Abstract
Background
The data on incidence, clinical presentation, and outcomes of ventilator-associated pneumonia (VAP) in patients with severe coronavirus disease 2019 (COVID-19) pneumonia requiring mechanical ventilation (MV) are limited. We performed this retrospective cohort study to assess frequency, clinical characteristics, responsible pathogens, and outcomes of VAP in patients COVID-19 pneumonia requiring MV between March 12th and April 24th, 2020 (all had RT-PCR-confirmed SARS-CoV-2 infection). Patients with COVID-19-associated acute respiratory distress syndrome (ARDS) requiring ECMO were compared with an historical cohort of 45 patients with severe influenza-associated ARDS requiring ECMO admitted to the same ICU during the preceding three winter seasons.
Results
Among 50 consecutive patients with Covid-19-associated ARDS requiring ECMO included [median (IQR) age 48 (42–56) years; 72% male], 43 (86%) developed VAP [median (IQR) MV duration before the first episode, 10 (8–16) days]. VAP-causative pathogens were predominantly Enterobacteriaceae (70%), particularly inducible AmpC-cephalosporinase producers (40%), followed by Pseudomonas aeruginosa (37%). VAP recurred in 34 (79%) patients and 17 (34%) died. Most recurrences were relapses (i.e., infection with the same pathogen), with a high percentage occurring on adequate antimicrobial treatment. Estimated cumulative incidence of VAP, taking into account death and extubation as competing events, was significantly higher in Covid-19 patients than in influenza patients (p = 0.002). Despite a high P. aeruginosa-VAP rate in patients with influenza-associated ARDS (54%), the pulmonary infection recurrence rate was significantly lower than in Covid-19 patients. Overall mortality was similar for the two groups.
Conclusions
Patients with severe Covid-19-associated ARDS requiring ECMO had a very high late-onset VAP rate. Inducible AmpC-cephalosporinase-producing Enterobacteriaceae and Pseudomonas aeruginosa frequently caused VAP, with multiple recurrences and difficulties eradicating the pathogen from the lung.
Similar content being viewed by others
Background
The emergence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and its ensuing pandemic has strained healthcare systems worldwide, particularly intensive care units (ICUs), with large numbers of patients requiring mechanical ventilation (MV) for severe coronavirus-infection disease 2019 (Covid-19)-associated pneumonia and acute respiratory distress syndrome (ARDS). Most of these patients require prolonged MV, including prone-positioning, heavy sedation, and muscle blockers for several weeks, and; thus, are at high risk of developing bacterial ventilator-associated pneumonia (VAP) [1]. However, only limited information is available regarding VAP frequency, characteristics and outcomes in patients with Covid-19 ARDS requiring MV [2]. Owing to Covid-19’s particular pathophysiology, with some evidence of prolonged immunocompromised status including profound lymphopenia [3], and the potential use of glucocorticoids or immunosuppressants to treat Covid-19 patients [4, 5], we hypothesized that such patients would frequently develop VAP and that they would have worse outcomes than patients with ARDS of other causes, especially higher rates of clinical failure and VAP recurrence [6].
We therefore conducted a retrospective study to evaluate VAP frequency, characteristics and outcomes for all patients sequentially admitted to our ICU (a tertiary referral center for extracorporeal membrane oxygenation (ECMO)) for virologically confirmed Covid-19 ARDS requiring ECMO between 12 March and 24 April 2020, and compared their data with those obtained from a historical cohort of patients with influenza-associated ARDS requiring ECMO.
Methods
Patients
All consecutive ICU-admitted patients, with reverse-transcriptase-polymerase-chain reaction-confirmed Covid-19 pneumonia, based on a respiratory specimen, between 12 March and 24 April 2020, having developed ARDS according to the Berlin definition [7] and requiring ECMO, were included. Patients with influenza-associated ARDS requiring ECMO and admitted to our ICU during the 2017–2018, 2018–2019, and 2019–2020 winters (hereafter called influenza group) served as controls [8].
VAP diagnosis
All ventilated Covid-19 patients suspected of developing VAP based on clinical criteria immediately underwent fiberoptic bronchoscopy, using bronchoalveolar lavage (BAL) to collect distal respiratory secretions from the area in which purulent secretions were most abundant, before new antibiotics were administered. Because it may be difficult to diagnose VAP in patients with acute respiratory distress syndrome (ARDS) and/or ECMO-treated patients, a heightened clinical suspicion of VAP was maintained throughout the study period and bronchoscopic samples were obtained as soon as a patient became febrile, had purulent tracheal secretions and/or deteriorated clinically, even when no progression of lung infiltration could be ascertained. Thus, distal respiratory secretions were collected bronchoscopically when: (1) unexplained hemodynamic instability required higher vasopressor doses or their introduction, (2) an unexplained increase of minute ventilation and/or deterioration of blood gases, or (3) an intercurrent event imposed an urgent change of antibiotic therapy, regardless of the reason. Because performing bronchoscopy in Covid-19 patients may expose healthcare workers to a high risk of SARS-CoV-2 infection, strict full-barrier precautions were implemented, including appropriate personal protective equipment, closed-system suction, and use of a disposable single-use bronchoscope. BAL fluid (BALF) was sent to the bacteriology laboratory for direct microscopic examination with Gram staining, quantitative microbiologic culture, and susceptibility testing of retrieved pathogens. Empirical antimicrobial treatment was started according to the recent French and international guidelines [9,10,11].
VAP was diagnosed in patients having received MV for at least 48 h when the following two criteria were met: (1) clinically suspected VAP, defined as a new and persistent pulmonary infiltrate on chest radiograph associated with at least two of the following: temperature ≥ 38 °C, white blood cell count ≥ 10 Giga/L, purulent tracheal secretions, increased minute ventilation, arterial oxygenation decline requiring modifications of the ventilator settings, and/or need for increased vasopressor infusion. For patients with ARDS, for whom demonstration of radiologic deterioration is difficult, at least two of the preceding criteria sufficed; and (2) significant quantitative growth (≥ 104 colony-forming units/mL) of distal BALF samples [12, 13].
Extreme vigilance for VAP recurrence was maintained throughout the study to detect any possible relapse or new episodes, and fiberoptic bronchoscopy was again performed as soon as any signs of clinical deterioration appeared, as indicated above, or when an intercurrent event imposed an urgent change of antibiotic therapy, regardless of the reason. The same criteria and VAP-diagnostic strategy were also applied during the previous years by our intensivist team for patients who developed influenza-associated ARDS [14].
Therapeutic drug monitoring was part of routine care and antibiotic levels were determined for patients with at least one VAP recurrence [15].
Outcomes
Primary outcome measurement was occurrence of VAP (first VAP episode, as described above. occurring before or after ECMO start), and secondary outcome measurement was VAP recurrence rate.
Definitions
Empiric therapy, defined as antibiotic(s) given between sampling and microbiologic results, was considered adequate when the patient received at least one antibiotic active against the responsible pathogen(s) at optimized dose(s). Definitive treatment was defined as antibiotic(s) given after susceptibility test results were obtained [16].
Patients were considered to have microbiologically documented VAP recurrence when the clinical signs reappeared after a first period of partial or complete resolution, either before or after the end of the initial antimicrobial regimen, and at least one bacterial species grew at a significant concentration from samples collected during a second bronchoscopy. Recurrence was considered a relapse if at least one of the initial causative bacterial strains (i.e., same genus and species) grew at a significant concentration from a second distal sample; otherwise, it was considered a superinfection [14].
Data collection and analysis
The following data were prospectively recorded in each patient’s medical chart: age, sex, Simplified Acute Physiology Score (SAPS) II and Sequential Organ-Failure Assessment (SOFA) score at ICU admission, date SARS-CoV-2 symptoms started, date of MV onset, presence or not of ARDS according to Berlin definition [7], need for venovenous (VV)-ECMO, antiviral agents potentially targeting SARS-CoV-2, use of immunomodulator(s), antibiotics received before VAP onset, antimicrobial regimen for each VAP episode (including empiric and definitive treatment(s)), SOFA-score kinetics during the first VAP episode, and procalcitonin levels at the end of antimicrobial therapy. Outcomes were assessed for patients discharged or those who had died at the study endpoint (24 June 2020). Moreover, the modified Clinical Pulmonary Infection Score (mCPIS) was calculated at infection onset and the end of antimicrobial treatment (Additional file 1: Table S1) [17].
Statistical analyses
The data are expressed as median (IQR) or n (%). Between-group comparisons were analyzed using Student’s t test or Mann–Whitney U tests according to variable’s distribution, i.e., normal or not, respectively, for continuous variables. Between-group differences were assessed with chi-square test or Fisher’s exact test for nominal variables. Incidence of VAP in the 2 groups (primary outcome) was compared using an estimated cumulative incidence function to take into account competing factors (death or extubation), as previously described [18]: cumulative incidence of VAP, extubation, and death were estimated in each group, taking into account only the first event, and compared. All reported p values are two-sided, and p < 0.05 was considered statistically significant. Analyses were computed using SPSS Version 23 (IBM SPSS, Chicago, IL) and R software, version 3.5.1 (R Foundation).
Ethics
In accordance with the current French law, informed written consent for demographic, physiologic and hospital-outcome data analyses was not obtained because this observational study did not modify the existing diagnostic or therapeutic strategies. Nonetheless, patients and/or relatives were informed about the anonymous data collection and told that they could decline inclusion. The protocol was approved by our institution’s ethics committee (CER-Sorbonne Université, no. CER-SU-2020-46), and the database is registered with the Commission Nationale l’Informatique et des Libertés (CNIL, registration no. 1950673).
Results
During the study period, among 58 patients with SARS-CoV-2-associated ARF admitted to our ICU, 54 were mechanically ventilated and 50 had ARDS requiring VV-ECMO constituted the Covid-19 group (Fig. 1). Their characteristics at ICU admission are reported in Table 1. Briefly, they were young [median (IQR) age, 48 (42–56) years]. Although fewer 20% had documented bacterial coinfection at ICU admission, all received antimicrobials for a median (IQR) of 5 (4–6) days. The median (IQR) interval between Covid-19 symptom onset and ICU admission was 11 (7–14) days.
Flow chart of the study. ARDS acute respiratory distress syndrome, ARF acute respiratory failure, Covid-19 coronavirus-infection disease 2019, ICU intensive care unit, MV mechanical ventilation, SARS-CoV-2 severe acute respiratory syndrome coronavirus-2, ECMO extracorporeal membrane oxygenation, VAP ventilator-associated pneumonia
Among these 50 patients, 43 developed at least one VAP episode, after a median (IQR) of 10 (8–16) days on MV (Table 2). Among the seven patients who did not develop VAP, four died before the end of the first week on MV, two were discharged from the ICU on day 52 or 59 after MV onset, and one died without VAP 36 days after starting MV. Only four or 3 patients, respectively, received glucocorticoids or immunomodulators before developing VAP.
Pathogens responsible for the first VAP episode, antimicrobial treatment of VAP and clinical characteristics at the end of that regimen are reported in Table 2. Thirty (70%) episodes were due to Enterobacteriaceae, 17 (57%) of them producing chromosomally inducible Amp-C cephalosporinases, with Klebsiella aerogenes being the most frequently recovered (11/17, 65%). Pseudomonas aeruginosa, the second most frequently isolated microorganism, caused VAP in 16 (37%) patients, while Staphylococcus aureus was isolated from only three patients.
VAP recurrence despite appropriate antimicrobial treatment was microbiologically documented for 34 (79%) of the 43 VAP patients, before or after the initial antibiotics were discontinued for 9 or 25 patients, respectively (all patients whose recurrence occurred before the end of antimicrobial treatment had initial improvement with no evidence of persistent infection, and then reappearance of signs of infection). Microorganisms responsible for subsequent VAP episodes are listed in Table 3. The infection was caused by the same pathogen as the initial episode in 26 (76%) patients with a median (IQR) interval of 2 (1–3) days between the end of the first episode and relapse. Although P. aeruginosa was the predominant causative pathogen of recurrent VAP, Enterobacteriaceae (mostly species with inducible Amp-C cephalosporinase) were also largely responsible for VAP relapse. Enterococcus faecalis, which is not a common VAP bacterium, was responsible for one patients’ recurrent episode. As part of antimicrobial stewardship program in our unit, patients were mainly treated with a beta-lactam monotherapy, this latter chosen according to pathogen susceptibility as having the narrowest-possible spectrum. There were no differences in antibiotic treatment of first VAP episode in patients with and without subsequent recurrences. Among the 34 patients who had a recurrent VAP episode, 21 had blood level determination of antibiotic trough level during recurrence. In all of them, antibiotic trough level was above the EUCAST breakpoint of the antibiotic for the responsible pathogen, and above 4 times the EUCAST breakpoint for 15/21 (71%).
Among the 20 patients with three or more VAP episodes, 16 had relapses, caused by inducible AmpC-cephalosporinase-producing Enterobacteriaceae for 9 of them (Klebsiella aerogenes for 8 and Serratia marcescens for one) (Table 3). For eight of these 9 patients, the Enterobacteriaceae remained wild type—ie, without selection of a de-repressed AmpC strain—despite the use of antibiotics that could have potentially selected it.
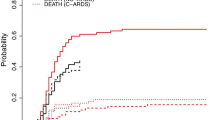
Among the 60 controls with influenza-associated ARF admitted to our ICU during the three preceding winters, 51 had received MV, 45 required VV-ECMO and were included (Fig. 1 and Table 1). When compared with Covid-19 patients, those with influenza were significantly older (p = 0.002), had shorter symptom-onset-to-ICU-admission intervals (p = 0.008), higher SAPS II and SOFA scores (p < 0.0001 and 0.02, respectively), higher rates of documented initial bacterial coinfection (p = 0.02), but less frequent VAP. Estimated cumulative incidence of VAP (taking into account death and extubation as competing factors) was significantly lower in influenza patients than Covid-19 patients (p = 0.002), whereas death and extubation did not differ between these 2 groups (Fig. 2). Despite influenza patients’ lower SOFA scores at VAP onset (Additional file 1: Figure S1), score kinetics was similar for the two groups over the following 7 days.
Estimated cumulative incidence of ventilator-associated pneumonia (VAP), extubation or death in Covid-19 and influenza patients, taking into account only the first event that occurred. p values for differences between Covid-19 and influenza patients were 0.002 for VAP, 0.11 for death and 0.07 for extubation
Unlike Covid-19 patients’ VAP-causing microorganisms, pathogens responsible for VAP in influenza controls were more frequently a P. aeruginosa strain and rarely an Enterobacteriaceae (Table 2). The rates of appropriate empiric treatment and antimicrobial-therapy duration were similar for the two groups.
Despite a high rate of P. aeruginosa VAP in patients with influenza-associated ARDS, their VAP recurrence was significantly less frequent than that of patients with Covid-19-associated ARDS (p = 0.03, Table 1). The overall mortality was similar for the two groups of VAP patients (34% for Covid-19 versus 40% for influenza, p = 0.54).
Discussion
To our knowledge, the characteristics and early outcomes have not yet been reported for a large case-series of sequentially hospitalized patients with severe confirmed Covid-19 ARF/ARDS requiring MV and almost always VV-ECMO. A very high rate of Covid-19-associated late-onset VAP was observed, well above the usual rates for patients with other causes of ARDS, including influenza [1]. Chromosomally inducible AmpC-cephalosporinase-producing Enterobacteriaceae and Pseudomonas aeruginosa were the pathogens most frequently responsible for VAP, with multiple recurrences and difficulties eradicating the microorganism(s) from the lung. Strikingly, for most patients with inducible AmpC-cephalosporinase-producing Enterobacteriaceae-infection recurrence, the pathogen remained the wild type, despite the use of antibiotics that could have potentially selected a de-repressed AmpC strain (eg, third-generation cephalosporins).
Several explanations can be advanced for Covid-19 patients’ high VAP and VAP-recurrence rates: firstly, most of our patients had the most severe form of Covid-19 ARDS requiring VV-ECMO support. They required longer MV durations than ARDS patients not requiring ECMO, and were therefore at higher risk of developing multiple VAP episodes [19]. However, our influenza-associated ARDS controls, with similar or even greater disease severity, similar ECMO rate and prolonged MV duration, had lower VAP and VAP-recurrence rates, as was also observed in the recent EOLIA trial [20]. Secondly, antimicrobial treatment duration might have been too short, despite being in agreement with the recent international guidelines [10, 11]. Notably, patients with influenza-associated ARDS had the same antimicrobial treatment duration and a lower VAP-recurrence rate. Moreover, a high percentage of Covid-19 patients’ VAP recurrences occurred even before the end of the initial antimicrobial therapy. Thirdly, the frequent VV-ECMO use and/or drug–drug interaction(s) in our Covid-19 patients might have impacted VAP outcome by altering antibiotic pharmacokinetics, even though the antibiotic levels of all the patients subjected to therapeutic drug monitoring were above the EUCAST breakpoint for the responsible pathogen [21, 22]. Fourthly, the administration of adjunctive immunomodulatory/immunosuppressant agents to a small fraction of Covid-19 patients could also have facilitated infectious complications [23].
There may be other explanations for the high VAP and VAP-recurrence rates. The pathophysiology of Covid-19 in ICU patients includes pulmonary vasculopathy with endothelial dysfunction and endothelialitis [24, 25]. These features, associated with dysregulated lung inflammation and diffuse alveolar damage, might enhance susceptibility to secondary bacterial infection, and/or decrease antibiotic availability in the lung parenchyma. Indeed, the antimicrobial treatment failure rate was high, with patients developing new VAP episodes with the same susceptible pathogen despite appropriate and adequate antimicrobial regimens.
Our study has several limitations that should be underlined. Firstly, its retrospective monocenter design that included the most severe Covid-19 patients, all of whom requiring VV-ECMO, making our results difficult to extrapolate to other ICUs with different case mixes. Particularly, whether patients with Covid-19-associated ARDS without ECMO have similar VAP and VAP recurrence rate remain to be determined. The small size of our study (only 50 patients with Covid-19 were included) is a second limitation. Third, our patients’ VAP-causing pathogens might essentially reflect our local ecology. Whether or not the same microorganism distribution would be found in other ICUs remains to be explored. Particularly, the high rate of Klebsiella aerogenes may raise the issue of cross-contamination between patients. Since we did not compare bacterial strains genetically, we cannot formally rule out this hypothesis. However, the rate of Klebsiella aerogenes VAP decreased dramatically in our unit after Covid-19 pandemic, rending this hypothesis unlikely. Fourth, Covid-19 and influenza patients were not strictly comparable and the differences observed in the VAP characteristics of the two populations should be viewed with caution.
Conclusion
In conclusion, patients with severe Covid-19-associated ARDS requiring ECMO are particularly prone to develop late-onset VAP, frequently caused by inducible AmpC-cephalosporinase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Once VAP is diagnosed and treated, clinicians should be aware that patients are at high risk of its recurrence/relapse, despite appropriate and adequate antimicrobial therapy.
Availability of data and materials
The datasets generated during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- ARF:
-
Acute respiratory failure
- BAL:
-
Bronchoalveolar lavage
- COVID-19:
-
Coronavirus infection disease 2019
- CPIS:
-
Clinical pulmonary infection score
- ECMO:
-
Extracorporeal membrane oxygenation
- ICU:
-
Intensive care unit
- MV:
-
Mechanical ventilation
- SAPS:
-
Simplified Acute Physiology Score
- SARS-CoV-2:
-
Severe Acute Respiratory Syndrome coronavirus 2
- SOFA:
-
Sequential organ failure assessment
- VAP:
-
Ventilator-associated pneumonia
- WBC:
-
White blood cell
References
Papazian L, Klompas M, Luyt C-E. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46:888–906.
Rawson TM, Moore LSP, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020. (in press).
Lescure F-X, Bouadma L, Nguyen D, Parisey M, Wicky P-H, Behillil S, et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697–706.
Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020;48:e440–69.
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–43.
François B, Laterre P-F, Luyt C-E, Chastre J. The challenge of ventilator-associated pneumonia diagnosis in COVID-19 patients. Crit Care. 2020;24:289.
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33.
Rozencwajg S, Bréchot N, Schmidt M, Hékimian G, Lebreton G, Besset S, et al. Co-infection with influenza-associated acute respiratory distress syndrome requiring extracorporeal membrane oxygenation. Int J Antimicrob Agents. 2018;51:427–33.
Leone M, Bouadma L, Bouhemad B, Brissaud O, Dauger S, Gibot S, et al. Brief summary of French guidelines for the prevention, diagnosis and treatment of hospital-acquired pneumonia in ICU. Ann Intensive Care. 2018;8:104.
Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur Respir J. 2017;50:1700582.
Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-111.
Chastre J, Fagon J-Y. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903.
Chastre J, Luyt C-E. Does this patient have VAP? Intensive Care Med. 2016;42:1159–63.
Chastre J, Wolff M, Fagon J-Y, Chevret S, Thomas F, Wermert D, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588–98.
Abdul-Aziz MH, Alffenaar J-WC, Bassetti M, Bracht H, Dimopoulos G, Marriott D, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med. 2020;46:1127–53.
Luyt C-E, Faure M, Bonnet I, Besset S, Huang F, Junot H, et al. Use of non-carbapenem antibiotics to treat severe extended-spectrum β-lactamase-producing Enterobacteriaceae infections in intensive care unit patients. Int J Antimicrob Agents. 2019;53:547–52.
Niederman MS, Alder J, Bassetti M, Boateng F, Cao B, Corkery K, et al. Inhaled amikacin adjunctive to intravenous standard-of-care antibiotics in mechanically ventilated patients with Gram-negative pneumonia (INHALE): a double-blind, randomised, placebo-controlled, phase 3, superiority trial. Lancet Infect Dis. 2020;20:330–40.
Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54.
Chastre J, Fagon JY, Soler P, Bornet M, Domart Y, Trouillet JL, et al. Diagnosis of nosocomial bacterial pneumonia in intubated patients undergoing ventilation: comparison of the usefulness of bronchoalveolar lavage and the protected specimen brush. Am J Med. 1988;85:499–506.
Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–75.
Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14:498–509.
Bouglé A, Dujardin O, Lepère V, Ait Hamou N, Vidal C, Lebreton G, et al. PHARMECMO: Therapeutic drug monitoring and adequacy of current dosing regimens of antibiotics in patients on Extracorporeal Life Support. Anaesth Crit Care Pain Med. 2019;38:493–7.
Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117:10970–5.
Leisman DE, Deutschman CS, Legrand M. Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. 2020;46:1105–8.
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–8.
Acknowledgements
The authors thank Janet Jacobson for her help during the preparation of the manuscript, and David Hajage for his help for statistical analysis.
Funding
None.
Author information
Authors and Affiliations
Contributions
CEL designed the study, collected, compiled, analysed and interpreted the data and wrote the manuscript. TS, MG, MPdC, JC, CD, JA, AN, NB, MS, GH and AC collected data. PV and JR performed the bacteriological analysis. SB and DB performed the virological analysis. JC analyzed and interpreted the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In accordance with the current French law, informed written consent for demographic, physiologic and hospital-outcome data analyses was not obtained because this observational study did not modify existing diagnostic or therapeutic strategies. Nonetheless, patients and/or relatives were informed about the anonymous data collection and told that they could decline inclusion. The protocol was approved by our institution’s ethics committee (CER-Sorbonne Université, no. CER-SU-2020-46) and the database is registered with the Commission Nationale l’Informatique et des Libertés (CNIL, registration no. 1950673).
Consent for publication
Not applicable.
Competing interests
C.-E. L. has served as consultant for Bayer Healthcare, Carmat and Thermo Fisher Brahms, and received lecture fees from MSD, Aerogen and BioMérieux, outside the submitted work. The other authors have no conflicts of interest to declare in relationship to this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
13613_2020_775_MOESM1_ESM.docx
Additional file 1: Table S1. Modified Clinical pulmonary infection score. Figure S1. Sequential Organ-Failure Assessment (SOFA) score kinetics from ventilator-associated pneumonia onset (day 1) to day 7. Results are expressed as means ± standard deviation. Covid-19 = coronavirus disease 2019. *p < 0.05 for between-group comparisons.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luyt, CE., Sahnoun, T., Gautier, M. et al. Ventilator-associated pneumonia in patients with SARS-CoV-2-associated acute respiratory distress syndrome requiring ECMO: a retrospective cohort study. Ann. Intensive Care 10, 158 (2020). https://doi.org/10.1186/s13613-020-00775-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-020-00775-4