Abstract
Introduction
Survivors of viral ARDS are at risk of long-term physical, functional and neuropsychological complications resulting from the lung injury itself, but also from potential multiorgan dysfunction, and the long stay in the intensive care unit (ICU). Recovery profiles after severe SARS-CoV-2 pneumonia in intensive care unit survivors have yet to be clearly defined.
Material and methods
The goal of this single-center, prospective, observational study was to systematically evaluate pulmonary and extrapulmonary function at 12 months after a stay in the ICU, in a prospectively identified cohort of patients who survived SARS-CoV-2 pneumonia. Eligible patients were assessed at 3, 6 and 12 months after onset of SARS-CoV-2. Patients underwent physical examination, pulmonary function testing, chest computed tomography (CT) scan, a standardized six-minute walk test with continuous oximetry, overnight home respiratory polygraphy and have completed quality of life questionnaire. The primary endpoint was alteration of the alveolar–capillary barrier compared to reference values as measured by DLCO, at 12 months after onset of SARS-CoV-2 symptoms.
Results
In total, 85 patients (median age 68.4 years, (interquartile range [IQR] = 60.1–72.9 years), 78.8% male) participated in the trial. The median length of hospital stay was 44 days (IQR: 20–60) including 17 days in ICU (IQR: 11–26). Pulmonary function tests were completed at 3 months (n = 85), 6 months (n = 80), and 12 months (n = 73) after onset of symptoms. Most patients showed an improvement in DLCO at each timepoint (3, 6, and 12 months). All patients who normalized their DLCO did not subsequently deteriorate, except one. Chest CT scans were abnormal in 77 patients (96.3%) at 3 months and although the proportion was the same at 12 months, but patterns have changed.
Conclusion
We report the results of a comprehensive evaluation of 85 patients admitted to the ICU for SARS-CoV-2, at one-year follow-up after symptom onset. We show that most patients had an improvement in DLCO at each timepoint.
Trial registration: Clinical trial registration number: NCT04519320.
Key Points
The most interesting findings were that most patients showed an improvement in their DLCO at 3, 6, and 12 months, and all patients but one who normalized their DLCO did not deteriorate afterwards. Only 11% of patients had persistent impairment of DLCO at 1 year.
Similar content being viewed by others
Introduction
In late December 2019, an outbreak of pneumonia started in Wuhan, China, caused by a novel coronavirus, which was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1], due to the occurrence of severe acute respiratory distress syndrome (ARDS) in 29% of hospitalized patients.
Although data to accurately estimate the extent of post-SARS-CoV-2 sequelae are lacking, survivors of viral ARDS are at risk of long-term physical, functional and neuropsychological complications resulting from the lung injury itself, but also from potential multiorgan dysfunction, and the long stay in the intensive care unit (ICU) [2,3,4]. Post-viral syndromes are well documented following other viral infections, including previous coronavirus outbreaks such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). SARS resulted in significant repercussions on pulmonary function, chronic musculoskeletal pain, and long-term mental disorders in survivors [5]. Chen et al. followed up 56 patients with H7N9 avian influenza to analyze pulmonary function and imaging changes up to 2 years after infection. Their results showed that despite interstitial changes and fibrosis on imaging, ventilation and diffusion dysfunction improved during the first 3 months and the improvement was associated with the sequelae observed at 2 years [6]. Wu et al. recently reported serial pulmonary function, exercise capacity, and chest high-resolution computed tomography (HRCT) changes in non-intubated patients hospitalized in Wuhan with severe SARS-CoV-2 pneumonia at 3, 6, 9, and 12 months following hospital discharge. They found evidence of persistent physiological and radiographic changes in a subgroup of patients [7]. To the best of our knowledge, there is no European report focusing exclusively on the most severe patients, as defined by the World Health Organization (WHO) categories. The long-term effect of SARS-CoV-2 on lung parenchyma and pulmonary function remains an open question.
With 295 patients hospitalized in the ICU at the peak of the epidemic in April, the region of Bourgogne-Franche-Comté in Eastern France was one of the regions with the highest incidence rates and ICU admissions for SARS-CoV-2 in France [8]. The goal of this study was to describe one-year recovery profiles, defined by repeated respiratory and exercise function, and quality of life evaluations, in a prospectively identified cohort of ICU patients who survived severe pneumonia.
Methods
Patients and study design of COV-RECUP
This single-center, prospective, observational study was performed in the French University Hospital of Besançon from April 2020 to June 2021 (first wave). All SARS-CoV-2 ICU survivors were contacted upon discharge from ICU and invited to participate in the trial. Patients were eligible if they had SARS-CoV-2 infection diagnosed by viral RNA detection by quantitative RT-PCR on nasal swabs or bronchoalveolar lavage. Patients had to have been admitted to the ICU with SpO2 < 92% and evidence of air-space changes in 25% of lung parenchyma on chest CT scan. For fear of non-compliance with follow-up procedures due to increased morbidity–mortality, patients were excluded if they were older than 79 years. Other exclusion criteria were the following: chronic respiratory insufficiency, long-term oxygen therapy, interstitial lung disease, significant psychiatric disorders, or a life expectancy estimated at less than one year. The study consisted in follow-up visits, including outpatient evaluation at 3, 6 and 12 months after symptom onset. Only for patients with sequelae, annual follow-up was planned up to a maximum of 5 years. After discharge from the ICU, all inpatients underwent targeted exercise rehabilitation twice daily for at least 20 min with a physiotherapist. Exercise rehabilitation consisted of passive range of motion, active range of motion, electrical muscle stimulation, sitting, tilting, standing, ambulation, and other mobilization techniques depending on the patient’s condition. Cardiopulmonary rehabilitation was performed with aerobic physical activity. All patients included in the study were systematically offered early psychological follow-up.
Written consent was obtained before the first visit at 3 months and the protocol was approved by the ethics committee (Comité de Protection des Personnes (CPP) Grand-Est) on 21/04/2020. The COV-RECUP study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines (Clinical trial registration number: NCT04519320).
Follow-up procedures
At 3, 6 and 12 months (± 3 weeks) after onset of SARS-CoV-2 symptoms, patients underwent a physical examination, pulmonary function testing, blood gas analysis, non-contrast enhanced chest millimeter section CT scan, resting oximetry, and a standardized six-minute walk test (6MWT) with continuous oximetry. At 3 months only, because of risk factors common to severe COVID-19 infection and sleep apnea syndrome, a complete overnight home respiratory polygraphy was performed to evaluate the frequency of sleep apnea syndrome. Routine spirometry, and single breath hemoglobin-adjusted DLCO were performed using Global Lung Function Initiative reference values [9, 10]. Maximum expiratory (MEP) and inspiratory pressures (MIP) (Platinum Elite; MGC Diagnostics Corporation, Saint Paul, Minnesota, USA) were performed using healthy subjects reference values [11]. For single breath hemoglobin-adjusted DLCO measurements, patients were instructed to hold their breath for 10 s followed by a complete and consistent exhalation, at which time an alveolar sample of exhaled gas was analyzed for calculation of uptake of CO. Six-minute walk distance (6MWD) was assessed according to established guidelines. Symptom-limited incremental cardiopulmonary exercise testing (CPET) was performed on an electronically braked cycle ergometer (Ergometrics 900, Ergoline, Bitz, Germany) and physiological data were obtained breath by breath (MGC-CPX System; MGC Diagnostics).
Chest CT scans were performed on a Revolution CT (GE Healthcare, Milwaukee, WI, USA). Imaging results were reviewed by two chest radiologists (J.B. and F.G. with, respectively, 11 and 6 years of experience) (Carestream Health, Rochester, NY, USA). Readers were blinded to the patient’s status, clinical and biological features. Readers were asked to assess presence or absence of abnormalities. The extent of lesions was graded from 0 to 4 as follows: 0 = 0%, 1 = [1–24%], 2 = [25–49%], 3 = [50–74%], 4 = [75–100%] of whole lung surface. The topography of each lesion was also assessed.
Patients were asked to complete the Hospital Anxiety and Depression Scale (HADS), to assess symptoms of anxiety and depression; and the Medical Outcomes Study 36-item Short-Form general health survey (SF-36), which measures health-related quality of life (HRQoL). The SF-36 includes eight multiple-item scales that assess physical functioning, social functioning, role physical, role emotional, mental health, pain, vitality, and general health. Scores for each dimension range from 0 (worst) to 100 (best) [12]. The HADS was developed to detect states of depression and anxiety in adults aged 16–65 years [13]. It contains an anxiety subscale (HADS-A) and a depression subscale (HADS-D), each consisting of 7 items, rated on a four-point Likert scale (0–3). A maximum count of 21 points per subscale is possible. A score of 0–7 is considered as normal, 8–10 as a borderline case, and 11–21 as a case (anxiety or depression). The questionnaire is designed to assess the participants' state over the past 2 weeks.
Statistical analysis
Our working hypothesis was that we would observe persistent pulmonary function changes in a subgroup of patients. To describe the recovery profile of the patients at 12 months, a primary endpoint of respiratory function was chosen, namely the hemoglobin-adjusted DLCO compared to reference values. This measure provides a standardized, objective, integrated assessment of the capillary alveolus barrier and therefore pulmonary function. The primary endpoint was alteration of the alveolo-capillary barrier at 12 months after symptom onset as measured by DLCO. Continuous variables are expressed as median (interquartile range) and were compared using Mann–Whitney U test or Wilcoxon signed rank test; categorical variables are expressed as number (percentage) and were compared using chi square or Fisher’s exact test or McNemar's test, as appropriate. Comparison of parameters over time was analyzed using linear mixed models in case of a linear evolution. Unconditional logistic regression models were performed to estimate the odds ratio (OR) and 95% confidence interval (CI) for factors associated with altered DLCO at 3 months. The relation between baseline clinical and biological parameters and altered DLCO was first assessed by univariate analyses. Continuous variables were transformed into categorical variables using the median, tertiles and receiving operating characteristic (ROC) curves to identify the best cutoff. Collinearity among variables was assessed using a correlation matrix. For variables with significant correlations (defined by a correlation coefficient ≥ 0.3 associated with a p-value < 0.001), only one variable was selected for inclusion in the multivariable model. The Concato rule (1 variable for at least 10 events) was applied. The most relevant clinical variables with a p-value < 0.05 by univariate analysis were selected for inclusion in the multivariate analysis.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary NC) and R (version 4.0.5). p values of less than 0.05 were considered statistically significant, and all tests were two-sided. No adjustment was performed for multiple testing.
Results
Characteristics of the study population at ICU admission
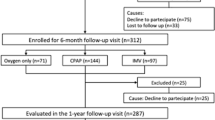
A total of 149 patients with an initial diagnosis of severe SARS-CoV-2 pneumonia were admitted to the ICU; among these, 90 were eligible for the study and 85 participated (Fig. 1). Fifty-nine patients were ineligible for the following reasons: 28 deaths, 5 negatives for SARS-CoV-2 (negative quantitative RT-PCR), 12 were aged > 79 years, and 14 for other reasons (Fig. 1). Among the 90 eligible patients, 3 refused to participate, 1 did not show up for the appointment and 1 patient was outside the time limits for inclusion. The median follow-up for the full cohort was 12 months (interquartile range (IQR): 11.5–12.3). Twelve patients were lost to follow-up, 5 between 3 and 6 months (1 died, 2 declined to continue follow-up, 1 lost to follow-up and 1 hospitalized for intercurrent disease), and 7 between 6 and 12 months (5 declined to continue follow-up and 2 patients did not show up for their appointment).
The demographic characteristics of study population (N = 85) are detailed in Table 1. Median age was 68.4 years (IQR: 60.1–72.9 years). Sixty-seven patients (78.8%) were males. Only one patient was a current smoker, 57.7% were former smokers and 41.2% never-smokers. A large majority of patients had known comorbidities (92.9%), including mostly respiratory and cardiovascular disorders. Twenty-eight patients were obese (32.9%), 44 had arterial hypertension (51.8%), 21 had diabetes (24.7%) and 27 had dyslipidemia (31.8%). The median length of hospital stay was 44 days (IQR: 20–60) and the median length of stay in the ICU was 17 days (IQR: 11.0–26.5). Fifty-seven patients (67.1%) required admission to a rehabilitation facility at hospital discharge. Thirty-six patients received steroids (45.9%), 33 during hospitalization and 3 after the 3-month evaluation.
Recovery profiles
Pulmonary function tests were completed in all patients at 3 months (n = 85), in 80 patients at 6 months, and in 73 patients at 12 months following onset of SARS-CoV-2. Most patients showed an improvement in their DLCO at each timepoint and patients who normalized their DLCO did not subsequently deteriorate. Forty-nine patients had returned to normal DLCO at 3 months (58%), 66 (85%) at 6 months, and 63 (89%) at 12 months (Additional file 1: Fig. S1). Median DLCO was 80% of predicted (IQR 64–91) at 3 months, 91% of predicted (79–104) at 6 months, and increased to 98% of predicted (88–107) at 12 months (p < 0.0001). Eight patients (11%) presented a DLCO below the lower limit of normal (LLN) at 12 months. Among patients with a DLCO below the LLN at 12 months, a mild-to-moderate reduction in DLCO was observed, with median DLCO at 62.0% of predicted (IQR 52.0 to 69.1 percent of the predicted values) (Table 2).
On the 6MWT, 50 patients (63.3%) had walk distances below their age-adjusted predicted values at 3 months (overall median walk distance = 481 m (IQR: 400–564 m)), and 18 (27.3%) at 12 months (overall median walk distance = 542 m (IQR: 495–600 m)) (p < 0.0001). The proportion of patients whose arterial oxygen saturation fell below 88 percent during the 6MWT was 11.4% at 3 months and 6.1% at 12 months. VO2 peak in incremental CPET was a median of 99% of predicted at 12 months (IQR: 88–106). Sixty-eight patients had an overnight polygraphy recording. Sixty-two patients presented on obstructive sleep apnea syndrome with an apnea–hypopnea index (AHI) ≥ 5 (91.2) (Table 2).
Chest CT scans were abnormal in 77 patients (96.3%) at 3 months. When present, radiologic changes included ground glass opacities in most cases (72.7%), atelectases, nodules and alveolar consolidations. At 12 months, the proportion of abnormal CT scan was the same, but there was a change in the patterns (96% vs 95% were abnormal CT Scan). The proportion of scans with ground glass dropped (73% to 53%), but there were more reticulations (10% vs 86%) and traction bronchiectases (69% vs 72%). The extent of each type of lesion decreased during follow-up (Table 2).
Quality of life assessment
All 85 patients completed the SF-36 questionnaire at 3 months. The scores for all domains of the SF-36 remained slightly below 100, three months after the first symptoms of SARS-CoV-2 except for one, namely impairment of work or other regular daily activities as a result of any emotional problem, where scores were normal (Fig. 2). The two domains with the lowest scores were vitality (55) and role-physical (25). In the 18 patients (27.7%) who had walk distances below their age-adjusted predicted values on the 6MWT at 3 months, the median in the vitality domain was 55 (IQR: 45–80). Most patients showed an improvement in their quality of life assessments at each timepoint (3, 6, and 12 months). The domain with the greatest improvement was the role-physical scale, which increased from 25 to 100 (Fig. 3).
Temporal changes in pulmonary function of ICU survivors of severe SARS-CoV-2 infection at 3, 6 and 12 months after symptoms onset. Graphs shows temporal changes in DLCO z-score (A), 6MWT walked distance (B) or SF36 General health (C) at 3, 6 and 12 months after SARS-CoV-2 symptoms onset. Data are median (IQR). Horizontal dotted line indicate the normal cutoff of z-score < LLN. DLCO diffusing capacity of the lung for carbon monoxide; 6MWT six-minute-walk test; SF36 Short Form 36-item questionnaire
Regarding the evaluation using the HADS, 3 patients were found to have symptoms of depression (3.5%) and 7 had symptoms of anxiety (8.2%).
Association with DLCO impairment
Table 3 presents the follow-up parameters and their relation with altered DLCO at 3, 6, 12 months. At 12 months, emphysema on CT scan was significantly associated with altered DLCO. Walked distance < theoretical distance (%) on the 6MWT was associated with altered DLCO at 3 and 6 months. No association was found between patient-reported outcomes and DLCO alteration (Table 3). For 6 of the 8 patients with an alteration of their DLCO, we concluded they had emphysema, which presumably existed before SARS-CoV-2. For one patient, after checking previous CT scans, we concluded that there had been a flare of pre-existing undiagnosed interstitial lung disease concurrent with the SARS-CoV-2 infection. For the last patient, there was persistent interstitial lung disease at 12 months, but the patient had no CT scans dating from before SARS-Cov-2 that could be used for comparison.
Risk factors associated with impaired DLCO
Univariate analysis identified three factors that were significantly associated with impaired DLCO at 3 months, namely length of stay in ICU (days), creatininemia and obesity. No risk factor for altered DLCO at 6 and 12 months was identified (Tables 4, 5).
Discussion
In this study, we report the results of a longitudinal evaluation of 85 patients admitted to the ICU for SARS-CoV-2, with a follow-up of one year. To the best of our knowledge, no reports have described such a large European cohort of SARS-CoV-2 survivors after ICU, with systematic comprehensive evaluation including lung function testing, 6MWT, incremental CPET, thoracic CT-Scan, polygraphy and HRQoL, with an excellent compliance of 86% at 1 year. A strength of our study is the complete longitudinal follow-up with repeated measures, making it possible to qualify the recovery of our patients as a function of time.
The most interesting findings were that only 8 patients (11%) having persistent impairment of DLCO at 1 year. Among these 8 patients, due to pre-existing, undiagnosed respiratory comorbidities in 7 patients, only 1 patient was likely to actually have COVID-19-related DLCO alteration at 1 year. The proportion of patients with abnormal DLCO in our study is lower than that reported by Wu et al., which was 32.5% [7]. This might be explained by the use of different reference values compared than in Wu’s cohort who used the American Thoracic Society guidelines of 1994 [14]. Compared with their DLCO reference values for adults, the most recent GLI DLCO reference values are notably lower [15]. Another explanation for this difference may consist in the fact that none of the patient in Wu’s cohort have received mechanical ventilation likewise in the cohort of Huang (< 1%) [7, 16]. This may be a message in favor of the implementation of lung protective ventilation in COVID-19 related ARDS. Compared to the population with all-cause ARDS followed by Herridge et al., in whom 3-month DLCO was 63% of predicted (IQR: 54–77) [2], respiratory function recovered more quickly in our cohort of patients with severe SARS-CoV-2.
Although the 6MWT showed significant recovery between 3 and 12 months in most patients, the walked distance remained below the predicted distance in 18 patients (27.3%), and 37.9% of patients had a drop of 4% or more in SpO2 at 12 months. Our results are concordant with the cohort of Wu et al. who reported around 14% of patients with 6MWT distance below the predicted value.
SARS-Cov-2 infection may have caused myopathic changes, also represented by the MIP (% of pred) or MEP (% of pred), which showed a constant improvement over the 12 months of follow-up [17].
Creatinine increased at the time of ICU hospitalization was shown as a predictive of low DLCO in the cohort follow-up. The association between creatinine and DLCO can be explained by the high prevalence of thromboembolic events. Reinforcing this hypothesis, the median level of D-dimers was high at the time of hospitalization with a frequency of pulmonary embolisms of 24%. We believe that these patients may have abnormal tiny blood vessels or microthrombus formation, which can be implicated in the reduction of DLCO over time or kidney injury. Other studies have demonstrated a higher rate of thromboembolic events in survivors of COVID-19 at 6 months follow-up, raising the question of the number of undiagnosed cases of segmental or subsegmental pulmonary embolism [18, 19]. The endothelial inflammation classically described in COVID-19 remains a good hypothesis for the increased incidence of pulmonary embolism, and potentially the leading cause of the persistent reduction in DLCO and kidney function over time with a specific increase in D-dimer levels [20]. In our study, D-dimer at the time of hospitalization was not showed to be correlated with KCO measurement at 3 months. Thus, other hypotheses can be put forward on the initial reduction of DLCO with normal KCO apart from microthrombus formation. Considering the number of patients with a history of chronic lung disease and obesity in our cohort, a re-distribution of the regional ventilation/perfusion ratio might have partially compensated the gas transfer efficiency, maintaining satisfactory KCO levels in some patients. Moreover, KCO may have been overestimated due to a functional restrictive pattern [21] that may have masked the possible persistence of damage to the vascular component [22].
At the 12-month CT scan, reticulations were observed in 86% of patients, traction bronchiectasis in 72% and ground glass opacities in 53%. These abnormalities were not significantly associated with DLCO alteration because they only concerned a small percentage of the lung area (< 25%) in most patients. Wu et al. reported abnormal chest imaging in 24% of patients, including interstitial thickening in 5% and reticular opacity in 4%. This higher proportion of persistent CT abnormalities at 1 year could be explained by the greater severity of our patients (85.2% of patients intubated in our cohort vs none in the series by Wu et al.).
Our analyses showed a slight-to-moderate deterioration in HRQoL in all domains except for the role-emotional domain. The most altered domains were vitality and role-physical, with a median score of 55 for vitality at 3 months. The only domain that improved over time was the role-physical domain. In an observational cohort by Vlake et al., 118 COVID-19 ICU survivors were evaluated using the same questionnaire [23]. These authors observed that mental HRQOL increased between 6 weeks and 3 months, and remained stable thereafter. Only the role-physical domain improved constantly over time, as in our cohort. A possible explanation might be that for all components except for the physical domain, improvements occur before 3 months, with a ceiling effect already reached by the time the study questionnaires were administered [24]. Compared to the population with all-cause ARDS followed by Herridge et al., HRQoL increased over time for each domain, and particularly the role physical domain, as in our cohort. At the 3-month evaluation, HRQoL scores were lower in the cohort of all-cause ARDS followed by Herridge et al. [2]. These results suggest, as in the paper by Vlake et al. comparing HRQOL results with those of a historical non-COVID-19 post-ICU population, that HRQOL is less deteriorated after an ICU stay for SARS-COV2 than after other causes of ARDS. When we investigated the relation between HRQoL and objective parameters, such as the 6MWT or CT scan findings, there were no significant relations, suggesting a discrepancy between the patient's perception and the functional parameters measured in our study. At 3 months, among the 50 patients (63.3%) who had walk distances below their age-adjusted predicted values, the median (min–max) score in the vitality domain was 55 (5–100), which was the same score as in patients with no alteration on the 6MWT. Vaes et al. reported survey results of post-discharge SARS-CoV-2 patients and they showed significant improvements in work productivity and functional status, although a proportion of patients had persistent symptom, moderate-to-poor health and impaired quality of life, as in our data [25]. No explanation was given for the persistence of an alteration in these domains, and some authors have characterized these patients with the term "long COVID" [26].
The last point to highlight from our cohort is that there were few indicators of psychological disorders, such as depression or anxiety, according to the self-reported HADS score. Using the HADS score at 3 months after hospital discharge, the results reported by Vlake et al. in their cohort of 118 post-ICU patients showed a higher frequency of depression and anxiety [23]. However, in our study, patients had early psychological follow-up and were encouraged to use new technologies to interact with their loved ones, as suggested by Kennedy et al. [27]. It has been reported that survivors of infectious diseases, such as SARS-CoV-2, are exposed to psychological risk, due to contagiousness, extensive isolation measures and public fear of the disease [28]. PTSD would have been interesting to evaluate. Indeed, in the cohort by Vlake et al., 23% of the 57 patients who responded to the IES-R questionnaire had psychological distress, including probable posttraumatic stress disorder (PTSD) in 7% of cases at 6 weeks after hospital discharge.
Our prospective study has some limitations. The severity of the epidemic and the significant mobilization of medical teams made it impossible to consider the constitution of a multicenter cohort at such short notice. Furthermore, some practices have changed based on the lessons learned from the epidemic. For example, the role of dexamethasone in hospitalized patients with COVID-19 has been established [29]. No effects of steroid use, ventilation supports, neuromuscular blocking agents, or prone position on DLCO could be demonstrated but this may be due to the too small size of our cohort. It is recommended to perform contrast-enhanced chest CT scan. Another potential bias is the lack of data regarding the patients’ respiratory function or existence of possible obstructive sleep apnea before contracting SARS-CoV-2. To minimize this potential bias, patients known to have chronic respiratory insufficiency, those on long-term oxygen therapy or those followed for interstitial lung disease were excluded from the cohort.
Conclusion
In this large cohort of ICU survivors of SARS-CoV-2 infection, systematic multidimensional evaluation up to one year after symptom onset showed that most patients had an improvement in DLCO at 3, 6, and 12 months, and patients who normalized their DLCO did not subsequently deteriorate.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- CPET:
-
Cardiopulmonary exercise testing
- CT scan:
-
Computed tomography scan
- DLCO:
-
Lung diffusing capacity for CO
- HADS:
-
Hospital Anxiety and Depression Scale
- HRQoL:
-
Health-related quality of life
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- LLN:
-
Lower limit of normal
- MEP:
-
Maximal expiratory pressure
- MIP:
-
Maximal inspiratory pressure
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SF-36:
-
Medical outcomes study (MOS) Short-Form 36-item questionnaire (SF36)
- 6MWD:
-
Six-minute walk distance
- 6MWT:
-
Six-minute walk test
References
Wuhan Municipal Health Commission. Report of clustering pneumonia of unknown etiology in Wuhan City. http://wjw.wuhan.gov.cn/front/web/showDetail/2019123108989. Accessed 24 Feb 2020.
Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–93.
Rousseau AF, Minguet P, Colson C, et al. Post-intensive care syndrome after a critical COVID-19: cohort study from a Belgian follow-up clinic. Ann Intensive Care. 2021;11:118.
Huang C, Huang L, Wang Y, Li X, Ren L, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–32.
Zhang P, Li J, Liu H, et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020;8:8.
Chen J, Wu J, Hao S, et al. Long term outcomes in survivors of epidemic Influenza A (H7N9) virus infection. Sci Rep. 2017;7:17275.
Wu X, Liu X, Zhou Y, et al. 3-Month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021. https://doi.org/10.1016/S2213-2600(21)00174-0.
santepubliquefrance.fr. COVID-19 point épidémiologique du 28avril 2020. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/bulletin-national/covid-19-point-epidemiologique-du-28-avril-2020.
Stanojevic S, Graham BL, Cooper BG, Thompson BR, et al. Global lung function initiative TLCO working group; Global Lung Function Initiative (GLI) TLCO. Eur Respir J. 2017. https://doi.org/10.1183/13993003.00010-2017.
Quanjer PH, Hall GL, Stanojevic S, et al. Age-and height-based prediction bias in spirometry reference equations. Eur Respir J. 2012;40(1):190–7.
Fitting JW, Héritier F, Uldry C. Evaluation of the inspiratory muscle strength using the nasal pressure of the sniff. Rev Mal Respir. 1996;5:479–84.
McHorney CA, Ware JE Jr, Lu JFR, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70.
Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200:e70-88.
Wardyn PM, de Broucker V, Chenivesse C, et al. Assessing the applicability of the new Global Lung Function Initiative reference values for the diffusing capacity of the lung for carbon monoxide in a large population set. PLoS ONE. 2021;16(1): e0245434.
Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
Islam MF, Cotler J, Jason LA. Post-viral fatigue and COVID-19: lessons from past epidemics. Fatigue Biomed Health Behav. 2020;8(2):61–9.
Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259–64.
Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology. 2020;296(3):E186–8.
Radermecker C, Detrembleur N, Guiot J, Cavalier E, et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J Exp Med. 2020;217(12): e20201012.
Johnson DC. Importance of adjusting carbon monoxide diffusing capacity (DLCO) and carbon monoxide transfer coefficient (K CO) for alveolar volume. Respir Med. 2000;94(1):28–37.
Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25: e100463.
Vlake JH, Van Bommel J, Hellemons ME, et al. Psychologic distress and quality of life after ICU treatment for coronavirus disease 2019: a multicenter, observational cohort study. Crit Care Explor. 2021. https://doi.org/10.1097/CCE.0000000000000497.
Fayers PM, Machin D. Quality of life: the assessment, analysis and reporting of patient-reported outcomes. New Jersey: Wiley; 2015.
Vaes AW, Goërtz YM, Van Herck M, et al. Recovery from COVID-19: a sprint or marathon? 6-month follow-up data from online long COVID-19 support group members. ERJ Open Res. 2021. https://doi.org/10.1183/23120541.00141-2021.
Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–31.
Kennedy NR, Steinberg A, Arnold RM, et al. Perspectives on telephone and video communication in the ICU during COVID-19. Ann Am Thorac Soc. 2020. https://doi.org/10.1513/AnnalsATS.202006-729OC.
Lettinga KD, Nieuwkerk PT, Jonkers RE. Health-related quality of life and post-traumatic stress disorder among survivors of an outbreak of Legionnaires disease. Clin Infect Dis. 2002;35:11–7.
Matthay MA, Thompson BT. Dexamethasone in hospitalised patients with COVID-19: addressing uncertainties. Lancet Respir Med. 2020;8:1170–2.
Funding
This study was supported by a grant from the Don Du Souffle and SOS Oxygene for institutional support.
Author information
Authors and Affiliations
Contributions
GE, FC, PR, LL, DV, AM, SPB, JB, FG, OR, VW, GC, contributed to the design and implementation of the research. GE, FC, PR, LL, OR, VW, GC, CB, KB, CC, JB, FG, SPF, HW, ES, SK, PD performed the measurements and were involved in planning and supervised the work. GE, FC, PR, LL, DV, AM, SPB, OR, GC, SK, PD, VW processed the experimental data, to the analysis of the results and to the writing of the manuscript. All authors discussed the results and commented on the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written consent was obtained before the first visit at 3 months and the protocol was approved by the ethics committee (Comité de Protection des Personnes (CPP) Grand-Est) on 21/04/2020. The COV-RECUP study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines (Clinical trial registration number: NCT04519320).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Flow chart of the COV-RECUP population according to DLCO status. Patient 77 had borderline data at each visit.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eberst, G., Claudé, F., Laurent, L. et al. Result of one-year, prospective follow-up of intensive care unit survivors after SARS-CoV-2 pneumonia. Ann. Intensive Care 12, 23 (2022). https://doi.org/10.1186/s13613-022-00997-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-022-00997-8