Abstract
Background
Transient and persistent acute kidney injury (AKI) could share similar physiopathological mechanisms. The objective of our study was to assess prognostic impact of AKI duration on ICU mortality.
Design
Retrospective analysis of a prospective database via cause-specific model, with 28-day ICU mortality as primary end point, considering discharge alive as a competing event and taking into account time-dependent nature of renal recovery. Renal recovery was defined as a decrease of at least one KDIGO class compared to the previous day.
Setting
23 French ICUs.
Patients
Patients of a French multicentric observational cohort were included if they suffered from AKI at ICU admission between 1996 and 2015.
Intervention
None.
Results
A total of 5242 patients were included. Initial severity according to KDIGO creatinine definition was AKI stage 1 for 2458 patients (46.89%), AKI stage 2 for 1181 (22.53%) and AKI stage 3 for 1603 (30.58%). Crude 28-day ICU mortality according to AKI severity was 22.74% (n = 559), 27.69% (n = 327) and 26.26% (n = 421), respectively. Renal recovery was experienced by 3085 patients (58.85%), and its rate was significantly different between AKI severity stages (P < 0.01). Twenty-eight-day ICU mortality was independently lower in patients experiencing renal recovery [CSHR 0.54 (95% CI 0.46–0.63), P < 0.01]. Lastly, RRT requirement was strongly associated with persistent AKI whichever threshold was chosen between day 2 and 7 to delineate transient from persistent AKI.
Conclusions
Short-term renal recovery, according to several definitions, was independently associated with higher mortality and RRT requirement. Moreover, distinction between transient and persistent AKI is consequently a clinically relevant surrogate outcome variable for diagnostic testing in critically ill patients.
Similar content being viewed by others
Introduction
One out of two intensive care unit (ICU) patients will experience an acute kidney injury (AKI) during his ICU stay [1]. This complication is responsible for a high burden: drastic short- and long-term mortality increase [2, 3] and persistent renal dysfunction [4]. Classifications such as the risk, injury, failure, loss of kidney function, and end-stage renal disease (RIFLE) one [5], shortly followed by acute kidney injury network (AKIN) [6] and at last by kidney disease: improving global outcomes (KDIGO) were developed in order to allow a better description of AKI spectrum [7]. They provided a consensual definition for AKI diagnosis and staging and enabled comparability between studies. However, these classifications do not integrate AKI duration in their criteria. The ADQI proposed a classification according to timing of recovery, relying, however, on expert opinion and requiring validation [8].
Transient AKI was classically thought to be due to pre-renal azotemia, whereas persistent AKI was considered as a consequence of acute tubular necrosis (ATN) [9]. These last years, several studies have contributed to question this paradigm [10,11,12,13]. AKI duration appears rather to be linked to AKI severity than to distinct physiopathological mechanism [11]. In a previous study [14] with unselected ICU patients, persistent AKI was far more frequent than transient AKI, associated with more severe AKI and more likely to fulfill both serum creatinine and diuresis criteria. When AKI severity was introduced into the model, the association between AKI duration and patients’ outcome disappeared, leading to the hypothesis that transient and persistent AKI could share similar pathophysiological mechanisms. However, our results could have failed to demonstrate an association between AKI duration and outcome due to an insufficient statistical power. Additionally, in this previous study, time-dependent nature of renal recovery (i.e., dead patients will never recover from their AKI) was only partly taken into account. Thus, a new and larger study was performed in a prospective multicentric French ICU cohort. By using a cause-specific model, the aim was to take into account competitive risk arising from discharged alive patients and time-dependent nature of renal recovery.
The primary objective of this study was to assess prognostic impact of AKI duration on 28-day ICU mortality. Secondary objective was to assess relationship between renal recovery at specific time frames and need for renal replacement therapy.
Patients and methods
Study population
Patients of the OUTCOMEREA™ cohort were included in the study if they suffered from AKI at ICU admission during the period ranging from 1996 to 2015. OUTCOMEREA™ database has already been described in some details [15] (see Additional file 1: quality of the database). Briefly, patients over 16 years of age admitted to 23 French ICUs were included in this retrospective analysis of an observational prospective multicenter cohort. Patients’ demographic, clinical and biological data were collected at baseline and daily during their ICU stay. The database was approved by CCTIRS and CNIL (number 999262), respectively the French Advisory Committee for Data Processing in Health Research and the French Informatics and Liberty commission. The study was approved by the ethics committee of Clermont-Ferrand (number 5891), France, and was performed in accordance with the Declaration of Helsinki.
Exclusion criteria were: chronic kidney disease at ICU admission and absence of creatinine value recorded in the database on the first day of ICU stay. In case of readmission, only the first ICU stay was considered.
Definitions
AKI at ICU admission was defined according to KDIGO classification [7]. Since 6- and 12-h diureses were not available in the database, only the creatinine component of this classification was used. Similarly, initial AKI and changes in renal dysfunction severity were assessed according to KDIGO creatinine criteria.
Baseline creatinine value was estimated via inverse Modification of Diet in Renal Disease (MDRD) formula, considering normal baseline GFR (75 ml/min/1.73 m2) [7] for all included patients.
Renal recovery was considered as a decrease of at least one KDIGO class compared to the previous day. For sensitive analysis purpose, an alternative definition considering renal recovery as full recovery of AKI according to KDIGO criteria was used.
Patients requiring RRT were classified as AKI stage 3 and considered as being weaned from RRT only if not requiring RRT for at least 5 days.
Discharge alive was defined as survival at discharge from the ICU.
Initial severity was assessed according to Simplified Acute Physiology Score II (SAPS II) [16] and Sequential Organ Failure Assessment (SOFA) Score [17]. Septic shock was defined according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) [18].
Chronic kidney disease was defined according to usual definitions [19] and reported in the dataset by investigator using ICD-10 code (N18-N18, N18.0, N18.8, N18.9, I12.0, N11-N11, N11.0, N11.1, N11.8, N11.9, N03-NO3, N03.0, N03.1, N03.2, N03.3, N03.4, N03.5, N03.6, N03.7, N03.8, N03.9, N19, N08.3).
Statistical analysis
Quantitative variables are presented as median and interquartile range and compared between groups with the Wilcoxon test. Qualitative variables are presented as frequency and corresponding percentage and compared with the Chi-square test.
In our first model, we aimed to assess the impact of renal recovery on 28-day ICU mortality. In this situation, discharge alive was considered as a competing event for the outcome. Cause-specific models are survival models used in the presence of competing risk. They allow fitting separate Cox model for each endpoint. Hence, cause-specific hazard ratio (= CSHR) obtained for the two endpoints can be concurrently interpreted for each model [20]. Renal recovery status was introduced as a time-dependent variable [21]. Variables identified in the literature as potential confounding factors were introduced into the model for adjustment. Baseline variables were: shock and initial AKI severity class. Time-dependent variables were Sequential Organ Failure Assessment (SOFA) score components, except renal one, and nephrotoxic drug administration in the five previous days. Subgroup analyses were conducted in patients suffering from diabetes, hypertension or septic shock during the first 24 h.
A sensitivity analysis was conducted considering a threshold of 3 days to distinguish transient and persistent AKI: a transient AKI was a renal recovery occurring within the first 3 days; otherwise, it was a persistent AKI. Only patients still alive and in ICU for at least 72 h were kept in the analysis, since the transient or persistent nature of AKI could not be determined before. A Cox model was used to assess the impact of AKI duration on 28-day ICU mortality, with adjustment on the worst value of the confounding risk factors during the first 3 days of ICU stay for patients with persistent AKI and before renal recovery for patients with transient AKI.
Last, performances of various definitions of persistent AKI (defined as a lack of renal recovery between day 2 and day 7) in predicting need for renal replacement therapy during ICU stay were evaluated in patients staying at least 8 days in ICU.
A P value of 0.05 was retained for statistical significance.
All statistical analyses were conducted with SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Initial characteristics
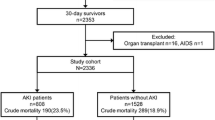
Of the 18,684 patients screened, 5242 patients were finally included in the study (Fig. 1).
The initial severity according to KDIGO definition was AKI stage 1 for 2458 patients (46.89%), AKI stage 2 for 1181 (22.53%) and AKI stage 3 for 1603 (30.58%) (Table 1).
Median age in the population was 70.2 years [58.0–78.9]. Diabetes was the most frequent comorbidity (20.95%). Population initial severity was 50 [37–67] assessed by SAPSII score and 5 [3,4,5,6,7,8,9,10] by SOFA score. The majority of the included patients were admitted due to a medical condition (77.15%). Shock and respiratory failure were the main organ dysfunctions at ICU admission (respectively, 40.98% and 22.57% of the included patients). Overall, 1355 patients (25.85%) had received nephrotoxic agent at ICU admission and aminoglycoside was the main nephrotoxic in this study (18.20%). A total of 330 (6.30%) patients received iodinated contrast agents.
Renal recovery impact on 28-day ICU mortality
Crude 28-day ICU mortality according to AKI severity was 22.74% (n = 559), 27.69% (n = 327) and 26.26% (n = 421) for patients with AKI stages 1, 2 and 3, respectively.
Rate of renal recovery was significantly different between AKI severity stages (65.62, 62.74 and 45.60% for AKI stages 1, 2 and 3, respectively; P < 0.01). AKI lasted longer in patients with AKI stage 3 (3 days [2,3,4,5,6] vs. 2 days [1,2,3,4] for stage 1 and 2). Among patients who experienced renal recovery, 431 (13.97%) died, versus 876 (40.61%) patients without recovery. Maximum AKI stage was stage 1 for 1829 patients (34.89%), stage 2 for 1155 (22.03%) and stage 3 for 2258 (43.08%) patients (P < 0.01). After adjustment for confounding factors, 28-day ICU mortality was independently lower in patients experiencing renal recovery [CSHR 0.54 (95% CI 0.46–0.63), P < 0.01; Table 2], whereas 28-day ICU discharge was significantly higher [CSHR 1.85 (95% CI 1.72–1.99), P < 0.01; Table 2].
These results were consistent among the different subgroups considered (Additional file 1: Table E1). In particular, among patients with septic shock, renal recovery occurrence was associated with a dramatically increase in discharge alive status (CSHR: 2.71 (95% CI 2.32–3.16), P < 0.01).
Sensitivity analysis
A sensitivity analysis was performed assessing influence of renal recovery when defined by full recovery of AKI. According to this definition, 2184 (41.66%) patients experienced renal recovery. Rate of renal recovery was significantly different across class of renal dysfunction severity [1432 (58.26%), 418 (35.39%) and 334 (20.84%), respectively, in patients with AKI stages 1, 2 and 3; P < 0.01)]. Using this definition, renal recovery remains independently associated with decreased 28-day ICU mortality [CSHR 0.55 (95% CI 0.47–0.66), P < 0.01; Additional file 1: Table E2].
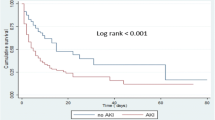
Last, in order to compare our results with previous studies in this field, influence of recovery within 72 h was assessed. After adjustment for confounding factors and AKI maximum severity in the first 3 days, transient AKI was independently associated with a decreased 28-day ICU mortality [HR 0.80 (95% CI 0.67–0.95), P = 0.01; Additional file 1: Table E3]. Corresponding survival curve is reported in Fig. 2.
AKI duration and prediction of RRT requirement
Rate of renal replacement therapy according to renal recovery at various time frames is reported in Fig. 3. Since day-2 threshold, persistent AKI appeared as a strong predictor of RRT requirement. Sensitivity decreased when choosing a higher threshold (from 93% at day 2 to 67% at day 7), whereas specificity increased (from 30% at day 2 to 83% at day 7).
Discussion
To the best of our knowledge, this is the first study based on a large multicentric ICU cohort assessing the prognostic impact of renal recovery when taking into account its time-dependent nature. Reversibility of AKI appeared as a strong predictor of enhanced survival in a time-dependent cause-specific model while considering discharge alive as a competing event. Secondly, an incremental AKI duration was associated with a poorer prognosis when persistent AKI was defined as a lack of renal recovery within 3 days, as compared to transient AKI. Lastly, RRT requirement was drastically increased in patients experiencing persistent AKI whichever threshold between 2 and 7 days was chosen to delineate transient and persistent AKI.
In our study, lack of renal recovery was associated with a significantly higher mortality and this result was confirmed in the different subgroups of patients at higher risk of kidney injury. Interestingly, this effect persisted even after adjustment on initial AKI severity. Up until now, studies based on recent AKI classifications and aiming to assess AKI prognostic impact on short- and long-term mortality have mainly focused on the effect of the maximum severity class reached [1, 22, 23] or AKI severity at ICU admission [3]. Whichever classification was considered, AKI occurrence was associated with a decreased survival. Of note, several studies found a similar risk for patients experiencing AKI-injury and AKI-failure class according to RIFLE classification [3, 24, 25], indicating that the maximum mortality risk was potentially reached as soon as a patient experiences AKI-injury class.
Several studies suggested that AKI duration and severity could be associated [11, 26]. One of the striking results of our previous study was that patients with persistent AKI, defined as an absence of renal recovery within 3 days, were more likely to meet both oliguria and serum creatinine elevation criteria for AKI and tended to experience more severe AKI than patients with transient AKI [14]. In a large cohort of 30,000 patients, short- and long-term outcomes appeared to be dramatically worse when a severity stage was reached by both criteria [27]. These findings were, however, unadjusted for time dependency of renal recovery [27].
Transient and persistent AKI were classically thought to be due to distinct physiopathological mechanisms, namely pre-renal azotemia and acute tubular necrosis [9]. This concept has been challenged these last years by experimental and clinical findings demonstrating the paucity of ATN features on renal biopsy [10] or its focal nature [12]. Urinary biomarker seemed also inefficient to predict an early renal recovery [13, 26, 28]. Hence, in accordance with these recent findings, transient and persistent AKI should rather be considered as a continuum of a same pathology with increasing severity [29].
Surprisingly, even though some data suggested that AKI duration could be a marker of severity, its impact, independent of those of AKI severity, and consequences of AKI reversible nature have poorly been studied in the literature. In a large cohort of 20,126 patients, Uchino et al. [30] showed an increasing mortality with AKI duration, this risk existing even for 1-day-lasting AKI. Similar results were found in postoperative contexts [31] and in ICU settings [26]. Interestingly, in a large multicentric cohort of diabetic patients who underwent non-cardiac surgery, in each strata of AKI duration, mortality was no longer influenced by AKIN severity classes [32]. These studies are yet insufficient to conclude due to consequent limitations concerning study population and limiting external generalizability of their conclusions (specific ICU patients’ subset [33, 34], use of monocentric cohorts [33, 35]). In a previous study [14], by including unselected critically ill patients from a multicentric cohort, we were able to demonstrate a lower hospital survival in the presence of persistent AKI, but this effect disappeared after adjustment on AKI severity. However, these results could have been influenced by a lack of statistical power and lead us to conduct another trial based on the high-quality multicentric cohort OUTCOMEREA™.
Statistical tools used in previous studies are also questionable. A consequent methodological limitation is linked to competing risk resulting from patients discharged alive from ICUs. In ICU settings, discharge alive is an informative censoring because censored patients are different from patients staying in ICU. It modifies the probability to observe the outcome, i.e., ICU death in the population staying in the unit. Standard survival methods in this case can no longer be used [36]. By using a cause-specific model, we were able to bypass this limitation and to estimate simultaneously a cause-specific hazard ratio for each outcome, ICU mortality and discharge alive. Another limitation arises from assumption in most studies that AKI reversibility was known since admission, even in largest trials [27], leading to a time-dependent bias [37]. In a study of Kellum et al. [38], patients without renal recovery had a decreased survival when compared to patients with partial or full recovery. In a second study, they identified several recovery patterns according to the delay before renal recovery and the occurrence of a relapse with or without a subsequent recovery, which were associated with different 1-year prognoses [39]. These findings were, however, probably influenced by the time dependency of renal recovery; patients dying before the occurrence of renal recovery will never experience this event. As a consequence, the absence of renal recovery can falsely be associated with mortality. Thus, in our study, this variable was introduced as time dependent. Another advantage to use cause-specific model is to adjust on confounding factors, i.e., patients’ severity represented by SOFA score component and nephrotoxic exposure, not only not only considering them at baseline but taking into account their evolution with respect to time sequence. Lastly, as in our previous work [14], persistent AKI was associated with a much higher rate of RRT requirement than patients experiencing a renal recovery. A threshold between day 2 and day 7 for defining AKI duration could thus be used as a surrogate marker for further need of RRT during ICU stay. The threshold should be chosen according to clinician preference, a greater sensitivity (day 2) or specificity (day 7).
Recently, studies have pointed out the importance of the renal recovery definition considered [40,41,42]. In a recent position paper, the ADQI group recommends defining recovery as full renal recovery, early recovery being defined by recovery within 48 h and acute kidney disease by a failure to recover within 7 days [8]. Although our findings confirm full recovery to be associated with outcome, they also demonstrate that incomplete recovery, as defined by decrease of at least one KDIGO class, is also associated with improved outcome. This finding may help to refine definition proposed by ADQI group and is a plea to further research to validate this definition. But more importantly, this finding is in keeping with known delay between improved glomerular filtration rate and serum creatinine decrease [43] and suggests that reduction of at least one KDIGO severity class may be a clinically relevant objective.
Several limitations in our study should be acknowledged. First of all, baseline creatinine was not available, so we had to estimate this value thanks to MDRD equation. Even if this method is suggested in KDIGO [7], it can lead to an excess in AKI diagnosis and reduce renal recovery probability [44]. Secondly, AKI staging was only based on creatinine criteria because hourly diuresis was not available in our database. As previously explained, reaching an AKI stage with both criteria could be associated with more severe AKI [14, 27] and it could have been interesting to include this data into the model. Due to muscle loss during ICU stay, renal recovery could have been over diagnosed. Only the first AKI episode was taken into account, and no conclusion could be inferred concerning the influence of a further relapse. The endpoint for analysis was limited at day 28. Hence, at this time, 92% of the tested patients either died or were discharged precluding further follow-up. Whether definition of renal recovery may influence longer-term outcome is unanswered by this study and may deserve to be further studied. Lastly, although multicentric, our population was mainly admitted for medical condition, limiting potentially the extension of these conclusions to surgical patients.
Conclusions
This study, taking time dependency of renal recovery into account confirms the prognostic impact of early renal recovery and the clinical relevancy of recovery definition based on timing. Distinction between transient AKI/rapid reversal and persistent AKI appears to be clinically relevant as surrogate outcome variable for diagnostic testing in critically ill. Our results suggest also that partial recovery, rather than full renal recovery, may be also a clinically relevant signal which may deserve further research in this field.
References
Hoste EAJ, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–23.
Gammelager H, Christiansen CF, Johansen MB, Tønnesen E, Jespersen B, Sørensen HT. Three-year risk of cardiovascular disease among intensive care patients with acute kidney injury: a population-based cohort study. Crit Care. 2014;18:492.
Gammelager H, Christiansen CF, Johansen MB, Tønnesen E, Jespersen B, Sørensen HT. One-year mortality among Danish intensive care patients with acute kidney injury: a cohort study. Crit Care. 2012;16:R124.
Rimes-Stigare C, Frumento P, Bottai M, Mårtensson J, Martling C-R, Walther SM, et al. Evolution of chronic renal impairment and long-term mortality after de novo acute kidney injury in the critically ill; a Swedish multi-centre cohort study. Crit Care. 2015;19:221.
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31.
Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17:204.
Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241–57.
Parikh CR, Coca SG. Acute kidney injury: defining prerenal azotemia in clinical practice and research. Nat Rev Nephrol. 2010;6:641–2.
Langenberg C, Gobe G, Hood S, May CN, Bellomo R. Renal histopathology during experimental septic acute kidney injury and recovery. Crit Care Med. 2014;42:e58–67.
Schneider AG, Bellomo R. Urinalysis and pre-renal acute kidney injury: time to move on. Crit Care. 2013;17:141.
Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187:509–17.
Darmon M, Vincent F, Dellamonica J, Schortgen F, Gonzalez F, Das V, et al. Diagnostic performance of fractional excretion of urea in the evaluation of critically ill patients with acute kidney injury: a multicenter cohort study. Crit Care. 2011;15:R178.
Perinel S, Vincent F, Lautrette A, Dellamonica J, Mariat C, Zeni F, et al. Transient and persistent acute kidney injury and the risk of hospital mortality in critically ill patients: results of a multicenter cohort study. Crit Care Med. 2015;43:e269–75.
Clec’h C, Alberti C, Vincent F, Garrouste-Orgeas M, de Lassence A, Toledano D, et al. Tracheostomy does not improve the outcome of patients requiring prolonged mechanical ventilation: a propensity analysis. Crit Care Med. 2007;35:132–8.
Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO. Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2012;2013(3):1–150.
Tai BC, Machin D, White I, Gebski V, EOI (The European Osteosarcoma Intergroup). Competing risks analysis of patients with osteosarcoma: a comparison of four different approaches. Stat Med. 2001;20:661–84.
Wolkewitz M, Vonberg RP, Grundmann H, Beyersmann J, Gastmeier P, Bärwolff S, et al. Risk factors for the development of nosocomial pneumonia and mortality on intensive care units: application of competing risks models. Crit Care. 2008;12:R44.
Nisula S, Kaukonen K-M, Vaara ST, Korhonen A-M, Poukkanen M, Karlsson S, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420–8.
Fuchs L, Lee J, Novack V, Baumfeld Y, Scott D, Celi L, et al. Severity of acute kidney injury and 2-year outcomes in critically ill patients. Chest. 2013;144:866–75.
Clec’h C, Gonzalez F, Lautrette A, Nguile-Makao M, Garrouste-Orgeas M, Jamali S, et al. Multiple-center evaluation of mortality associated with acute kidney injury in critically ill patients: a competing risks analysis. Crit Care. 2011;15:R128.
Ostermann M, Chang RWS. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35:1837–43 (quiz 1852).
Nejat M, Pickering JW, Devarajan P, Bonventre JV, Edelstein CL, Walker RJ, et al. Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int. 2012;81:1254–62.
Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015;26:2231–8.
Pons B, Lautrette A, Oziel J, Dellamonica J, Vermesch R, Ezingeard E, et al. Diagnostic accuracy of early urinary index changes in differentiating transient from persistent acute kidney injury in critically ill patients: multicenter cohort study. Crit Care. 2013;17:R56.
Bellomo R, Bagshaw S, Langenberg C, Ronco C. Pre-renal azotemia: A flawed paradigm in critically ill septic patients? Contrib Nephrol. 2007;156:1–9.
Uchino S, Bellomo R, Bagshaw SM, Goldsmith D. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transpl. 2010;25:1833–9.
Brown JR, Kramer RS, Coca SG, Parikh CR. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg. 2010;90:1142–8.
Coca SG, King JT, Rosenthal RA, Perkal MF, Parikh CR. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int. 2010;78:926–33.
Wu H-C, Wang W-J, Chen Y-W, Chen H-H. The association between the duration of postoperative acute kidney injury and in-hospital mortality in critically ill patients after non-cardiac surgery: an observational cohort study. Ren Fail. 2015;37:985–93.
Sood MM, Shafer LA, Ho J, Reslerova M, Martinka G, Keenan S, et al. Early reversible acute kidney injury is associated with improved survival in septic shock. J Crit Care. 2014;29:711–7.
Han SS, Kim S, Ahn SY, Lee J, Kim DK, Chin HJ, et al. Duration of acute kidney injury and mortality in critically ill patients: a retrospective observational study. BMC Nephrol. 2013;14:133.
Resche-Rigon M, Azoulay E, Chevret S. Evaluating mortality in intensive care units: contribution of competing risks analyses. Crit Care. 2006;10:R5.
van Walraven C, Davis D, Forster AJ, Wells GA. Time-dependent bias was common in survival analyses published in leading clinical journals. J Clin Epidemiol. 2004;57:672–82.
Kellum JA, Chawla LS, Keener C, Singbartl K, Palevsky PM, Pike FL, et al. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care Med. 2016;193:281–7.
Kellum JA, Sileanu FE, Bihorac A, Hoste EAJ, Chawla LS. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017;195:784–91.
Schortgen F. Defining renal recovery: pitfalls to be avoided. Intensive Care Med. 2015;41:1993–5.
Schetz M, Gunst J, De Vlieger G, Van den Berghe G. Recovery from AKI in the critically ill: potential confounders in the evaluation. Intensive Care Med. 2015;41:1648–57.
Kellum JA. How can we define recovery after acute kidney injury? Considerations from epidemiology and clinical trial design. Nephron Clin Pract. 2014;127:81–8.
Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20:672–9.
Siew ED, Matheny ME. Choice of reference serum creatinine in defining acute kidney injury. Nephron. 2015;131:107–12.
Authors’ contributions
AST participated in acquisition of data, data analysis, interpretation of data, drafting of the manuscript and critical revision of the manuscript. SPR participated in acquisition of data, interpretation of data, drafting of the manuscript and critical revision of the manuscript. BS participated in study design, interpretation of data and critical revision of the manuscript. SB participated in acquisition of data, interpretation of data, drafting of the manuscript and critical revision of the manuscript. LZ participated in acquisition of data, interpretation of data, drafting of the manuscript and critical revision of the manuscript. LB participated in acquisition of data, interpretation of data, drafting of the manuscript and critical revision of the manuscript. CC participated in acquisition of data, interpretation of data, drafting of the manuscript and critical revision of the manuscript. MGO participated in acquisition of data, interpretation of data, drafting of the manuscript and critical revision of the manuscript. GL participated in acquisition of data, interpretation of data, drafting of the manuscript and critical revision of the manuscript. CS participated in acquisition of data, interpretation of data, drafting of the manuscript and critical revision of the manuscript. FGE participated in acquisition of data, interpretation of data, drafting of the manuscript and critical revision of the manuscript. CA participated in acquisition of data, interpretation of data, drafting of the manuscript and critical revision of the manuscript. ASD participated in acquisition of data, interpretation of data, drafting of the manuscript and critical revision of the manuscript. PZ participated in acquisition of data, interpretation of data, drafting of the manuscript and critical revision of the manuscript. LA participated in acquisition of data, interpretation of data, drafting of the manuscript and critical revision of the manuscript. SJ participated in acquisition of data, interpretation of data, drafting of the manuscript and critical revision of the manuscript. DGT participated in statistical analysis, interpretation of data, drafting of the manuscript and critical revision of the manuscript. GM participated in statistical analysis, interpretation of data, drafting of the manuscript and critical revision of the manuscript. JFT participated in study design and coordination, statistical analysis, interpretation of data, drafting of the manuscript and critical revision of the manuscript. MD conceived the study, participated in study design and coordination, acquisition of data, interpretation of data, drafting of the manuscript and critical revision of the manuscript. MD had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. MD affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as registered have been explained. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Members of the OUTCOMEREA study group
Scientific Committee: Jean-François Timsit (Medical and Infectious Diseases ICU, Bichat-Claude Bernard Hospital, Paris, France; UMR 1137 Inserm–Paris Diderot university IAME, F75018, Paris); Elie Azoulay (Medical ICU, Saint Louis Hospital, Paris, France); Maïté Garrouste-Orgeas (ICU, Saint-Joseph Hospital, Paris, France); Jean-Ralph Zahar (Infection Control Unit, Angers Hospital, Angers, France); Christophe Adrie (ICU, Delafontaine Hospital, Saint Denis, and Physiology, Cochin Hospital, Paris, France); Michael Darmon (Medical ICU, Saint Etienne University Hospital, St Etienne, France); and Christophe Clec’h (ICU, Avicenne Hospital, Bobigny, and UMR 1137 Inserm–Paris Diderot university IAME, F75018, Paris, France). Biostatistical and information system expertise: Jean-Francois Timsit (Medical and Infectious Diseases ICU, Bichat-Claude Bernard Hospital, Paris, France; UMR 1137 Inserm–Paris Diderot university IAME, F75018, Paris); Corinne Alberti (Medical Computer Sciences and Biostatistics Department, Robert Debré Hospital, Paris, France); Adrien Français (Integrated Research Center U823, Grenoble, France); Aurélien Vesin (OUTCOMEREA organization and Integrated Research Center U823, Grenoble, France); Stephane Ruckly (OUTCOMEREA organization and Inserm UMR 1137 IAME, F75018, Paris); Sébastien Bailly (Grenoble university hospital Inserm UMR 1137 IAME, F75018, Paris) and Christophe Clec’h (ICU, Avicenne Hospital, Bobigny, and Inserm UMR 1137 IAME, F75018, Paris, France); Frederik Lecorre (Supelec, France); Didier Nakache (Conservatoire National des Arts et Métiers, Paris, France); and Aurélien Vannieuwenhuyze (Tourcoing, France). Investigators of the OUTCOMEREA database: Christophe Adrie (ICU, Delafontaine Hospital, Saint Denis, and Physiology, Cochin Hospital, Paris, France); Bernard Allaouchiche (ICU, Pierre benite Hospital, Lyon, France); Laurent Argaud (Medical ICU, Hospices Civils de Lyon, Lyon, France); Claire Ara-Somohano (Medical ICU, University Hospital, Grenoble, France); Elie Azoulay (Medical ICU, Saint Louis Hospital, Paris, France); Francois Barbier (medical-surgical ICU, Orleans, France), Jean-Pierre Bedos (ICU, Versailles Hospital, Versailles, France); Julien Bohé (ICU, Hôpital Pierre Benite, Lyon France), Lila Bouadma (ICU, Bichat Hospital, Paris, France); Christine Cheval (ICU, Hyeres Hospital, Hyeres, France); Christophe Clec’h (ICU, Avicenne Hospital, Bobigny, France); Michael Darmon (ICU, Saint Etienne Hospital, Saint Etienne, France); Anne-Sylvie Dumenil (Antoine Béclère Hospital, Clamart, France); Claire Dupuis (Bichat hospital and UMR 1137 Inserm–Paris Diderot university IAME, F75018, Paris, France), Marc Gainier hôpital la Timone, Marseille, France), Akim Haouache (Surgical ICU, H Mondor Hospital, Creteil, France); Samir Jamali (ICU, Dourdan, Dourdan Hospital, Dourdan, France); Hatem Khallel (ICU, Cayenne General Hospital, Cayenne, France); Alexandre Lautrette (ICU, G Montpied Hospital, Clermont-Ferrand, France); Guillaume Marcotte (Surgical ICU, Hospices Civils de Lyon, Lyon, France); Eric Le Miere (ICU, Louis Mourier Hospital, Colombes, France); Maxime Lugosi (Medical ICU, University Hospital Grenoble, Grenoble, France); Bruno Mourvillier (ICU, Bichat Hospital, Paris, France); Benoît Misset (ICU, Saint-Joseph Hospital, Paris, France); Delphine Moreau (ICU, Saint Louis Hospital, Paris, France); Bruno Mourvillier (ICU, Bichat Hospital, Paris, France); Laurent Papazian (Hopital Nord, Marseille, France), Benjamin Planquette (pulmonology ICU, George Pompidou Hospital, Versailles, France); Bertrand Souweine (ICU, G Montpied Hospital, Clermont-Ferrand, France); Carole Schwebel (ICU, A Michallon Hospital, Grenoble, France); Gilles Troché (ICU, Antoine Béclère Hospital, Clamart, France); Marie Thuong (ICU, Delafontaine Hospital, Saint Denis, France); Guillaume Thierry (ICU, Saint Louis Hospital, Paris, France); Dany Toledano (ICU, Gonesse Hospital, Gonesse, France); and Eric Vantalon (SICU, Saint-Joseph Hospital, Paris, France). Study monitors: Julien Fournier, Caroline Tournegros, Stéphanie Bagur, Mireille Adda, Vanessa Vindrieux, Loic Ferrand, Nadira Kaddour, Boris Berthe, Samir Bekkhouche, Kaouttar Mellouk, Sylvie Conrozier, Igor Theodose, Veronique Deiler, and Sophie Letrou.
Availability of data and materials
The data that support the findings of this study are available from OUTCOMEREA study group, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The database was approved by CCTIRS and CNIL (number 999262), respectively the French Advisory Committee for Data Processing in Health Research and the French Informatics and Liberty commission. The study was approved by the ethics committee of Clermont-Ferrand (number 5891), France, and was performed in accordance with the Declaration of Helsinki.
Funding
The study was entirely funded by the OUTCOMEREA™ research network. AST received an educational grant from the French Kidney Foundation under the aegis of the French Medical Research Foundation, code DEA2014FDR/FRM04_FdR-SdN-SFD_FRM_TRUCHE.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1.
Quality of the dataset. Table S1: Discharge alive and 28-day mortality cause-specific model according to renal recovery defined as a decrease of at least one KDIGO class compared to the previous day. Table S2: Discharge alive and 28-day mortality cause specific model according to renal recovery defined as full recovery of AKI Table S3: Cox model of 28 day-mortality according to transient AKI defined as renal recovery occurring within the first 3 days as compared to persistent AKI.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Truche, A.S., Ragey, S.P., Souweine, B. et al. ICU survival and need of renal replacement therapy with respect to AKI duration in critically ill patients. Ann. Intensive Care 8, 127 (2018). https://doi.org/10.1186/s13613-018-0467-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-018-0467-6