Abstract
Purpose
To describe health-related quality of life (HRQoL) and dyspnea of COVID-19, 2 and 12 months after an intensive care unit (ICU) stay.
Methods
Patients discharged from the ICU between April and June 2020 and subsequently transferred to an inpatient rehabilitation facility were assessed 2 months and 12 months after ICU admission. HRQoL was assessed by the EuroQoL EQ-5D-3L (visual analog scale and time trade-off normalized to the French population algorithm) and dyspnea was assessed by the modified Medical Research Council (mMRC) dyspnea scale.
Results
We enrolled 94 patients. Median EQ-5D-3L time trade-off was 0.80 (interquartile range, 0.36–0.91) at 2 months and 0.91 (0.52–1.00) at 12 months (P = 0.12). EQ-5D-3L visual analog scale was 70 (60–85) at 2 months and 70 (60–85) at 12 months (P = 0.07). The mMRC dyspnea scale was 3 (2–4) at ICU discharge, 1 (0–2), P < 0.001 at 2 months and 1 (1–2) at 12 months. At 12 months, 68 (76%) patients reported at least one symptom that was not present prior to ICU admission and 27 (61%) of the 44 patients who were previously working had returned to work. On multiple linear regression, factors associated with EQ-5D-3L were body mass index on ICU admission, tracheostomy, male gender and active smoking.
Conclusions
Twelve months after ICU admission for COVID-19 and subsequent rehabilitation, a substantial proportion of patients reported alterations of HRQoL, dyspnea and symptoms that were not present prior to admission and a substantial proportion of these patients had not returned to work. Factors associated with a risk of poorer 12-month quality of life, may help to identify at-risk patients.
Similar content being viewed by others
Introduction
The medium-term and long-term impact of COVID-19 on respiratory function and health-related quality of life are increasingly described [1,2,3,4,5,6,7]. However, most published studies have included patients with varying degrees of severity, have evaluated patients up to 6 months after the COVID-19 episode, and have not specifically focused on patients admitted to the intensive care unit (ICU) for a severe form of COVID-19. It is therefore unclear to what extent COVID-19 impacts health-related quality of life (HRQoL) and breathing comfort up to 12 months after ICU admission in this specific population, although sequelae are likely to be more pronounced in ICU survivors compared to patients who did not require ICU admission or who even did not require supplemental oxygen [1]. A better knowledge of these sequelae may help to improve the management of ICU survivors, which has consequences for all of society.
The high frequency and severity of sequelae, such as respiratory impairment and limb muscle weakness in patients with non-COVID-19-related acute respiratory distress syndrome (ARDS) [8,9,10] has stimulated rehabilitation programs that are still under development [11, 12]. Given the spread of the COVID-19 pandemic and the high proportion of patients requiring ICU admission, the rehabilitation community has called for specific action in relation to COVID-19 patients [13, 14], but very little information is currently available concerning the outcome of these programs [14].
The aim of the present study was to report temporal trends in dyspnea and HRQoL in COVID-19 patients admitted to the ICU for a severe form of COVID-19 and subsequently transferred to an inpatient rehabilitation unit. We also sought to identify the factors associated with alterations of respiratory comfort and HRQoL, including exercise capacity limitation and pulmonary function tests.
Methods
Study design
This study was conducted at La Pitié-Salpêtrière University Hospital in Paris, from 16 April 2020 to 25 June 2020. Patients admitted to an ICU of the great Paris area for acute respiratory failure with laboratory-confirmed SARS-CoV-2 infection and subsequently discharged and transferred to the inpatient respiratory rehabilitation facility were included. Laboratory-confirmed SARS-CoV-2 infection was defined as a positive result of real-time reverse transcriptase-polymerase chain reaction assay of nasal and pharyngeal swabs. Patients aged < 18 years were excluded. The study was approved by the French Pulmonary Medicine Society ethics committee (CEPRO No. 2020-070). Patients gave their informed consent. The study complied with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement guidelines (http://www.equator-network.org).
Rehabilitation was conducted in an inpatient unit with a multidisciplinary approach including oxygen and respiratory devices adaptation and education, nutritional support, psychiatric and psychologic evaluation and treatment, occupational therapist, especially for walking tools and plexopathy consequences, physiotherapists for neuromuscular electrostimulation. When possible, patients had stretching exercises and endurance training on treadmill or ergocycle, always with oxygen, twice a day.
Data collection
Demographic data, medical history and data on the current episode of acute respiratory failure were abstracted from the patients' medical charts and electronic reports. These data included age, gender, body mass index, respiratory or cardiac comorbidity, Simplified Acute Physiologic Score (SAPS) 2 [15], oxygenation strategy, occurrence and severity of Acute Respiratory Distress Syndrome according to the Berlin definition [16], need for prone positioning, and organ support such as vasopressors, renal replacement therapy and extracorporeal lung support. Lengths of stay in the ICU, weaning unit (when applicable) and inpatient rehabilitation facility were also recorded.
Patients were evaluated in an outpatient clinic one to two months after ICU discharge. This evaluation included pulmonary function tests and arterial blood gases. Restrictive syndrome was defined as total lung capacity < 80% of predicted [17] obstructive syndrome was defined as forced expiratory volume in one second to forced vital capacity < 70%, diffusing capacity for carbon monoxide was considered to be abnormal when < 80% [18] and respiratory muscle weakness was defined as maximal inspiratory pressure < 80% [19]. A standardized six-minute walk test with continuous oximetry was performed. Walk distance was normalized to predicted distance, which was calculated based on age, sex, height and weight age, gender, height and weight [20]. The patient also completed a HRQoL assessment using the EQ-5D-3L [21] (EuroQol Research Foundation https://euroqol.org), which collates responses into five domains of HRQoL (mobility, self-care, usual activities, pain or discomfort, and anxiety or depression) with a three-level score (no problems, moderate problems, extreme problems or unable). EQ-5D-3L states were then converted into a single summary number or index value, which reflects how good or bad a health state is according to the preferences of the general population of a country or region. This approach ensures that the values represent the societal perspective. Here, we used the quality of life time trade-off utility values (EQ-5D-TTO), calculated using the French value set, with values generally ranging between 0 (death) to 1 (perfect health) [22]. Values less than 0 represent health states considered to be worse than death. Respondents were also asked to rate their perceived health on a visual analog scale (VAS) from 0 (worst) to 100 (best). Dyspnea was assessed by the modified Medical Research Council (mMRC) dyspnea scale [23] that grades the effect of breathlessness on daily activities from 0 “no limitations” to 4 “unable to leave home because of breathlessness”.
Twelve months after ICU discharge, patients were either evaluated in an outpatient clinic or contacted by telephone by a physician as a part of their post-ICU follow-up and a questionnaire including HRQoL assessment using the EQ-5D-3L and the mMRC dyspnea scale were administered and the patient's current weight was recorded. Patients were asked to list symptoms not present prior to COVID-19 (dichotomous outcome) such as: shoulder or arm weakness, persistent pain or dysesthesia, decreased range of motion of large joints, skin damage, especially in the neck, upper airway symptoms, including voice change and swallowing issues, anxiety or depression symptoms that needed medications. They were also asked whether or not they had returned to work. Pulmonary function tests and the six-minute walk test were performed at the physician's discretion, based on the patient's respiratory symptoms.
Statistical analysis
The sample size was defined by the total number of patients admitted to the rehabilitation facility from an ICU, from the beginning to the end of the first wave of the COVID-19 epidemic in Paris, France. Quantitative variables were described as median (interquartile range) and qualitative variables were described as frequency (percentage).
Univariate linear regression models were used to identify factors associated with the six following dependent outcome variables: normalized 6-min walk test at 2 months, EQ-5D-3L (time trade-off and visual analog scale) at 2 months and 12 months and dyspnea mMRC at 12 months. Then, multivariate linear regression analysis were performed to identify factors indecently associated with these six dependent variables. All independent variables with P < 0.20 on univariate analysis were used to select the final model. We used a forward stepwise method based on Fisher Snedecor test for selection of the final model. Collinearity between variables and residuals were checked. Goodness of fit of the model was assessed with R2 and global F-test.
Statistical analyses were carried out using R v3.6.6 [24] (https://www.R-project.org/). P values ≤ 0.05 were considered to be statistically significant.
Results
Patients
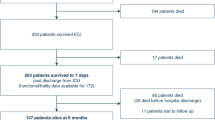
Over the 3-month recruitment period, we enrolled 94 COVID-19 survivors, who were discharged from ICU and transferred to the inpatient rehabilitation facility (Table 1). Median age of these patients was 63 (49–70) years. Table 2 describes patient characteristics and management in the ICU. Among the 73 (78%) patients who were intubated, one (1%) developed mild ARDS, 42 (58%) developed moderate ARDS and 30 (41%) developed severe ARDS. Among intubated patients, 11 (15%) received extracorporeal lung support, and 24 (33%) were tracheostomized. Median ICU length of stay was 25 (15–46) days.
Figure 1 displays the patient flowchart. Patients came from 18 ICUs of the great Paris area (1073 admission over the study period, 26% mortality). Among the 744 patients who were discharges to a medical ward, 52 (55%) were transferred directly to the inpatient rehabilitation facility. In addition 42 patients (45%) were first transferred to a weaning unit (median length of stay: 12 [9,10,11,12,13,14,15,16] days) and then subsequently transferred to the inpatient rehabilitation facility. The median duration of mechanical ventilation was 30 (20–41) days.
On admission to the inpatient rehabilitation facility, the mMRC dyspnea scale was 3 (2–4) and weight loss was 11 (7–15) %. Patients spent 12 (8–20) days in the inpatient rehabilitation facility (Table 2). After discharge from the inpatient rehabilitation facility, 14 (15%) patients were transferred to another facility (12 to another rehabilitation unit and 2 to a nursing home) and 80 (85%) were discharged home, including 10 patients who required home hospitalization. No patient died or was transferred to an ICU. Overall, patients spent 50 (35–74) days in hospital (Fig. 1).
Two-month follow-up assessment
The real follow-up at the time of the 2-month visit was 43 (34–55) days after ICU discharge.
Two months after ICU admission, patients who survived COVID-19 were 9 (5–15) % below their baseline body weight. At the 2-month visit, 12 (13%) patients were still receiving supplemental oxygen. The mMRC dyspnea scale was 1 (0–2) (P < 0.001 vs. ICU discharge) (Fig. 2). Pulmonary function tests were performed in 89 (95%) patients and arterial blood gases were determined in 75 (80%) patients (Table 3). Briefly, 37 (47%) patients had a restrictive syndrome, 9 (10%) had an obstructive syndrome, 54 (69%) had a decreased diffusing capacity for carbon monoxide and 44 (56%) had respiratory muscle weakness. Twenty-four (26%) patients were unable to perform 6-min walk test due to obvious muscle weakness. 6-min walk test was 392 m (322–484), which represented 58 (47–69) % of predicted normal value.
Temporal changes in health-related quality of life (panel A and B) and dyspnea (Panel C) 2 months and 12 months after intensive care unit (ICU) discharge for severe COVID-19. EQ-5D VAS (panel A): patients rated their perceived health on a visual scale from 0 (worst) to 100 (best) called visual analog scale. EQ-5D TTO (panel B): Quality of life “time trade-off” utility values were calculated using the French algorithm with values generally ranging between 0 (death) to 1 (perfect health) [22]. mMRC dyspnea scale (panel C): modified Medical Research Council dyspnea scale. EQ-5D VAS and EQ-5D TTO are represented as box and whiskers where the red center line denotes the median value (50th percentile), the box contains the 25th to 75th percentiles and the whiskers mark the 5th and 95th percentiles
Seventy-seven patients (82%) completed the five questions of the EQ-5D-3L, 69 (73%) patients completed the EQ-5D-3L VAS and 61 (65%) completed both instruments. Table 4 shows the answers to the EQ-5D-3L with a sum of 7 (5–9) and 0.80 (0.36–0.91) after normalization to the French population using the specific TTO value set for France [25] (Fig. 2). Finally, the EQ-5D VAS was 70 (60–85).
In Additional file 1: Tables S1, S2, S3 show the factors associated with reduced normalized six-minute walking distance and reduced HRQoL identified on univariate analysis. On multivariate analysis, the only factor associated with a reduced normalized six-minute walk test was length of stay prior to rehabilitation unit admission (linear regression coefficient − 2.14, 95% confidence interval [95% CI] − 3.24 to − 1.03, P < 0.001) (p F-statistic < 0.001, R2 = 0.17). Factors independently associated with EQ-5D-3L TTO were male gender (linear regression coefficient 0.26, 95% CI 0.11–0.42, P = 0.001) and immunosuppression (linear regression coefficient 0.23, 95%CI 0.02–0.44, P = 0.031) (p F-statistic < 0.001, R2 = 0.20). The only factor independently associated with EQ-5D-3L VAS was dyspnea on admission to the inpatient rehabilitation facility (linear regression coefficient − 5.31, 95%CI − 8.86 – − 1.77, P = 0.005) (p F-statistic = 0.005, R2 = 0.12). On multivariate analysis, pulmonary function tests were not associated with six-minute walking distance and reduced HRQoL.
12-month follow-up assessment
The 12-month follow-up was performed in 89 (91%) patients. Four patients were lost to follow-up and one patient died as the result of a car accident. The real follow-up at the time of the 12-month visit was 11.9 (11.3–12.2) months. The 12-month assessment was performed at the outpatient clinic in 53 patients (60%) and by phone only in the remaining 36 patients (40%).
Twelve months after ICU admission, patients who survived COVID-19 were 3 (− 1 to 7) % below their baseline body weight. A plexopathy was present in 17 (19%) patients, confirmed by an electromyography. Fourteen (16%) patients reported persistent pain or dysesthesia, 14 (16%) patients reported decreased range of motion of large joints (mostly the shoulders), 24 (27%) patients reported an altered appearance of their neck skin and 20 (22%) patients reported upper airway symptoms, including voice change. Twenty-two (25%) patients were receiving medications for anxiety or depression symptoms. Thirteen patients (15%) reported “slow thinking”. Overall, 58 (65%) patients reported at least one symptom not present prior to ICU admission (Fig. 3). Among the 44 patients who were working prior to ICU admission, 27 (61%) had returned to work (12 [44%] part-time).
Representation of symptoms not present before COVID-19 in the 89 patients assessed 12 months after intensive care unit admission. Numbers represent patients with symptoms: A plexopathy; (n = 17); B persistent pain or dysesthesia (n = 14); C decreased range of motion of large joints, mostly the shoulders (n = 14); D altered appearance of the skin of the neck (n = 24); E upper airway symptoms (n = 20); F anxiety or depression requiring medication (n = 22); G reported “slow thinking” (n = 13). 31 patients did not report any of these symptoms
The mMRC dyspnea scale was 1 (1–2) (p = 0.809 vs. 2-month assessment). Pulmonary function tests were performed in 42 (45%) patients (Aditional file 1: Table S4). Forced vital capacity and diffusing capacity for carbon monoxide improved. Six-minute walk test was 506 m (440–562) (p < 0.001 vs. 2-month assessment), which represented 80 (72–82) % of predicted normal value (p < 0.001 vs. 2-month assessment).
Eighty-six patients (90%) completed the five questions of the EQ-5D-3L, 82 (85%) patients completed the EQ-5D-3L VAS and 86 (90%) completed both instruments. Table 4 shows the answers to the EQ-5D-3L with a sum of 6 (5–8) and 0.91 (0.52–1.00) after normalization to the French population (p = 0.12 vs. 2-month assessment) [25]. Finally, the score on the EQ-5D analog scale was 70 (60–85) (p = 0.114 vs. 2-month assessment).
Tables 1, 2 and 3 show the factors associated with dyspnea and HRQoL identified on univariate analysis. On multivariate analysis, factors associated with a EQ-5D-3L TTO were body mass index on ICU admission (linear regression coefficient − 0.01, 95%CI − 0.02–0.00, p = 0.005) and tracheostomy (linear regression coefficient − 0.22, 95%CI − 0.36 to − 0.08, p = 0.002) (p F-statistic < 0.001, R2 = 0.20). Factors independently associated with EQ-5D-3L VAS were male gender (linear regression coefficient − 8.74, 95%CI 1.37–16.11, p = 0.023) and active smoking (linear regression coefficient − 3.00, 95%CI − 5.88–0.12, p = 0.044) (p F-statistic < 0.001, R2 = 0.20). The only factor independently associated with mMRC dyspnea scale was active smoking (linear regression coefficient 0.50, 95%CI 0.04–0.95, p = 0.036) (p F-statistic = 0.036, R2 = 0.056). On multivariate analysis, 2-month pulmonary function tests were not associated with 12-month HRQoL or dyspnea mMRC.
Discussion
The main and major findings of our study are as follows. In patients who survived a severe form of COVID-19 that had required ICU admission and were subsequently transferred to an inpatient rehabilitation facility: (1) HRQoL was still altered 12 months after ICU admission, with a substantial proportion of patients reporting at least one symptom related to the ICU stay; (2) some variables may predict earlier the alteration of health-related quality of life.
Because the survival of patients with ARDS has dramatically improved, the morbidity and sequelae in survivors have now become a major subject of interest. Over the past 20 years, several investigators have evaluated morbidity among survivors and have reported alteration of lung function, exercise capacity and HRQoL after ICU discharge [8, 9, 26,27,28]. To date, most data available for COVID-19 patients who required ICU admission come from 2 months and up to 6 months follow-up [1, 4,5,6,7]. These data are consistent with our results, showing similar alterations of six-minute walking test, pulmonary function tests and exercise capacity. Data on 12 months follow-up are scarce and, to date, no study has been devoted to ICU patient [4]. In contrast, in our study, all patients were admitted to the ICU and 78% of them were intubated. It is not clear to what extent COVID-19 will leave sequelae, such as persistent limitations in respiratory, physical, and functional outcomes, but such sequelae are likely to be more pronounced in the subgroup of ICU survivors [14, 29]. A better knowledge of these sequelae may help to improve the management of ICU survivors.
A strikingly high proportion of patients reported at least one symptom that was not present prior to ICU admission. This proportion seems to be higher than that reported in a previous cohort of non-COVID ARDS patients with a similar ICU length of stay and a high proportion of tracheostomized patients [8]. This difference could be partly explained by the high rate of prone positioning that is likely to alter the skin and cause brachial plexus injuries, although factors directly related to SARS-CoV-2 infection cannot be ruled out. There are a number of differences between SARS-CoV-2 and other infectious agents that target the lungs, such as the intense proinflammatory cytokine storm and marked endothelial dysfunction [30]. Finally, 12 months after ICU admission, HRQoL had not returned to normal, as EQ-5D visual analog scale and TTO were below the normal range of the French population [22]. While pulmonary function tests at 2 months were moderately, but not dramatically altered, and given the fact that a continuous improvement over the first year has been reported [1, 4] altered lung function may not explain the decreased HRQoL. On the other hand, the persistence of symptoms that were not present prior to ICU admission might contribute to this decreased HRQoL.
A previous study in COVID-19 patients showed improvement of dyspnea and exercise capacity between two months and 6 months [4], while we did not observe any change in dyspnea and HRQoL between these two time-points. In our cohort, dyspnea improved between ICU discharge and 2-month, but not thereafter, which could be explained by the fact that all patients in our cohort were rehabilitated, which may have rapidly improved dyspnea after ICU discharge.
Identifying, as early as possible, those patients at risk of poor long-term quality of life constitutes a major challenge, as, after discharge from the rehabilitation unit, these patients could integrate an outpatient rehabilitation program in order to improve their 12-month quality of life as much as possible [14]. Unfortunately, few factors in our cohort were independently associated with poorer 12-month quality of life. However, obese patients, active smokers, tracheostomized patients and patients with a long ICU length of stay were at higher risk of poorer HRQoL at 12 months. Surprisingly, markers of severity such as hypoxemia and SAPS 2 were not associated with 12-month HRQoL.
This study has several limitations. First, we limited the 12-month evaluation to dyspnea and HRQoL and did not perform pulmonary function tests and six-minute walk test in all patients. These tests were requested by the patient’s physician, based on his/her clinical judgment. However, as these tests were performed in only very few patients, and exclusively in those who remained severely symptomatic, we did not report the results of these tests. Second, this study did not comprise a control group, which precludes comparison of the prevalence and severity of symptoms with patients admitted to the ICU for acute respiratory failure due to a cause other than COVID-19. Third, because patients were admitted during the first wave of the epidemic, many did not receive corticosteroids, which are now an integral part of the treatment of severe forms of COVID-19. Fourth, all patients were admitted to the inpatient rehabilitation facility after discharge from ICU, which precludes assessment of the impact of rehabilitation on 12-month HRQoL. Last, evaluation were performed by phone, which is less reliable than in an outpatient clinics.
Conclusion
Twelve months after ICU admission for COVID-19 and subsequent rehabilitation, a substantial proportion of patients reported alterations of HRQoL, dyspnea and symptoms that were not present prior to admission and a substantial proportion of these patients had not returned to work. Our study identifies factors associated with a risk of poorer 12-month quality of life, which may help to identify at-risk patients. Our findings highlight the importance of follow-up of patients who have experienced severe forms of COVID-19. Further studies are necessary to determine whether an early outpatient rehabilitation program after discharge from an inpatient rehabilitation facility can help to improve one-year HRQoL in at-risk patients. Longer follow-up is also necessary.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- ICU:
-
Intensive care unit
- HRQoL:
-
Health-related quality of life
- ARDS:
-
Acute respiratory distress syndrome
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SAPS:
-
Simplified Acute Physiologic Score
- mMRC:
-
Modified Medical Research Council dyspnea scale
- COPD:
-
Chronic obstructive pulmonary disease
- HFNC:
-
High-flow nasal cannula
- NIV:
-
Non-invasive ventilation
- CPAP:
-
Continuous positive airway pressure
References
Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet Lond Engl. 2021;397:220–32.
Writing Committee for the COMEBAC Study Group, Morin L, Savale L, Pham T, Colle R, Figueiredo S, et al. Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA. 2021;325:1525–34.
Laveneziana P, Straus C, Meiners S. How and to What Extent Immunological Responses to SARS-CoV-2 Shape Pulmonary Function in COVID-19 Patients. Front Physiol. 2021;12:628288.
Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9:747–54.
Carenzo L, Protti A, Dalla Corte F, Aceto R, Iapichino G, Milani A, et al. Short-term health-related quality of life, physical function and psychological consequences of severe COVID-19. Ann Intensive Care. 2021;11:91.
Gamberini L, Mazzoli CA, Sintonen H, Colombo D, Scaramuzzo G, Allegri D, et al. Quality of life of COVID-19 critically ill survivors after ICU discharge: 90 days follow-up. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2021;56:9.
van Brugge S, Talman S, Boonman-de-Winter L, de Mol M, Hoefman E, van Etten RW, et al. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respir Med. 2021;176:106272.
Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–93.
Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–304.
Needham DM, Wozniak AW, Hough CL, Morris PE, Dinglas VD, Jackson JC, et al. Risk factors for physical impairment after acute lung injury in a national, Multicenter Study. Am J Respir Crit Care Med. 2014;189:1214–24.
Brown SM, Bose S, Banner-Goodspeed V, Beesley SJ, Dinglas VD, Hopkins RO, et al. Approaches to Addressing Post-Intensive Care Syndrome among Intensive Care Unit Survivors: A Narrative Review. Ann Am Thorac Soc. 2019;16:947–56.
Kiekens C. Follow-up services for improving long-term outcomes in intensive care unit (ICU) survivors - A Cochrane Review summary with commentary. J Rehabil Med. 2019;51:879–82.
Thornon J. Covid-19: the challenge of patient rehabilitation after intensive care. BMJ. 2020;369:1787.
Polastri M, Nava S, Clini E, Vitacca M, Gosselink R. COVID-19 and pulmonary rehabilitation: preparing for phase three. Eur Respir J. 2020;55:9.
Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63.
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33.
Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Eur Respir J. 1993;6(Suppl 16):5–40.
Cotes JE, Chinn DJ, Quanjer PH, Roca J, Yernault JC. Standardization of the measurement of transfer factor (diffusing capacity). Eur Respir J. 1993;6(Suppl 16):41–52.
Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis. 1969;99:696–702.
Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J. 1999;14:270–4.
Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2013;22:1717–27.
Andrade LF, Ludwig K, Goni JMR, Oppe M, de Pouvourville G. A French Value Set for the EQ-5D-5L. Pharmacoeconomics. 2020;38:413–25.
Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–6.
R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing. 2020.
Chevalier J, de Pouvourville G. Valuing EQ-5D using time trade-off in France. Eur J Health Econ HEPAC Health Econ Prev Care. 2013;14:57–66.
Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde-Zwirble WT, Dremsizov TT, et al. Quality-adjusted Survival in the First Year after the Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2001;163:1389–94.
Pfoh ER, Wozniak AW, Colantuoni E, Dinglas VD, Mendez-Tellez PA, Shanholtz C, et al. Physical declines occurring after hospital discharge in ARDS survivors: a 5-year longitudinal study. Intensive Care Med. 2016;42:1557–66.
Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, et al. Physical Complications in Acute Lung Injury Survivors: A Two-Year Longitudinal Prospective Study. Crit Care Med. 2014;42:849–59.
Belli S, Balbi B, Prince I, Cattaneo D, Masocco F, Zaccaria S, et al. Low physical functioning and impaired performance of activities of daily life in COVID-19 patients who survived hospitalisation. Eur Respir J. 2020;56:90.
Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care Lond Engl. 2020;24:353.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
AD, EM, MD, and JG contributed to study design, data collection, data acquisition, analysis, interpretation and to drafting the manuscript. RO, RM, MAGJ contributed to data acquisition, data interpretation, and to drafting the manuscript. PL, CMP and TS contributed to data interpretation and to drafting the manuscript. YDR contributed to data analysis, interpretation and to drafting the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study was approved by the ethics committee of the French Pulmonary Medicine Society ethics committee (CEPRO No. 2020-070). Patients gave their informed consent.
Consent for publication
Not applicable.
Competing interests
A. Demoule reports grants, personal fees and non-financial support from Philips, personal fees from Baxter, personal fees and non-financial support from Fisher & Paykel, grants from French Ministry of Health, personal fees from Getinge, grants, personal fees and non-financial support from Respinor, grants, personal fees and non-financial support from Lungpacer, personal fees from Lowenstein, personal fees from Gilead, outside the submitted work. Elise Morawiec has no conflict of interest to report. Maxens Decavele reports non-financial support (congress registration) from ISIS Medical, outside the submitted work. Raphaelle Ohayon has no conflict of interest to report. Roxane Malrin has no conflict of interest to report. Maria Alejandra Galarza-Jimenez has no conflict of interest to report. Pierantonio Laveneziana has no conflict of interest to report. Capucine Morelot-Panzini has no conflict of interest to report. Thomas Similowski has no conflict of interest to report. Yann De Rycke has no conflict of interest to report. Jesus Gonzalez-Bermejo has no conflict of interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Tables S1, S2, S3.
Factors associated with reduced six-minute walking distance and reduced HRQoL two months after intensive care unit admission identified on univariate analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Demoule, A., Morawiec, E., Decavele, M. et al. Health-related quality of life of COVID-19 two and 12 months after intensive care unit admission. Ann. Intensive Care 12, 16 (2022). https://doi.org/10.1186/s13613-022-00991-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-022-00991-0