Abstract
Background
Ventilator-associated pneumonia (VAP) caused by Stenotrophomonas maltophilia is poorly described in the literature. However, it has been shown to be associated with increased morbidity and mortality. Probabilistic antibiotic therapy against S. maltophilia is often ineffective as this pathogen is resistant to many antibiotics. There is no consensus at present on the best therapeutic strategy to adopt (class of antibiotics, antibiotic combination, dosage, treatment duration). The aim of this study was to evaluate the effect of antibiotic therapy strategy on the prognosis of patients with VAP caused by S. maltophilia.
Results
This retrospective study evaluated all consecutive patients who developed VAP caused by S. maltophilia between 2010 and 2018 while hospitalized in the intensive care unit (ICU) of a French university hospital in Reunion Island, in the Indian Ocean region. A total of 130 patients with a median Simplified Acute Physiology Score II of 58 [43–73] had VAP caused by S. maltophilia after a median duration of mechanical ventilation of 12 [5–18] days. Ventilator-associated pneumonia was polymicrobial in 44.6% of cases, and ICU mortality was 50.0%. After multivariate Cox regression analysis, the factors associated with increased ICU mortality were older age (hazard ratio (HR): 1.03; 95% CI 1.01–1.04, p = 0.001) and high Sequential Organ Failure Assessment score on the day of VAP onset (HR: 1.08; 95% CI 1.03–1.14, p = 0.002).
Appropriate antibiotic therapy, and in particular trimethoprim–sulfamethoxazole, was associated with decreased ICU mortality (HR: 0.42; 95% CI 0.24–0.74, p = 0.003) and decreased hospital mortality (HR: 0.47; 95% CI 0.28–0.79, p = 0.04).
Time to start of appropriate antibiotic therapy, combination therapy, and duration of appropriate antibiotic therapy had no effect on ICU mortality (p > 0.5).
Conclusion
In our study, appropriate antibiotic therapy, and in particular trimethoprim–sulfamethoxazole, was associated with decreased ICU and hospital mortality in patients with VAP caused by S. maltophilia.
Similar content being viewed by others
Introduction
Ventilator-associated pneumonia (VAP) caused by Stenotrophomonas maltophilia is relatively common, with the latest report of the European Centre for Disease Prevention and Control stating that this microorganism is one of the 10 most frequently isolated germs in respiratory samples [1]. Depending on the study, the incidence S. maltophilia in patients with VAP varies between from 0.3 and 2.0% [2, 3]. According to some authors, however, S. maltophilia is of limited pathogenicity and should not be considered as an infectious agent in the majority of VAP cases [4,5,6]. Admittedly, the link between colonization by S. maltophilia and respiratory infection is often difficult to establish. On the one hand, severely ill patients can be colonized with S. maltophilia in the respiratory tract without presenting respiratory symptoms [4, 5]. On the other hand, half of respiratory samples containing strains of S. maltophilia show significant growth of other microorganisms with known pathogenicity [2, 7]. Several studies nonetheless suggest that VAP caused by S. maltophilia is associated with high morbidity and mortality [3, 4, 7], in particular due to inappropriate probabilistic treatment [7, 8]. In some studies, the mortality rate attributable to S. maltophilia colonization or infection was found to vary between 20.0 and 38.0% [8].
There is no consensus at present on the therapeutic strategy to adopt in cases of VAP caused by S. maltophilia (class of antibiotics, antibiotic combination, dosage, treatment duration). Some authors recommend high doses of trimethoprim–sulfamethoxazole based on clinical data, while others favor combination therapy based on in vitro data [9, 10]. In view of this, our study aimed to evaluate the effect of antibiotic therapy strategy on the prognosis of patients with VAP caused by S. maltophilia.
Methods
This single-center retrospective study was conducted in the Multi-Purpose Intensive Care Unit (ICU) of Félix Guyon University Hospital in Reunion Island, a French overseas department located in the Indian Ocean.
All patients hospitalized in ICU with a blood or respiratory culture positive for S. maltophilia between 1 January 2010 and 31 December 2018 were evaluated and considered for inclusion. Patients aged over 18 years who developed VAP caused by S. maltophilia during their ICU stay were included in the study (Fig. 1).
In accordance with the French legislation on non-interventional studies [11], this study was registered with the National Institute of Health Data under the number MR 5611200420 and was approved by the Ethics Committee of the French Society of Infectious Disease and Tropical Medicine (CER-MIT 2021-0302). This study complies with the Strengthening the Reporting of Observational studies in Epidemiology recommendations statement [12].
Definitions
Ventilator-associated pneumonia caused by S. maltophilia was defined by: new or progressive infiltrate; temperature > 38.0 °C or < 36.5 °C; white blood cell count > 12G/L or < 4G/L; purulent secretions; drop in oxygenation; positive respiratory culture obtained by bronchoalveolar lavage (threshold: 104 colony forming unit (CFU)/mL), protected distal sampling (threshold: 103 CFU/mL) or endotracheal aspiration (threshold: 106 CFU/mL); and occurrence of infection 48 h or more after tracheal intubation. [13].
All patients with a Clinical Pulmonary Infection Score (CPIS) > 6 and those with a CPIS ≤ 6 who were treated by a clinician for VAP were evaluated [14].
Pharmacological management
In accordance with our protocol, all patients with VAP caused by S. maltophilia were treated with: trimethoprim–sulfamethoxazole (1200 mg/240 mg)/6 h and/or ciprofloxacin 400 mg/8 h and/or moxifloxacin and/or ticarcillin–clavulanate 4 g/8 h and/or ceftazidime 2 g/6 h.
Dosages were adjusted to renal function if necessary, and the choice of antibiotics was left to the discretion of the clinician.
Data collection
The following information was collected:
-
Demographic data (age, sex), organ failure during ICU stay and on the day of VAP onset, and comorbidities [diabetes, hypertension, chronic heart failure, chronic respiratory failure, chronic liver failure, chronic renal failure, immunodeficiency, recent or ongoing chemotherapy, chronic alcohol abuse, body mass index (BMI) > 30 kg/m2, malnourishment (BMI < 18.5 kg/m2 or weight loss > 10% over the previous 6 months)].
-
Use of extracorporeal organ support such as extracorporeal membrane oxygenation and/or renal replacement therapy, use of catecholamines, mechanical ventilation (MV) settings, Glasgow score.
-
Highest bilirubin level, lowest platelet level, lowest prothrombin level, and lowest PaO2/FiO2 ratio during ICU stay and PaO2/FiO2 ratio on the day of VAP onset.
-
Simplified Acute Physiology Score II (SAPS II) within 48 h of admission and Sequential Organ Failure Assessment (SOFA) scores on admission and on the day of VAP onset.
-
Data on infection: type of respiratory sample, type of infection (monomicrobial or polymicrobial), duration of previous MV, known rectal or respiratory colonization with S. maltophilia, and CPIS on the day of microbiological sampling.
-
Susceptibility of S. maltophilia strains to selected antimicrobial agents, as determined using the disk-diffusion method and the minimum inhibitory concentrations defined by the European Committee on Antimicrobial Susceptibility Testing [15].
-
Data on treatment of VAP caused by S. maltophilia: class of antibiotics, type of therapy (monotherapy or combination therapy), as well as time to start and duration of appropriate antibiotic therapy.
-
Prognosis data: MV duration, length of stay in ICU and in hospital, ICU and hospital mortality.
Statistical analyses
Patient characteristics were described as frequency and percentage for categorical variables and as median and interquartile range for quantitative variables. Qualitative variables were compared using the Chi-square test, the Kruskal–Wallis test or Fisher’s exact test, as appropriate. Continuous variables were compared using the nonparametric Mann–Whitney test. Bivariate analysis of the main outcome (ICU mortality) was performed using the Kaplan–Meier method and comparisons were performed using the log-rank test. A multivariate analysis was conducted with a Cox model. A competing risk analysis of the main outcome was also performed for patients who underwent Withholding of Life-Sustaining Treatment (WLST) versus those who did not. Adjusted hazard ratios (HR) and their 95% confidence intervals (CI) were calculated. The proportional risk hypothesis was tested by introducing an interaction between groups (treated patients versus non-treated patients) and time. All tests were performed at a 2-tailed type I error of 5% using SAS 9.4 software (SAS Institute Inc., Cary, NC).
Results
Population
Between January 2010 and December 2018, 9542 patients were hospitalized in our ICU, 130 (1.4%) of whom presented with VAP caused by S. maltophilia. Our study population was predominantly male (63.8%), with a median age of 61 [51–70] years. Median severity scores on admission were 9 [7–12] for SOFA and 58 [43–73] for SAPS II. Reasons for admission to ICU were acute respiratory failure in almost half of cases (48.5%), followed by septic shock (23.1%), post-operative management of cardiac surgery (21.5%), neurological causes, cardiogenic shock, and polytrauma. Lastly, 36.2% of our patients had chronic heart failure, 20.8% had chronic respiratory failure, 30.7% had chronic alcohol abuse, and 30.7% were malnourished (Table 1).
Sampling methods and microorganism distribution
Respiratory samples were obtained through endotracheal aspiration in 46.2% of cases, protected distal sampling in 34.6% of cases, and bronchoalveolar lavage in 19.2% of cases.
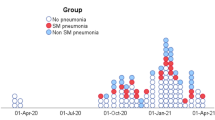
Infection was polymicrobial in 44.6% of cases. The microorganism most frequently associated with S. maltophilia was Pseudomonas aeruginosa (Table 3).
Median CPIS was 8 [7–9], and MV duration before onset of VAP was 12 [5–18] days (Table 2).
Susceptibility of Stenotrophomonas maltophilia strains and type of antibiotic therapy
Identified strains of S. maltophilia were susceptible to trimethoprim–sulfamethoxazole in 86.2% of cases, to fluoroquinolones in 85.4% of cases, to ticarcillin–clavulanate in 58.5% of cases, and to ceftazidime in 40.0% of cases. Appropriate antibiotic therapy was initiated after a median of 2 [1–3] days.
Dual antibiotic therapy was initiated in 53 (40.8%) patients, 43 (81.1%) of whom received trimethoprim–sulfamethoxazole and fluoroquinolone. Triple antibiotic therapy was initiated in 35 (26.9%) patients, 32 (91.4%) of whom received ticarcillin–clavulanate, trimethoprim–sulfamethoxazole, and fluoroquinolone (Table 3). A total of 38 patients (29.2%) received no treatment for S. maltophilia. There was no significant difference between treated and untreated patients with respect to the CPIS (p = 0.17), the SOFA score on the day of VAP onset (p = 0.43), the polymicrobial character of the infection (p = 0.12), or the presence of WLST (p = 0.55). Probabilistic antibiotic therapy was effective against the co-infecting microorganism(s) in 86.2% of cases (50/58) and in 100% of cases when the co-infecting microorganism was P. aeruginosa.
Prognosis and risk factors associated with ICU mortality
Median MV duration was 21 [14–37] days and median MV duration after onset of VAP was 7 [4–15] days. Tracheotomy was performed in 31.5% of patients, with a statistically higher incidence in surviving patients [41.5% in survivors versus 21.5% in non-survivors (p = 0.01)]. ICU mortality was 50.0% and hospital mortality was 56.2%.
The Kaplan–Meier method using the log-rank test found a significant difference in ICU survival and hospital survival between treated and non-treated patients (p = 0.009 and p = 0.02, respectively) (Fig. 2). The only antibiotic therapy associated with a significant difference between treated and non-treated patients was trimethoprim–sulfamethoxazole (p = 0.02) (Fig. 3). For the others, there was no difference between treated and non-treated patients: fluoroquinolones (p = 0.54), ticarcillin–clavulanate (p = 0.14), and ceftazidime (p = 0.64).
Kaplan–Meier estimates of the probability of ICU Survival. a ICU survival according to the antibiotic treatment with trimethoprim/sulfamethoxazole. b ICU survival according to antibiotic treatment with fluoroquinolone. c ICU survival according to antibiotic treatment with ticarcillin/clavulanate. d ICU survival according to antibiotic treatment ceftazidime
After adjustment for confounding factors (Table 4), appropriate antibiotic therapy was associated with decreased ICU mortality (HR: 0.42; 95% CI 0.24–0.74, p = 0.003) and decreased hospital mortality (HR: 0.47; 95% CI 0.28–0.79, p = 0.04). When taking competing risks into consideration (Table 5), appropriate antibiotic therapy was associated with a similar significant decrease in ICU mortality in patients who underwent WLST (HR: 0.47; 95%CI 0.24–0.90; p = 0.02) and in patients who did not (HR: 0.33; 95% CI 0.11–0.97; p = 0.04).
Discussion
We performed a search of the literature in English and French using the terms pneumonia, S. maltophilia, intensive care unit, and outcome. We found three articles on VAP caused by S. maltophilia, two of which were conducted in adult populations. The first was the multicenter study by Guerci et al. (236 cases) and the second the study by Ibn Saied et al. (102 cases from the OUTCOMEREA database) [2, 16]. Both studies examined the impact of therapeutic modalities (antibiotic combination, treatment duration, etc.) on the prognosis of patients, but neither of them evaluated the association between prognosis and lack of appropriate treatment.
As in other studies of VAP caused by S. maltophilia [4, 16], the risk factors associated with increased ICU mortality were older age and high SOFA score on the day of VAP onset.
An original finding of our study was that appropriate antibiotic therapy, and in particular trimethoprim–sulfamethoxazole, is associated with decreased ICU mortality (HR: 0.42; 95% CI 0.24–0.74, p = 0.003) and hospital mortality (HR: 0.47; 95% CI 0.28–0.79, p = 0.04). However, time to start of appropriate antibiotic therapy and duration of appropriate antibiotic therapy had no effect on mortality.
Most studies of ICU patients with pneumonia caused by S. maltophilia found no association between mortality and antibiotic therapy strategy [2, 16]. The only exceptions are the study by Tseng et al. [17] and Hanes et al. [7], in which mortality was associated with delayed initiation of appropriate antibiotic therapy. We found no such association in our study, even though appropriate antibiotic therapy was initiated 2 days after VAP onset. The discrepancy between our results and those of Hanes et al. may be partly explained by the fact that the latter found a higher rate of co-infection in their cohort, and in particular a higher rate of co-infection with P. aeruginosa (92.3% of patients with a co-infection and 34.6% with a co-infection with P. aeruginosa versus 44.6% with a co-infection and 18.5% with a co-infection with P. aeruginosa in our study) [7]. Likewise, in the study by Tseng et al., the association between mortality and delayed initiation of appropriate antibiotic therapy was especially strong in cases of co-infection [17]. In line with these findings, Yin et al. observed that co-infection with P. aeruginosa, which is common in cases of S. maltophilia pneumonia, is a poor prognostic factor [18]. In our study, however, the rate of co-infection was the same in survivors and non-survivors (p = 0.29).
Studies examining VAPs caused by non-fermenting Gram-negative bacilli (GNB) found no effect of treatment duration on mortality, MV duration, or length of stay in ICU. However, in the randomized prospective study by Chastre et al., prolonged antibiotic therapy (15 days) was associated with fewer relapses than short antibiotic therapy (8 days) in the subgroup of patients infected with non-fermenting GNB (41.0% of relapses in patients who received 8 days of treatment versus 25.0% in patients who received 15 days of treatment) [19]. Similarly, in a systematic review of prolonged treatment for hospital-acquired pneumonia, Pugh et al. observed fewer relapses in patients infected with non-fermenting GNB who had received prolonged treatment (OR: 2.18; 95% CI 1.1–4.2) [20]. It should be noted, however, that the most frequently implicated non-fermenting GNB in the evaluated studies was P. aeruginosa [19, 20]. A randomized study is currently underway that evaluates the effect of treatment duration on the prognosis of patients with VAP caused by P. aeruginosa [21].
In our study, patients were treated mainly with combination therapy, with no difference in effect on mortality between dual and triple therapy. The difference in effect on mortality between combination therapy and monotherapy could not be evaluated, as only 4 of our patients received monotherapy. Our study suggests that trimethoprim–sulfamethoxazole has a protective effect in patients with VAP caused by S. maltophilia. This finding is in line with other studies of patients with S. maltophilia pneumonia, in which trimethoprim–sulfamethoxazole was not associated with excess mortality or the emergence of resistance, even when used as monotherapy [2, 22,23,24]. One in vitro study did find that combination therapy broadens the spectrum of antibiotics and constitutes as such a more effective probabilistic treatment for patients with sepsis, particularly in cases of non-fermenting GNB infection [25]. However, clinical studies found no superiority of combination therapy over monotherapy in cases of sepsis, whether in terms of clinical cure or mortality, and this even in cases of non-fermenting GNB infection [26, 27].
The incidence of VAP caused by S. maltophilia was relatively high (1.4%) in our ICU compared to what has been reported in the literature. Ibn Saied et al. and Guerci et al. reported an incidence of 0.5% and 0.3%, respectively—keeping in mind that the latter study examined all cases of hospital-acquired S. maltophilia pneumonia [2, 16]. The study by Nseir et al. found an incidence of 2.0%, but it included cases of colonization with S. maltophilia in addition to infection cases [3]. The discrepancy between these and our results may be explained by the fact that our study was conducted in a tropical environment, which has been shown to favor the development of the parent microorganism Acinetobacter baumannii [28]. Another potential explanation is that our patients were diagnosed using the highly sensitive sampling technique of endotracheal aspiration.
In our study, ICU mortality was 50.0% and hospital mortality was 56.2%. These findings are in line with the study by Saugel et al., in which ICU mortality was 50.0% [4]. While Guerci et al. found a hospital mortality of 50.0%, their study included all cases of hospital-acquired pneumonia caused by S. maltophilia and not just those of VAP [2]. The study by Ibn Saied et al., in which the median SOFA score on the day of VAP onset was the same as in our study, found an ICU mortality of 40.0% despite the fact that 68.0% of patients received no treatment [16]. It may be that Ibn Saied et al. included cases of colonization with S. maltophilia in addition to cases of infection in their sample, which could explain this lower ICU mortality [16]. The study by Saugel et al., in which ICU mortality was 29.0% in colonized patients, would tend to support this hypothesis [4].
Our study has some limitations. The single center and retrospective nature of the study may have led to biases. In addition, the relatively small size of our sample limits the statistical power of the results. The size of our sample stems from our decision to exclude patients with a CPIS ≤ 6 who were not treated by a clinician for VAP, as we assumed these to be cases of colonization with S. maltophilia or ventilator-associated tracheobronchitis [4, 29]. It should be noted, however, that the retrospective studies by Guerci et al. and Ibn Saied et al. included a comparable number of patients: 228 (80.0% of whom had VAP) and 102, respectively [2, 16].
Another limitation of our study is that we did not collect data on relapse or emergence of resistance. Lastly, we used mostly endotracheal aspiration with quantitative cultures, which may have led us to overestimate the incidence of S. maltophilia in our population due to heightened sensitivity. Current guidelines for the management of VAP allow the use of endotracheal aspirates (in addition to invasive respiratory specimens), but with semi-quantitative as opposed to quantitative cultures. In France, however, endotracheal aspiration with quantitative cultures is the most commonly used technique for the diagnosis of VAP [30]. Thus, in the study by Guerci et al., which represents the largest sample of patients with VAP caused by S. maltophilia to date, 30.0% of cases were diagnosed using this technique [2].
Conclusion
This is the third retrospective study of VAP caused by S. maltophilia to use a large sample of patients and the first to focus primarily on the effect of antibiotic therapy on the prognosis of infected patients.
In our study, appropriate antibiotic therapy, and in particular trimethoprim–sulfamethoxazole, was associated with decreased ICU and hospital mortality in patients with VAP caused by S. maltophilia.
Availability of data and materials
The datasets used and/or analyzed for this study are available from the corresponding author on reasonable request.
Abbreviations
- VAP:
-
Ventilator-associated pneumonia
- ICU:
-
Intensive care unit
- CFU:
-
Colony forming unit
- CPIS:
-
Clinical Pulmonary Infection Score
- BMI:
-
Body mass index
- MV:
-
Mechanical ventilation
- SAPS II:
-
Simplified Acute Physiology Score II
- SOFA:
-
Sequential Organ Failure Assessment
- HZ:
-
Hazard ratio
- CI:
-
Confidence interval
- WLST:
-
Withholding of Life-Sustaining Treatment
- GNB:
-
Gram-negative bacilli
References
Healthcare-associated infections in intensive care units—annual epidemiological report for 2017. European Centre for Disease Prevention and Control. http://ecdc.europa.eu/en/publications-data/healthcare-associated-infections-intensive-care-units-annual-epidemiological. Accessed 2019 Oct.
Guerci P, Bellut H. Outcomes of Stenotrophomonas maltophilia hospital-acquired pneumonia in intensive care unit: a nationwide retrospective study. Crit Care. 2019. https://doi.org/10.1186/s13054-019-2649-5.
Nseir S, Di Pompeo C. Intensive care unit-acquired Stenotrophomonas maltophilia: incidence, risk factors, and outcome. Crit Care. 2006. https://doi.org/10.1186/cc5063.
Saugel B, Eschermann K. Stenotrophomonas maltophilia in the respiratory tract of medical intensive care unit patients. Eur J Clin Microbiol Infect Dis. 2012. https://doi.org/10.1007/s10096-011-1459-8.
Pathmanathan A, Waterer GW. Significance of positive Stenotrophomonas maltophilia culture in acute respiratory tract infection. Eur Respir J. 2005. https://doi.org/10.1183/09031936.05.00096704.
Scholte JB, Zhou TL. Stenotrophomonas maltophilia ventilator-associated pneumonia. A retrospective matched case-control study. Infect Dis. 2016. https://doi.org/10.1080/23744235.2016.1185534.
Hanes SD, Demirkan K. Risk factors for late-onset nosocomial pneumonia caused by Stenotrophomonas maltophilia in critically ill trauma patients. Clin Infect Dis. 2002. https://doi.org/10.1086/341022.
Falagas ME, Kastoris AC. Attributable mortality of Stenotrophomonas maltophilia infections: a systematic review of the literature. Future Microbiol. 2009. https://doi.org/10.2217/fmb.09.84.
El Chakhtoura NG, Saade E. Therapies for multidrug resistant and extensively drug-resistant non-fermenting gram-negative bacteria causing nosocomial infections: a perilous journey toward “molecularly targeted” therapy. Expert Rev Anti Infect Ther. 2018. https://doi.org/10.1080/14787210.2018.1425139.
Shio-Shin J, Yin-Chun C. Epidemiology, treatment, and prevention of nosocomial bacterial pneumonia. J Clin Med. 2020;9(1):275. https://doi.org/10.3390/jcm9010275.
Toulouse E, Masseguin C. French legal approach to clinical research. Anaesth Crit Care Pain Med. 2018. https://doi.org/10.1016/j.accpm.2018.10.013.
Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7.
Koenig SM, Truwit JD. Ventilator-associated pneumonia: diagnosis, treatment, and prevention. Clin Microbiol Rev. 2006;19(4):637–57. https://doi.org/10.1128/CMR.00051-05.
Torres A, Niederman MS. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur Respir J. 2017. https://doi.org/10.1183/13993003.00582-2017.
Recommandations 2019 du comité de l’antibiogramme de la société française de microbiologie. https://www.sfm-microbiologie.org/2019/01/07/casfm-eucast-2019/. Accessed Jan 2019.
Ibn Saied W, Merceron S. Ventilator-associated pneumonia due to Stenotrophomonas maltophilia: risk factors and outcome. J Infect. 2019. https://doi.org/10.1016/j.jinf.2019.10.021.
Tseng CC, Fang WF. Risk factors for mortality in patients with nosocomial Stenotrophomonas maltophilia pneumonia. Infect Control Hosp Epidemiol. 2009. https://doi.org/10.1086/648455.
Yin C, Yang W. Co-infection of Pseudomonas aeruginosa and Stenotrophomonas maltophilia in hospitalised pneumonia patients has a synergic and significant impact on clinical outcomes. Eur J Clin Microbiol Infect Dis. 2017. https://doi.org/10.1007/s10096-017-3050-4.
Chastre J, Wolff M. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003. https://doi.org/10.1001/jama.290.19.2588.
Pugh R, Grant C. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev. 2015. https://doi.org/10.1002/14651858.CD007577.pub3.
Bouglé A, Foucrier A, Dupont H, et al. Impact of the duration of antibiotics on clinical events in patients with Pseudomonas aeruginosa ventilator-associated pneumonia: study protocol for a randomized controlled study. Trials. 2017. https://doi.org/10.1186/s13063-017-1780-3.
Shah MD, Coe KE. Efficacy of combination therapy versus monotherapy in the treatment of Stenotrophomonas maltophilia pneumonia. J Antimicrob Chemother. 2019. https://doi.org/10.1093/jac/dkz116.
Ko JH, Kang CI, Cornejo-Juárez P. Fluoroquinolones versus trimethoprim-sulfamethoxazole for the treatment of Stenotrophomonas maltophilia infections: a systematic review and meta-analysis. Clin Microbiol Infect. 2019. https://doi.org/10.1016/j.cmi.2018.11.008.
Wang YL, Scipione MR. Monotherapy with fluoroquinolone or trimethoprim-sulfamethoxazole for treatment of Stenotrophomonas maltophilia infections. Antimicrob Agents Chemother. 2014. https://doi.org/10.1128/AAC.01324-13.
Foster RA, Troficanto C. Utility of combination antimicrobial therapy in adults with bloodstream infections due to Enterobacteriaceae and non-fermenting gram-negative bacilli based on in vitro analysis at two community hospitals. Antibiotics. 2019. https://doi.org/10.3390/antibiotics8010015.
Paul M, Lador A. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev. 2014. https://doi.org/10.1002/14651858.CD003344.pub3.
Vardakas KZ, Tansarli GS. β-Lactam plus aminoglycoside or fluoroquinolone combination versus β-lactam monotherapy for Pseudomonas aeruginosa infections: a meta-analysis. Int J Antimicrob Agents. 2013. https://doi.org/10.1016/j.ijantimicag.2012.12.006.
Dexter C, Murray GL. Community-acquired Acinetobacter baumannii: clinical characteristics, epidemiology and pathogenesis. Expert Rev Anti Infect Ther. 2015. https://doi.org/10.1586/14787210.2015.1025055.
Gaudet A, Martin-Loeches I. Accuracy of the clinical pulmonary infection score to differentiate ventilator-associated tracheobronchitis from ventilator-associated pneumonia. Ann Intensive Care. 2020. https://doi.org/10.1186/s13613-020-00721-4.
Kalil AC, Metersky ML. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin Infect Dis. 2016. https://doi.org/10.1093/cid/ciw353.1717.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
BP, NA, and CF had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: BP and NA; acquisition of data: BP and CC; analysis and interpretation of data: BP, NA, CF and JA; drafting of the manuscript: BP, NA, and JJ; critical revision of the manuscript for important intellectual content: NA and JJ; statistical analysis: NA and CF; administrative, technical, or material support: BP, NA, CC, LT, CC, MC, and TA; study supervision: BP and NA. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the French Society of Infectious Disease and Tropical Medicine (CER-MIT 2021-0302) and was registered with the National Institute of Health Data under the number MR 5611200420.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Puech, B., Canivet, C., Teysseyre, L. et al. Effect of antibiotic therapy on the prognosis of ventilator-associated pneumonia caused by Stenotrophomonas maltophilia. Ann. Intensive Care 11, 160 (2021). https://doi.org/10.1186/s13613-021-00950-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-021-00950-1