. Our results highlight further investigation and development of flow index as a new ventilator monitoring modality for inspiratory effort assessment.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- FiO2 :
-
Inspired oxygen fraction
- GCS:
-
Glasgow Coma Scale
- ICC:
-
Interclass correlation coefficient
- IE:
-
Ineffective effort
- MV:
-
Minute ventilation
- NPV:
-
Negative predictive values
- OR:
-
Odds ratio
- Paw :
-
Airway pressure
- PBW:
-
Predicted body weight
- Pcw :
-
The static recoil pressure of the chest wall
- PEEP:
-
Positive end-expiratory pressure
- PEEPi:
-
Intrinsic positive end-expiratory pressure
- Pes :
-
Esophageal pressure
- PMI:
-
Pressure muscle index
- Pmus :
-
Inspiratory muscle pressure
- PPV:
-
Positive predictive values
- PSV:
-
Pressure support ventilation
- PTP:
-
Pressure–time product
- PTPes :
-
Esophageal pressure–time product
- P0.1:
-
Airway occlusion pressure
- ΔPes :
-
Tidal swing of esophageal pressure
- ∆POCC :
-
The swing in Paw generated by the patient’s respiratory effort
- ROC:
-
Receiver operating characteristic curve
- RR:
-
Respiratory rate
- RSBI:
-
Rapid shallow breathing index
- VT :
-
Tidal volume
- WOB:
-
Work of breathing
References
Esteban A, Frutos-Vivar F, Muriel A, Ferguson ND, Penuelas O, Abraira V, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med. 2013;188:220–30.
Brochard LJ, Lellouche F. Pressure support ventilation. In: Tobin MJ, editor. Principles and Practice of Mechanical Ventilation. New York: McGraw Hill Companies; 2013. p. 199–227.
Goligher EC, Jonkman AH, Dianti J, Vaporidi K, Beitler JR, Patel BK, et al. Clinical strategies for implementing lung and diaphragm-protective ventilation: avoiding insufficient and excessive effort. Intensive Care Med. 2020;46:2314–26.
Hudson MB, Smuder AJ, Nelson WB, Bruells CS, Levine S, Powers SK. Both high level pressure support ventilation and controlled mechanical ventilation induce diaphragm dysfunction and atrophy. Crit Care Med. 2012;40(4):1254–60.
Vitacca M, Bianchi L, Zanotti E, Vianello A, Barbano L, Porta R, et al. Assessment of physiologic variables and subjective comfort under different levels of pressure support ventilation. Chest. 2004;126:851–9.
Perez J, Dorado JH, Papazian AC, Berastegui M, Gilgado DI, Cardoso GP, et al. Titration and characteristics of pressure-support ventilation use in Argentina: an online cross-sectional survey study. Rev Bras Ter Intensiva. 2020;32:81–91.
Telias I, Spadaro S. Techniques to monitor respiratory drive and inspiratory effort. Curr Opin Crit Care. 2020;26:3–10.
de Vries H, Jonkman A, Shi ZH, Spoelstra-de Man A, Heunks L. Assessing breathing effort in mechanical ventilation: physiology and clinical implications. Ann Transl Med. 2018;6:387.
Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189:520–31.
Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, et al. Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med. 2016;42:1360–73.
Telias I, Junhasavasdikul D, Rittayamai N, Piquilloud L, Chen L, Ferguson ND, et al. Airway occlusion pressure as an estimate of respiratory drive and inspiratory effort during assisted ventilation. Am J Respir Crit Care Med. 2020;201:1086–98.
Bertoni M, Telias I, Urner M, Long M, Del Sorbo L, Fan E, et al. A novel non-invasive method to detect excessively high respiratory effort and dynamic transpulmonary driving pressure during mechanical ventilation. Crit Care. 2019;23:346.
Kyogoku M, Shimatani T, Hotz JC, Newth CJL, Bellani G, Takeuchi M, et al. Direction and magnitude of change in plateau from peak pressure during inspiratory holds can identify the degree of spontaneous effort and elastic workload in ventilated patients. Crit Care Med. 2020;49:517–26.
Albani F, Pisani L, Ciabatti G, Fusina F, Buizza B, Granato A, et al. Flow Index: a novel, non-invasive, continuous, quantitative method to evaluate patient inspiratory effort during pressure support ventilation. Crit Care. 2021;25:196.
Albani F, Fusina F, Ciabatti G, Pisani L, Lippolis V, Franceschetti ME, et al. Flow Index accurately identifies breaths with low or high inspiratory effort during pressure support ventilation. Crit Care. 2021;25:427.
Luo XY, He X, Zhou YM, Wang YM, Chen JR, Chen GQ, et al. Patient-ventilator asynchrony in acute brain-injured patients: a prospective observational study. Ann Intensive Care. 2020;10:144.
Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis. 1982;126:788–91.
Foti G, Cereda M, Banfi G, Pelosi P, Fumagalli R, Pesenti A. End-inspiratory airway occlusion: a method to assess the pressure developed by inspiratory muscles in patients with acute lung injury undergoing pressure support. Am J Respir Crit Care Med. 1997;156:1210–6.
Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991;324:1445–50.
Sassoon CS, Light RW, Lodia R, Sieck GC, Mahutte CK. Pressure-time product during continuous positive airway pressure, pressure support ventilation, and T-piece during weaning from mechanical ventilation. Am Rev Respir Dis. 1991;143:469–75.
Cabello B, Mancebo J. Work of breathing. Intensive Care Med. 2006;32:1311–4.
Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med. 2006;32:1515–22.
Pletsch-Assuncao R, Caleffi Pereira M, Ferreira JG, Cardenas LZ, de Albuquerque ALP, de Carvalho CRR, et al. Accuracy of invasive and noninvasive parameters for diagnosing ventilatory overassistance during pressure support ventilation. Crit Care Med. 2018;46:411–7.
Pham T, Telias I, Piraino T, Yoshida T, Brochard LJ. Asynchrony Consequences and Management. Crit Care Clin. 2018;34:325–41.
Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–42.
Pelosi P, Ferguson ND, Frutos-Vivar F, Anzueto A, Putensen C, Raymondos K, et al. Management and outcome of mechanically ventilated neurologic patients. Crit Care Med. 2011;39:1482–92.
Tejerina EE, Pelosi P, Robba C, Penuelas O, Muriel A, Barrios D, et al. Evolution over time of ventilatory management and outcome of patients with neurologic disease. Crit Care Med. 2021;49:1095–106.
Acknowledgements
Not applicable.
Funding
This work is supported by a grant from the Beijing Engineering Research Center of Digital Healthcare for Neurological Diseases (JFG-2021–1358). The sponsor had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
MYM and JXZ contributed to the study design. MYM, WC, YMZ and JXZ participated in the data measurement and analysis. MYM and JXZ conducted the statistical analysis. MYM, LZ, and JXZ drafted the manuscript. MMY, RG, DJS, SPW, YLY, LZ and JXZ revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The use of data in this study was approved by the Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University (KY 2017–028-02).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miao, MY., Chen, W., Zhou, YM. et al. Validation of the flow index to detect low inspiratory effort during pressure support ventilation. Ann. Intensive Care 12, 89 (2022). https://doi.org/10.1186/s13613-022-01063-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-022-01063-z