Abstract
Background
This study aimed to investigate the association between preadmission glucocorticoid (GC) therapy and 30-day mortality in critically ill patients following admission to an intensive care unit (ICU). We aimed to determine whether this association differed according to daily GC dosage and type. We conducted a retrospective cohort study of adult patients admitted to a single tertiary academic hospital ICU from January 2012 to December 2017. We classified the patients regularly undergoing oral GC therapy as preadmission GC users, and those with no history of GC use were classified as non-GC users.
Results
The study included 24,929 patients, of whom 816 (3.3%) were preadmission GC users. Thirty-day mortality in preadmission GC users (173 of 816 patients) was 21.2% compared to 8.8% (2113 of 24,113 patients) in non-GC users. Multivariate Cox regression analysis showed that preadmission GC users had a 1.62-fold increase in 30-day mortality compared to non-GC users [hazard ratio (HR) 1.62, 95% confidence interval (CI) 1.29–2.03, P < 0.001]. When comparing preadmission GC users with diabetes mellitus to non-GC users, a 2.29-fold increase in 30-day mortality was noted (HR 2.29, 95% CI 1.08–4.86, P = 0.031). In the sensitivity analysis, compared to non-GC users, daily dosages of ≤ 5 and > 5 mg of prednisolone in preadmission GC users showed 1.45-fold (HR 1.45, 95% CI 1.03–2.03, P = 0.033) and 1.67-fold (HR 1.67, 95% CI 1.25–2.24, P = 0.001) increases, respectively, in 30-day mortality after ICU admission. Moreover, prednisolone, methylprednisolone, and dexamethasone users in the preadmission GC users group showed 1.56-fold (HR 1.56, 95% CI 1.21–2.01, P = 0.001), 1.90-fold (HR 1.90, 95% CI 1.12–3.25, P = 0.018), and 1.30-fold (HR 1.30, 95% CI 1.05–1.50, P = 0.042) increases, respectively, in 30-day mortality compared to non-GC users.
Conclusion
Preadmission GC use among critically ill patients was associated with an increased 30-day mortality after ICU admission compared to non-GC use. This association was more prevalent in preadmission GC users with diabetes mellitus and in preadmission GC users who took > 5 mg/day of prednisolone and methylprednisolone.
Similar content being viewed by others
Background
The steroid hormone glucocorticoid (GC) is a pleiotropic hormone that is commonly prescribed for 1.2% [1] and 1.0% [2] of patients with chronic conditions in the USA and in the UK, respectively. GC has both immunosuppressive and potent anti-inflammatory effects [3], and GC inhibits the immune response and the production of prostaglandins and leukotrienes, the two main products of inflammation [4]. GC has been reported to be a useful treatment option worldwide for patients who suffer from diseases such as allergies [5], chronic obstructive lung disease or asthma [6], autoimmune disease [7], and rheumatic disease [8], due to its immunosuppressive and anti-inflammatory effects. However, chronic GC use is known to cause various side effects [9], of which patient susceptibility to infection due to its immunosuppressive effects is the most fatal [10]. Moreover, chronic GC use has a direct immunosuppressive effect that leads to an increased risk of infection [10], and chronic GC use can also lead to central adrenal failure [11]. In this regard, chronic GC use prior to admission to an intensive care unit (ICU) could increase the mortality risk, especially in critically ill patients more susceptible to infection and mortality due to sepsis [12]. Many patients admitted to the ICU experience newly ICU-acquired immunosuppression for various reasons [13]; therefore, preadmission GC use that can reduce immunity in patients may be associated with a poor prognosis in critically ill patients. One cohort study has reported that preadmission GC use increased 30-day mortality after stroke [14], and another cohort study showed a decrease in the incidence of acute respiratory distress syndrome (ARDS) in sepsis patients [15]. However, few studies have investigated the effects of preadmission GC use in critically ill patients; therefore, this issue remains challenging.
This study aimed to investigate the association between preadmission GC use and 30-day mortality after ICU admission among adult patients. Additionally, we aimed to determine whether this association differed according to daily dosage, types of GC, or the main diagnosis at the time of ICU admission.
Materials and methods
Study design and participants
This retrospective cohort study investigated the medical records of adult patients aged > 18 years who had been admitted to an ICU from January 2012 to December 2017. If a patient had been admitted to the ICU more than once during the study period, only details of the last ICU admission were included for analysis. Patients with incomplete or missing medical records detailing medication history were excluded from the analysis. All data collection and classifications were performed by a medical records technician from the institution who was blinded to the study purpose.
Ethics
This study was conducted with the approval of the Seoul National University Bundang Hospital Institutional Review Board (IRB) (IRB approval number: B-1806/474-105). The requirement for informed consent was waived by the IRB as this was a retrospective study.
Preadmission chronic GC use (independent variable)
Patients were classified as preadmission GC users if they had been taking a daily dose of oral GC prior to ICU admission. Conversely, patients were defined as non-GC users if they had no history of GC use. Oral GCs were classified into three types, namely prednisolone, methylprednisolone, and dexamethasone, and daily GC dosages were divided into two groups, namely > 5 mg/day and ≤ 5 mg/day of prednisolone. Using this dosage calculation, 4 mg/day of methylprednisolone and 0.75 mg/day of dexamethasone were considered the same as a dosage of 5 mg/day of prednisolone, given the potency of GCs [16]. Our reasoning for dividing the groups according to a 5 mg/day cutoff was that previous studies have reported no significant association with doses < 5 mg prednisolone for all-cause mortality in patients with rheumatoid arthritis [17, 18].
Covariates
Data collected for this study included demographic information such as sex, age (in years), body mass index (kg/m2), and medication history at ICU admission, and the Acute Physiology and Chronic Health Evaluation II score at ICU admission. Comorbidity data at ICU admission concerning hypertension, diabetes mellitus, ischemic heart disease, cerebrovascular disease, chronic obstructive lung disease, liver disease (liver cirrhosis, hepatitis, and fatty liver disease), chronic kidney disease, anemia (hemoglobin < 10 g/dl), and cancer were collected. Information concerning whether admission occurred through the emergency department was also obtained. Additionally, the main diagnosis on ICU admission, for example, septic shock, cardiac disease, neurologic disease, kidney failure, respiratory insufficiency or failure, drug intoxication, trauma, gastrointestinal bleeding/ischemia/or perforation, hemorrhagic shock, post-cardiac arrest, and others [liver failure, urinary tract infection, peripheral vascular disease, and ICU admission for close monitoring], was also recorded. The main diagnosis on ICU admission was classified using ICU records completed by the primary physician at the time of ICU admission. If, on ICU admission, one patient had multiple main diagnoses, for example, septic shock with kidney failure, then the patient was assigned to two groups (septic shock and kidney failure) in duplicate.
Outcome measure (dependent variable)
Thirty-day mortality was defined as any death that occurred within 30 days of ICU admission. The accurate death date for all patients, including patients who had been discharged, was obtained up until May 16, 2018, with approval from the Korean Ministry of the Interior and Safety.
End points
The primary end point was to identify the association between preadmission GC therapy and 30-day mortality after ICU admission. Thirty-day mortality due to diagnosed infection was confirmed in cases where the pathogen had been confirmed through culture. Additionally, we investigated whether this association changed according to dosage, types of GC, and main diagnosis on ICU admission.
Statistical analysis
To compare the clinical and demographic characteristics based on preadmission GC use, sample t-tests for continuous variables and Chi-square tests for categorical variables were used. First, a univariate Cox regression analysis was performed to identify the individual correlation between the covariates and 30-day mortality. From this univariate analysis, covariates with a P value < 0.2 were selected for adjustment in the final multivariate Cox regression analysis.
Additionally, sensitivity analyses according to daily dosage and type of GC were performed to investigate whether the association between chronic GC use and 30-day mortality might differ according to daily dosage and type of GC taken by the patients in the chronic GC user group. Finally, a subgroup analysis of 11 groups, according to the main diagnosis at the time of ICU admission, was performed to confirm whether an association between chronic GC use and 30-day mortality might differ according to the main diagnosis at the time of ICU admission. In this subgroup analysis, Bonferroni correction was applied to reduce type I errors in multiple comparisons [19]. We tested the interaction of chronic GC use and a function of time, implemented as time-dependent chronic GC use, to confirm that the proportional hazards assumption had been satisfied (P > 0.05). All statistical analyses were performed using IBM SPSS version 24.0. (IBM Corp., Armonk, NY). A P-value < 0.005 was considered statistically significant for the subgroup analysis using Bonferroni correction, and P < 0.05 was considered statistically significant for all other analyses.
Results
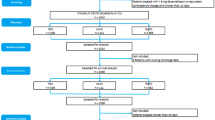
From January 2012 to December 2017, a total of 30,398 patients had been admitted to the ICU on 40,533 occasions. Of them, 10,135 ICU admissions were excluded because they represented multiple admissions, and only the last episode of ICU admission was considered for each patient. Next, 5440 pediatric patients < 18 years of age, and 29 patients with incomplete or missing medical records with regard to chronic GC use, were excluded. In total, 24,929 patients were included in the analysis, and of them, 819 (3.3%) patients were preadmission GC users (Fig. 1). The 30-day mortality was 21.2% (173 of 816 patients) in the preadmission GC users group and 8.8% (2113 of 24,113 patients) in the non-GC users. Thirty-day mortality due to diagnosed infection occurred in 31 of 816 (3.8%) preadmission GC users and in 260 of 24,113 (1.1%) non-GC users.
Thirty-day mortality after ICU admission
Table 1 shows the differences in baseline characteristics between preadmission GC users and non-GC users, and 30-day mortality was significantly higher in preadmission GC users (173 of 816 patients, 21.2%) than in non-GC users (2113 of 24,113 patients, 8.8%) (P < 0.001). Additional file 1: Table S1 shows the univariate Cox regression analysis of the association between covariates and 30-day mortality after ICU admission. Table 2 shows the results of the Cox regression analysis for the association between 30-day mortality and preadmission GC use before and after adjustment for the covariates selected from the univariate analysis. In the adjusted multivariate Cox regression model, there was a 1.62-fold increase in 30-day mortality when comparing preadmission GC users and non-GC users [hazard ratio (HR) 1.62, 95% confidence interval (CI); 1.29–2.03, P < 0.001]. Additionally, there was a 2.29-fold increase in the 30-day mortality when preadmission GC users with diabetes mellitus and non-GC users were compared (HR 2.29, 95% CI 1.08–4.86, P = 0.031).
Sensitivity analysis according to dosage and types of GCs
The results of sensitivity analysis according to dosage and types of GCs are presented in Table 3. Compared to non-GC users, daily dosages of ≤ 5 mg and > 5 mg in preadmission GC users showed 1.45-fold (HR 1.45, 95% CI 1.03–2.03, P = 0.033) and 1.67-fold (HR 1.67, 95% CI 1.25–2.24, P = 0.001) increases in 30-day mortality after ICU admission. Additionally, prednisolone users, methylprednisolone users, and dexamethasone users from within the preadmission GC users group showed 1.56-fold (HR 1.56, 95% CI 1.21–2.01, P = 0.001), 1.90-fold (HR 1.90, 95% CI 1.12–3.25, P = 0.018), and 1.30-fold (HR 1.30, 95% CI 1.05–1.50, P = 0.042) increases in 30-day mortality after ICU admission compared to non-GC users.
Subgroup analysis according to the main diagnosis on ICU admission
The results of subgroup analysis according to the main diagnosis at the time of ICU admission are presented in Table 4. Thirty-day mortality was 40.1% (164 of 409 patients) for septic shock, 4.2% (340 of 8192) for cardiac disease, 4.7% (156 of 3348) for neurologic disease, 27.1% (468 of 1725) for kidney failure, 25.5% (740 of 2906) for respiratory insufficiency or failure, 6.2% (11 of 176) for drug intoxication, 7.9% (15 of 191) for trauma, 8.7% (96 of 1108) for gastrointestinal bleeding/ischemia/or perforation, 6.5% (7 of 107) for hemorrhagic shock, and 54.4% (180 of 331) for post-cardiac arrest.
In multivariate Cox regression analysis, preadmission GC users showed 3.21-fold (HR 3.21, 95% CI 1.72–5.97, P < 0.001), 1.90-fold (HR 1.90, 95% CI 1.67–5.40), and 2.04-fold (HR 2.04, 95% CI 1.47–2.82, P < 0.001) increases in 30-day mortality after ICU admission in septic shock patients, neurologic disease, and respiratory insufficiency or failure patients compared to non-GC users, respectively.
Discussion
This study showed that preadmission GC use was significantly associated with an increase in 30-day mortality among critically ill adult patients. This association was more prevalent in preadmission GC users with diabetes mellitus and in preadmission GC users who took a higher daily dosage, such as > 5 mg/day of prednisolone. Additionally, in the subgroup analysis, preadmission GC users who had been diagnosed with septic shock or respiratory insufficiency or failure, and neurologic disease on ICU admission showed an associated increase in 30-day mortality after ICU admission. This study is the first to investigate the association between preadmission GC use and 30-day mortality in a mixed ICU population.
First, our findings reflect those of a previous study that showed a direct immunosuppressive effect due to preadmission chronic GC use might be associated with 30-day mortality in critically ill patients [10]. This possible association is supported by the results of our subgroup analysis indicating that critically ill patients with septic shock or respiratory failure or insufficiency were more affected if they had been chronic GC users. Septic shock or respiratory insufficiency might be due to infection such as pneumonia [20], and the immunosuppressive effect of chronic GC use on 30-day mortality could be significant in these patients. Second, adrenal insufficiency, commonly due to chronic GC administration [21], might affect 30-day mortality in critically ill patients. A recently published systematic review has reported that the median incidence of adrenal insufficiency among chronic GC users was 37.4% (interquartile range 13–63%) [22]. Given that mortality has been reported to increase in patients with adrenal insufficiency [23], adrenal insufficiency due to chronic GC use may have affected the results in this study.
Previous studies have not shown consistent results regarding the effects of chronic GC use on the mortality of critically ill patients. Sundbøll et al. [14] reported that preadmission GC use was associated with an increase in 30-day mortality among patients with ischemic stroke, intracranial hemorrhage, and subarachnoid hemorrhage, and their results are similar to the findings in our study that showed 30-day mortality increased in patients with neurologic disease on ICU admission (Table 4). However, McKown et al. [15] reported that there was no significant association between preadmission GC use and hospital mortality among sepsis patients, although it was associated with a lower incidence of early acute respiratory distress syndrome (ARDS). The contrasting conclusions in McKown et al.’s study and in our study may be due to differing population characteristics. In McKown et al.’s study, patients admitted to ICU with sepsis were included for analysis, whereas we included a mixed ICU population for analysis; therefore, the effects of preadmission GC use may have differed.
The results of sensitivity analysis according to daily dosage were also noteworthy in this study. Two previous studies showed that a low daily GC dose (≤ 5 mg of prednisolone) did not affect mortality in patients with rheumatoid arthritis [17, 18]. However, a daily GC dose of ≤ 5 mg of prednisolone was associated with an increase in 30-day mortality in critically ill patients in our study. There are two possible explanations for this. First, critically ill patients are more severely ill than are patients with rheumatoid arthritis, and the immunosuppressive effect of chronic GC administration might be a significant risk factor for mortality in those taking a low daily dose of GC (≤ 5 mg of prednisolone). Second, the systematic review reported that adrenal insufficiency could have occurred in patients receiving a daily dose of < 5 mg prednisolone, which might have affected 30-day mortality among critically ill patients in that study [22].
Preadmission GC users with diabetes mellitus showed a relatively higher risk of 30-day mortality after ICU admission than other preadmission GC users compared to non-GC users. Chronic GC administration is known to be a risk factor for the development of diabetes mellitus [24]. Moreover, GC administration usually causes hyperglycemia [25], and management of hyperglycemia in chronic GC users is a challenging issue facing clinicians. A previous study reported that hyperglycemia in hospitalized patients who received GC administration was common, and it was associated with higher mortality or morbidity in the patients [26]. Therefore, the findings in our study suggest that preadmission GC users with diabetes mellitus on ICU admission should be treated even more carefully because these patients have an increased risk of 30-day mortality.
This study has several limitations. First, due to the study’s retrospective cohort design, selection bias may have been present. Second, this cohort study was conducted at a single center; therefore, the generalizability of our findings may be limited. Third, having to perform a dose conversion for the different GCs to conduct a sensitivity analysis according to daily dosage when considering the potency of each GC could have introduced bias into this study. Finally, since this study used records from multiple physicians determining the main diagnosis on ICU admission, there may have been inconsistencies or inaccuracy in determining the diagnoses.
In conclusion, the present study showed that preadmission GC use among critically ill patients was associated with increased 30-day mortality after ICU admission compared to non-GC use. This association was more prevalent in preadmission GC users with diabetes mellitus and preadmission GC users taking > 5 mg/day of prednisolone or methylprednisolone. Additionally, this association was significant in patients who had been admitted for septic shock, respiratory insufficiency or failure, and neurologic disease.
Abbreviations
- APACHE:
-
Acute Physiology and Chronic Health Evaluation
- ARDS:
-
acute respiratory distress syndrome
- CI:
-
confidence interval
- GC:
-
glucocorticoid
- GI:
-
gastrointestinal
- HPA:
-
hypothalamic pituitary adrenal
- HR:
-
hazard ratio
- ICU:
-
intensive care unit
- IRB:
-
institutional review board
References
Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res. 2013;65(2):294–8.
van Staa TP, Leufkens HG, Abenhaim L, Begaud B, Zhang B, Cooper C. Use of oral corticosteroids in the United Kingdom. QJM. 2000;93(2):105–11.
Boumpas DT, Chrousos GP, Wilder RL, Cupps TR, Balow JE. Glucocorticoid therapy for immune-mediated diseases: basic and clinical correlates. Ann Intern Med. 1993;119(12):1198–208.
Goppelt-Struebe M, Wolter D, Resch K. Glucocorticoids inhibit prostaglandin synthesis not only at the level of phospholipase A2 but also at the level of cyclo-oxygenase/PGE isomerase. Br J Pharmacol. 1989;98(4):1287–95.
Dimeloe S, Nanzer A, Ryanna K, Hawrylowicz C. Regulatory T cells, inflammation and the allergic response-The role of glucocorticoids and Vitamin D. J Steroid Biochem Mol Biol. 2010;120(2–3):86–95.
Keatings VM, Jatakanon A, Worsdell YM, Barnes PJ. Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPD. Am J Respir Crit Care Med. 1997;155(2):542–8.
Tait AS, Butts CL, Sternberg EM. The role of glucocorticoids and progestins in inflammatory, autoimmune, and infectious disease. J Leukoc Biol. 2008;84(4):924–31.
Berthelot JM. Comments about the article by Mouterde et al. entitled “Indications of glucocorticoids in early arthritis and rheumatoid arthritis: recommendations for clinical practice based on data from the literature and expert opinions”. Joint Bone Spine. 2010;77:597–603. Low-dose prednisone and biologics: allies rather than competitors? Joint Bone Spine. 2012;79(1):103–4 (author reply 4–6).
Bloechliger M, Reinau D, Spoendlin J, Chang SC, Kuhlbusch K, Heaney LG, et al. Adverse events profile of oral corticosteroids among asthma patients in the UK: cohort study with a nested case-control analysis. Respir Res. 2018;19(1):75.
Klein NC, Go CH, Cunha BA. Infections associated with steroid use. Infect Dis Clin North Am. 2001;15(2):423–32.
Crowley RK, Argese N, Tomlinson JW, Stewart PM. Central hypoadrenalism. J Clin Endocrinol Metab. 2014;99(11):4027–36.
Hranjec T, Sawyer RG. Management of infections in critically ill patients. Surg Infect (Larchmt). 2014;15(5):474–8.
Pene F, Pickkers P, Hotchkiss RS. Is this critically ill patient immunocompromised? Intensive Care Med. 2016;42(6):1051–4.
Sundbøll J, Horvath-Puho E, Schmidt M, Dekkers OM, Christiansen CF, Pedersen L, et al. Preadmission use of glucocorticoids and 30-day mortality after stroke. Stroke. 2016;47(3):829–35.
McKown AC, McGuinn EM, Ware LB, Wang L, Janz DR, Rice TW, et al. Preadmission oral corticosteroids are associated with reduced risk of acute respiratory distress syndrome in critically ill adults with sepsis. Crit Care Med. 2017;45(5):774–80.
Meikle AW, Tyler FH. Potency and duration of action of glucocorticoids. Effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. Am J Med. 1977;63(2):200–7.
del Rincon I, Battafarano DF, Restrepo JF, Erikson JM, Escalante A. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):264–72.
Movahedi M, Costello R, Lunt M, Pye SR, Sergeant JC, Dixon WG. Oral glucocorticoid therapy and all-cause and cause-specific mortality in patients with rheumatoid arthritis: a retrospective cohort study. Eur J Epidemiol. 2016;31(10):1045–55.
Armstrong RA. When to use the Bonferroni correction. Ophthalmic Physiol Opt. 2014;34(5):502–8.
Montull B, Menendez R, Torres A, Reyes S, Mendez R, Zalacain R, et al. Predictors of severe sepsis among patients hospitalized for community-acquired pneumonia. PLoS ONE. 2016;11(1):e0145929.
Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet. 2014;383(9935):2152–67.
Joseph RM, Hunter AL, Ray DW, Dixon WG. Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin Arthritis Rheum. 2016;46(1):133–41.
Quinkler M, Ekman B, Zhang P, Isidori AM, Murray RD, Investigators E-A. Mortality data from the European Adrenal Insufficiency Registry-Patient characterization and associations. Clin Endocrinol. 2018;89(1):30–5.
Suh S, Park MK. Glucocorticoid-induced diabetes mellitus: an important but overlooked problem. Endocrinol Metab. 2017;32(2):180–9.
Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15(5):469–74.
Donihi AC, Raval D, Saul M, Korytkowski MT, DeVita MA. Prevalence and predictors of corticosteroid-related hyperglycemia in hospitalized patients. Endocr Pract. 2006;12(4):358–62.
Authors’ contributions
TKO designed the study, analyzed the data, and drafted the manuscript. IAS, JHL, CL, HJB, YTJ, and YHJ contributed to the data acquisition and interpretation. All authors read and approved the final manuscript.
Acknowledgements
The authors thank the Medical Research Collaborating Center at Seoul National University Bundang Hospital for revision regarding statistical analyses.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This retrospective study was approved by the institutional review board of the Seoul National University Bundang Hospital (IRB Approval Number: B-1806/474-105) and waived the need for informed consent because of the retrospective study design.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1.
Table S1. Univariate Cox regression analysis of covariates for 30-day mortality after ICU admission.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Oh, T.K., Song, IA., Lee, J.H. et al. Effect of preadmission glucocorticoid therapy on 30-day mortality in critically ill patients: a retrospective study of a mixed ICU population in a tertiary hospital. Ann. Intensive Care 9, 8 (2019). https://doi.org/10.1186/s13613-019-0489-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-019-0489-8