Abstract
Background
Passive immunization against RSV (Respiratory Syncytial Virus) is given in most western countries (including Israel) to infants of high risk groups such as premature babies, and infants with Congenital Heart Disease or Congenital Lung Disease. However, immunoprophylaxis costs are extremely high ($2800–$4200 per infant). Using cost-utility analysis criteria, we evaluate whether it is justified to expand, continue or restrict nationwide immunoprophylaxis using palivizumab of high risk infants against RSV.
Methods
Epidemiological, demographic, health service utilisation and economic data were integrated from primary (National Hospitalization Data, etc.) and secondary data sources (ie: from published articles) into a spread-sheet to calculate the cost per averted disability-adjusted life year (DALY) of vaccinating various infant risk groups. Costs of intervention included antibody plus administration costs. Treatment savings and DALYs averted were estimated from applying vaccine efficacy data to relative risks of being hospitalised and treated for RSV, including possible long-term sequelae like asthma and wheezing.
Results
For all the groups RSV immunoprophylaxis is clearly not cost effective as its cost per averted DALY exceeds the $105,986 guideline representing thrice the per capita Gross Domestic Product. Vaccine price would have to fall by 48.1% in order to justify vaccinating Congenital Heart Disease or Congenital Lung Disease risk groups respectively on pure cost-effectiveness grounds. For premature babies of < 29 weeks, 29–32 and 33–36 weeks gestation, decreases of 36.8%, 54.5% and 83.3% respectively in vaccine price are required.
Conclusions
Based solely on cost-utility analysis, at current price levels it is difficult to justify the current indications for passive vaccination with Palivizumab against RSV. However, if the manufacturers would reduce the price by 54.5% then it would be cost-effective to vaccinate the Congenital Heart Disease or Congenital Lung Disease risk groups as well as premature babies born before the 33rd week of gestation.
Similar content being viewed by others
Key points
- Question:
-
Should we continue using Palivizumab immunoprophylaxis for at-risk infants against Respiratory Syncytial Virus?
- Findings:
-
A cost-utility analysis which modelled the costs, resultant treatment savings and improvements in quality of life as a result of decreased morbidity from passive immunization, found for all risk-groups that RSV immunoprophylaxis is clearly not cost effective, unless vaccine prices fall considerably.
- Meaning:
-
Based solely on cost-utility analysis, at current price levels, it is difficult to justify the current indications for immunoprophylaxis against RSV.
Background
In infants and young children the most common cause of severe lower respiratory tract disease is Respiratory syncytial virus (RSV). Most new-borns are infected before they are one year old, and virtually everyone gets an RSV infection by the age of two [1].
In Israel (Population 8.75 million [2]), RSV accounts for thousands of hospitalization days annually in children under two years old. The almost solitary identified chronic sequelae are possibly wheezing and asthma.
Since the disease course in high risk children is much more severe, and since no active vaccine is available, passive immunization with five sequential monthly injections of anti-RSV monoclonal antibodies (Palivizumab) is given during the RSV season (November – March). This schedule has proven to decrease hospitalization in high risk groups [3].
An RSV passive immunoprophylaxis course (costing around $6,300) is over a hundred times more expensive than courses of prophylaxis in the form of vaccinations against other infectious diseases such as measles, mumps, rubella, polio, diphtheria, pertussis, hepatitis or Haemophilus influenzae type B.
In 2001, despite its extremely high costs, passive vaccination using RSV was introduced in the high risk group of extreme premature babies (<30 Gestational Age in Weeks [GAW]), without any prior evaluation based on cost-effectiveness analysis. During the following years the indications for the passive vaccination were steadily expanded to include older premature babies. Currently, immunoprophylaxis is provided to infants with <35 GAW, as well as to infants at high risk such as those with CHD (Congenital Heart Disease) or CLD (Congenital Lung Disease), again with no underpinning cost-effectiveness analysis.
However, recently the American Academy of Pediatrics (AAP) narrowed the indication to those born with <29 GAW [3]. In response, the Israeli Association of Pediatrics decided to examine the application of restricting the guidelines for RSV immunoprophylaxis in Israel. An important component of this decision, although not the sole one, is a cost utility analysis.
Therefore we carried out a cost utility analysis of passive immunization with palmivizumab against RSV to see if the DALY (Disability Adjusted life Year) gains justify the high RSV immunoprophylactic costs in various at-risk groups.
Methods
Cost-utility analysis: Basic model
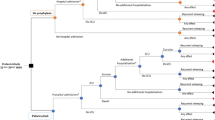
A Microsoft Excel spread-sheet model was constructed, incorporating vaccine efficacy, epidemiological, health service utilization, demographic and economic data (listed with sources in Table 4 in Appendix 1). The effect of vaccinations against RSV was modelled on incidence, chronic sequelae and mortality over a 100 year time horizon as is standard practice in order to capture the full effects of the intervention. The cost utility ratio calculated the net costs per averted Disability Adjusted Life Year (DALY) added as a result of passive immunization against RSV by means of palivizumab, using the formula:
Costs are viewed from a societal perspective at mid-2015 price levels at the average annual exchange rate of 3.89 shekels to the US dollar [4]. Besides direct health service costs, we also included from a social perspective, costs due to work absences and transport costs to receive treatment. All future costs and DALYs were discounted at an annual rate of 3% as is the standard practice in Israel. While DALYs averted from reduced caregiver burden were available, data on out-of-pocket expenses was however not available.
The cost-utility ratios of immunoprophylaxis for the following various risk groups was calculated:-
-
a)
Congenital Heart Disease (CHD)
-
b)
Congenital Lung Disease (CLD)
-
c)
Prematures under 29 weeks gestation
-
d)
Prematures 29–32 weeks gestation
-
e)
Prematures 33–36 weeks gestation
-
f)
Not a member of any of the above risk groups
Evidence from studies relating to Bronchopulmonary Dysplasia (BPD) were included under the category of Congenital Lung Disease (CLD).
Decision rules
Taking into account the resources available in Israel, an intervention was defined as being very cost-effective and cost-effective if the cost per averted DALY is less than the per capita GDP (gross Domestic product) of $35,329 in 2015 [2, 4] or between 1 and 3 times the per capita GDP ($35,329 – $105,987) respectively. If the cost per averted DALY is more than three times the GDP per capita ($105,987) then the intervention was regarded as not being cost-effective [5].
Immunoprophylaxis
We assumed a five dose passive immunoprophylaxis schedule, using palivizumab, in which there would be a take up of 4.90 (for CLD) and 4.93 shots (for others), which was achieved in the clinical trials of palivizimab and hence influenced the overall effectiveness of the immunoprophylaxis schedule. Data from the “IMPACT” study [6] led us to assume there were no significant adverse palvizimab related events apart from minor effects. Immunoprophylaxis wastage was assumed to fall from 5.8% levels in 2008 to around 3.3% based on the implementation of improved delivery systems [7].
Intervention costs
We used the current vaccine price, of $520 and $957 for 50 mg and 100 mg vials respectively, as a baseline price (excluding Value Added Tax as this is just a transfer payment). The unit immunoprophylaxis costs were applied to the average age-specific weights of the immunized children. Since at each point of the immunoprophylaxis schedule the infant received no other concurrent vaccinations, we included costs arising from transport and work losses. Provision was also made for treatment costs, transport costs and work losses arising from the visits to health service providers for minor side effects from the palivizimab passive immunization. Also included were the costs (and DALY losses) of long-term chronic sequelae from RSV from increased incidence of asthma and a more controversial possible increased incidence of wheezing.
Immunoprophylactic efficacy and its impact on hospitalizations and mortality from RSV by the risk groups were obtained by combining data from the literature (Table 4 in Appendix 1). Interpolations and extrapolations were extensively used due to the lack of homogeneity in reporting results by age and gestational age groups. Hospitalization rates and data on lengths of stay on account of RSV in Israel were based on data from the Ministry of Health’s National Hospitalization data base while mortality data was based on the National Deaths Registry (Personal Communication Ziona Haklaii and Nehama Goldberger).
Besides confirmed cases (of pneumonia and bronchiolitis) caused by RSV, we estimated that 13.2% [8,9,10] of hospitalizations recorded with an Otitis Media diagnosis were caused by RSV, and similarly that RSV was responsible for 40% of cases [11] of acute bronchitis (AB) recorded as being of unknown origin.
Treatment costs
Acute care costs were calculated by multiplying the expected number of hospitalization days or visits by the unit costs of the respective ambulatory (ie: family practitioner and out-patient visits), emergency room and hospital services that were used.
Costs of sequelae (wheezing, asthma) were taken from the literature [12, 13] and adjusted to Israeli price levels, with 80% of costs (mainly labor costs) converted using purchasing power parity rates and the remaining 20% on exchange rates.
Disability weights
Disability Weights (DW) associated with the pre-hospital, post-hospital and chronic phases (up to half a year) were obtained from the literature for both the patient [14]and the caregiver [14]. Additional DW for chronic sequelae after the chronic phase were based on five episodes a year of severe wheezing [13]and a similar number of annual asthma attacks [12]. All these DW were adjusted by the age specific DW of a healthy person.
Averted DALY losses
Morbidity losses (with and without the intervention) were calculated from the product of changes in incidence (derived from the RR of the prophylaxis), the specific DW and the duration of the disability. Mortality losses were calculated by multiplying mortality rates (with and without the intervention – derived from the RR of the prophylaxis) by gender-specific the HALE (Healthy adjusted Life Expectancy) of the deceased.
Total DALY losses averted were based on the sum of the morbidity and mortality DALY losses, as a result of the passive vaccination lowering the incidence of RSV and Chronic sequelae. DALY losses resulting from caregiver burden were also included [14].
Sensitivity analyses
One way sensitivity analyses were carried out by:- varying the number of hospitalizations attributable to RSV between 2,700-3,200 :- by excluding effects of long-term asthma, :- by varying the % of cases of otitis media and of Acute Bronchitis of unknown origin attributable to RSV: and finally by varying the values of the major input cost driver of immunoprophylaxis costs.
Results
Because of the low prevalence of CHD and CLD in 2015 (0.16% of all births or 267 infants), passive immunization costs for these two risk groups would only total $1.67 million (Table 1). The costs of immunoprophylaxis of premature babies or children not at risk are considerably higher, being $83 and $1,037 million respectively. However, decisions should obviously not be made on the basis of cost alone and this justifies our cost-utility analysis that combines economic with epidemiologic data.
In our baseline situation, RSV caused approximately 2,945 hospitalizations each year in under two years of age babies. For all the risk groups and hospitalization ranges, even when the long-term effects of Asthma are included, passive immunization against RSV is clearly not cost effective as its cost per DALY is well in excess of the $105,986 guideline (Table 2). Of particular interest is the 29-32 GAW infants as the AAP does not recommend providing immunoprophylaxis to this age group. For this group the cost per DALY ratio is around ten times the GNP per capita level in Israel, meaning that giving pulmizamub to this group is clearly not justified on grounds of cost effectiveness, all the more so on those with 33-36 GAW (Table 2).
RSV incidence would have to increase by between 56%-424% (depending on the at-risk group), to between 4,581-15,457 annual attributable hospitalizations in order that immunoprophylaxis would become cost- effective to specific at-risk groups (Table 2). Even if three-quarters of all the otitis media and unknown AB hospitalizations were attributable to RSV (instead of the estimated 13.2% and 40% respectively), this would only amount to 4,452 hospitalizations annually that could be attributable to RSV.
In the baseline situation, there would have to be a decrease in vaccine price of around 48% in order to justify passively immunizing CHD and CLD risk groups on pure cost-effectiveness grounds (Table 3). For premature babies of <29 weeks, 29-32 and 33-36 weeks gestation, decreases of 36.8%, 54.5% and 83.3% respectively in vaccine price would be required. Omission of long-term asthma effects, results in even higher cost per DALY ratios (Table 3) and even lower vaccine prices required to attain cost-effectiveness.
Discussion
For all the groups and hospitalization ranges, passive immunoprophylaxis against RSV is clearly not cost effective. Based only on cost-effectiveness criteria, the current immunoprophylactic RSV policy should be stopped or modified and resources may be more efficaciously devoted to elsewhere in the health system.
However, due to their potentially harsh individual morbidity profiles, small numbers and hence far smaller budget impact, consideration could be given to continuing the passive immunization of infants belonging to the CHD and CLD risk groups, even at the current vaccine price levels.
If pressure could be asserted on the manufacturers to reduce the vaccine price by around 48.1% then it would be cost-effective to provide palvizimab only to the CHD and CLD risk groups. If the palvizimab price were to be reduced by 54.5% then it would also be cost-effective to provide passive immunization to the larger numbers of premature infants, born before the 33rd week.Our study’s finding that passive immunoprophylaxis of RSV is not cost-effective affirms the findings of numerous other studies in infant risk-groups [15,16,17,18,19,20,21] (Appendix 2). On the other hand, there are also many studies which reported that immunoprophylaxis was cost-effective [15, 19,20,21,22,23,24,25,26,27,28,29,30,31,32] (Appendix 2) or even cost-saving in some risk groups [19, 21, 33,34,35,36,37] (Appendix 2). Many studies [16, 17, 38,39,40,41,42,43,44,45,46,47,48,49] reported that Palivizumab infant immunoprophylactic costs exceeded the resultant savings in hospitalization costs (Appendix 2).
So is immunoprophylaxis cost-effective or not cost-effective (as our study shows)? Comparisons with studies in other countries have to be made with caution not only on account of differences in intervention costs, incidence rates, treatment modalities and costs, but also due to differing model specifications [50] and especially the funding source. Several studies tend towards showing lower net costs [51], especially those incorporating indirect costs due to valuing premature mortality by discounting future years productivity losses [15, 22,23,24,25,26,27,28] instead of using the method of friction costing [52] (which would be minimal in the event of infant or child deaths).
Our study was based on the acceptable practice of valuing premature morbidity using friction costing which take into account only the premature burial costs and marginal costs of possibly training a person to fill the job vacancy caused by the deceased person. The loss of the deceased person is captured mainly in terms of loss of disability adjusted life years as the monetary loss to society is minimal. We conclude that the major explanation of the existence of two large contradictory reports relating to the potential cost-effectiveness of immunoprophylaxis against RSV, is that the research results are dependent on the nature of the different funding sources.
An extensive Health Technology Assessment [53], which like this paper integrated data from many studies, concluded that prophylaxis with Palivizumab does not justify its cost. Nevertheless the study defined a few cost-effective groups (based on a threshold of 30,000 sterling, about 1.3 times the GDP per capita) such as in children under 6 weeks old at the start of the RSV season who had at least two risk factors and a < 25 GAW, or children with CHD or CLD under 6 weeks old and with < 25 GAW or < 29 GAW respectively.
The estimates in our study were fortunate to be based on quality of life estimates not only of the infant but also of the caregiver, a luxury not always enjoyed in most published cost utility analyses, outside the realm of dementia.
Costs per DALY could be considered to be overestimated since it could be possible to still further reduce vaccine wastage to around 1.5%, where large volumes are used [7]. On the other hand the cost utility ratio could be underestimated because we excluded the (negligible) room overheads for vaccination and publicity outreach costs.
In 2014, the risk groups of infants that received RSV immunoprophylaxis in Israel were expanded to include infants born prematurely between 33-34 weeks. As demonstrated in our study this decision (like the initial decision in 2001 to supply RSV vaccinations) was not based on any cost utility or cost effectiveness analyses.
A critical question is whether the introduction or expansion of medical technologies should be based only, mainly or partly on cost utility criteria. Pure cost utility based on comprehensive meta-analyses of available economic, medical and epidemiological information may dilute unwanted effects such as political pressure and lobbing by industry, by providing the decision makers with a clear “standard” for their decision.
On the other hand, there could be several reasons for the avoidance of using the gold-standard metric of cost-utility analysis such as the case of very rare diseases where the medical costs do not have significant economic impacts or societal consensus of providing priorities for specific groups such as neonates or pregnant women. However, even in these cases, cost utility analyses may provide alternatives for investments in these specific populations to get the best yield in terms of saving lives and reducing morbidities.
It is surely in the pharmaceutical industry's interest (and in the interest of free competition) that interventions should be objectively compared using cost-utility analysis (as per the National Institute for Clinical Excellence in the United Kingdom). The ministry could also use the results of cost-utility analyses to sometimes request decreases in unit costs so as to turn an intervention that is not cost-effective into one that is cost-effective or very cost-effective, as was achieved by the NHS regarding the recent meningococcal B vaccination in the United Kingdom [54].
We hope that this cost-utility analysis will provide the decision makers with a powerful and transparent tool to aid in logical decision for determining the extent of implementing technologies such as RSV prophylaxis. Only time will tell whether or not the results of our RSV analysis will modify the policy for the provision of immunoprophylaxis against RSV or another alternatives will be agreed on for improving the health of premature infants.
Conclusions
Based on cost-utility analysis, at current price levels it is difficult to justify the current immunoprophylaxis program against RSV in Israel. However, if the manufacturers would reduce the price of the passive vaccine by 55.4% then it would be cost—effective to vaccinate the CHD and CLD risk groups as well as premature babies born before the 33rd week.
Abbreviations
- AAP:
-
American Academy of Pediatrics
- AB:
-
Acute Bronchitis
- CHD:
-
Congenital Heart Disease
- CLD:
-
Congenital Lung Disease
- DALY:
-
Disability-adjusted life year(s)
- DW:
-
Disability Weights
- GAW:
-
Gestational Age in Weeks
- GDP:
-
Gross Domestic Product
- HALE:
-
Healthy Adjusted Life expectancy
- NHS:
-
National Health Service (UK)
- RSV:
-
Respiratory Syncytial virus
- USD:
-
United States Dollars
References
Centers for Disease Control and Prevention. CDC Data and Statistics: Respiratory Synocitial Virus (RSV). http://www.cdc.gov/rsv/research/us-surveillance.html. Accessed 23 Sept 2018.
Central Bureau of Statistics. Monthly bulletin of statistics - July 2016, Jerusalem 2016. http://www.cbs.gov.il/webpub/pub/text_page_eng.html?publ=93. Accessed 23 Sept 2018.
American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(2):415–20 Erratum in: Pediatrics. 2014; 134(6):1221.
Central Bureau of Statistics. Statistical Abstract of Israel 2015 no 66. Jerusalem 2015. http://www.cbs.gov.il/reader/shnaton/shnatone_new.htm?CYear=2015&Vol=66&CSubject=30. Accessed 23 Sept 2018.
WHO Commission on MacroEconomics and Health. Macroeconomics and health: investing in health for economic development. Report of the Commission on Macroeconomics and Health. Geneva: World Health Organization; 2001.
The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(3pt1):531–7.
Arad L, Kron O, Goldman D. Management of Vaccinations against RSV. Presented to the 11th Annual Health Policy Conference, National Centre of Health Service Policy Research, May 13th 2015. Dan Panorama, Tel Aviv. Book of Abstracts Page 55 (in Hebrew). http://www.israelhpr.org.il/e/101/. Accessed 23 Sept 2018.
Arola M, Ruuskanen O, Ziegler T, et al. Clinical role of respiratory virus infection in acute otitis media. Pediatrics. 1990;86(6):848–55.
Gomaa MA, Galal O, Mahmoud MS. Risk of acute otitis media in relation to acute bronchiolitis in children. Int J Ped Otrorhinolaryngology. 2012;76(1):49–51.
Heikkinen T, Thint M, Chonmaitree T. Prevalence of various respiratory viruses in the middle ear during acute otitis media. New Engl J Med. 1999;340(4):260–4.
Kafetzis D, Tsolia AM, Liapi G, Mathioudakis J, Kallergi K. Otitis and respiratory distress episodes following a respiratory syncytial virus infection. Clin Microbiol Infect. 2003;9(10):1006–10.
Jang J, Chan KCG, Huang H, Sullivan SD. Trends in cost and outcomes among adult and pediatric patients with asthma: 2000-2009. Ann Allergy Asthma Immunol. 2013;111(6):516–22.
Prosser LA, Meltzer MI, Fiore A, et al. Effects of adverse events on the projected population benefits and cost-effectiveness of using live attenuated influenza vaccine in children aged 6 months to 4 years. Arch Pediatr Adolesc Med. 2011;165(2):112–8.
Leidy NK, Margolis MK, Marcin JP, et al. The impact of severe respiratory syncytial virus on the child, caregiver, and family during hospitalization and recovery. Pediatrics. 2005;115(6):1536–46.
Tam DY, Banerji A, Paes BA, et al. The cost effectiveness of palivizumab in term Inuit infants in the eastern Canadian Arctic. J Med Econ. 2009;12(4):361–70.
Hampp C, Kauf TL, Saidi AS, Winterstein AG. Cost-effectiveness of respiratory syncytial virus prophylaxis in various indications. Arch Pediatr Adolesc Med. 2011;165(6):498–505.
Yount LE, Mahle WT. Economic analysis of Palivizumab in infants with congenital heart disease. Pediatrics. 2004;114(6):1606–11.
Elhassan NO, Sorbero MES, Hall CB, Stevens TP, Dick AW. Cost-effectiveness analysis of Palivizumab in premature infants without congenital lung disease. Arch Pediatr Adolesc Med. 2006;160(10):1070–6.
Weiner LB, Masaquel AS, Polak MJ, Mahadevia PJ. Cost-effectiveness analysis of palivizumab among pre-term infant populations covered by Medicaid in the United States. J Med Econ. 2012;15(5):997–1018.
Joffe S, Ray GT, Escobar GJ, Black SB, Lieu TA. Cost-effectiveness of respiratory Synctial virus prophylaxis among preterm infants. Pediatrics. 1999;104(3):419–27.
Mahadevia PJ, Masaquel AS, Polak MJ. Cost utility of palivizumab prophylaxis among pre-term infants in the United States: a national policy perspective. J Med Econ. 2012;15(5):987–96.
Lancto KL, Masoud ST, Paes BA, et al. The cost-effectiveness of palivizumab for respiratory syncytial virus prophylaxis in premature infants with a gestational age of 32–35 weeks: a Canadian-based analysis. Curr Med Res Opin. 2008;24(11):3223–37.
Lazaro y de Mercado P, Aloy JF, Martinez ED, et al. La eficiencia (coste-efectivad) de palivizumab como profilaxis para la infeccion por virus respiratorio sincitial en prematuros de 32-35 semanas en Espana. An Pediatr (Barc). 2006;65(4):316–24 (In Spanish).
Neovius K, Buesch K, Sandström K, Neovius M. Cost-effectiveness analysis of palivizumab as respiratory syncytial virus prophylaxis in preterm infants in Sweden. Acta Paediatr. 2011;100(10):1306–14.
Nuijten MJ, Wittenberg W. Cost effectiveness of palivizumab in Spain: an analysis using observational data. Eur J Health Econ. 2010;11(1):105–15.
Nuijten MJ, Wittenberg W, Lebmeier M. Cost effectiveness of Palivizumab for respiratory syncytial virus prophylaxis in high-risk children, A UK Analysis. Pharmacoeconomics. 2007;25(1):55–71.
Nuijten MJ, Lebmeier M, Wittenberg W. Cost effectiveness of palivizumab for RSV prevention in high-risk children in the Netherlands. J Med Econ. 2009;12(4):291–300.
Nuijten MJ, Lebmeier M, Wittenberg W. Cost effectiveness of palivizumab in children with congenital heart disease in Germany. J Med Econ. 2009;12(4):301–8.
Resch B, Gusenleiter W, Nuijten MJC, Lebmeier M, Wittenberg W. Cost-effectiveness of Palivizumub against respiratory syncytial viral infection in high-risk children in Austria. Clin Ther. 2008;30(4):749–60.
Chirico G, Ravasio R, Sbarigia U. Cost-utility analysis of palivizumab in Italy: results from a simulation model in the prophylaxis of respiratory syncytial virus infection (RSV) among high-risk preterm infants. Ital J Pediatr. 2009;35(1):4. https://doi.org/10.1186/1824-7288-35-4.
Resch B, Sommer C, Nuijten MJC, et al. Cost-effectiveness of Palivizumab for respiratory syncytial virus infection in high-risk children, based on long-term epidemiologic data from Austria. Pediatr Infect Dis J. 2012;31(1):e1–8. https://doi.org/10.1097/INF.0b013e318235455b.
Salinas-Escudero G, Martinez-Valverde S, Reyes-Lopez A, et al. Cost-effectiveness analysis of the use of palivizumab in the prophylaxis of preterm patients in Mexico. Salud Publica Mex. 2012;54(1):47–59.
Thomas M, Bedford-Russell A, Sharland M. Hospitalisation for RSV infection in ex-preterm infants—implications for use of RSV immune globulin. Arch Dis Child. 2000;83(2):122–7.
Marchetti A, Lau H, Magar R, Wang L, Devercelli G. Impact of Palivizumab on expected costs of respiratory syncytial virus infection in preterm infants: potential for savings. Clin Ther. 1999;21(4):752–66.
Chan PW, Abdel-Latif ME. Cost of hospitalization for respiratory syncytial virus chest infection and implications for passive immunization strategies in a developing nation. Acta Paediatr. 2003;92(4):481–5.
Lofland JH, O'Connor JP, Chatterton ML, et al. Palivizumab for respiratory syncytial virus prophylaxis in high-risk infants: a cost-effectiveness analysis. Clin Ther. 2000;22(11):1357–69.
Banerji A, Lanctot KL, Bosco A, et al. Comparison of the cost of hospitalization for respiratory syncytial virus disease versus Palivizumab prophylaxis in Canadian Inuit infants. CMAJ. 2001;164(13):1847–50.
Reeve CA, Whitehall JS, Buettner PG, Norton R, Reeve DM, Francis F. Cost-effectiveness of respiratory syncytial virus prophylaxis with palivizumab. J Paediatr Child Health. 2006;42(5):253–8.
Farina D, Rodriguez SP, Bauer G, et al. Respiratory syncytial virus prophylaxis: cost-effective analysis in Argentina. Pediatr Infect Dis J. 2002;21(4):287–91.
Clark SJ, Beresford MW, Subhedar NV, Shaw NJ. Respiratory syncytial virus infection in high risk infants and the potential impact of prophylaxis in a United Kingdom cohort. Arch Dis Child. 2000;83(4):313–6.
Harris KC, Anis AH, Crosby MC, Cender LM, Potts JE, Human DG. Economic evaluation of Palivizumab in children with congenital heart disease: a Canadian perspective. Can J Cardiol. 2011;27(4):523.e11–5. https://doi.org/10.1016/j.cjca.2010.12.064.
Rietveld E, Steyerberg EW, Polder JJ, et al. Passive immunisation against respiratory syncytial virus: a cost-effectiveness analysis. Arch Dis Child. 2010;95(7):493–8.
Rodrıguez SP, Farina D, Bauer G. Respiratory syncytial virus prophylaxis in a high-risk population in Argentina. A cost-effectiveness analysis. Pediatr Infect Dis J. 2008;27(7):660–1.
Shireman TI, Braman KS. Impact and cost-effectiveness of respiratory syncytial virus prophylaxis for Kansas medicaid's high-risk children. Arch Pediatr Adolesc Med. 2002;156(12):1251–5.
Rackham OJ, Thorburn K, Kerr SJ. The potential impact of prophylaxis against bronchiolitis due to the respiratory syncytial virus in children with congenital cardiac malformations. Cardiol Young. 2005;15(3):251–5.
Stevens TP, Sinkin RA, Hall CB, Maniscalco WM, McConnochie KM. Respiratory syncytial virus and premature infants born at 32 weeks’ gestation or earlier: hospitalization and economic implications of prophylaxis. Arch Pediatr Adolesc Med. 2000;154(1):55–61.
Roeckl-Wiedmann I, Liese JG, Grill E, Fischer B, Carr D, Belohradsky BH. Economic evaluation of possible prevention of RSV-related hospitalizations in premature infants in Germany. Eur J Pediatr. 2003;162(4):237–44.
Vogel AM, McKinlay MJ, Ashton T, et al. Cost-effectiveness of palivizumab in New Zealand. J Paediatr Child Health. 2002;38(4):352–7.
Numa A. Outcome of respiratory syncytial virus infection and a cost-benefit analysis of prophylaxis. J Paediatr Child Health. 2000;36(5):422–7.
Ginsberg GM. Generalizability of cost-utility analyses across countries and settings. Best Pract Res Clin Gastroenterol. 2013;27(6):845–52.
Meissner HC, Kimberlin DW. RSV Immunoprophylaxis: does the benefit justify the cost? Pediatrics. 2013;132(5):915–8.
Meijboom MJ, Rozenbaum MH, Bennedictus A, et al. Cost-effectiveness of potential infant vaccination against respiratory syncytial virus infection in the Netherlands. Vaccine. 2012;30(31):4691–700.
Wang D, Bayliss S, Meads C. Palivizumab for immunoprophylaxis of respiratory syncytial virus (RSV) bronchiolitis in high-risk infants and young children: a systematic review and additional economic modelling of subgroup analyses. Health Technol Assess. 2011;15(5):iii–v.
Christensen H, Trotter CL, Hickman M, Edmunds WJ. Re-evaluating cost effectiveness of universal meningitis vaccination (Bexero) in England: modelling study. BMJ. 2014;349:g5725. https://doi.org/10.1136/bmj.g5725.
The Israel Society of Pediatrics. Recommendations for choosing indications in Israel for passiove immunization with Palivizumab (“Synagis”) against: Respiratory Syncytial Virus. 2014. (In Hebrew).
Israel Ministry of Health, Israel Center for Disease Control, Publication No. 354. Health 2013, February 2014, Jerusalem. page 54 (In Hebrew). http://www.health.gov.il/PublicationsFiles/health2013.pdf. Accessed 22 Sept 2018.
Bamberger E, Srugo I, Abu Raya B, et al. What is the clinical relevance of respiratory syncytial virus bronchiolitis?: findings from a multi-center, prospective study. Eur J Clin Microbiol Infect Dis. 2012;31(12):3323–30.
Greenberg D, Dagan R, Shany E, Bar-Ziv J, Givon-Lavi N. Increased risk for respiratory syncytial virus-associated,Community-acquired alveolar pneumonia in infants born at 31–36 weeks of gestation. Pediatr Infect Dis J. 2014;33(4):381–6.
Forbes ML, Hall CB, Jackson A, Masaquel AS, Mahadevia PJ. Comparative costs of hospitalisation among infants at high risk for respiratory syncytial virus lower respiratory tract infection during the first year of life. J Med Econ. 2010;13(1):136–41.
Boyce TG, Mellen BG, Mitchel EF Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr. 2000;137(6):865–70.
Feltes TF, Cabalka MD, Meissner HC, for the Cardiac Synagis Study Group, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory Synctial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143(4):532–40.
Prais D, Danino D, Schonfeld T, Amir J, for the Israeli RSV Monitoring Group. Impact of Palivizumab on admission to the ICU for respiratory syncytial virus bronchiolitis: A National Survey. Chest. 2005;128(4):2765–71.
Pedraz C, Carbonell-Estrany X, Figuueraz-Aloy J, Quero J, the Iris study Group. Effect of palivizumab prophylaxis in decreasing respiratory syncytial virus hospitalizations in premature infants. Pediatr Infect Dis J. 2003;22(9):823–7.
Stewart DL, Romero JR, Buysman EK, et al. Total healthcare costs in the US for preterm infants with respiratory syncytial virus lower respiratory infection in the first year of life requiring medical attention. Curr Med Res Opin. 2009;25(11):2795–804.
Breese Hall C, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–98.
Paramore LC, Ciuryla V, Ciesla G, Liu L. Economic impact of respiratory syncytial virus-related illness in the US. An Analysis of National Databases. Pharmacoeconomics. 2004;22(5):275–84.
Levitzky O, Lerner-Geva L, Dollberg S, Reichman B. The Israel National Very Low Birth Weight Infant Database. Harefuah. 2016;155(1):32–6. [Article in Hebrew]
Escobar GJ, Masaquel AS, Li SX, Walsh EM, Kipnis P. Persistent recurring wheezing in the fifth year of life after laboratory-confirmed, medically attended respiratory syncytial virus infection in infancy. BMC Pediatr. 2013;13:97. https://doi.org/10.1186/1471-2431-13-97.
Weber MW, Milligan P, Giadom B, et al. Respiratory illness after severe respiratory syncytial virus disease in infancy in the Gambia. J Pediatr. 1999;135(6):683–8.
Schauer U, Hoffjan S, Bittscheidt J, et al. RSV bronchiolitis and risk of wheeze and allergic sensitisation in the first year of life. Eur Respir J. 2002;20(5):1277–83.
Juntti H, Kokkonen J, Dunder T, Renko M, Niinimaki A, Uhari M. Association of an early respiratory syncytial virus infection and atopic allergy. Allergy. 2003;58(9):878–84.
Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65(12):1045e1052. https://doi.org/10.1136/thx.2009.121582.
Carbonell-Estrany X, Pérez-Yarza EG, García LS, IRIS (Infección Respiratoria Infantil por Virus Respiratorio Sincitial) Study Group, et al. Long-term burden and respiratory effects of respiratory syncytial virus hospitalization in preterm infants—the SPRING study. PLoS One. 2015;10(5):e0125422. https://doi.org/10.1371/journal.pone.0125422.
Singleton RJ, Redding GJ, Lewis TC, et al. Sequelae of severe respiratory syncytial virus infection in infancy and early childhood among Alaska native children. Pediatrics. 2003;112(2):285–90.
Fjærli H-O, Farstad T, Rød G, Ufert GK, Gulbrandsen P, Nakstad B. Acute bronchiolitis in infancy as risk factor for wheezing and reduced pulmonary function by seven years in Akershus County, Norway. BMC Pediatr. 2005 Aug 18;5:31. https://doi.org/10.1186/1471-2431-5-31.
Mok JQ, Simpson H. Outcome of acute lower respiratory tract infection in infants: preliminary report of seven-year follow-up study. Br Med J (Clin Res Ed). 1982 Jul 31;285(6338):333–7.
Henderson J, Hilliard TN, Sherriff A, Stalker D, Shammari NA, Thomas HM, the ALSPAC Study Team. Hospitalization for RSV bronchiolitis before12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16(5):386–92.
Korppi M, Piippo-Savolainen E, Korhonen K, Remes S. Respiratory morbidity 20 years after RSV infection in infancy. Pediatr Pulmonol. 2004;38(2):155–60.
Pullan CR, Hey EN. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. Br Med J (Clin Res Ed). 1982;284(6630):1665–9.
Mikalsen IB, Halvorsen T, Øymar K. The outcome after severe bronchiolitis is related to gender and virus. Pediatr Allergy Immunol. 2012; Jun;23(4):391–8.
Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354(9178):541–5.
Romero JR, Stewart DL, Buysman EK, Fernandes AW, Jafri HS, Mahadevia PJ. Serious early childhood wheezing after respiratory syncytial virus lower respiratory tract illness in preterm infants. Clin Ther. 2010;32(14):2422–32.
Simoes EA, Groothuis JR, Carbonell-Estrany X, the Palivizumab Long-Term Respiratory Outcomes Study Group. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151(1):34–42.
Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the global burden of disease study 2010. Lancet. 2012;380(9859):2129–43.
Ginsberg G, Block C, Stein-Zamir C. Cost-utility analysis of a Nationwide vaccination program against serogroup B meningococcal disease in Israel. Int J Public Health. 2016;61(6):683–92.
Ministry of Health Price List for Ambulatory and Hospitalization Services. Onset date: 01/06/16. https://www.health.gov.il/subjects/finance/taarifon/pages/pricelist.aspx. Accessed 23 Sept 2018.
Ontario Ministry of Health and Long-Term Care. Schedule A2016/17 Ontario Hospital Interprovincial per diem rates for inpatient services Effective April 1, 2016. http://www.health.gov.on.ca/en/pro/programs/ohip/bulletins/na_67/na_67.pdf. Accessed 23 Sept 2016.
WHO. Israel Statistical Profile. http://www.who.int/gho/countries/isr.pdf?ua=1. Accessed 23 Sept 2018.
Acknowledgements
To Dr. Nehama Goldberger and Ziona Haklaii of the Health Ministry’s Statistical Unit for supplying essential raw mortality and hospitalization data for RSV and associated diagnoses.
Availability of data and materials
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
Dr. GMG conceptualized and designed the study, collected the data, built the model, drafted the initial manuscript, and approved the final manuscript as submitted. Prof. ES provided medical know-how to input into the model, critically reviewed and revised the initial manuscript, and approved the final manuscript as submitted. Prof. YS conceptualized the study, provided data on low birth prevalence, medical know-how to input into the model, critically reviewed and revised the initial manuscript, and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
As the study is based on published literature and a built spreadsheet, no human subjects were involved – hence there is no need for ethical approval or consent to participate.
Consent for publication
Not applicable.
Competing interests
We confirm that we have read BioMed Central’s guidance on competing interests and all the authors are salaried staff of the Ministry of Health or Shaare Zedek Medical Center and there are no competing interests to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
Appendix 2
Cost-Effectiveness and Cost-Containment Studies
Cost-effectiveness Studies
-
a)
Passive immunoprophylaxis is not cost-effective.
Our study’s finding that passive immunization of RSV is not cost-effective affirms the findings of many other studies in infants with CHD [17], CLD [16], 26–32 GAW non CLD [18], 32–44 GAW [19], < 33 GAW [16, 20], < 33 GAW with > 27 days in Neonatal Intensive Care Unit, discharged between December and August [20], 32–35 GAW with less than two AAP2006 risk factors [19.21], 33–36 GAW [20] and Innuit ethnicity living in low-risk urban areas regardless of GAW [15].
-
b)
Passive immunoprophylaxis is cost-effective.
There are many studies which reported that immunoprophylaxis was cost-effective in BPD [26, 27, 29,30,31], CHD [26,27,28,29, 31] < 29 GAW [24, 32], 29–32 GAW [32], < 33 GAW and > 27 days in the Neonatal Intensive Care Unit, discharged between September and November [20], < 32 GAW [19, 21],32–34 GAW with risk factors [21], 32–35 GAW with risk factors [19, 21,22,23], < 33 GAW [25, 29, 31], 33–35 GAW [29,30,31], < 34 GAW [30], < 36 GAW (ie: all preterm) [26, 27, 29, 31], < 36 GAW with risk factors [19] and Innuit heritage living in rural and high-risk urban areas regardless of GAW [15].
Cost-Containment Studies
-
c)
Passive immunoprophylaxis is cost-saving
Palivisumab was actually found to be cost-saving and added QALYs among infants < 32 GAW and under 6 months old [19, 21]. Three other industry funded studies [34,35,36] reported a wide range of net costs, which included cost-savings. Another industry study suggested there will be net cost savings if infants under six months old living in rural or high risk Arctic Canadian communities received palivisumab [37]. A lone publicly funded study [33] showed cost savings would occur in CLD patients who received oxygen in their home setting.
-
d)
Immunoprophylactic intervention costs exceeded savings in hospitalization costs.
A lone industry funded study with a non-directional grant [38] and many publically funded economic studies [16, 17, 39,40,41,42,43,44,45,46,47,48,49], reported that Palivizumab infant immunoprophylactic costs exceeded the resultant savings in hospitalization costs due to decreased morbidity.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ginsberg, G.M., Somekh, E. & Schlesinger, Y. Should we use Palivizumab immunoprophylaxis for infants against respiratory syncytial virus? – a cost-utility analysis. Isr J Health Policy Res 7, 63 (2018). https://doi.org/10.1186/s13584-018-0258-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13584-018-0258-4