Abstract
The tetraspanins (TSPANs) are a family of four-transmembrane proteins with 33 members in mammals. They are variably expressed on the cell surface, various intracellular organelles and vesicles in nearly all cell types. Different from the majority of cell membrane proteins, TSPANs do not have natural ligands. TSPANs typically organize laterally with other membrane proteins to form tetraspanin-enriched microdomains (TEMs) to influence cell adhesion, migration, invasion, survival and induce downstream signaling. Emerging evidence shows that TSPANs can regulate not only cancer cell growth, metastasis, stemness, drug resistance, but also biogenesis of extracellular vesicles (exosomes and migrasomes), and immunomicroenvironment. This review summarizes recent studies that have shown the versatile function of TSPANs in cancer development and progression, or the molecular mechanism of TSPANs. These findings support the potential of TSPANs as novel therapeutic targets against cancer.
Similar content being viewed by others
Introduction
The tetraspanins (TSPANs) are a family of proteins with four transmembrane domains (TM1, TM2, TM3, and TM4), two extracellular loops (ECL1 and ECL2), and one intracellular loop (ICL) [1]. In Homo sapiens, the TSPANs family has 33 members, namely TSPAN1-TSPAN33 (Table 1). Some members have their common-used names, such as CD9 for TSPAN29, CD151 for TSPAN24, and CD81 for TSPAN28 (Table 1). 65 to 95% of amino acids are highly conserved among the TSPAN family members. The four transmembrane domains form a compact bundle in the membrane and facilitate interactions with other proteins [2]. TM domains contain many polar residues that can stabilize TSPAN protein structure with the help of ECL2 disulfide crosslinks. ECL2 is essential to the functions of TSPANs since most of protein–protein interaction sites have been mapped to ECL2. ECL2 consists of a conserved domain and a variable domain. The conserved domain facilitates interactions between different TSPAN molecules, while the variable domain determines interactions with other non-TSPAN proteins. There are also some highly conserved motifs in ECL2, such as CCG (Cys-Cys-Gly), PXSC (Phe-X-Ser-Cys) and EGC (Glu-Gly-Cys) [3]. These conserved motifs are basic structures for the interaction with other proteins. However, the structure and function of ECL1 and ICL have remained unclear so far [4]. The crystal structure of TSPAN proteins remains unknown until Rie Umeda and colleagues recently revealed the crystal structure of CD9 (TSPAN29) [5]. They found that the reversed cone-like molecular shape of CD9 in the crystalline lipid layers, giving reasons to the CD9 localization in regions with high membrane curvature and its implications in membrane remodeling [5].
TSPANs are expressed on the surface of most nucleated cells and play important roles in cell proliferation, differentiation, adhesion, migration, and cell–cell crosstalk [6]. Recent studies have revealed that TSPANs are indispensable for cancer initiation and progression [3]. These members have pro-tumor or anti-tumor functions in a context-dependent manner [3]. Although mainly located on cell membrane, TSPANs have no natural ligands. They affect different biological processes mainly via interacting with different partner molecules to form tetraspanin-enriched microdomains (TEMs). Tetraspanins can further influence the distribution and function of their partners. Integrins are the most prominent partner of TSPANs [3]. For instance, CD151 (TSPAN24) can enhance integrin-mediated adhesion to laminin and downstream signaling [7]. CD151 can form a complex with integrin α3β1 to activate PI3K or PI4K signaling pathway, and finally impacts cancer cell migration via remodeling actin cytoskeleton or inducing matrix metalloproteinase (MMP) secretion. Furthermore, CD151–α3β1/α6β4 integrin complexes can recruit and activate small G proteins (RAS, RAC1, and CDC42) in melanoma cell lines [8]. Other partners of TSPANs include growth factor receptors (EGFR [9], mtTGF-β [10]), transporters (ASCT2 [11], FATP1 [12], MDR1 [13]), membrane-linked kinases (BTRC [14], SOCSS3 [15], ATXN3 [16]), other transmembrane proteins (ADAM10 [17], CD44 [18], p120 [19]) or some nonproteins such as cholesterol [20]. For example, TSPAN6 could bind with EGFR and inhibit its downstream KRAS-ERK1/2 signaling to suppress KRAS-driven cancer initiation and metastasis [20](Fig. 1).
The intracellular signaling of TSPANs in cancer. Although mainly located on cell membrane, TSPANs have no natural ligands. They affect different biological processes mainly via interacting with different partner molecules to form TEMs. Integrins are the most prominent partner of TSPANs. Other partners of TSPANs include growth factor receptors (EGFR [9], mtTGF-β [10]), transporters (ASCT2 [11], FATP1 [12], MDR1 [13]), membrane-linked kinases (BTRC [14], SOCSS3 [15], ATXN3 [16]), other transmembrane proteins (ADAM10[17], CD44 [18], p120 [19]). Thus, TSPANs can affect several signaling pathways, including PI3K/AKT, Wnt/β-catenin, ERK1/2, STAT3/5, Src, Notch pathways
Moreover, the post-translational modifications at specific sites in TSPAN proteins are important for the protein–protein interaction and the subsequent downstream pathways. These modifications include N-glycosylations at the ECL2 domain, palmitoylation at the N- and C-terminal tails and ubiquitination at the N-terminal domain [3]. For example, glycosylation of CD63 (TSPAN30) in breast cancer cells by RPN2, part of the N-oligosaccharyle transferase complex, could stabilize CD63 on the cell membrane [13]. Glycosylation of TSPAN1 promotes its transition through the endoplasmic reticulum in ovarian cancer cells [21], while glycosylation of CD82 by the glycosyltransferase MGAT3 is pivotal to disrupt integrin α5β1-mediated cell migration [22].
The multiple possible combinations between different TSPANs and their interacting proteins could generate an enormous variability of biological function. In addition to the traditional role of TSPANs in cancer growth, invasion and metastasis, recent studies have revealed that these proteins also participate in cancer stemness, cell–cell communication, drug resistance and cancer immunology [16, 23,24,25,26] (Table 2). Specifically, TSPANs have been shown to regulate the biogenesis, cell-specific attachment of exosomes[19], and TSPAN4 can even promote the formation of migrasomes [27]. This review summarizes recent studies that have shown the versatile role of tetraspanins in cancer biology.
TSPANs in cancer invasion and metastasis
Metastasis is the leading cause of cancer-related deaths and the failure of cancer treatment [28]. The development of metastases requires cancer cells to leave their primary site (local invasion), intravasate, circulate and survival in the bloodstream, extravasate, acclimate in a secondary site, and finally form new colonization foci [29, 30]. Indeed, all cellular behaviors, including cancer cell migration, invasion, proliferation, apoptosis, should be concisely coordinated to ensure cancer metastasis [31, 32]. The function of TSPAN members in cancer cell invasion and metastasis will be first worth summarizing.
Most TSPAN proteins are reported to promote cancer invasion and metastasis, such as TSPAN1, TSPAN8, TSPAN12, TSPAN15, CD151, CD81, CD9, TSPAN31, and TSPAN13. TSPAN1 is mainly expressed on the plasma membrane, some intracellular vesicles and organelles (e.g., exosomes, lysosomes and endoplasmic reticulum), and perinuclear membrane [3]. TSPAN1 is frequently upregulated in cholangiocarcinoma, pancreatic cancer, and gastric cancer [33,34,35]. High level of TSPAN1 correlates with advanced tumor stage and metastasis [33, 36]. TSPAN1 could promote cholangiocarcinoma growth, metastasis, and induce epithelial-to-mesenchymal transition (EMT) by interacting with integrin α6β1 to amplify the PI3K/AKT/GSK-3β/Snail/PTEN feedback loop [33]. Meanwhile, TSPAN1 promotes pancreatic cancer cell migration and invasion by upregulating MMP2 [34]. The ablation of TSPAN1 suppressed the growth and motility of breast cancer cells by inhibiting the EMT process and the PI3K/Akt pathway [37]. TSPAN1 also significantly promotes the proliferation and invasion of colon cancer cells [38] and gastric cancer cells [35] with unrevealed mechanism. Jennifer and colleagues also revealed that TSPAN1 could promote the migration of prostate cancer cells [39]. Furthermore, TSPAN-1 was found to interact with and stabilize the human thiamine transporter-1 (hTHTR-1) to facilitate thiamine intake in colon cancer and epithelial cells [40].
TSPAN8 is reported to be highly expressed in colorectal cancer, pancreatic cancer tissues and melanoma [41,42,43,44]. TSPAN8 promotes the proliferation, migration and EMT process of colorectal cancer cells [41]. TSPAN8 also facilitates metastasis of pancreatic cancer cells in vivo and in vitro [42], while it promotes metastasis of rat pancreatic cancer cells through recruiting integrins out of adhesion into motility promoting complexes [45]. Meanwhile, TSPAN8 could induce the EMT process and enhance cell–cell adhesion of breast cancer cells via interacting with p120 [19]. TSPAN8 is more frequently expressed in metastatic melanoma species and correlates with the presence of a BRAF-V600E mutation, a higher propensity to form distant metastases and an increased risk of death [44]. TSPAN8 stabilizes β-catenin, which in turn directly triggers the transcription of TSPAN8, leading to melanoma invasion [43]. TSPAN8+ melanoma cells have elevated active MMP-3 and low TIMP-1 levels to promote keratinocyte-originated proMMP-9 activation process, collagen IV degradation and dermal colonization [46]. Interestingly, the nuclear localization of TSPAN8 can be detected in multiple cancer cells, which involves the formation of TSPAN8-cholesterol-14-3-3θ-importin β complex after being palmitoylated [47]. The same group further demonstrated that nuclear TSPAN8 could interact with STAT3 to enhance its chromatin occupancy [9]. The authors further revealed that blocking the translocation of TSPAN8 using a humanized monoclonal antibody hT8Ab4 can remarkably inhibit breast cancer growth in vitro and in vivo [9].
TSPAN12 is highly expressed in both colorectal cancer and non-small lung cancer tissues [48,49,50]. High TSPAN12 expression is significantly correlated with TNM stage, tumor size and lymph node metastasis in colorectal cancer patients. Knockdown of TSPAN12 significantly could suppress cell proliferation, migration and invasion, in vivo tumor growth, while induce cell apoptosis of both colorectal cancer and non-small cell llung cancer cells [48, 50]. In contrast, TSPAN12 promotes breast cancer cell growth, but depresses tumor-endothelial interactions and metastasis to mouse lungs. Mechanistic study demonstrated that TSPAN12 stabilizes FZD4–LRP5 association to activate the canonical Wnt-pathway signaling [49].
CD151 expression has been reported to be associated with advanced cancer stage, cancer invasiveness and poor prognosis in endometrial cancer, hepatocellular carcinoma, breast cancer and non-small cell lung cancer patients [45, 51,52,53,54,55]. CD151 ablation markedly reduces breast cancer cell migration, invasion by inhibiting FAK-Rac1 signaling and disrupting EGFR-α6 integrin collaboration [53]. Meanwhile, CD151 could promote non-small cell lung cancer cell proliferation, migration, and invasion by interacting with integrin α3β1 to enhance EGFR signaling [55]. Moreover, CD151 recruits and activates MMP9 and MMP13 to create a path for invasion and metastasis of rat pancreatic cancer cells [45].
High TSPAN15 expression in esophageal squamous cell carcinoma tissues is significantly associated with lymph node and distant metastasis, and poor prognosis [14, 56]. TSPAN15 could augment metastatic capabilities but not proliferation of esophageal squamous cell carcinoma cells. It specifically interacts with β-transducin repeat containing E3 ubiquitin protein ligase (BTRC) to promote the ubiquitination and proteasomal degradation of p-IκBα, and thereby triggers NF-κB nuclear translocation and initiates transcription of several metastasis-related genes [14]. TSPAN15 can also increase the ADAM10 on the cell surface, the soluble N-Cadherin secretion and β-catenin nuclear translocation in esophagus cancer cells [57].
CD81, CD9, TSPAN31, and TSPAN13 are also reported to facilitate cancer cell invasion and metastasis. Mice with C81 deficient develop fewer breast cancer metastases compared to their wild-type counterparts. The same group showed that a unique anti-human CD81 antibody (5A6) effectively halts invasion and metastasis of triple-negative breast cancer cell lines [58]. CD9 is highly expressed in the bone metastases versus primary breast cancer tissues [59]. It is reported to promote breast cancer migration [60]. However, CD9 deletion in the MMTV/PyMT mouse model impaired tumor growth, but did not affect tumor initiation or metastasis [60]. CD9 depletion or anti-CD9 antibody could result in polynucleation and multipolar mitoses [61]. CD9 on lung adenocarcinoma cells is also necessary for the pro-invasion effect of the secreted TIMP-1 from cancer-associated fibroblasts, probably depending on the direct interaction between these two proteins [62]. TSPAN31 is highly expressed in gastric cancer tissues and correlates with poor prognosis of gastric cancer patients. It could promote the gastric cancer proliferation and migration via activating PI3K/AKT signaling [63, 64]. In contrast, TSPAN31 facilitates the migration and invasion, but has less impact on proliferation of hepatocellular carcinoma cells [65]. Furthermore, knockdown of TSAPN13 in U2OS sarcoma cells increased cell apoptosis and also suppressed EMT process [66].
On the other hand, some TSPAN family members, such as CD82, TSPAN6, TSPAN9 and CD63, exhibit tumor suppressor properties. For example, CD82 directly associates with EGFR and suppressed EGF-induced lamellipodial extensions and cell migration in non-small cell lung cancer cells. CD82 could specifically increase EGFR endocytosis after EGF stimulation but not the initial activation of EGFR [67]. It also inhibits cell migration by enhancing focal adhesion through promoting YAP nuclear translocation in breast epithelial cells [68]. In addition, CD82 binds with EWI2 in Du145 metastatic prostate cancer cells and inhibits cell migration on both fibronectin- and laminin-coated substratum [69]. The other member TSPAN6 could suppress tumor growth and metastasis of human RAS activating mutant pancreatic cancer xenografts. Whole-body knockout as well as tumor cell autonomous inactivation using floxed alleles of TSPAN6 in mice enhanced KrasG12D-driven lung tumor initiation and malignant progression. Similar to the function in lung cancer cells, TSPAN6 binds to the EGFR and blocks EGFR-induced RAS activation, thus inhibiting EMT process and cell migration [20]. The proliferation, migration and invasion of human gastric cancer SGC7901 cells were significantly inhibited by overexpression of TSPAN9, which is mediated by the inhibition of ERK1/2 signaling and MMP-9 expression [70]. Finally, CD63-silenced melanoma cells showed enhanced motility, invasiveness, EMT and in vivo tumor growth [71].
Evidence has shown the context-dependent role of TSPAN7 in cancer. TSPAN7 is highly expressed in primary osteosarcomas and promote osteosarcoma cell growth, EMT process, and in vivo metastasis. Mechanistically, the authors demonstrated that TSPAN7 interacted with β1 integrin to activate FAK-Src-Ras-ERK1/2 signaling [72]. TSPAN7 could also promote non-small cell lung cancer cell proliferation, migration, and EMT process [73]. However, the tumor-suppressing effect of TSPAN7 has been reported in myeloma and bladder cancer. TSPAN7 significantly reduced tumor burden in 5TGM1/KaLwRij mice 4 weeks after intravenous injection of the murine myeloma cell line 5TGM1 by increasing cell adhesion to stromal cells and transendothelial migration, with no impact on cell proliferation [74]. Additionally, Xi Yu et al. showed low TSPAN7 expression level is associated with higher tumor stage and poor prognosis in bladder cancer and TSPAN7 inhibits both cell migration and proliferation through suppressing the PTEN/PI3K/AKT Pathway [75].
In summary, most TSPAN proteins (TSPAN1, TSPAN8, TSPAN12, TSPAN15, CD151, CD81, CD9, TSPAN31, TSPAN13) could promote cancer invasion and metastasis, while few members (CD82, TSPAN6, TSPAN9 and CD63) have the opposite function. Meanwhile, the function of TSPAN7 in cancer invasion and metastasis is context-dependent.
TSPANs in cancer proliferation and growth
As mentioned above, several TSPAN family members could regulate the invasion and metastasis of cancer cells. Cell growth and proliferation are also essential for cancer metastasis or progression besides invasion. These biological processes should coordinate with each other to achieve cancer progression. We can see that TSPAN1, TSPAN12, TSPAN13, TSPAN6, TSPAN8, TSPAN9, CD151, CD63 can promote or inhibit both cancer cell invasion and proliferation. However, the function of TSPAN molecules seems to be context-dependent. For example, TSPAN31 has been reported to promote migration of hepatocellular carcinoma, but not affect cell proliferation [65]. Even more, TSPAN12 could inhibit growth of breast cancer cells, but enhance metastasis [49]. In contrast, TSPAN12 has been found to promote the proliferation of small cell lung cancer cells and colorectal cancer cells [48, 76]. Larger discrepancy exists as Hu Z et al. reported that TSPAN12 could promote the proliferation of non-small lung cancer cells [50], while another group found an opposite effect [77]. We herein summarize several studies that have focused on the function of TSPAN members in cancer cell proliferation and growth.
On the one hand, some TSPAN members could promote cancer cell growth. Pancreatic cancer is one of the most aggressive malignancy, with a 5-year survival rate of less than 5% [78]. High TSPAN1 expression was correlated with poor overall survival of pancreatic cancer patients, and TSPAN1 promote the proliferation of pancreatic cancer cells [79]. The authors further revealed that TSPAN1 promoted autophagy maturation via direct binding to LC3 by two conserved LC3-interacting regions in the two extracellular loops [79]. Moreover, TSPAN15 has been reported to promote the proliferation of hepatocellular carcinoma cells via activating ERK1/2 signaling [17]. However, it does not significantly affect the proliferation of esophagus carcinoma cells [55]. It can associate with a molecular scissor, ADAM10, but the effect is unknown [17, 57]. A latest study reported that CD151 could stabilize the oncogene c-Myc to activate the transcription of SPTLC1, the first rate-limiting enzyme in sphingolipid biosynthesis, thus fueling osteosarcoma cell growth [80]. Another recent study reported that TSPAN29 associates with ADAM10 to increase its cell surface trafficking and α-secretase activity, which further produces more cleaved Notch1 to support growth of colorectal cancer cells [81].
On the other hand, some other TSPAN members could inhibit cancer cell growth. TSPAN31 serves as a natural antisense transcript to inhibit CDK4 protein expression in human cervical cancer and hepatocellular carcinoma by targeting the 3ʹ-untranslated region of the CDK4 mRNA, thus suppressing cell proliferation [65, 82]. The expression of TSPAN6 is frequently decreased or even lost in colorectal cancer tissues, and correlates with favorable survival. TSPAN6 deletion facilitates colorectal cancer development and results in the activation of EGF-dependent signaling pathways through increased production of the transmembrane form of TGF-α (tmTGF-α) associated with extracellular vesicles [83]. Liang G. and colleagues found that TSPAN12 could inhibit tumor growth of non-small lung cancer cells [77]. CD9 associates with transmembrane TGF-α to enhance the ligand-induced activation of the EGFR, and thus promoted Madin-Darby Canine Kidney cells (MDCK) cell proliferation [84]. CD37, whose expression correlates with favorable prognosis, can protect against the development of B cell lymphoma by interacting with the suppressor of cytokine signaling 3 (SOCS3) to inhibit IL-6 signaling [15, 85]. Most recently, CD37 has been demonstrated to inhibit fatty acid metabolism in aggressive B-cell lymphoma through interacting with fatty acid transporter protein 1 (FATP1) in the plasma membrane, and inhibiting the uptake and processing of exogenous palmitate [12].
Altogether, TSPAN1, TSPAN15, TSPAN29, and CD151 could support cancer cell growth, while TSPAN31, TSPAN6, CD9, CD37 could inhibit cancer growth.
TSPANs in cancer cell stemness
Stem cells are capable of both self-renewing and multilineage differentiating. Tumor heterogeneity is now recognized as a hallmark of tumors [86]. Only a distinct population of cancer cells has the capabilities of self-renewal, drug-resistance, metastasis and tumorigenicity, called cancer stem cells (CSCs) [87,88,89]. Our understanding of the biology and therapeutic implication of CSCs is still evolving since the establishment of this concept.
Several TSPAN members, such as CD9, TSPAN8, TSPAN3, CD82, CD81, and TSPAN1, have been implicated in the regulation of CSCs. CD9 has been reported to be specifically expressed on leukemia stem cells. CD9positive cells exhibit more resistance to chemotherapy drugs, higher migration potential, and stronger tumorigenicity [90]. CD9 was also identified as a marker of pancreatic cancer-initiating cells. CD9high pancreatic cancer cells have increased organoid formation capability and in vivo carcinogenesis. Mechanistically, CD9 enhances glutamine uptake in pancreatic cancer cells via promoting the plasma membrane localization of the glutamine transporter ASCT2 [11]. TSPAN8 expression is upregulated in breast CSCs. It could upregulate the stemness gene NANOG, OCT4, and ALDHA1, and enhance both tumor formation and drug resistance. TSPAN8 interacts with the Hedgehog receptor PTCH1 and inhibits the degradation of the SHH/PTCH1 complex through recruitment of deubiquitinating enzyme ATXN3, thus inducing downstream gene expression [16]. In addition, TSPAN3 knockout impaired leukemia stem cell self-renewal and disease propagation, and significantly improved survival in mouse models of acute myelocytic leukemia. This effect is at least partially mediated by disabling homing within in the niche in responses to CXCL12 [91]. CD82 was up-regulated in CD34+/CD38+ acute myelocytic leukemia stem cells and increased the phosphorylation of transcription factor STAT5 to transactivate IL-10 transcription [92]. More recently, CD81 has been revealed to interact with CD44 to enhance the stemness of triple-negative breast cancer cells, and high CD81 expression can be found in circulating tumor cells [18]. Furthermore, TSPAN1 is found to be elevated in CSCs from head and neck squamous cell carcinoma cells and lead to drug resistance [93].
CD63 and CD81 have also been reported to play important roles in stemness maintenance of non-malignant cells. CD63 could confer hematopoietic stem cells with more quiescent status, more robust self-renewal and myeloid differentiation abilities than those with negative/low CD63 expression. Knockout of CD63 in mice reduced the number of hematopoietic stem cells in bone marrow and CD63-deficient hematopoietic stem cells exhibit impaired quiescence and long-term repopulating capacity, and increased sensitivity to irradiation or 5-fluorouracil treatment. CD63 was found to interact with TGF-β receptors I and II to sustain TGF-β signaling activity [70]. A CD81+/PDGFRAlow population present just below crypts is sufficient to expand intestinal stem cells in vitro and contribute to stemness maintenance in vivo via secreting the BMP antagonist Gremlin1 [94].
TSPANs in therapy resistance
Despite significant advances in cancer treatment, the development of resistance almost invariably emerges [95]. Multiple studies have revealed that cancer cells utilize a plethora of distinct mechanisms to survive under chemotherapy or radiotherapy [96, 97]. The following TSPANs have been involved in enhancing cancer therapy resistance: CD9, CD81, TSPAN1, TSPAN3, TSPAN31, CD82 and CD63.
CD9 mediates chemoresistance in acute myeloid leukemia [90] and small cell lung cancer [98]. As mentioned above, CD9 enhances the stemness and chemoresistance of acute myeloid leukemia cells. In addition, CD9 is expressed preferentially in relapsed small cell lung cancers but not chemo-responsive primary tumors. CD9 renders small cell lung cancer cells resistant to cisplatin or etoposide, and increases cell adherence to fibronectin via β1 integrin. A specific monoclonal antibody against CD9, ALB6, triggered apoptosis in the chemoresistant cells [98]. CD81 has been reported to enhance both chemoresistance and radioresistance. CD81 knockout induces chemosensitivity, reduces cellular adhesion, and disrupts in vivo bone marrow homing and engraftment in acute lymphoblastic leukemia cells. This chemosensitization is mediated through control of Bruton tyrosine kinase (BTK) signaling and induction of p53-mediated cell death [99]. Accordingly, suppressing CD81 by siRNA/shRNA could enhance radiation-induced cell killing and DNA damage of γ-H2AX formation, and delaye tumor xenograft growth of glioblastoma. Knockdown of CD81 significantly decreased radiation-induced expression of nuclear Rad51, a key protein for homologous recombination repair [100].
TSPAN1, TSPAN3, TSPAN12, TSPAN31, CD82 and CD63 have also been reported to reduce chemosensitivity of cancer cells. TSPAN1 is found to be upregulated in cisplatin-resistant head and neck squamous cell carcinoma cells. TSPAN1 depletion reduces cell proliferation, induces apoptosis, decreases autophagy, sensitizes to chemotherapeutic agents and inhibits the phosphorylation of SRC signaling [93]. Moreover, TSPAN3 is up-regulated in adriamycin-resistant acute myeloid leukemia samples and cells. It increases adriamycin resistance, proliferation, migration and invasion and reduces apoptosis in adriamycin-resistant cells [101]. TSPAN12 elevation in small cell lung cancer specimens correlates with poor pathologic stage and shorter survival time. It could enhance cells chemoresistance, proliferation and tumor growth [76]. Knockdown of TSPAN31 improves chemosensitivity to cisplatin through the suppression of ABCC2 in gastric cancer cells [64]. Furthermore, CD63 silencing reduces the chemoresistance of breast cancer cells by stabilizing MDR1 on cell surface [13]. CD82 could significantly reduce cell death in response to daunorubicin in acute myeloid leukemia cells. The underlying mechanism involves the activation of protein kinase c alpha (PKCα)-β1 integrin-p38 signaling [102].
TSPANs in extracellular vesicles
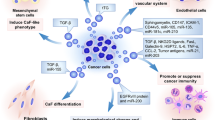
Extracellular vesicles (EV) are of utmost importance in intercellular communication under physiological and pathological conditions, allowing cells to exchange proteins, lipids, and genetic material [93]. Exosomes are a subpopulation of small 40–100 nm EVs, which can be recovered in all body fluids. Exosomes are build-up by a transmembrane protein-containing lipid bilayer and proteins, coding and noncoding RNA, and DNA in the vesicle lumen. Exosome biogenesis starts with early endosomes (EE) formation, which originate from the trans-Golgi network or internalized membrane microdomains [104]. EE are then guided towards multivesicular bodies (MVB) to receive their cargo during inward budding of intraluminal vesicles [105]. Several TSPANs such as CD9, CD81, and CD63 are major constituents and canonical markers of EVs. Moreover, they can regulate the biogenesis of exosomes in the following aspects: (1) TSPAN-enriched microdomains (TEMs) are prone for internalization or curvature [106, 107], and proteins in TEMs have been proposed to be carried by EVs [108]; (2) TSPANs contribute to EE traffic towards MVB [109]. (3) The exosome TSPAN web strengthens binding avidity by clustering TSPAN-associated molecules [110] (Fig. 2).
The function of TSPANs in EV biogenesis. TSPANs can regulate the biogenesis of EVs in the following aspects: (1) TEMs are prone for internalization or curvature [106, 107], and proteins in TEMs have been proposed to be carried by EVs [108]; (2) TSPANs contribute to EE traffic towards MVB [109]. (3) The exosome TSPAN web strengthens binding avidity by clustering TSPAN-associated molecules. TSPAN8 could promote EV production and the attachment to target cells. In contrast, TSPAN6 can reduce EV production and the contents in EVs in cancer cells. CD151 facilitates secretion of ribosomal proteins while reducing complement proteins to promote the migration and invasion of triple-negative breast cancer cells [111]. CD81 ensures the membrane integrity of exosomes, which are capable of inducing stemness in triple-negative breast cancer [18]. CD9 on the surface of EVs facilitates the uptake of EVs from cancer-associated fibroblasts by pancreatic cancer cells [117]
TSPAN8 has been reported to promote EV production and the attachment to target cells. TSPAN8 mediated a several-fold increase in EV number in breast cancer cell culture and the circulation of tumour-bearing animals [19]. Rat PDAC cells with CD151 or TSPAN8 knockdown poorly metastasize, but regain metastatic capacity when rats are pretreated with exosomes from parental cells. Both exosomal CD151 and TSPAN8 contribute to host matrix remodelling due to exosomal TSPAN-integrin and TSPAN-protease associations, and stroma cell activation [112]. Extracellular vesicles originated from TSPAN8high expression pancreatic cancer cells remarkably promotes maturation, activation of endothelial cells and fibroblasts [103]. Tspan8-enriched EVs exhibit stronger attachment to the target cells including breast cancer cells or fibroblast, by molecular adhesion [25]. TSPAN8-overexpressing EVs increases invasion of non-small lung cancer cells and elevates TSPAN8 expression on serum EVs correlated with reduced distant metastasis-free survival [113].
In contrast, TSPAN6 can reduce EV production and the contents in EVs in cancer cells. TSPN6 acts as a suppressor of exosome release by facilitating the lysosomal degradation of SDC4 and syntenin in breast cancer cells [114]. In addition, TSPAN6 deletion promotes colorectal cancer organoid growth in an EV-dependent manner, as EV-depleted media could not support proliferation and viability of TSPAN6-expressing organoids [83]. Furthermore, TSPAN6 in colorectal cancer cells might decrease the TGF-α content in the EVs, which could activate EGFR signaling in target cells. However, TSPAN6 recruits the cytosolic exosome-forming adaptor syntenin to increase secretion of exosomes that contain amyloid precursor protein-C-terminal fragments in brain [115].
CD151, TSPAN1, CD81, CD9 have also been implicated in the EV biogenesis. CD151 expression level in triple-negative breast cancer-derived serum exosomes is significantly higher than those from healthy subjects [111]. CD151 facilitates secretion of ribosomal proteins while reducing complement proteins to promote the migration and invasion of triple-negative breast cancer cells [111]. TSPAN1 was found to be upregulated in plasma EVs from colon cancer patients compared to those from healthy controls [116]. In addition, CD81 has been reported to ensure the membrane integrity of exosomes, which are capable of inducing stemness in triple-negative breast cancer [18]. Recently, Jérémy Nigri and colleagues showed that CD9 on the surface of EVs facilitates the uptake of EVs from cancer-associated fibroblasts by pancreatic cancer cells [117].
TSPAN proteins also regulate the formation of migrasomes [27]. Migrasomes are large vesicle-like structures that are released from cells during migration, providing spatiotemporal chemical information for cell–cell communication. The authors found that 14 out of the 33 known TSPANs could enhance migrasome formation [27]. TSPAN4, one of the most powerful promoters of migrasome formation, could elevate the membrane stiffness of the TEMs to facilitate micron-scale macrodomain assembly [27]. The same group further reported that TSPAN4 promotes membrane repair by mediating assembly of micron-scale macrodomains in gastric cancer cell, rat kidney cell and mouse fibroblast [118].
Tetraspanins in cancer immunology
Immunotherapy has revolutionized and rejuvenated cancer treatment. The immune system plays a pivotal role in immunosurveillance, as immune cells of the adaptive and innate immune systems infiltrate into the tumor microenvironment (TME) and modulate cancer progression. Due to their powerful tumor-killing capability, T cells are the focus of tumor immunology [119]. Antigen presentation is necessary for T cell immune surveillance of cancer cells. CD8 + T cell activation is primarily driven by the presentation of peptides from endogenously expressed proteins on MHC class I molecules (MHC-I), while CD4 + T cells activation is driven by MHC II molecules (MHC-II) [78, 79]. Professional antigen-presenting cells (APCs), including dendritic cells (DCs), monocytes, and B cells, internalize and process antigens, producing immunogenic peptides that enable antigen presentation to T lymphocytes. Antigen-specific T cell stimulation is initiated by direct contact of the T cell receptor (TCR) with the immunogenic peptide-bound MHC complexes (pMHC) on antigen presenting cells (APCs) [121]. Some TSPANs have relevant roles during immune responses, including antigen presentation and cell migration.
CD9, CD82, CD37, CD151, CD63, and TSPAN5 have been revealed to interact with MHC complex in APCs. CD9 is reported to associated with MHC II molecules in dendritic cells (DCs) and B cells, which might facilitate the formation of MHC II multimers [122]. Deletion of CD9 in mice enhanced macrophage infiltration and TNF-α production in the lung after administration of Lipopolysaccharide [123]. CD9 knockout bone marrow-derived DCs (BMDCs) induces lower levels of T cell activation than wild-type DCs. CD9 causes MHC-II retention on cell surface by facilitating MHC II trafficking and reducing MHC II endocytosis and recycling [84]. CD82 is upregulated upon activation of BMDCs and monocyte-derived DCs, supporting MHC class II maturation and stable interactions between T cells and splenic DCs or BMDCs through inhibiting RhoA activation [124]. On contradictory, CD37, CD151 and CD63 exhibit inhibitory effect on T cell activation. DCs lacking either CD37 or CD151 expression were hyper-stimulatory to T cells. CD151 inhibits co-stimulation of T cells whereas CD37 dampens peptide/MHC presentation [125, 126]. In addition, knockdown of CD63 in B lymphoblastoid cells consistently activated the CD4 + T-cells via enhancing exosome production [127]. However, it should be noted that CD63 influences neither the amount nor dimerization of MHC II in these cells [87]. CD53, CD81, and CD82 have been revealed to bind with MHC class II molecules in B cell lymphoma cells, but the effect needs further study [81]. Less is known about the association between TSPAN proteins and MHC I complex. One latest study demonstrated that TSPAN5 associates with MHC I molecules to induce more intense MHC I clusters for CD8 + T cell activation. This interaction starts in the endoplasmic reticulum and is maintained on the cell surface [88].
Moreover, CD37, CD81, CD82, CD53 and TSPAN33 regulate the adhesion or migration of APCs. CD37 ablation impairs chemo-tactic migration and in vivo priming of adoptively transferred naive T cells of DCs via activating Rac-1 [128, 129]. CD81 is required for the lamellipodia formation of DCs during migration [130]. CD81 increases adhesion strengthening in monocytes and primary murine B cells, thus facilitating both leukocyte rolling and arrest on VCAM-1 under shear flow as well as adhesion to fibronectin during short stationary contacts [131]. CD82 is upregulated upon activation of BMDCs and monocyte-derived DCs, and restrains migration of BMDCs [129]. Accordingly, CD82 restrains the migration of neutrophils and macrophages into tissues [132]. CD53 could enhance the degranulation of rat NK cells in response to tumor cells, and reduce the IFN-γ response, while decrease homotypic adhesion by activating the β2 integrin LFA-1 [133]. CD53 could also impede the adhesion of both B and T cells [134]. TSPAN33 is reported to promote protrusion formation and invasion in B cells, meanwhile reducing cell adhesion [135]. The effect of TSPANs on T cells remains poorly known. One recent study reported that CD53 could stabilize CD45 on T cell membrane and is required for optimal phosphatase activity and subsequent activation [136].
Although emerging evidence demonstrated that TSPAN proteins are important regulator of immune cells, there lacks direct evidence showing the function of TSPAN proteins in cancer immunology. Further work needs to be done to explore the potential functions. Interestingly, Daniel and colleagues identified that TSPAN8 can be used as a specific target candidate for chimeric antigen receptor T cells (CAR-T) against pancreatic cancer among 371 antigens. CAR-T cells specific for TSPAN8 can significantly decrease the tumor burden in a subcutaneous xenograft model [77].
Discussion and conclusion
The TSPANs are a family of 33 four-transmembrane proteins in Homo sapiens. TSPANs are mainly expressed on the surface of most nucleated cells and play important roles in cell proliferation, differentiation, adhesion, migration, and cell–cell crosstalk. Recent studies have revealed that TSPANs are indispensable for cancer initiation and progression. TSPANs affect different biological processes mainly via interacting with different partner molecules to form TEMs, including integrins, EGFR, mtTGF-β, EWI2, ASCT2, LC3, PTCH1, P120 and others. We herein summarized the recent studies revealing the versatile role of TSPAN family members in cancer cell invasion, metastasis, proliferation, stemness maintenance, drug resistance, and EV biogenesis. However, other proteins with four transmembrane domains are not included in the TSPAN family, such as TM4SF5 [137].
Most TSPAN proteins are reported to promote cancer invasion and metastasis, such as TSPAN1, TSPAN7, TSPAN8, TSPAN12, TSPAN15, CD151, CD81, CD9, TSPAN31, TSPAN13 and TSPAN9, while CD82, CD63 and TSPAN6 can inhibit cancer invasion or metastasis. The function of TSPAN7 is context-dependent. On the one hand, TSPAN7 can promote the invasion and metastasis of myeloma, non-small lung cancer, and osteosarcoma [72,73,74]. Conversely, TSPAN7 is downregulated in bladder cancer tissues and inhibits cell proliferation, invasion and in vivo tumor growth [75]. TSPAN15 could promote the proliferation of hepatocellular carcinoma cells [17], but not esophagus carcinoma cells [55]. There are also some discrepancies in the function of specific TSPAN members in cancer cell proliferation and invasion. TSPAN31 and TSPAN7 can promote migration of hepatocellular carcinoma and myeloma cells respectively, but do not affect cell proliferation [30, 66]. More interestingly, TSPAN12 could inhibit the growth of breast cancer cells, but facilitate metastasis [44]. These results showed the complex roles of TSPAN members in cancer metastasis.
CSCs are a distinct population of cancer cells with the capabilities of self-renewal, drug-resistance, metastasis and tumorigenicity. TSPAN1, TSPAN8, TSPAN3, CD9, CD82 have been shown to enhance the stemness of cancer cells. CD63 and CD81 could contribute to the maintenance of hematopoietic stem cells and intestinal stem cells, respectively. Nevertheless, their role in CSCs remains to be explored. TSPAN1, TSPAN3, TSPAN12, CD9, CD81, TSPAN31 and CD82 can mediate chemoresistance in multi cancer types. Meanwhile, CD81 could enhance glioblastoma survival after radiation treatment [100]. We then summarize recent studies showing the essential roles of TSPAN family members in EV-driven cell–cell communication. TSPAN proteins are abundantly enriched in exosomes and can regulate the biogenesis of exosomes, the uptake of exosomes by target cells, and the cargo within exosomes. TSPAN8 has been reported to promote exosome production and the attachment to target cells [103], while TSPAN6 suppresses exosome production and regulate the contents in EVs [115]. TSPANs can regulate the formation of migrasomes, which are smaller vesicles released during cell migration [138]. Currently, the migrasomes exhibit three modes of action: release of signaling molecules through rupturing or leaking, carriers of damaged mitochondria, and lateral transfer of mRNA or proteins [139]. It has been reported that 14 out of the 33 known TSPANs could enhance migrasome formation, but only TSPAN4 has been comprehensively studies. The function of TSPANs on migrasome formation and uptake needs further study.
The immune system is critical in immunosurveillance against cancer. T cells are the focus of tumor immunology as they can efficiently kill cancer cells. The activation of T cells relies on the antigen presentation process [119]. CD9, CD82 have been revealed to interact with MHC II complex in APCs, which might facilitate the formation of MHC II multimers and subsequent CD4 + T cell activation [122]. One latest study demonstrated that TSPAN5 could promote the formation of intense MHC I clusters for CD8 + T cell activation [24]. On the contrary, CD37, CD151, CD63 on APCs exhibit suppressors of MHC presentation [125]. In addition, several TSPAN members, including CD37, CD81 and CD82, are required for the migration of APCs [129]. It should be noted that TSPANs can also regulate attachment, entry, and internalization of viruses, including SARS-CoV-2 [140,141,142], implying that TSPANs might impact the development of virus-related cancers. Although emerging evidence showed that TSPAN proteins are important for the proper function of APCs, the function of these molecules in cancer immunology is yet to be elucidated. Further, TSPAN proteins may also affect the function of other stroma cells, as TSPAN12 in fibroblasts promotes cancer cell proliferation and invasion through direct cancer-to-stromal cell contact with unknown mechanism [122].
Increasing studies have revealed the regulatory mechanism of TSPAN expression in cancer. The regulation of TSPAN expression can be divided into transcriptional regulation, post-transcriptional regulation, and post-translational regulation. (1) Several transcription factor, like SOX9 and β-cantenin, and promoter demethylase LSD1 have been reported to regulate TSPAN8 transcription. SOX9 could directly enhance the transcription of TSPAN8 expression in response to EGF stimulation [42], while β-cantenin could trigger the direct transcriptional activation of TSPAN8 in melanoma cells [43]. Lysine Specific Demethylase 1 (LSD1) could up-regulate TSPAN8 expression by reducing H3K9me2 occupancy on the TSPAN8 promoter in colorectal cancer cells [41]. (2) The post-transcriptional regulation of TSPANs expression mainly involves microRNAs (miRNAs) and RNA binding proteins. miR-518f-5p could inhibit the expression of CD9 in breast cancer cells [60]. miR-573 and miR-454 could suppress the expression of TSPAN1 in gastric cancer and pancreatic cancer cells, respectively [35, 79]. Meanwhile, TSPAN12 can be the target of miR-495 and miR-196b-5p in lung cancer cells [76, 77]. miR-339-5p has been reported to inhibit TSPAN15 expression in esophagus cancer cells [14], while miR-193a-3p targets TSPAN3 in acute myeloid leukemia [101]. Moreover, miR-135b could depress TSPAN31 expression in hepatocellular carcinoma cells [65]. Finally, RNA binding protein Musashi 2 has been reported to bind with the TSPAN3 mRNA and increase its expression [91]. (3) The post-translational regulation of TSPAN proteins includes glycosylation, palmitoylation and phosphorylation. Glycosylation of TSPAN proteins seems to augment their function. Glycosylation of CD63 in breast cancer cells by RPN2 could increase the cell membrane localization of CD63 [13]. Glycosylation of TSPAN-1 at four distinct sites promotes its correct folding and transition through the endoplasmic reticulum in ovarian cancer cells [21]. Glycosylation of CD82 by the glycosyltransferase MGAT3 is pivotal to disrupt integrin α5β1-mediated cellular adhesion and cytoskeleton rearrangements [22]. Palmitoylation and phosphorylation of TSPAN8 have been reported to facilitate its nucleus translocation, which enhances the chromatin occupancy of STAT3 transcription factor [47].
In conclusion, emerging data has elucidated the critical role of TSPANs in cancer development. Although TSPANs have no natural ligands, they interact with other proteins to elicit their function on cancer cells, ranging from proliferation, apoptosis, migration, invasion, chemoresistance, stemness, to exosome biogenesis. Future work can be done in the following aspects: (1) Elucidate the function of other TSPAN members in carcinogenesis; (2) Demonstrate the upstream and downstream molecular mechanism of TSPANs; (3)Validate the therapeutic efficiency of TSPAN-based strategies, including developing specific antibodies, gene therapy, specific CAR-T cells and others; (4) Reveal the role of TSPANs in the uptake of exosomes; (5) Explore the role of TSPANs in cancer immunology or other stroma cells in the TME; (6) Resolve the structure of TSPAN proteins.
Availability of data and materials
Not applicable.
Abbreviations
- TSPAN:
-
Tetraspanin
- TEM:
-
Tetraspanin-enriched microdomains
- EV:
-
Extracellular vesicle
- TM:
-
Transmembrane domain
- ECL:
-
Extracellular loop
- ICL:
-
Intracellular loop
- mtTGF-β:
-
membrane type TGF-β
- ASCT2:
-
Sodium-Dependent Neutral Amino Acid Transporter Type 2
- EFGR:
-
Epidermal growth factor receptor
- FATP1:
-
Fatty acid transporter protein 1
- MDR1:
-
Multidrug resistance protein 1
- SOCSS3:
-
Suppressor of cytokine signaling 3
- ATXN3:
-
Ataxin 3
- ADAM10:
-
ADAM metallopeptidase domain 10
- BTRC:
-
Beta-transducin repeat containing E3 ubiquitin protein ligase
- EWI2:
-
Glu-Trp-Ile EWI motif-containing protein 2
- NF-κB:
-
Nuclear factor kappa B subunit 1
- IκBα:
-
NF-kappa-B inhibitor alpha
- EMT:
-
Epithelial-mesenchymal transition
- FAK:
-
Focal adhesion kinase 1
- Src:
-
SRC Proto-oncogene
- PTEN:
-
Phosphatase and tensin homolog
- LC3:
-
Microtubule associated protein 1 light chain 3 alpha
- SPTLC1:
-
Serine palmitoyltransferase long chain base subunit 1
- CDK4:
-
Cyclin dependent kinase 4
- MDCK:
-
Madin-Darby canine kidney cells
- CSC:
-
Cancer stem cell
- ALDHA1:
-
Aldehyde dehydrogenase 1 family member A1
- IL-10:
-
Interleukin 10
- BTK:
-
Bruton tyrosine kinase
- Rad51:
-
Recombination protein A
- EE:
-
Early endosomes
- MVB:
-
Multivesicular bodies
- MHC-I:
-
MHC class I molecules
- MHC-II:
-
MHC class II molecules
- pMHC:
-
Peptide-bound MHC complexes
- APCs:
-
Antigen presenting cells
- DCs:
-
Dendritic cells
- SOX9:
-
SRY-box transcription factor 9
- LSD1:
-
Lysine specific demethylase 1
- BC:
-
Breast cancer
- GC:
-
Gastric cancer
- PDAC:
-
Pancreatic ductal carcinoma
- OVC:
-
Ovarian carcinoma
- TNBC:
-
Triple-negative carcinoma
- ESCC:
-
Esophagus squamous cell carcinoma
- NSCLC:
-
Non-small cell lung cancer
- SCLC:
-
Small cell lung cancer
- AML:
-
Acute myeloid leukemia
- BCL:
-
B cell lymphoma
- TCL:
-
T cell leukemia
- HNSCC:
-
Head and neck squamous cell carcinoma
- CCA:
-
Cholangiocarcinoma
- PC:
-
Prostate cancer
- FA:
-
Fatty acid
References
Hemler ME. Tetraspanin proteins promote multiple cancer stages. Nat Rev Cancer. 2014;14:49–60.
Bonnet M, Maisonial-Besset A, Zhu Y, Witkowski T, Roche G, Boucheix C, et al. Targeting the tetraspanins with monoclonal antibodies in oncology: focus on Tspan8/Co-029. Cancers. 2019;11:179.
Garcia-Mayea Y, Mir C, Carballo L, Sánchez-García A, Bataller M, LLeonart ME. TSPAN1, a novel tetraspanin member highly involved in carcinogenesis and chemoresistance. Biochim Biophys Acta Rev Cancer. 2022. https://doi.org/10.1016/j.bbcan.2021.188674.
Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–11.
Umeda R, Satouh Y, Takemoto M, Nakada-Nakura Y, Liu K, Yokoyama T, et al. Structural insights into tetraspanin CD9 function. Nat Commun. 2020;11:1606.
Cai S, Deng Y, Peng H, Shen J. Role of tetraspanins in hepatocellular carcinoma. Front Oncol. 2021. https://doi.org/10.3389/fonc.2021.723341.
Lammerding J, Kazarov AR, Huang H, Lee RT, Hemler ME. Tetraspanin CD151 regulates alpha6beta1 integrin adhesion strengthening. Proc Natl Acad Sci USA. 2003;100:7616–21.
Hong IK, Jeoung D-I, Ha KS, Kim YM, Lee H. Tetraspanin CD151 stimulates adhesion-dependent activation of Ras, Rac, and Cdc42 by facilitating molecular association between β1 integrins and small GTPases. J Biol Chem. 2012;287:32027–39.
Lu X, An L, Fan G, Zang L, Huang W, Li J, et al. EGFR signaling promotes nuclear translocation of plasma membrane protein TSPAN8 to enhance tumor progression via STAT3-mediated transcription. Cell Res. 2022;32:359–74.
Hu M, Lu Y, Wang S, Zhang Z, Qi Y, Chen N, et al. CD63 acts as a functional marker in maintaining hematopoietic stem cell quiescence through supporting TGFβ signaling in mice. Cell Death Differ. 2021;29:178–91.
Wang VMY, Ferreira RMM, Almagro J, Evan T, Legrave N, Zaw Thin M, et al. CD9 identifies pancreatic cancer stem cells and modulates glutamine metabolism to fuel tumour growth. Nat Cell Biol. 2019;21:1425–35.
Peeters R, Cuenca-Escalona J, Zaal EA, Hoekstra AT, Balvert ACG, Vidal-Manrique M, et al. Fatty acid metabolism in aggressive B-cell lymphoma is inhibited by tetraspanin CD37. Nature Commun. 2022;13:1–18.
Tominaga N, Hagiwara K, Kosaka N, Honma K, Nakagama H, Ochiya T. RPN2-mediated glycosylation of tetraspanin CD63 regulates breast cancer cell malignancy. Mol Cancer. 2014;13:134.
Zhang B, Zhang Z, Li L, Qin YR, Liu H, Jiang C, et al. TSPAN15 interacts with BTRC to promote oesophageal squamous cell carcinoma metastasis via activating NF-κB signaling. Nat Commun. 2018;9:1423.
de Winde CM, Veenbergen S, Young KH, Xu-Monette ZY, Wang XX, Xia Y, et al. Tetraspanin CD37 protects against the development of B cell lymphoma. J Clin Invest. 2016;126:653–66.
Zhu R, Gires O, Zhu L, Liu J, Li J, Yang H, et al. TSPAN8 promotes cancer cell stemness via activation of sonic Hedgehog signaling. Nat Commun. 2019;10:2863.
Sidahmed-Adrar N, Ottavi JF, Benzoubir N, Ait Saadi T, Bou Saleh M, Mauduit P, et al. Tspan15 is a new stemness-related marker in hepatocellular carcinoma. Proteomics. 2019;19:e1900025.
Ramos EK, Tsai CF, Jia Y, Cao Y, Manu M, Taftaf R, et al. Machine learning-assisted elucidation of CD81-CD44 interactions in promoting cancer stemness and extracellular vesicle integrity. Elife. 2022;11:e82669.
Voglstaetter M, Thomsen AR, Nouvel J, Koch A, Jank P, Navarro EG, et al. Tspan8 is expressed in breast cancer and regulates E-cadherin/catenin signalling and metastasis accompanied by increased circulating extracellular vesicles. J Pathol. 2019;248:421–37. https://doi.org/10.1002/path.5281.
Humbert PO, Pryjda TZ, Pranjic B, Farrell A, Fujikura K, de Matos SR, et al. TSPAN6 is a suppressor of Ras-driven cancer. Oncogene. 2022;41:2095–105.
Scholz C-J, Sauer G, Deissler H. Glycosylation of tetraspanin Tspan-1 at four distinct sites promotes its transition through the endoplasmic reticulum. Protein Pept Lett. 2009;16:1244–8.
Li J, Xu J, Li L, Ianni A, Kumari P, Liu S, et al. MGAT3-mediated glycosylation of tetraspanin CD82 at asparagine 157 suppresses ovarian cancer metastasis by inhibiting the integrin signaling pathway. Theranostics. 2020;10:6467–82.
Vences-Catalan F, Levy S. Tetraspanins in cell stemness and cancer initiation: markers or active players? Trends Cell Biol. 2022;32:377–9.
Colbert JD, Cruz FM, Baer CE, Rock KL. Tetraspanin-5-mediated MHC class I clustering is required for optimal CD8 T cell activation. Proc Natl Acad Sci. 2022;119(42):e2122188119. https://doi.org/10.1073/pnas.2122188119.
Wang T, Wang X, Wang H, Li L, Zhang C, Xiang R, et al. High TSPAN8 expression in epithelial cancer cell-derived small extracellular vesicles promote confined diffusion and pronounced uptake. J Extracell Vesicles. 2021;10:e12167.
Kaur S, Livak F, Daaboul G, Anderson L, Roberts DD. Single vesicle analysis of CD47 association with integrins and tetraspanins on extracellular vesicles released by T lymphoblast and prostate carcinoma cells. J Extracell Vesicles. 2022;11:e12265.
Huang Y, Zucker B, Zhang S, Elias S, Zhu Y, Chen H, et al. Migrasome formation is mediated by assembly of micron-scale tetraspanin macrodomains. Nature Cell Biol. 2019;21:991–1002.
Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5:28.
Mao LH, Chen SY, Li XQ, Xu F, Lei J, Wang QL, et al. LncRNA-LALR1 upregulates small nucleolar RNA SNORD72 to promote growth and invasion of hepatocellular carcinoma. Aging. 2020;12:4527–46.
Yang Z, Zhou L, Si T, Chen S, Liu C, Ng KK, et al. Lysyl hydroxylase LH1 promotes confined migration and metastasis of cancer cells by stabilizing Septin2 to enhance actin network. Mol Cancer. 2023;22:21.
Klein CA. Cancer progression and the invisible phase of metastatic colonization. Nat Rev Cancer. 2020;20:681–94.
Zhao R, He B, Bie Q, Cao J, Lu H, Zhang Z, et al. AQP5 complements LGR5 to determine the fates of gastric cancer stem cells through regulating ULK1 ubiquitination. J Exp Clin Cancer Res. 2022;41:322.
Wang Y, Liang Y, Yang G, Lan Y, Han J, Wang J, et al. Tetraspanin 1 promotes epithelial-to-mesenchymal transition and metastasis of cholangiocarcinoma via PI3K/AKT signaling. J Exp Clin Cancer Res. 2018;37:300.
Zhang X, Shi G, Gao F, Liu P, Wang H, Tan X. TSPAN1 upregulates MMP2 to promote pancreatic cancer cell migration and invasion via PLCγ. Oncol Rep. 2019;41:2117–25.
Lu Z, Luo T, Nie M, Pang T, Zhang X, Shen X, et al. TSPAN1 functions as an oncogene in gastric cancer and is downregulated by miR-573. FEBS Lett. 2015;589:1988–94. https://doi.org/10.1016/j.febslet.2015.05.044.
Scholz CJ, Kurzeder C, Koretz K, Windisch J, Kreienberg R, Sauer G, et al. Tspan-1 is a tetraspanin preferentially expressed by mucinous and endometrioid subtypes of human ovarian carcinomas. Cancer Lett. 2009;275:198–203.
Wu Y, Chen W, Gong Y, Liu H, Zhang B. Tetraspanin 1 (TSPAN1) promotes growth and transferation of breast cancer cells via mediating PI3K/Akt pathway. Bioengineered. 2021;12:10761–70. https://doi.org/10.1080/21655979.2021.2003130.
Chen L, Yuan D, Zhao R, Li H, Zhu J. Suppression of TSPAN1 by RNA interference inhibits proliferation and invasion of colon cancer cells in vitro. Tumori. 2010;96:744–50.
Munkley J, McClurg UL, Livermore KE, Ehrmann I, Knight B, McCullagh P, et al. The cancer-associated cell migration protein TSPAN1 is under control of androgens and its upregulation increases prostate cancer cell migration. Sci Rep. 2017;7:5249.
Nabokina SM, Senthilkumar SR, Said HM. Tspan-1 interacts with the thiamine transporter-1 in human intestinal epithelial cells and modulates its stability. Am J Physiol Gastrointest Liver Physiol. 2011;301:808–13. https://doi.org/10.1152/ajpgi.00269.2011.
Zhang HS, Liu HY, Zhou Z, Sun HL, Liu MY. TSPAN8 promotes colorectal cancer cell growth and migration in LSD1-dependent manner. Life Sci. 2020;241:117114.
Li J, Chen X, Zhu L, Lao Z, Zhou T, Zang L, et al. SOX9 is a critical regulator of TSPAN8-mediated metastasis in pancreatic cancer. Oncogene. 2021;40:4884–93.
el Kharbili M, Agaësse G, Barbollat-Boutrand L, Pommier RM, de la Fouchardière A, Larue L, et al. Tspan8-β-catenin positive feedback loop promotes melanoma invasion. Oncogene. 2019;38:3781–93.
Berthier-Vergnes O, Barbollat-Boutrand L, Pommier RM, de la Fouchardière A, Combemale P, Grimont M, et al. Tetraspanin8 expression predicts an increased metastatic risk and is associated with cancer-related death in human cutaneous melanoma. Mol Cancer. 2021. https://doi.org/10.1186/s12943-021-01429-0.
Yue S, Mu W, Zöller M. Tspan8 and CD151 promote metastasis by distinct mechanisms. Eur J Cancer. 2013;49:2934–48.
el Kharbili M, Cario M, Béchetoille N, Pain C, Boucheix C, Degoul F, et al. Tspan8 drives melanoma dermal invasion by promoting ProMMP-9 activation and basement membrane proteolysis in a keratinocyte-dependent manner. Cancers. 2020;12:1297.
Huang Y, Li J, Du W, Li S, Li Y, Qu H, et al. Nuclear translocation of the 4-pass transmembrane protein Tspan8. Cell Res. 2021;31:1218–21.
Liu J, Chen C, Li G, Chen D, Zhou Q. Upregulation of TSPAN12 is associated with the colorectal cancer growth and metastasis. Am J Transl Res. 2017;9:812.
Knoblich K, Wang HX, Sharma C, Fletcher AL, Turley SJ, Hemler ME. Tetraspanin TSPAN12 regulates tumor growth and metastasis and inhibits β-catenin degradation. Cell Mol Life Sci. 2014;71:1305–14. https://doi.org/10.1007/s00018-013-1444-8.
Hu Z, Hou D, Wang X, You Z, Cao X. TSPAN12 is overexpressed in NSCLC via p53 inhibition and promotes NSCLC cell growth in vitro and in vivo. Onco Targets Ther. 2018;11:1095–103.
Romanska HM, Potemski P, Kusinska R, Kopczynski J, Sadej R, Kordek R. Expression of CD151/Tspan24 and integrin alpha 3 complex in aid of prognostication of HER2-negative high-grade ductal carcinoma in situ. Int J Clin Exp Pathol. 2015;8:9471–8.
Voss MA, Gordon N, Maloney S, Ganesan R, Ludeman L, McCarthy K, et al. Tetraspanin CD151 is a novel prognostic marker in poor outcome endometrial cancer. Br J Cancer. 2011;104:1611–8.
Yang XH, Richardson AL, Torres-Arzayus MI, Zhou P, Sharma C, Kazarov AR, et al. CD151 accelerates breast cancer by regulating α6 integrin function, signaling, and molecular organization. Cancer Res. 2008;68:3204–13.
Ke AW, Shi GM, Zhou J, Wu FZ, Ding ZB, Hu MY, et al. Role of overexpression of CD151 and/or c-Met in predicting prognosis of hepatocellular carcinoma. Hepatology. 2009;49:491–503. https://doi.org/10.1002/hep.22639.
Zhu J, Cai T, Zhou J, Du W, Zeng Y, Liu T, et al. CD151 drives cancer progression depending on integrin α3β1 through EGFR signaling in non-small cell lung cancer. J Exp Clin Cancer Res. 2021;40:1–18. https://doi.org/10.1186/s13046-021-01998-4.
Hiroshima K, Shiiba M, Oka N, Hayashi F, Ishida S, Fukushima R, et al. Tspan15 plays a crucial role in metastasis in oral squamous cell carcinoma. Exp Cell Res. 2019;384:111622.
Koo CZ, Harrison N, Noy PJ, Szyroka J, Matthews AL, Hsia HE, et al. The tetraspanin Tspan15 is an essential subunit of an ADAM10 scissor complex. J Biol Chem. 2020;295:12822–39.
Vences-Catalán F, Rajapaksa R, Kuo CC, Miller CL, Lee A, Ramani VC, et al. Targeting the tetraspanin CD81 reduces cancer invasion and metastasis. Proc Natl Acad Sci USA. 2021;118:e2018961118.
Kischel P, Bellahcene A, Deux B, Lamour V, Dobson R, de Pauw E, et al. Overexpression of CD9 in human breast cancer cells promotes the development of bone metastases. Anticancer Res. 2012;32:5211–20.
Bond DR, Kahl R, Brzozowski JS, Jankowski H, Naudin C, Pariyar M, et al. Tetraspanin CD9 is regulated by miR-518f-5p and functions in breast cell migration and in vivo tumor growth. Cancers. 2020;12:795.
Rappa G, Green TM, Lorico A. The nuclear pool of tetraspanin CD9 contributes to mitotic processes in human breast carcinoma. Mol Cancer Res. 2014;12:1840–50.
Duch P, Díaz-Valdivia N, Ikemori R, Gabasa M, Radisky ES, Arshakyan M, et al. Aberrant TIMP-1 overexpression in tumor-associated fibroblasts drives tumor progression through CD63 in lung adenocarcinoma. Matrix Biol. 2022;111:207–25.
Ma X, Qiu S, Tang X, Song Q, Wang P, Wang J, et al. TSPAN31 regulates the proliferation, migration, and apoptosis of gastric cancer cells through the METTL1/CCT2 pathway. Transl Oncol. 2022;20:101423.
Takashima Y, Komatsu S, Ohashi T, Kiuchi J, Kamiya H, Shimizu H, et al. Overexpression of tetraspanin31 contributes to malignant potential and poor outcomes in gastric cancer. Cancer Sci. 2022;113:1984–98.
Wang J, Zhou Y, Li D, Sun X, Deng Y, Zhao Q. TSPAN31 is a critical regulator on transduction of survival and apoptotic signals in hepatocellular carcinoma cells. FEBS Lett. 2017;591:2905–18. https://doi.org/10.1002/1873-3468.12737.
Jaiswal RK, Kumar P, Kumar M, Yadava PK. hTERT promotes tumor progression by enhancing TSPAN13 expression in osteosarcoma cells. Mol Carcinog. 2018;57:1038–54. https://doi.org/10.1002/mc.22824.
Odintsova E, Sugiura T, Berditchevski F. Attenuation of EGF receptor signaling by a metastasis suppressor, the tetraspanin CD82/KAI-1. Curr Biol. 2000;10:1009–12.
Ordas L, Costa L, Lozano A, Chevillard C, Calovoulos A, Kantar D, et al. Mechanical control of cell migration by the metastasis suppressor tetraspanin CD82/KAI1. Cells. 2021;10:1545.
Zhang XA, Lane WS, Charrin S, Rubinstein E, Liu L. EWI2/PGRL associates with the metastasis suppressor KAI1/CD82 and inhibits the migration of prostate cancer cells. Cancer Res. 2003;63:2665–74.
Li PY, Lv J, Qi WW, Zhao SF, Sun L-B, Liu N, et al. Tspan9 inhibits the proliferation, migration and invasion of human gastric cancer SGC7901 cells via the ERK1/2 pathway. Oncol Rep. 2016;36:448–54. https://doi.org/10.3892/or.2016.4805/abstract.
Lupia A, Peppicelli S, Witort E, Bianchini F, Carloni V, Pimpinelli N, et al. CD63 tetraspanin is a negative driver of epithelial-to-mesenchymal transition in human melanoma cells. J Invest Dermatol. 2014;134:2947–56.
Shao S, Piao L, Guo L, Wang J, Wang L, Wang J, et al. Tetraspanin 7 promotes osteosarcoma cell invasion and metastasis by inducing EMT and activating the FAK-Src-Ras-ERK1/2 signaling pathway. Cancer Cell Int. 2022;22:183.
Wang X, Lin M, Zhao J, Zhu S, Xu M, Zhou X. TSPAN7 promotes the migration and proliferation of lung cancer cells via epithelial-to-mesenchymal transition. Onco Targets Ther. 2018;11:8815–22.
Cheong CM, Chow AWS, Fitter S, Hewett DR, Martin SK, Williams SA, et al. Tetraspanin 7 (TSPAN7) expression is upregulated in multiple myeloma patients and inhibits myeloma tumour development in vivo. Exp Cell Res. 2015;332:24–38.
Yu X, Li S, Pang M, Du Y, Xu T, Bai T, et al. TSPAN7 exerts anti-tumor effects in bladder cancer through the PTEN/PI3K/AKT pathway. Front Oncol. 2021;10:3040.
Ye M, Wei T, Wang Q, Sun Y, Tang R, Guo L, et al. TSPAN12 promotes chemoresistance and proliferation of SCLC under the regulation of miR-495. Biochem Biophys Res Commun. 2017;486:349–56.
Liang G, Meng W, Huang X, Zhu W, Yin C, Wang C, et al. miR-196b-5p-mediated downregulation of TSPAN12 and GATA6 promotes tumor progression in non-small cell lung cancer. Proc Natl Acad Sci USA. 2020;117:4347–57.
Chen S, Ning B, Song J, Yang Z, Zhou L, Chen Z, et al. Enhanced pentose phosphate pathway activity promotes pancreatic ductal adenocarcinoma progression via activating YAP/MMP1 axis under chronic acidosis. Int J Biol Sci. 2022;18:2304–16.
Zhou C, Liang Y, Zhou L, Yan Y, Liu N, Zhang R, et al. TSPAN1 promotes autophagy flux and mediates cooperation between WNT-CTNNB1 signaling and autophagy via the MIR454-FAM83A-TSPAN1 axis in pancreatic cancer. Autophagy. 2021;17:3175–95.
Wang H, Jin X, Zhang Y, Wang Z, Zhang T, Xu J, et al. Inhibition of sphingolipid metabolism in osteosarcoma protects against CD151-mediated tumorigenicity. Cell Biosci. 2022;12:169.
Yuan S, Yin Y, Wang K, Zhou H, Qian C. Tetraspanin-29 activates Notch signaling by interacting with ADAM10 to enhance its activity in colorectal cancer. Biochem Cell Biol. 2022;100:292–300.
Xia Y, Deng Y, Zhou Y, Li D, Sun X, Gu L, et al. TSPAN31 suppresses cell proliferation in human cervical cancer through down-regulation of its antisense pairing with CDK4. Cell Biochem Funct. 2020;38:660–8. https://doi.org/10.1002/cbf.3526.
Andrijes R, Hejmadi RK, Pugh M, Rajesh S, Novitskaya V, Ibrahim M, et al. Tetraspanin 6 is a regulator of carcinogenesis in colorectal cancer. Proc Natl Acad Sci USA. 2021;118:e2011411118.
Shi W, Fan H, Shum L, Derynck R. The tetraspanin Cd9 associates with transmembrane TGF-α and regulates TGF-α–induced egf receptor activation and cell proliferation. J Cell Biol. 2000;148:591–602.
Xu-Monette ZY, Li L, Byrd JC, Jabbar KJ, Manyam GC, de Winde CM, et al. Assessment of CD37 B-cell antigen and cell of origin significantly improves risk prediction in diffuse large B-cell lymphoma. Blood. 2016;128:3083–100.
Marusyk A, Janiszewska M, Polyak K. Intratumor heterogeneity: the rosetta stone of therapy resistance. Cancer Cell. 2020;37:471–84.
Prasetyanti PR, Medema JP. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer. 2017;16:41.
Paul R, Dorsey JF, Fan Y. Cell plasticity, senescence, and quiescence in cancer stem cells: Biological and therapeutic implications. Pharmacol Ther. 2022;231:107985.
Rao J, Zhou Z-H, Yang J, Shi Y, Xu S-L, Wang B, et al. Semaphorin-3F suppresses the stemness of colorectal cancer cells by inactivating Rac1. Cancer Lett. 2015;358:76–84.
Liu Y, Wang G, Zhang J, Chen X, Xu H, Heng G, et al. CD9, a potential leukemia stem cell marker, regulates drug resistance and leukemia development in acute myeloid leukemia. Stem Cell Res Ther. 2021;12:1–13. https://doi.org/10.1186/s13287-021-02155-6.
Kwon HY, Bajaj J, Ito T, Blevins A, Konuma T, Weeks J, et al. Tetraspanin 3 is required for the development and propagation of acute myelogenous leukemia. Cell Stem Cell. 2015;17:152–64.
Nishioka C, Ikezoe T, Yang J, Nobumoto A, Kataoka S, Tsuda M, et al. CD82 regulates STAT5/IL-10 and supports survival of acute myelogenous leukemia cells. Int J Cancer. 2014;134:55–64. https://doi.org/10.1002/ijc.28348.
Garcia-Mayea Y, Mir C, Carballo L, Castellvi J, Temprana-Salvador J, Lorente J, et al. TSPAN1: a novel protein involved in head and neck squamous cell carcinoma chemoresistance. Cancers. 2020;12:1–20.
McCarthy N, Manieri E, Storm EE, Saadatpour A, Luoma AM, Kapoor VN, et al. Distinct mesenchymal cell populations generate the essential intestinal bmp signaling gradient. Cell Stem Cell. 2020;26:391-402.e5.
Zhang C, Liu X, Jin S, Chen Y, Guo R. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer. 2022;21:1–12. https://doi.org/10.1186/s12943-022-01530-y.
Nussinov R, Tsai CJ, Jang H. Anticancer drug resistance: an update and perspective. Drug Resist Updat. 2021;59:100796.
Zhou L, Mao LH, Li X, Wang QL, Chen SY, Chen ZJ, et al. Transcriptional regulation of NDUFA4L2 by NFIB induces sorafenib resistance by decreasing reactive oxygen species in hepatocellular carcinoma. Cancer Sci. 2022. https://doi.org/10.1111/cas.15648.
Kohmo S, Kijima T, Otani Y, Mori M, Minami T, Takahashi R, et al. Cell surface tetraspanin CD9 mediates chemoresistance in small cell lung cancer. Cancer Res. 2010;70:8025–35.
Quagliano A, Gopalakrishnapillai A, Anders Kolb E, Barwe SP. CD81 knockout promotes chemosensitivity and disrupts in vivo homing and engraftment in acute lymphoblastic leukemia. Blood Adv. 2020;4:4393–405.
Zheng W, Chen Q, Liu H, Pan Y, Shao C, Hu S, et al. CD81 enhances radioresistance of glioblastoma by promoting nuclear translocation of Rad51. Cancers. 2021;13:1998.
Sun H, Sun Y, Chen Q, Xu Z. LncRNA KCNQ1OT1 contributes to the progression and chemoresistance in acute myeloid leukemia by modulating Tspan3 through suppressing miR-193a-3p. Life Sci. 2020;241:117161.
Floren M, Restrepo Cruz S, Termini CM, Marjon KD, Lidke KA, Gillette JM. Tetraspanin CD82 drives acute myeloid leukemia chemoresistance by modulating protein kinase C alpha and β1 integrin activation. Oncogene. 2020;39:3910–25.
Mu W, Provaznik J, Hackert T, Zöller M. Tspan8-tumor extracellular vesicle-induced endothelial cell and fibroblast remodeling relies on the target cell-selective response. Cells. 2020;9:319.
Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28.
Saiz ML, Rocha-Perugini V, Sánchez-Madrid F. Tetraspanins as organizers of antigen-presenting cell function. Front Immunol. 2018;9:1074.
Dharan R, Goren S, Cheppali SK, Shendrik P, Brand G, Vaknin A, et al. Transmembrane proteins tetraspanin 4 and CD9 sense membrane curvature. Proc Natl Acad Sci USA. 2022;119:e2208993119.
Yáñez-Mó M, Siljander PRM, Andreu Z, Zavec AB, Borràs FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:1–60.
Rocha-Perugini V, del Hoyo GM, González-Granado JM, Ramírez-Huesca M, Zorita V, Rubinstein E, et al. CD9 Regulates major histocompatibility complex class II trafficking in monocyte-derived dendritic cells. Mol Cell Biol. 2017;37:e00202-e217.
Vogt S, Stadlmayr G, Stadlbauer K, Sádio F, Andorfer P, Grillari J, et al. Stabilization of the CD81 large extracellular loop with de novo disulfide bonds improves its amenability for peptide grafting. Pharmaceutics. 2018;10:138.
Li S, Li X, Yang S, Pi H, Li Z, Yao P, et al. Proteomic landscape of exosomes reveals the functional contributions of CD151 in triple-negative breast cancer. Mol Cell Proteomics. 2021;20:100121.
Yue S, Mu W, Erb U, Zöller M. The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding. Oncotarget. 2015;6:2366–84.
Liu Y, Fan J, Xu T, Ahmadinejad N, Hess K, Lin SH, et al. Extracellular vesicle tetraspanin-8 level predicts distant metastasis in non-small cell lung cancer after concurrent chemoradiation. Sci Adv. 2020;6:eaaz6162.
Ghossoub R, Chéry M, Audebert S, Leblanc R, Egea-Jimenez AL, Lembo F, et al. Tetraspanin-6 negatively regulates exosome production. Proc Natl Acad Sci USA. 2020;117:5913–22. https://doi.org/10.1073/pnas.1922447117.
Guix FX, Sannerud R, Berditchevski F, Arranz AM, Horré K, Snellinx A, et al. Tetraspanin 6: a pivotal protein of the multiple vesicular body determining exosome release and lysosomal degradation of amyloid precursor protein fragments. Mol Neurodegener. 2017;12:1–21.
Lee CH, Im EJ, Moon PG, Baek MC. Discovery of a diagnostic biomarker for colon cancer through proteomic profiling of small extracellular vesicles. BMC Cancer. 2018;18:1.
Nigri J, Leca J, Tubiana SS, Finetti P, Guillaumond F, Martinez S, et al. CD9 mediates the uptake of extracellular vesicles from cancer-associated fibroblasts that promote pancreatic cancer cell aggressiveness. Sci Signal. 2022;15:eabg8191.
Huang Y, Zhang X, Wang HW, Yu L. Assembly of Tetraspanin-enriched macrodomains contains membrane damage to facilitate repair. Nat Cell Biol. 2022;24:825–32.
Coulie PG, van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14:135–46.
Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev. 2013;31:443–73. https://doi.org/10.1146/annurev-immunol-032712-095910.
Rock KL, Farfán-Arribas DJ, Colbert JD, Goldberg AL. Re-examining class-I presentation and the DRiP hypothesis. Trends Immunol. 2014;35:144–52.
Untemaehrer JJ, Chow A, Pypaert M, Inaba K, Mellman I. The tetraspanin CD9 mediates lateral association of MHC class II molecules on the dendritic cell surface. Proc Natl Acad Sci USA. 2007;104:234–9.
Suzuki M, Tachibana I, Takeda Y, He P, Minami S, Iwasaki T, et al. Tetraspanin CD9 negatively regulates lipopolysaccharide-induced macrophage activation and lung inflammation. J Immunol. 2009;182:6485–93.
Szöllósi J, Horejsí V, Bene L, Angelisová P, Damjanovich S. Supramolecular complexes of MHC class I, MHC class II, CD20, and tetraspan molecules (CD53, CD81, and CD82) at the surface of a B cell line JY. J Immunol. 1996;157:2939–46.
Sheng KC, van Spriel AB, Gartlan KH, Sofi M, Apostolopoulos V, Ashman L, et al. Tetraspanins CD37 and CD151 differentially regulate Ag presentation and T-cell co-stimulation by DC. Eur J Immunol. 2009;39:50–5. https://doi.org/10.1002/eji.200838798.
Gartlan KH, Belz GT, Tarrant JM, Minigo G, Katsara M, Sheng K-C, et al. A Complementary role for the tetraspanins CD37 and Tssc6 in cellular immunity. J Immunol. 2010;185:3158–66.
Petersen SH, Odintsova E, Haigh TA, Rickinson AB, Taylor GS, Berditchevski F. The role of tetraspanin CD63 in antigen presentation via MHC class II. Eur J Immunol. 2011;41:2556–61. https://doi.org/10.1002/eji.201141438.
Gartlan KH, Wee JL, Demaria MC, Nastovska R, Chang TM, Jones EL, et al. Tetraspanin CD37 contributes to the initiation of cellular immunity by promoting dendritic cell migration. Eur J Immunol. 2013;43:1208–19. https://doi.org/10.1002/eji.201242730.
Jones EL, Wee JL, Demaria MC, Blakeley J, Ho PK, Vega-Ramos J, et al. Dendritic cell migration and antigen presentation are coordinated by the opposing functions of the tetraspanins CD82 and CD37. J Immunol. 2016;196:978–87.
Quast T, Eppler F, Semmling V, Schild C, Homsi Y, Levy S, et al. CD81 is essential for the formation of membrane protrusions and regulates Rac1-activation in adhesion-dependent immune cell migration. Blood. 2011;118:1818–27.
Feigelson SW, Grabovsky V, Shamri R, Levy S, Alon R. The CD81 tetraspanin facilitates instantaneous leukocyte VLA-4 adhesion strengthening to vascular cell adhesion molecule 1 (VCAM-1) under shear flow. J Biol Chem. 2003;278:51203–12.
McGowan ENS, Wong O, Jones E, Nguyen J, Wee J, Demaria MC, et al. Tetraspanin CD82 restrains phagocyte migration but supports macrophage activation. iScience. 2022;25:104520.
Todros-Dawda I, Kveberg L, Vaage JT, Inngjerdingen M. The tetraspanin CD53 modulates responses from activating NK cell receptors, promoting LFA-1 activation and dampening NK cell effector functions. PLoS ONE. 2014;9:e97844. https://doi.org/10.1371/journal.pone.0097844.
Cao L, Yoshino T, Kawasaki N, Sakuma I, Takahashi K, Akagi T. Anti-CD53 monoclonal antibody induced LFA-1/ICAM-1 -dependent and -independent lymphocyte homotypic cell aggregation. Immunobiology. 1997;197:70–81.
Navarro-Hernandez IC, López-Ortega O, Acevedo-Ochoa E, Cervantes-Díaz R, Romero-Ramírez S, Sosa-Hernández VA, et al. Tetraspanin 33 (TSPAN33) regulates endocytosis and migration of human B lymphocytes by affecting the tension of the plasma membrane. FEBS J. 2020;287:3449–71. https://doi.org/10.1111/febs.15216.
Dunlock VME, Arp AB, Singh SP, Charrin S, Nguyen V, Jansen E, et al. Tetraspanin CD53 controls T cell immunity through regulation of CD45RO stability, mobility, and function. Cell Rep. 2022;39:111006.
Jung JW, Kim JE, Kim E, Lee JW. Amino acid transporters as tetraspanin TM4SF5 binding partners. Exp Mol Med. 2020;52:7–14.
Ma L, Li Y, Peng J, Wu D, Zhao X, Cui Y, et al. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 2015;25:24–38.
Yu S, Yu L. Migrasome biogenesis and functions. FEBS J. 2022;289:7246–54.
Florin L, Lang T. Tetraspanin assemblies in virus infection. Front Immunol. 2018;9:1140.
Malla R, Kamal MA. Tetraspanin-enriched microdomain containing CD151, CD9, and TSPAN 8—Potential mediators of entry and exit mechanisms in respiratory viruses including SARS-CoV-2. Curr Pharm Des. 2022;28:3649–57.
Lasswitz L, Zapatero-Belinchón FJ, Moeller R, Hülskötter K, Laurent T, Carlson LA, et al. The tetraspanin CD81 is a host factor for chikungunya virus replication. MBio. 2022;13:e00731-e822.
Acknowledgments
The figures were reated with BioRender.com.
Funding
This work was supported by the National Natural Science Fund (No. 81972285), the Natural Science Foundation of Chongqing (Chongqing, China, CSTB2022NSCQ-MSX1038, CSTB2022NSCQ-MSX0775), Senior Medical Talents Program of Chongqing for Young and Middle-aged and Kuanren Talents Program of the Second Affiliated Hospital of Chongqing Medical University (13-002-011, 13-004-009), Hetao Shenzhen-Hong Kong Science and Technology Innovation Cooperation Zone Shenzhen Park Project (HZQB-KCZYZ-2021017).
Author information
Authors and Affiliations
Contributions
ZHZ was a major contributor in collecting the information, designing the figures, and writing and harmonizing the manuscript. ZHY was a contributor in collecting the information, designing the figures, and writing and harmonizing the manuscript. LZ was a contributor in collecting the information, designing the figures, and writing the manuscript. MSY was a contributor in designing and reviewing the manuscript. SH was a major contributor in designing, reviewing, writing and harmonizing the manuscript, and designing the figures. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, Z., Yang, Z., Zhou, L. et al. The versatile roles of testrapanins in cancer from intracellular signaling to cell–cell communication: cell membrane proteins without ligands. Cell Biosci 13, 59 (2023). https://doi.org/10.1186/s13578-023-00995-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13578-023-00995-8