Abstract
Background
Stratifying patients with sepsis was the basis of the predisposition, infection, response and organ dysfunction (PIRO) concept, an attempt to resolve the heterogeneity in treatment response. The purpose of this study is to perform an independent validation of the PIRO staging system in an international cohort and explore its utility in the identification of patients in whom time to antibiotic treatment is particularly important.
Methods
Prospective international cohort study, conducted over a 6-month period in five Portuguese hospitals and one Australian institution. All consecutive adult patients admitted to selected wards or the intensive care, with infections that met the CDC criteria for lower respiratory tract, urinary, intra-abdominal and bloodstream infections were included.
Results
There were 1638 patients included in the study. Patients who died in hospital presented with a higher PIRO score (10 ± 3 vs 8 ± 4, p < 0.001). The observed mortality was 3%, 15%, 24% and 34% in stage I, II, III and IV, respectively, which was within the predicted intervals of the original model, except for stage IV patients that presented a lower mortality. The hospital survival rate was 84%. The application of the PIRO staging system to the validation cohort resulted in a positive predictive value of 97% for stage I, 91% for stage II, 85% for stage III and 66% for stage IV. The area under the receiver operating characteristics curve (AUROC) was 0.75 for the all cohort and 0.70 if only patients with bacteremia were considered. Patients in stage III and IV who did not have antibiotic therapy administered within the desired time frame had higher mortality rate than those who have timely administration of antibiotic.
Conclusions
To our knowledge, this is the first external validation of this PIRO staging system and it performed well on different patient wards within the hospital and in different types of hospitals. Future studies could apply the PIRO system to decision-making about specific therapeutic interventions and enrollment in clinical trials based on disease stage.
Similar content being viewed by others
Background
Infections are one of the five leading causes of death worldwide [1], representing a major contribution to the overall health care burden [2]. The worldwide “Surviving Sepsis Campaign” (SSC), achieved a reduction in mortality from severe sepsis and septic shock of 25% in 5 years by setting recommendations grouped into “bundles” based on time of performance. It included; early recognition, early use of antibiotics, early goal-directed therapy resuscitation protocol, and early supportive care in the ICU [3]. Nonetheless, the incidence of sepsis continues to increase as do the number of absolute deaths, despite improved survival rates [4, 5].
The development of new adjunctive therapies for sepsis, including molecular therapeutic or immunomodulatory agents, has unfortunately resulted in failure to demonstrate effectiveness which have been attributed to the inclusion of a very heterogeneous group of patients [6].
Stratifying patients with sepsis into a more homogeneous population that could benefit from specific therapies was the basis of the Predisposition, Infection, Response and Organ dysfunction (PIRO) concept [7]. This concept describes the phenotype of a patient with sepsis based on those four dimensions [8].
Several studies have been developed to test this concept in multiple settings with the ultimate goal of developing a score [9,10,11,12,13] or a model [14,15,16] that would predict mortality from sepsis. In 2013 we published a clinical staging system based on the PIRO concept [6], including in Predisposition age, several comorbidities, functional status and previous ATB therapy; in Infection, type of infection (either community, healthcare or hospital-acquired); in Response altered temperature, hyperglycemia, tachypnea and severity of infection and in Organ dysfunction, hypotension and SOFA score on the moment of diagnosis. The original study showed good discrimination in predicting hospital mortality, and allowed classification of patients according to their PIRO phenotype. That was a single center study. The purpose of the current study is to preform external validation of the PIRO staging system in an international prospective cohort of hospitalized patients with infection.
Methods
Study design and population
Prospective international cohort study, conducted over a 6-month period (1st October 2014 to 31st March 2015) in five Portuguese hospitals (three teaching and tertiary care and two secondary care hospitals) and one Australian institution (teaching and tertiary care hospital). Data were collected in individual centers and entered directly into a web-based electronic case report form.
All consecutive adult patients admitted to selected wards or the intensive care unit (ward selection was by convenience) of participating hospitals with selected infections were included (Additional file 1: Table S1). Infections included those that met the Centers for Disease Control and Prevention’s (CDC) criteria for lower respiratory tract, urinary, intra-abdominal and bloodstream infection [17]. Primary bloodstream infections included intravascular device-associated infections.
Definitions
Secondary bloodstream infection was defined as an infection when an organism isolated from a blood culture was related to an infection at another site.
Community-acquired infection (CAI) was defined as an infection detected within 48 h of hospital admission in patients who do not fit the criteria for a healthcare-associated infection (HCAI).
HCAI was defined a priori using the criteria of Friedman and colleagues published in 2002 [18].
Hospital-acquired infection (HAI) was defined as a localized or systemic infection that occurred 48 h or more after hospital admission and was not incubating at the time of hospital admission [19]. Infections arising in patients discharged from the hospital within the previous 2-week period were also included in this group.
Previous antibiotic therapy was defined as any antibiotic administered in the previous month with therapeutic intent.
Time to antibiotic therapy was calculated between infection diagnosis time and antibiotic administration time, and then categorized into antibiotic administration within the first 1, 3 and 6 h.
Severity of infection (infection, sepsis, severe sepsis or septic shock) was defined according to the criteria proposed by the American College of Chest Physicians/Society of Critical Care Medicine [20].
Staging system
The PIRO staging system is based on Predisposition, Infection, Response and Organ dysfunction scores. The original scores were developed according to the original proposal of the American Thoracic Society/Society of Critical care Medicine [7] and are shown in Table 1 [6]. Predisposition score [range, 0 (best) to 18 (worst) points] allows stratification into P1 (score 0–2), P2 (score 3–4) and P3 (score ≥ 5). The Infection score [range, 0 (best) to 2 (worst) points] allows stratification into I1 (score 0–1) and I2 (score 2). The Response score [range, − 1 (best) to 7 (worst) points] allows stratification into R1 (score – 1 to 3) and R2 (score ≥ 4). The Organ dysfunction score [range, 0 (best) to 4 (worst) points] allows stratification into O1 (score 0) and O2 (score ≥ 1).
The PIRO score was computed as soon as the patient was enrolled in the study, up to 24 h maximum after the clinical diagnosis of infection. Any missing value was attributed the value of 0 for the final calculus.
The phenotypes that compose each stage and the predicted mortality are shown in Table 2 [6].The original cohort published in 2013 did not contemplate all possible phenotypes, namely: P1I2R2O1 and P1I1R2O1 that present an hospital mortality rate of 0% and were included in stage I; P2I2R2O1, with a hospital mortality rate of 13% and P1I2R2O2 and P2I1R2O1, with an hospital mortality rate of 14%, that were included in stage II; and P3I1R2O1, with a mortality rate 33% and P3I2R2O1 with 50%, that were included in stage III.
The performance of the current model to predict hospital mortality was compared against SAPS II, a physiological score designed to predict hospital mortality among intensive care patients [21], extrapolated to the various settings were patients were recruited.
Statistical analysis
Categorical variables were described as proportions and compared using Chi-square or Fisher’s exact test. Continuous variables were described by mean and standard deviation. Comparisons of continuous variables were performed using Student’s t-test.
For the validation of each stage, the test was considered positive if the patient met the criteria for that stage. The outcome was survival at hospital discharge.
The area under the receiver operating characteristics curve was used to compare PIRO and SAPS II scores in different populations.
Statistical significance was defined as p < 0.05. The statistical analysis was performed in SPSS®24 (SPSS Inc., Chicago IL).
Results
During the study period 1638 patients met the inclusion criteria. General characteristics of these patients are shown in Additional file 1: Table S2 and compared with the original cohort [6].
In Predisposition, the validation cohort included older patients, more males, more patients with cancer, atherosclerosis and a Karnofsky score < 70, less patients with hematologic disease and overall a higher P score. In Infection, the validation cohort included a lower prevalence of community and hospital-acquired infection and a greater prevalence of HCAI, with no difference in the overall I score. In Response, the validation cohort included a higher prevalence of fever, less hypothermia, more tachypnea and more severe sepsis, with no difference in the overall R score. In Organ dysfunction, the validation cohort included more hypotension and higher SOFA scores, with higher overall O score. The total PIRO score was higher in the validation cohort. Patients that died presented with a higher PIRO score in both cohorts (Additional file 1: Table S2).
In the current cohort patients who died in hospital presented with a higher PIRO score than those who survived (10 ± 3 vs 8 ± 4, p < 0.001).
In Table 2 the distribution of patients according to the four PIRO stages is shown along with the predicted and observed mortality. In the validation cohort, the observed mortality was within the predicted mortality of the original model, with the exception of stage IV, which had a lower mortality than predicted. The hospital mortality in each stage for different focus of infection was also within the predicted intervals, with exception of those in stage IV that presented a lower mortality than predicted regardless of the focus of infection; patients in stage I with primary bacteremia showed an hospital mortality of 8%, slightly higher than predicted (0–5%) and those in stage III with urinary infection lower than predicted (21–50%)–15%.
The prevalence of hospital survival in this population was 84%. The performance of the model for predicting hospital survival at each stage is illustrated in Table 3.
The application of the PIRO staging system to the validation cohort resulted in a probability post-positive test (that is the probability of hospital survival after classification in each stage) of 97% for stage I, 91% for stage II, 85% for stage III and 66% for stage IV. Specificity was 95%, 66%, 12% and 88%, respectively, for stages I, II, III and IV (Table 3).
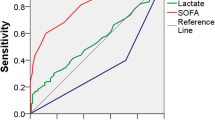
The area under the ROC curve (AUC) for hospital mortality was 0.75 for the PIRO model, and was similar if only patients with microbiological documentation of infection (0.74) or bacteremia (0.70) were considered. The AUC was higher for patients on the general ward (0.76) than in the ICU/HDU (0.68). The discrimination of the model was higher than SAPS II in all settings (Table 4).
In Table 5 hospital mortality rate in each stage according to time of antibiotic therapy administration is shown; there were no significant differences in outcome, but there was a consistent trend towards lower hospital mortality rate in patients with earlier administration of antibiotic therapy particularly in those in stage III and IV. There were seven patients in whom time of antibiotic administration was not registered and therefore not included in this analysis.
Discussion
In this study, we present the first external validation of the PIRO staging system [6]. In this international study, the observed hospital mortality rate in each stage was within the range predicted by our original work, with increasing mortality from stage I to stage III. Patients in stage IV exhibited the highest mortality rate, albeit lower than predicted, which may reflect the overall decrease in mortality attributed to sepsis and severe sepsis [4, 5]. The performance of this staging model was similar for all studied focus of infection, reinforcing its applicability in different infections.
There were some differences between the original cohort and the current one, namely in different components of predisposition, insult, response and organ dysfunction that would alter the distribution of patients in each phenotype and stage. Nevertheless, although the proportion of patients in each stage was different, the outcome remained within the previsions of the original work in each stage, except for stage IV that presented a lower mortality in the current study, suggesting that it would perform equally well in different populations.
As in the original cohort, we found that patients who died in hospital displayed higher PIRO scores when compared with those that survived. The performance of the original model in the validation cohort (AUC 0.75) was moderately good and similar to other validation studies of different PIRO models [9, 10, 22,23,24].
Although there have been several PIRO models and scores previously published, our work is the first to validate a true staging system based on the PIRO concept. Among previously developed scoring systems based on the PIRO concept the most studied are from Howell et al. [8], Rubulotta et al. [16] and Moreno et al. [15].
Howell et al. prospectively studied Emergency Department (ED) patients and their model performed very well in the original cohort with an AUC of 0.90, while subsequent studies showed an AUC of between 0.73 [25] and 0.86 [26] in ED patients and 0.71 [22] to 0.75 [23], in ICU patients.
Rubulotta et al. [16] performed a secondary analysis of a large database of patients with severe sepsis (PROWESS and PROGRESS). Subsequent studies on ICU patients showed an AUC of 0.71 [24] and 0.76 [27], for ED patients and 0.65 [23] and 0.71 [22], for ICU patients.
Moreno et al. [15] also performed a secondary analysis of a database of ICU patients (SAPS 3) with an AUC of 0.77 in the original cohort, while subsequent studies showed an AUC of 0.74 [23] and 0.84 [22], both in ICU patients.
Our model also showed that PIRO performed better than SAPS II in ICU and in the general ward patients, increasing its range of applicability. Importantly, all required variables for the model can be collected almost immediately at the bedside which is imperative for early treatment and possible enrollment in clinical trials.
This staging system is a way to stratify patients into a more homogeneous groups and test the value of interventions that would reduce disease progression, morbidity and mortality. The ultimate aim of the PIRO staging system is to identify the appropriate patient population more likely to benefit from new and/or specific therapeutic interventions [28].
Although this study was not designed specifically to evaluate the impact of different therapies in each stage, we have tried to see if patients in different stages respond differently to timely administration of antibiotic therapy and, although not reaching statistical significance, the mortality rates in patients from stages III and IV were consistently higher in the group with delayed antibiotic administration, regardless of the time interval considered (1, 3 or 6 h), suggesting that these phenotypes are particularly sensitive to this therapeutic intervention. This is the final objective of this staging system: to elect patients who benefit the most from specific therapies, may that be early antibiotic therapy or new immunomodulatory agents.
Our study has various limitations that need to be acknowledged. As a prospective observational study, ascertainment and informational bias cannot be excluded. Our study includes only one biomarker (C-reactive protein) although there are other biomarkers already in use, like procalcitonin that could assist with discrimination. Furthermore, 35% of patients did not have microbiological documentation of infection, however, the analysis of the subgroup with positive blood cultures revealed a similar performance. The small number of patients included in stage IV might explain the low sensibility of this group, a larger study or one focused on patients with severe infection might help to improve the sensibility of the staging system in this group.
Nonetheless, the strengths of this study are the fact that it is an international prospective cohort study, with a large cohort size, incorporating both community and university hospitals, and it includes patients from different wards in the hospital which increases its external applicability.
Patients with very similar mortality risk may have dramatically different responses to therapy based on their PIRO stage. It is hoped that this study makes a contribution towards translation of the PIRO concept into clinical practice.
Conclusions
To our knowledge, our study is the first external validation of the PIRO staging system and it showed good performance in different settings within the hospital and in different types of hospitals. Future studies could apply the PIRO system to decision-making with respect to primary and adjuvant treatment modalities based on disease stage at clinical presentation.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUROC:
-
Area under the receiver operating characteristics curve
- CAI:
-
Community-acquired infection
- CDC:
-
Centers for Disease Control and Prevention
- ED:
-
Emergency department
- HAI:
-
Hospital-acquired infection
- HCAI:
-
Healthcare-associated infection
- HDU:
-
High-dependency unit
- ICU:
-
Intensive care unit
- PIRO:
-
Predisposition, infection, response and organ dysfunction
- SAPS II:
-
Simplified Acute Physiological Score
- SOFA:
-
Sequential Organ Failure Assessment
- SSC:
-
Surviving Sepsis Campaign
References
WHO. The top 10 causes of death: WHO. 2016. https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death.
Chalupka AN, Talmor D. The economics of sepsis. Crit Care Clin. 2012;28(1):57–76.
Levy MM, Rhodes A, Phillips GS, Townsend SR, Schorr CA, Beale R, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Intensive Care Med. 2014;40(11):1623–33.
Stoller J, Halpin L, Weis M, Aplin B, Qu W, Georgescu C, et al. Epidemiology of severe sepsis: 2008–2012. J Crit Care. 2016;31(1):58–62.
Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311(13):1308–16.
Cardoso T, Teixeira-Pinto A, Rodrigues PP, Aragao I, Costa-Pereira A, Sarmento AE. Predisposition, insult/infection, response and organ dysfunction (PIRO): a pilot clinical staging system for hospital mortality in patients with infection. PLoS ONE. 2013;8(7): e70806.
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31(4):1250–6.
Howell MD, Talmor D, Schuetz P, Hunziker S, Jones AE, Shapiro NI. Proof of principle: the predisposition, infection, response, organ failure sepsis staging system. Crit Care Med. 2011;39(2):322–7.
Tsai JC, Weng SJ, Huang CY, Yen DH, Chen HL. Feasibility of using the predisposition, insult/infection, physiological response, and organ dysfunction concept of sepsis to predict the risk of deterioration and unplanned intensive care unit transfer after emergency department admission. J Chin Med Assoc. 2014;77(3):133–41.
Chen YX, Li CS. Risk stratification and prognostic performance of the predisposition, infection, response, and organ dysfunction (PIRO) scoring system in septic patients in the emergency department: a cohort study. Crit Care. 2014;18(2):R74.
de Groot B, Lameijer J, de Deckere ER, Vis A. The prognostic performance of the predisposition, infection, response and organ failure (PIRO) classification in high-risk and low-risk emergency department sepsis populations: comparison with clinical judgement and sepsis category. Emerg Med J. 2014;31(4):292–300.
Rathour S, Kumar S, Hadda V, Bhalla A, Sharma N, Varma S. PIRO concept: staging of sepsis. J Postgrad Med. 2015;61(4):235–42.
Chen YX, Li CS. Evaluation of community-acquired sepsis by PIRO system in the emergency department. Intern Emerg Med. 2013;8(6):521–7.
Granja C, Povoa P, Lobo C, Teixeira-Pinto A, Carneiro A, Costa-Pereira A. The predisposition, infection, response and organ failure (Piro) sepsis classification system: results of hospital mortality using a novel concept and methodological approach. PLoS ONE. 2013;8(1): e53885.
Moreno RP, Metnitz B, Adler L, Hoechtl A, Bauer P, Metnitz PG, et al. Sepsis mortality prediction based on predisposition, infection and response. Intensive Care Med. 2008;34(3):496–504.
Rubulotta F, Marshall JC, Ramsay G, Nelson D, Levy M, Williams M. Predisposition, insult/infection, response, and organ dysfunction: a new model for staging severe sepsis. Crit Care Med. 2009;37(4):1329–35.
Prevention CfDCa. CDC/NHSN surveillance definitions for specific types of infections. 2014; 13 August 2015.
Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137(10):791–7.
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections. Am J Infect Control. 1988;16:128–40.
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–55.
Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–63.
Songsangjinda T, Khwannimit B. Comparison of severity score models based on different sepsis definitions to predict in-hospital mortality among sepsis patients in the intensive care unit. Med Intensiva. 2019. https://doi.org/10.1016/j.medin.2018.12.004.
Tafelski S, Nachtigall I, Stengel S, Wernecke K, Spies C. Comparison of three models for sepsis patient discrimination according to PIRO: predisposition, infection, response and organ dysfunction. Minerva Anestesiol. 2015;81(3):264–71.
Nguyen HB, Van Ginkel C, Batech M, Banta J, Corbett SW. Comparison of predisposition, insult/infection, response, and organ dysfunction, acute physiology and chronic health evaluation II, and mortality in emergency department sepsis in patients meeting criteria for early goal-directed therapy and the severe sepsis resuscitation bundle. J Crit Care. 2012;27(4):362–9.
de Groot B, Stolwijk F, Warmerdam M, Lucke JA, Singh GK, Abbas M, et al. The most commonly used disease severity scores are inappropriate for risk stratification of older emergency department sepsis patients: an observational multi-centre study. Scand J Trauma Resusc Emerg Med. 2017;25(1):91.
Macdonald SP, Arendts G, Fatovich DM, Brown SG. Comparison of PIRO, SOFA, and MEDS scores for predicting mortality in emergency department patients with severe sepsis and septic shock. Acad Emerg Med. 2014;21(11):1257–63.
Quinten VM, van Meurs M, Wolffensperger AE, Ter Maaten JC, Ligtenberg JJM. Sepsis patients in the emergency department: stratification using the clinical impression score, predisposition, infection, response and organ dysfunction score or quick sequential organ failure assessment score? Eur J Emerg Med. 2018;25(5):328–34.
Rubulotta F, Ramsay D, Williams MD. PIRO score for community-acquired pneumonia: a new prediction rule for assessment of severity in intensive care unit patients with community-acquired pneumonia. Crit Care Med. 2010;38(4):1236 (author reply-7).
Funding
This study was supported by an unrestricted Grant from ASSUCIP—Associação de Cuidados Intensivos do Porto (Oporto Intensive Care Association). Teresa Cardoso is partially funded by a research Grant from the Teaching and Research Department (Departamento de Formação, Ensino e Investigação) of Oporto University Hospital Centre (2014/169). The funding sources had no role in the study design, the collection, analysis or interpretation of the data, or in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
TC and NDF conceived the study. TC coordinated data collection. TC and PPR performed the statistical analysis. All authors participated in the analysis and interpretation of the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Ethics and Institutional Review Board approval was obtained for all participating sites: Centro Hospitalar Universitário do Porto (2014.147-CES), Unidade Local de Saúde do Nordeste (25/07/2014), Hospital de Braga (14/10/2014), Unidade Local de Saúde de Matosinhos (2014 – 073/CE/JAS), Centro Hospitalar de São João (CES 249-14) and Barwon Health (14/131).
Consent for publication
Informed consent was waived due to the observational nature of the study.
Competing interests
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Characterization of participating hospitals and patients’ distribution by type of ward in each hospital. Table S2. Comparison of variables significantly associated with hospital mortality, within each of the four components of PIRO in the original and the validation cohorts.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cardoso, T., Rodrigues, P.P., Nunes, C. et al. Prospective international validation of the predisposition, infection, response and organ dysfunction (PIRO) clinical staging system among intensive care and general ward patients. Ann. Intensive Care 11, 180 (2021). https://doi.org/10.1186/s13613-021-00966-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-021-00966-7