Abstract
Background
Central nervous system (CNS) control of metabolism plays a pivotal role in maintaining energy balance. In the brain, Glucagon-like peptide 1 (GLP-1), encoded by the proglucagon ‘Gcg’ gene, produced in a distinct population of neurons in the nucleus tractus solitarius (NTS), has been shown to regulate feeding behavior leading to the suppression of appetite. However, neuronal networks that mediate endogenous GLP-1 action in the CNS on feeding and energy balance are not well understood.
Results
We analyzed the distribution of GLP-1R-expressing neurons and axonal projections of NTS GLP-1-producing neurons in the mouse brain. GLP-1R neurons were found to be broadly distributed in the brain and specific forebrain regions, particularly the hypothalamus, including the arcuate nucleus of the hypothalamus (ARC), a brain region known to regulate energy homeostasis and feeding behavior, that receives dense NTSGcg neuronal projections. The impact of GLP-1 signaling in the ARC GLP-1R-expressing neurons and the impact of activation of ARC GLP-1R on food intake was examined. Application of GLP-1R specific agonist Exendin-4 (Exn-4) enhanced a proportion of the ARC GLP-1R-expressing neurons and pro-opiomelanocortin (POMC) neuronal action potential firing rates. Chemogenetic activation of the ARC GLP-1R neurons by using Cre-dependent hM3Dq AAV in the GLP-1R-ires-Cre mice, established that acute activation of the ARC GLP-1R neurons significantly suppressed food intake but did not have a strong impact on glucose homeostasis.
Conclusions
These results highlight the importance of central GLP-1 signaling in the ARC that express GLP-1R that upon activation, regulate feeding behavior.
Similar content being viewed by others
Introduction
Glucagon-like peptide 1(GLP-1), an incretin hormone encoded by the proglucagon Gcg gene, is produced by intestinal enteroendocrine L cells and a subpopulation of hindbrain nucleus tractus solitarius (NTS) neurons in both rodents and humans (for review, see [1]). GLP-1 actions are mediated by the GLP-1 receptor (GLP-1R), a class B subfamily G-protein-couple-receptor, widely expressed in central and peripheral nervous systems [2,3,4]. There is now ample evidence suggesting that the endogenous GLP-1 signaling works on both peripheral organs and the brain to regulate energy homeostasis, including but not exclusively through glucose metabolism and food intake [5]. GLP-1R agonists are now widely used therapeutics to treat type 2 diabetes and more recently obesity [5, 6]. Studies in animals have demonstrated that central GLP-1-producing NTSGcg neurons project to various regions in the forebrain [7]. Still, the physiological role of endogenous GLP-1 signaling in the control of food intake and body weight remains enigmatic [5].
Despite the clear indication that acute intracerebroventricular injections of GLP-1R antagonist exendin-9 in rats increases food intake [8], various genetic perturbation of the GLP-1Rs, including a knockout of GLP-1R causes little effect on food intake or body weight [9,10,11]. While inhibition of GLP-1 expressing neurons in the brainstem increases feeding, food intake and meal size, and activation of these neurons suppress feeding; postnatal ablation of GLP-1 neurons does not impact food intake, meal size, energy expenditure, or body weight [12]. Although Burmeister et al. used multiple genetic approaches, including Nkx2-Cre, Pomc-Cre, Sim1-Cre, or nuclear receptor 5A1 (Nr5a)-Cre, to cross with floxed GLP-1R (i.e., GLP-1Rf/f) to broadly or selectively ablate the GLP-1R in mouse hypothalamus, these mice failed to show changes in the body weight [13, 14]. However, knockdown of GLP-1R within the lateral hypothalamus by RNAi in adult rats caused a significant increase in food intake, body weight, and fat mass [15]. Consistent with this notion, knockdown of GLP-1R in the dorsomedial hypothalamus increased food intake, decreased energy expenditure, and increased body weight over weeks of observation [16]. Also, the knockdown of the Gcg gene in the NTS increased food intake and body weight in high-fat-fed rats [17]. In addition, chemogenetic stimulation of Gcg neurons suppressed feeding when fed or fasted animals were fed chow or a high-fat diet [18]. On the other hand, acute chemogenetic inhibition of Gcg neurons did not increase ad libitum feeding but increased refeeding after fasting and blocked stress-induced hypophagia [19]. In our previous study, we found that NTS GLP-1 neurons project to the paraventricular hypothalamic nucleus (PVN) and enhance glutamatergic synaptic transmission, which is sufficient to suppress food intake and specific ablation of PVN GLP-1R causes overeating and obesity [20]. Nevertheless, the CNS GLP-1 signaling in regulating feeding behavior does not seem to be activated by peripherally secreted endogenous GLP-1 and, therefore, may be distinct from the peripheral GLP-1 system. Central and peripheral GLP-1 systems have been presumed to independently suppress the feeding [12]. These results highlight the complexity of GLP-1 regulation of energy homeostasis, but the cellular and circuit mechanisms mediating endogenous GLP-1 action in the CNS are still poorly understood. This is partly due to the presence of diverse neuronal subtypes and complex central neuronal connectivity in the brain.
The NTSGcg neurons send robust projections to multiple brain regions [12, 21, 22], especially to the mediobasal hypothalamic regions; PVN, lateral hypothalamus (LH), ventromedial nucleus of the hypothalamus (VMH) and arcuate nucleus of the hypothalamus (ARC) [7]. The ARC is an evolutionarily conserved brain region with essential roles in regulating the energy homeostasis [23]. The various ARC functions are supported by specialized neuronal subtypes, including two well-characterized neuronal populations that control feeding and insulin sensitivity: Agouti-related protein (AgRP) and Proopiomelanocortin (POMC) neurons [24,25,26]. However, a recent study that utilized the single-cell RNA-seq technology provided further evidence regarding the heterogeneous POMC cell populations within the ARC and revealed that the chemogenetic activation of POMC GLP-1R-expressing neurons could remarkably suppress feeding while activation of POMC Leptin receptor-expressing neurons cannot [27]. These results indicate that GLP-1 signaling plays an essential role within the ARC neurons.
Synaptic transmission mediates information flow in the brain to control behavior, including feeding and food intake [28]. Previous studies have shown that GLP-1R signaling may facilitate excitatory presynaptic release [29, 30] and increase presynaptic vesicle release probability in the mesolimbic dopamine system [31]. Our group has shown that GLP-1R activation suppresses excitatory synaptic strength in the mesolimbic pathway [32]. However, in the PVN, GLP-1R activation enhances AMPA receptor trafficking and the excitatory postsynaptic strength [20]. It also has been shown that the GLP-1R agonist, semaglutide/liraglutide, directly activates POMC neurons and indirectly inhibits NPY/AGRP neurons in the ARC [33, 34]. These results suggest that GLP-1 might play a distinctive role in different cell types and brain regions through the distinct mechanism to regulate neuronal functions. Nevertheless, the mechanistic regulation of GLP-1 signaling in modulating ARC neurons is not yet apparent.
To elucidate the involvement of GLP-1 signaling in the ARC neurons in regulating feeding behavior, we used GLP-1R-ires-Cre knock-in mice [35] and Gcg-Cre recombinase transgenic mice [18], which are unique genetic models for specifically investigating the functions of GLP-1R in the brain and the Gcg (proglucagon) GLP-1 releasing neurons located in the hindbrain, respectively. First, we systematically characterized the anatomy of the GLP-1R neuronal distribution in the whole brain using the GLP-1R-ires-Cre mice crossed with Ai14 reporter mice [36]. Next, we evaluated the GLP-1 neuronal (NTSGcg) projections in the mouse brain. Our data show that GLP-1R-expressing neurons are widely distributed in multiple brain regions, including mediobasal hypothalamic areas, such as the PVN, ARC, and VMH. Meanwhile, these brain regions also receive dense NTSGcg neuronal inputs. Since the ARC is an established significant target of the CNS GLP-1 system [37, 38], we focused on elucidating the ARC GLP-1 signaling in regulating synaptic transmission as well as feeding behavior. Our whole-cell patch-clamp recording analysis shows that Exn-4 enhances the action potential firing frequency in a proportion of ARC GLP-1R-expressing neurons and POMC neurons. Finally, acute chemogenetic activation of ARC GLP-1R neurons suppresses feeding dramatically but had no strong impact on glucose tolerance.
Results
Distributions of GLP-1R neurons in the mouse brain
To comprehensively investigate the whole-brain distribution of GLP-1R-expressing neurons, GLP-1R positive neurons were genetically labeled using GLP-1R-ires-Cre mice crossed to the tdTomato fluorescent reporter line Ai14 [36]. Consistent with previous reports, we found GLP-1R expression neurons, based on the expression of tdTomato (Fig. 1A). We systematically analyzed the cell number and cell density across the whole brain (Fig. 1B, C). In three animals, a total of 101,618 GLP-1R-expressing (tdTomato) neurons were counted from a series of 50 μm sections (every 6 sections). The majority of GLP-1R neurons were found in the hypothalamus (~ 29.59%), cortical regions (~ 20.71%), striatum (~ 11.54%), and midbrain (~ 9.6%). Within the hypothalamic region (Fig. 1D), dense populations of GLP-1R neurons were enriched in the subconical organ (SFO), ARC, supraoptic nucleus (SO), the suprachiasmatic nucleus (Sch), and paraventricular hypothalamic nucleus (PVN). Notably, the ARC region possesses a high abundance of GLP-1R neurons, consistent with a previous report on GLP-1R expression using a super-resolution fluorescent probe in the brain [39]. In addition, a high density of GLP-1R neurons are also found in the cortex, striatum [21], thalamus, and midbrain regions indicating that GLP-1 signaling also has essential functions in both cortical and subcortical neuronal networks (Fig. 1C; Additional file 1: Fig. S1 and Additional file 2: Fig. S2).
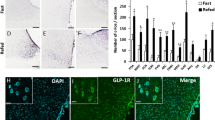
Quantification of GLP-1 receptor distribution in the whole brain. A Representative images of GLP-1R-ires-cre mouse crossed with Ai14 tdTomato (LSL-TdTomato) reporter mice in Suprachiasmatic nucleus (Sch), Paraventricular nucleus of the hypothalamus (PVN), Basomedial amygdaloid nucleus (BMA), anterior cortical amygdaloid area (ACo), medial amygdaloid nucleus (MeP). B Whole brain slice preparation of GLP-1R-ires-Cre::Ai14 tdTomato. C Percentage (%) of GLP-1R neuronal density in the brain. D Quantification of the total number of GLP-1R neurons area (density) in the brain. Data are presented as mean ± standard error of the mean (SEM), [n = 3]
Axonal projection patterns of NTSGcg neurons
A previous immunohistochemical study using custom-made GLP-1 monoclonal antibodies examined the distribution of GLP-1 expressing nerve terminals in the rat forebrain regions [7], but provided limited information about the overall GLP-1 neuronal projections in the mouse brain. To evaluate the NTSGcg neuronal projections in the mouse brain, we injected AAV-DIO-ChR2-EYFP into the NTS region of the Gcg-Cre mice [18] (Fig. 2A and B). The morphological analysis of the axonal terminals revealed dense GLP-1 neuronal terminals in the hypothalamus, especially in the PVN, DMH, ARC, and the medial vestibular parvicellular subfields nucleus (MvePC) (Fig. 2C and D). We also found dense projections in the median preoptic nucleus (MnPO), medial dorsal nucleus (MD), dorsal raphe nucleus (DR), and pontine reticular nucleus, oral part (PnO) (Additional file 3: Fig. S3 and Additional file 4: Fig. S4). These projections ascertain the prime brain regions involved in the GLP-1 signaling which regulate brain and body functions but are not limited to food intake, body weight, glucose metabolism, learning, and memory.
Axonal projection patterns of NTS Gcg endogenous GLP-1 neurons. A Gcg-Cre mouse injected with AAV-DIO-Chr2-EYFP in the NTS. B Representative image of the injection site in the Gcg-Cre mouse injected with AAV-DIO-Chr2-EYFP in the NTS. C Representative images of the axon terminals in the brain regions of Gcg-Cre mouse injected with AAV-DIO-ChR2-EYFP. D Identified brain regions with axon terminals from NTSGcg (GLP-1) neurons. A map representing the projection patterns of the GLP-1 neurons in the brain (n = 3)
Modulation of neuronal activity in ARC by GLP-1 signaling
The ARC region of the hypothalamus receives strong innervation from the NTS GLP-1 nerve terminals (Fig. 3A) and notes the abundance of the GLP-1R expressed in the ARC neurons (Fig. 3B and C). We next investigated the cellular impacts of GLP-1 signaling in the ARC neurons. We labeled ARC GLP-1R neurons by crossing GLP-1R-ires-Cre with Ai14 reporter mice (Fig. 3D). Whole-cell patch-clamp recording from the tdTomato-expressing GLP-1R neurons revealed the application of Exendin 4 (Exn4), a specific agonist of GLP-1R [40], regulates ARC neuronal firings. Our data suggested that GLP-1R signaling produces diverse cellular responses in the ARC GLP-1R-expressing neurons: ~ 75% of GLP-1R neurons showed depolarization (Fig. 3E), while the rest showed hyperpolarization (Fig. 3F and G). Moreover, Exn4 significantly increased the firing rates of neurons showing spontaneous action potentials (Fig. 3H and I). Next, we investigated the impact of Exn4 on one of the major neuronal types, POMC neurons in ARC. We labeled POMC neurons using a POMC-Cre mice [41] with Cre-dependent expression of GFP (AAV-DIO-eGFP) (Fig. 3J and K). Whole-cell patch-clamp recording from the POMC neurons revealed that Exn4 increased the AP firing of POMC neurons with a slight depolarization (Fig. 3L–N). The GLP-1R neurons recording data suggested a heterogenous regulation by GLP-1R mediated signaling, possibly due to the diverse cell types in the ARC.
Expression of GLP-1R in the ARC and the effects of Exn4 on ARC neurons. A GLP-1 neuronal axonal projections in the ARC region. AAV-DIO-Chr2-EYFP was injected into the NTS region of the Gcg-Cre mouse. B GLP-1R-expressing neurons in the ARC region revealed by tdTomato fluorescence in GLP-1R-ires-Cre::Ai14 (LSL-TdTomato) mice. C Expression of GLP-1R in the ARC using a conditional viral-mediated approach. AAV-DIO-eGFP was injected into the ARC region of the GLP-1R-ires-Cre mouse. D Experimental paradigm for recordings from GLP-1R-expressing neurons labeled with tdTomato in GLP-1R-ires-cre::Ai14 tdTomato (Lox-Stop-Lox-TdTomato) reporter mice. E and F The resting membrane potential (RMP) of ARC GLP-1R-expressing neurons in the absence or presence of Exn4. Paired t-test, *p < 0.05, ***p < 0.001. G Pie chart of the proportion of ARC GLP-1R-expressing neurons showing RMP changes in the presence of Exn-4. H Represent traces of membrane potentials with action potentials (APs) in the ARC GLP-1R neurons with or without Exn4. I Pooled data. Recordings were conducted in 4 animals. Paired t-test, *p < 0.05. J and K Experimental paradigm for recordings in POMC-neurons. POMC-Cre mice were injected with AAV-DIO-eYFP in the ARC. L Representative traces of APs in POMC neurons in the absence or presence of Exn4. M and N Pooled data. Data are presented as mean + S.E.M., n = 5/2 cells/animals. Paired t-test, *p < 0.05
Activation of ARC GLP-1R neurons does not regulate blood glucose and insulin secretion
Using pharmacological manipulations, it has been suggested that the ARC GLP-1 receptor-mediated signaling regulates glucose homeostasis, not food intake [2]. On the other hand, the role of central GLP-1 in regulating blood glucose and appetite has gained attention and has been reported in numerous studies. Hence, we next asked if chemogenetically activating the ARC GLP-1R neurons will affect glucose metabolism. To this end, we used Designer Receptor Exclusively Activated by Designer Drugs (DREADDs) [42] technology to activate ARC GLP-1R-expressing neurons. We injected AAV-DIO-hM3Dq-DREADD and AAV-FLEX-tdTomato (Control) into the ARC of the GLP-1-ires-Cre mice. 4 weeks post-injection, we administered DREADDs ligand clozapine-N-oxide (CNO, 1 mg/kg body weight) via intraperitoneal (i.p.) route. We confirmed that chemogenetic activation of GLP-1R neurons increased the expression of the c-Fos protein, an early-intermediate marker of neuronal activity (Fig. 4A–C). To evaluate the impact of the GLP-1R neurons mediated signaling on glucose metabolism, we performed a glucose tolerance test (GTT) and measured plasma levels of insulin. Thirty minutes after CNO injection, 1 g/kg glucose was administered i.p., however, the blood glucose levels show no difference (Fig. 4D–F). Similarly, no plasma insulin level changes were found at 1 h after CNO injection (Fig. 4G). Collectively, these data suggest that under the conditions studied, the ARC GLP-1R neurons in vivo does not mediate glucose tolerance and insulin secretions upon direct acute activation.
Acute activation response of ARC GLP-1R neurons on blood glucose. A Experimental paradigm for virus delivery and chemogenetic stimulation of the GLP-1R neurons in ARC. B Representative image of hM3Dq (red) and c-Fos (white) staining in the GLP-1R-ires-Cre ARC region. C Quantification of c-Fos and GLP-1R co-localized neurons in the ARC after chemogenetic activation (CNO) control n = 4, hM3Dq n = 5. D Average bodyweight of the control and hM3Dq groups after overnight fasting (12 h) control n = 7, hM3Dq n = 8. E Fasting glucose levels control n = 11, hM3Dq n = 12. F IP GTT glucose levels upon ARC GLP-1R neuronal activation, p-value = 0.0633 control n = 11, hM3Dq n = 12. G Insulin levels after 1 h ARC GLP-1R neuron activation via CNO delivery. Data are presented as mean ± standard error of the mean (SEM). Student’s t-test (Bar-graph); *p < 0.05; **p < 0.01, ***p < 0.001. Two-way Anova, with Geisser-Greenhouse correction
Activation of ARC GLP-1R neurons suppresses food intake behaviors
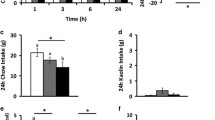
We next assessed the impact of activation of the ARC GLP-1R neurons on feeding behavior. We hypothesized that the activation of the GLP-1R neurons in the ARC might affect food intake. Animals (GLP-1R-ires-Cre mice with Cre-dependent expression of hM3Dq or tdTomato AAV vectors injected in the ARC) in this cohort were singly housed for 3 days, fasted overnight, and GLP-1R neurons were activated by i.p. CNO (Fig. 5A). Acute activation of ARC GLP-1R neurons led to significant decreases in food intake compared to control mice expressing mCherry alone (Fig. 5B). The effect of the DREADD ligand seemed to dissipate after 12 h; the activation had little impact on total food intake within 24 h after the application of CNO (Fig. 5C). This possibly could be due to the relatively short half-life of the DREADD ligand CNO [43].
Acute activation response of ARC GLP-1R neuron on feeding behavior. A Experimental paradigm for virus delivery and chemogenetic stimulation of the GLP-1R neurons in ARC. B Food intake consumption upon activation of the GLP-1R neurons in ARC (0.5 g food fed overnight), p value = 0.0261*, control n = 7, hM3Dq n = 8. C Food intake consumption after 24 h CNO injection. D In an exploratory open field test, the dependent locomotor activity of control and hM3Dq mice after CNO delivery, measuring various parameters. E Active count. F Total active time (min). G Total traveled distance (cm). H Time spent in the cage center (min). Data are presented as mean ± standard error of the mean (SEM). Student’s t-test; *p < 0.05; **p < 0.01, ***p < 0.001. Two-way Anova, with Geisser-Greenhouse correction
Because the behavioral state can influence feeding, we examined whether the inhibition of food intake was due to the presence of anxiety [44]. Mice from the previous experiments were placed in an open-field arena 30 min after injection of CNO. No differences were observed between mCherry and hM3Dq-expressing mice in the time spent in the center of the open field, providing evidence that activation of the ARC GLP-1R neurons does not cause overt anxiety (Fig. 5D–H). Overall, the data show that activation of the GLP-1R neurons in mice leads to repetitive behaviors that are not due to increased anxiety levels but remarkably suppressed feeding behavior.
Discussion
In this study, using viral-mediated labeling and genetically modified animal models, we surveyed the distribution of GLP-1R neurons and the overall projection patterns of the GLP-1-producing neurons in the mouse brain. In brain slices, activation of GLP-1R with specific agonist Exn-4 enhanced the firing rate of a proportion of the ARC neurons including the POMC-expressing neurons. We then found that activation of the ARC GLP-1R neurons suppressed food intake without a noticeable impact on glucose metabolism or causing any stress-related responses. This result highlights the importance of the central function of GLP-1 signaling within the ARC in regulating metabolism.
A widespread distribution of the GLP-1R neurons in the mouse brain was located, including the hypothalamus, cortical regions, striatum, and midbrain. It is interesting to note that NTSGCG neurons do not project to all these areas with the same density nor are equivalent to the distribution of the GLP-1R density in the brain (Fig. 2). For example, our previous research indicates that in the PVN [20] and DMH [45], the high density of GLP-1R-expressing neurons is consistent with a high density of GLP-1 fiber terminals, and the GLP-1 neurons from the NTS form synapses onto the postsynaptic neurons. On the other hand, although high-level of GLP-1R-expressing neurons are found in the hippocampus [46], and primary cortex [47], very few GLP-1 neuronal fiber projections were found (Fig. 1). The mismatched projections/receptors distribution is not unique to GLP-1 and has been described in other peptidergic or nonpeptidergic transmitter systems [48, 49]. This suggests that GLP-1 release may affect postsynaptic neuronal functionality and non-synaptic volume transmission via synaptic release or diffusion through the tissue to their target sites [50]. Another possibility is that circumventricular organs are located outside the blood–brain barrier, suggesting that GLP-1 of peripheral origin could be the ligand of these receptors. Indeed, the binding of peripherally administered GLP-1 to the SFO and the area postrema (AP) has been demonstrated by [51]. Given that some GLP-1 analogs are FDA-approved drugs to treat obesity [5], they may function via these widespread GLP-1R-expressing neurons. Interestingly, the ARC has a high-density distribution of GLP-1 nerve terminals as well as high density of GLP-1R neurons. The ARC is in close proximity to the third ventricle and with incomplete brain blood barriers [52], which makes it the prime target of both circulating peripheral GLP-1 (or pharmacological GLP-1 analogs) and central GLP-1 regulations. For example, it has been shown that peripheral application of fluorescently labeled liraglutide binds to neurons (presumably GLP-1R-expressing neurons) within the ARC [33].
Ample studies have demonstrated that various ARC functions are supported by specialized neuronal subtypes [22, 24,25,26]. For example, ARC POMC neurons are activated by energy surfeit, and their activations suppress feeding and promote weight loss [53]; while AgRP neuronal activations promote feeding [24, 54, 55]. Further, peripherally released ghrelin hormone suppresses food intake via ARC [56]. ARC POMC neurons mediate serotonin signaling regulation of the energy homeostasis [57]. A recent elegant study using mouse genetics revealed the profound and redundant function of ARC neurons in the obesity development [58]. ARC POMC neurons are known to be regulated by GLP-1 signaling [27, 33, 59]. It has been reported that POMC neurons express GLP-1R but not AgRP neurons [23]. Notably, a recent study defined distinct POMC neurons expressing leptin receptor and GLP-1R exhibiting a distinct anatomical distribution and electrophysiological properties [27], suggesting further investigation of the heterogeneous function mediated by these diverse cell types in the ARC is required. To that fact, when GLP-1R agonist, Exn4 is applied to this region, it enhances the ARC POMC neuronal spontaneous action potential while decreasing the trend membrane potential (Fig. 3L–N), which is consistent with the previous findings of GLP-1/Liraglutide modulating ARC POMC/CART neurons [33, 34, 60]. Meanwhile, Exn4 also showed a suppression of non-POMC neurons’ spontaneous action potential firing frequency, suggesting an enhanced inhibition, which is consistent with the recent findings in the ARC NPY neurons [34]. This data suggests that the GLP-1 can modulate cell-type-specific POMC neuronal activity within the ARC region.
Chemogenetic activation of the NTSGcg neurons in the brainstem promotes glucose homeostasis [22]. GLP-1R agonist CNS infusion has shown that the arcuate GLP-1 receptors are a crucial component of the GLP-1 system for improving glucose homeostasis by regulating insulin secretion and glucose production without affecting food intake [61]. Meanwhile, a previous study has demonstrated that the ARC AgRP neurons in mice rapidly coordinate hunger states with glucose homeostasis by stimulating the expression of muscle-related genes in brown adipose tissue [26]. These data strongly suggest that GLP-1 signaling in the ARC regulates glucose homeostasis. However, our study found that acute GLP-1R neuronal activation causes insignificant changes in blood glucose clearance and insulin secretion (Fig. 4). These data suggest that the blood glucose regulatory function of ARC GLP-1 signaling may not be via the acute activation of ARC GLP-1R neurons; or GLP-1 might modulate GLP-1R expressing presynaptic terminal projected to the ARC region (e.g., PVN, DMH). Interestingly, our recent study revealed that GLP-1R neurons in the dorsomedial hypothalamus are glucose-sensing neurons and are involved in regulating glucose metabolism [45]. Further investigations are needed to address the detailed mechanism of the GLP-1 signaling in regulating glucose metabolism and the involvement of postsynaptic GLP-1R in the ARC.
Despite the report that ARC GLP-1 signaling regulates glucose metabolism but not feeding behavior [61], it has also been shown that GLP-1R agonist liraglutide infusion in the ARC induces weight loss in rodents [33]. A recent study has demonstrated that the chemogenetic activation of POMCGlp1r+ neurons remarkably suppresses the feeding behavior [27]. Consistent with this notion, we found that the chemogenetic activation of the ARC GLP-1R neurons remarkably reduces food intake upon hM3Dq ligand injections (i.p., CNO). This suggests that acute activation of the GLP-1R neurons in the ARC robustly reduces food intake and might be a key player in regulating energy homeostasis (Fig. 5). While our data do not test GLP-1 signaling in the ARC directly, we show that the ARC POMC neurons are activated by GLP-1 signaling (Fig. 3), and the activation of the ARC GLP-1R neurons significantly suppresses feeding (Fig. 5). Here, we want to note that the chemogenetic activation of the ARC GLP-1R neurons is not equivalent to the GLP-1 signaling activation. These data suggest that the GLP-1R neuronal circuitry in the brain, including the ARC, plays a pivotal role in regulating energy homeostasis.
Conclusion
Taken together, these experiments revisit the distribution patterns of GLP-1R-expressing neurons and NTSGcg neuron projections in the mouse brain. Our study suggests that the GLP-1R mediated signaling regulates postsynaptic ARC neuronal excitability and activation of these GLP-1R neurons modulate energy homeostasis by regulation of appetite.
Materials and methods
Animals
All studies and procedures involving mice were approved by the Rutgers University Institutional Animal Care and Use Committee (IACUC) and by the National Institute of Health (NIH) guidelines. The animals used in this study were 5–7 weeks old, housed, and bred in the Child Health Institute of New Jersey facility. We used cell-type-specific expression of Cre-recombinase mouse lines; GLP-1R-ires-Cre [35], Gcg-Cre [18], and POMC-Cre [41]. For the whole-brain mapping and analyses, GLP-1R-ires-Cre was crossed with Ai14 JaxMice (#007914), Rosa-CAG-LSL-tdTomato-WPRE::ΔNeo [36] to obtain GLP-1R-ires-Cre::Ai14 mice. In all cases, mice were randomized according to body weight in each experimental group. The investigators were blinded to the treatment. The sample size required n = 7–12 per group was based on the previous studies examining the effects of GLP-1 signaling on feeding behavior [32].
Virus injection
The AAV virus (Adeno-Associated Virus) used include pAAV-EF1a-double floxed-hChR2 (H134R)-EYFP-WPRE-HGHpA (Catalog #20298-AAV1), pAAV-FLEX-tdTomato (Catalog #28305-AAV5) and pAAV-hSyn-DIO-hM3D (Gq)-mCherry (Catalog #44361-AAV9).
Electrophysiology
Brain slice electrophysiology was conducted, as described elsewhere [20]. Mice were anesthetized, decapitated, and brains were removed and quickly immersed in cold (4 °C) oxygenated cutting solution containing (in mM): 50 sucrose, 2.5 KCl, 0.625 CaCl2, 1.2 MgCl2, 1.25 NaH2PO4, 25 NaHCO3, and 2.5 glucose. Coronal cerebral cortex slices, 300 mm in thickness, were cut using a vibratome (VT 1200S; Leica). Brain slices were collected in artificial cerebrospinal fluid (ACSF) and bubbled with 5%CO2 and 95%O2. The ACSF contained (in mM): 125 NaCl, 2.5 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 2.5 glucose. After 1 h of recovery, slices were transferred to a recording chamber and constantly perfused with bath solution (30 °C) at a flow rate of 2 ml/min. Exn4 (10 nM) was added to the bath solution to stimulate the ARC GLP-1R neurons. Patch pipettes with a resistance of 8-10MΩ were made from borosilicate glass (World Precision Instruments) with a pipette puller (PC-10, Narishige) and filled with the pipette solution containing (in mM): 126 K-Gluconate, 4 KCl, 10 HEPES, 4 Mg-ATP, 0.3 Na2-GTP, 10 phosphocreatine (pH to 7.2 with KOH) for current and voltage-clamp recordings. All data were analyzed offline using ClampFit 10.2 (Molecular Devices, USA) software.
Histology and immunohistochemistry assay
Mice were anesthetized with Euthasol and transcardially perfused with 4% PFA in PBS, pH 7.4. The brains were kept in 4% PFA overnight and then moved to 30% sucrose. Coronal brain slices (50 μm) were prepared thereafter. The numbers of GLP-1R-expressing (tdTomato) neurons in GLP-1R-ires-Cre::Ai14 mice were counted from a series of 50 μm sections (5 section intervals from section to section) using ImageJ. Images were acquired with a Zeiss LSM700 confocal microscope using a 546 nm laser. Z-stack images captured the entire thickness of the section at 10 µm steps for images taken with a 20× objective. Gcg-expressing neurons and their axon terminals were labeled with ChR2-eYFP by injecting AAV vectors in the Gcg-Cre mice. The semi-quantitation of the projections was based on the fluorescent signal area and intensity.
For the c-Fos staining, only slices with the ARC region were prepared. The standard IHC protocol was followed. The primary antibodies used were anti-c-Fos (1:1000, Santa Cruz, SC271243). AlexaFluor secondary antibodies (488-goat anti-rabbit, 1:500, Life Technologies) were used to visualize the signals, and images were acquired through confocal microscopy (Zeiss, LSM700).
c-Fos quantification: Total GLP-1R (RFP) and total c-Fos (Green) labeled cells were counted using the Cell Counter plugin in ImageJ. Then the overlapping signals between RFP and Green were measured as c-Fos + cells. This quantification was done for the control and target (hM3Dq) group, and the comparison was presented accordingly.
Behavioral experiments
Chemogenetic activation of GLP-1R-expressing neurons
Male mice were caged into groups of either 3 or 4. For the stimulation of the GLP-1R-expression neurons in the ARC, we injected 300 nL of pAAV-FLEX-tdTomato (Catalog #28305-AAV5) and pAAV-hSyn-DIO-hM3D (Gq)-mCherry (Catalog #44361-AAV9) in ARC at coordinates: Anteroposterior (AP): − 1.3 mm from bregma; mediolateral (ML): ± 0.2 mm, dorsovental (DV): − 6.2 to 6.1 mm. After four weeks of recovery, glucose tolerance tests (GTT) and food intake behavior experiments were conducted after an i.p. DREADD ligand injection.
Glucose tolerance test
The mice were fasted overnight (0.5 g chow was provided). The same protocol was used as described before [62]. At Time 0, blood glucose was measured to set a baseline. 1 mg/kg body weight CNO was injected intra-peritoneally. After 30 min, 1 g/kg body weight of 20% dextrose solution was injected i.p., and blood samples were collected from the tail vein at 15, 30, 60, and 120 min. For GTT, injection volume was calculated as mentioned below:
Food intake
Mice were singly housed before the experiment in a 12 h light/dark cycle with ad libitum access to water and were fasted overnight for 12 h (9 pm–9 am); access to only 0.5 g of food was given. The following day, each animal's weight was measured, CNO was prepared, and 1 mg/kg BW CNO was injected i.p. into each mouse. After 30 min, food was added to their cage. Standard chow intake was measured at t = 0,1,2,3,4,5 and 24 h. After the experiment, animals were grouped together into their respective cages.
Open field locomotor activity
Mice were single housed the night before the experiment. To test whether there are any side effects of the chemogenetic agent used, 1 mg/kg BW CNO was i.p. injected. After 30 min, mice were allowed to explore a novel environment for 6 min. They were put in the open field “exploratory stage,” and their activity and movement were recorded via the center cage software. These experiments were performed under dim light during the light cycle [63].
Insulin measurements
After overnight fasting, CNO was injected as described before. After 120 min, Mice were anesthetized with Euthasol, and 1 ml of blood was collected from the heart and stored at − 80 °C. Plasma was separated by centrifugation at 6000 rpm for 10 min at 4 °C. Insulin levels were then analyzed using a mouse supersensitive insulin ELISA (ALPCO, Salem, NH, USA) [64].
Quantification and statistical analysis
Statistical analysis was performed using Excel (version 2019). In addition, statistical analysis for blood glucose and food intake behavior was also analyzed using GraphPad Prism 8.0. All the data is presented as mean ± standard error of the mean (SEM). Data were analyzed using Student’s t-test and Two-way ANOVA using Geisser-Greenhouse’s epsilon correction. A p-value of less than 0.05 was considered statistically significant.
Availability of data and materials
The data and datasets used and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
Abbreviations
- AgRP:
-
Agouti-related protein
- ARC:
-
The arcuate nucleus of the hypothalamus
- CNO:
-
Clozapine-N-oxide
- CNS:
-
Central nervous system
- CRH:
-
Corticotrophin-releasing hormone
- DIO:
-
Double inverted open reading frame
- DR:
-
Dorsal raphe nucleus
- DREADDs:
-
Designer Receptor Exclusively Activated by Designer Drugs
- eYFP:
-
Enhanced yellow fluorescent protein
- Enx4:
-
Exendin4
- GFP:
-
Green fluorescent protein
- RFP:
-
Red fluorescent protein
- GLP-1:
-
Glucagon-like peptide 1
- GLP-1R:
-
Glucagon-like peptide 1 receptor
- GTT:
-
Glucose tolerance test
- LH:
-
Lateral hypothalamus
- MD:
-
Medial dorsal nucleus
- MnPO:
-
Median preoptic nucleus
- MvePC:
-
Medial vestibular parvicellular subfields nucleus
- NTS:
-
Nucleus tractus solitarius
- PnO:
-
Pontine reticular nucleus, oral part
- POMC:
-
Pro-opiomelanocortin
- PVN:
-
Paraventricular hypothalamic nucleus
- SFO:
-
Subconical organ
- SO:
-
Supraoptic nucleus
- VMH:
-
Ventromedial nucleus of the hypothalamus
References
Muller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30:72–130.
Sandoval D. CNS GLP-1 regulation of peripheral glucose homeostasis. Physiol Behav. 2008;94(5):670–4.
Gromada J, Holst JJ, Rorsman P. Cellular regulation of islet hormone secretion by the incretin hormone glucagon-like peptide 1. Pflugers Arch. 1998;435(5):583–94.
Gromada J, Brock B, Schmitz O, Rorsman P. Glucagon-like peptide-1: regulation of insulin secretion and therapeutic potential. Basic Clin Pharmacol Toxicol. 2004;95(6):252–62.
Drucker DJ. GLP-1 physiology informs the pharmacotherapy of obesity. Mol Metab. 2022;57: 101351.
Niswender K, Pi-Sunyer X, Buse J, Jensen KH, Toft AD, Russell-Jones D, Zinman B. Weight change with liraglutide and comparator therapies: an analysis of seven phase 3 trials from the liraglutide diabetes development programme. Diabetes Obes Metab. 2013;15(1):42–54.
Gu G, Roland B, Tomaselli K, Dolman CS, Lowe C, Heilig JS. Glucagon-like peptide-1 in the rat brain: distribution of expression and functional implication. J Comp Neurol. 2013;521(10):2235–61.
Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69–72.
Scrocchi LA, Brown TJ, MaClusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med. 1996;2(11):1254–8.
Scrocchi LA, Hill ME, Saleh J, Perkins B, Drucker DJ. Elimination of glucagon-like peptide 1R signaling does not modify weight gain and islet adaptation in mice with combined disruption of leptin and GLP-1 action. Diabetes. 2000;49(9):1552–60.
Varin EM, Mulvihill EE, Baggio LL, Koehler JA, Cao X, Seeley RJ, Drucker DJ. Distinct neural sites of GLP-1R expression mediate physiological versus pharmacological control of incretin action. Cell Rep. 2019;27(11):3371-3384 e3373.
Brierley DI, Holt MK, Singh A, de Araujo A, McDougle M, Vergara M, Afaghani MH, Lee SJ, Scott K, Maske C, et al. Central and peripheral GLP-1 systems independently suppress eating. Nat Metab. 2021;3(2):258–73.
Burmeister MA, Ayala JE, Smouse H, Landivar-Rocha A, Brown JD, Drucker DJ, Stoffers DA, Sandoval DA, Seeley RJ, Ayala JE. The hypothalamic glucagon-like peptide 1 receptor is sufficient but not necessary for the regulation of energy balance and glucose homeostasis in mice. Diabetes. 2017;66(2):372–84.
Burmeister MA, Brown JD, Ayala JE, Stoffers DA, Sandoval DA, Seeley RJ, Ayala JE. The glucagon-like peptide-1 receptor in the ventromedial hypothalamus reduces short-term food intake in male mice by regulating nutrient sensor activity. Am J Physiol Endocrinol Metab. 2017;313(6):E651–62.
Lopez-Ferreras L, Richard JE, Noble EE, Eerola K, Anderberg RH, Olandersson K, Taing L, Kanoski SE, Hayes MR, Skibicka KP. Lateral hypothalamic GLP-1 receptors are critical for the control of food reinforcement, ingestive behavior and body weight. Mol Psychiatry. 2018;23(5):1157–68.
Lee SJ, Sanchez-Watts G, Krieger JP, Pignalosa A, Norell PN, Cortella A, Pettersen KG, Vrdoljak D, Hayes MR, Kanoski SE, et al. Loss of dorsomedial hypothalamic GLP-1 signaling reduces BAT thermogenesis and increases adiposity. Mol Metab. 2018;11:33–46.
Barrera JG, Jones KR, Herman JP, D’Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci. 2011;31(10):3904–13.
Gaykema RP, Newmyer BA, Ottolini M, Raje V, Warthen DM, Lambeth PS, Niccum M, Yao T, Huang Y, Schulman IG, et al. Activation of murine pre-proglucagon-producing neurons reduces food intake and body weight. J Clin Invest. 2017;127(3):1031–45.
Holt MK, Richards JE, Cook DR, Brierley DI, Williams DL, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP-1, mediate stress-induced hypophagia, and limit unusually large intakes of food. Diabetes. 2019;68(1):21–33.
Liu J, Conde K, Zhang P, Lilascharoen V, Xu Z, Lim BK, Seeley RJ, Zhu JJ, Scott MM, Pang ZP. Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon-like peptide-1 in the paraventricular hypothalamus. Neuron. 2017;96(4):897-909 e895.
Williams DL, Lilly NA, Edwards IJ, Yao P, Richards JE, Trapp S. GLP-1 action in the mouse bed nucleus of the stria terminalis. Neuropharmacology. 2018;131:83–95.
Shi X, Chacko S, Li F, Li D, Burrin D, Chan L, Guan X. Acute activation of GLP-1-expressing neurons promotes glucose homeostasis and insulin sensitivity. Mol Metab. 2017;6(11):1350–9.
Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, Goldman M, Verstegen AM, Resch JM, McCarroll SA, et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci. 2017;20(3):484–96.
Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14(3):351–5.
Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121(4):1424–8.
Steculorum SM, Ruud J, Karakasilioti I, Backes H, Engstrom Ruud L, Timper K, Hess ME, Tsaousidou E, Mauer J, Vogt MC, et al. AgRP neurons control systemic insulin sensitivity via myostatin expression in brown adipose tissue. Cell. 2016;165(1):125–38.
Biglari N, Gaziano I, Schumacher J, Radermacher J, Paeger L, Klemm P, Chen W, Corneliussen S, Wunderlich CM, Sue M, et al. Functionally distinct POMC-expressing neuron subpopulations in hypothalamus revealed by intersectional targeting. Nat Neurosci. 2021;24(7):913–29.
Pang ZP, Han W. Regulation of synaptic functions in central nervous system by endocrine hormones and the maintenance of energy homoeostasis. Biosci Rep. 2012;32(5):423–32.
Acuna-Goycolea C, van den Pol A. Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal. J Neurosci. 2004;24(37):8141–52.
Liu J, Pang ZP. Glucagon-like peptide-1 drives energy metabolism on the synaptic highway. FEBS J. 2016;283(24):4413–23.
Mietlicki-Baase EG, Ortinski PI, Reiner DJ, Sinon CG, McCutcheon JE, Pierce RC, Roitman MF, Hayes MR. Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. J Neurosci. 2014;34(20):6985–92.
Wang XF, Liu JJ, Xia J, Liu J, Mirabella V, Pang ZP. Endogenous glucagon-like peptide-1 suppresses high-fat food intake by reducing synaptic drive onto mesolimbic dopamine neurons. Cell Rep. 2015;12(5):726–33.
Secher A, Jelsing J, Baquero AF, Hecksher-Sorensen J, Cowley MA, Dalboge LS, Hansen G, Grove KL, Pyke C, Raun K, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124(10):4473–88.
Dong Y, Carty J, Goldstein N, He Z, Hwang E, Chau D, Wallace B, Kabahizi A, Lieu L, Peng Y, et al. Time and metabolic state-dependent effects of GLP-1R agonists on NPY/AgRP and POMC neuronal activity in vivo. Mol Metab. 2021;54: 101352.
Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory neurons that detect stretch and nutrients in the digestive system. Cell. 2016;166(1):209–21.
Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–40.
Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, Gribble FM, Reimann F. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63(4):1224–33.
Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S. Distribution and characterisation of glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol Metab. 2015;4(10):718–31.
Ast J, Arvaniti A, Fine NHF, Nasteska D, Ashford FB, Stamataki Z, Koszegi Z, Bacon A, Jones BJ, Lucey MA, et al. Super-resolution microscopy compatible fluorescent probes reveal endogenous glucagon-like peptide-1 receptor distribution and dynamics. Nat Commun. 2020;11(1):467.
Meeran K, O’Shea D, Edwards CM, Turton MD, Heath MM, Gunn I, Abusnana S, Rossi M, Small CJ, Goldstone AP, et al. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7-36) amide or exendin-(9-39) alters body weight in the rat. Endocrinology. 1999;140(1):244–50.
Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC Jr, Elmquist JK, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42(6):983–91.
Roth BL. DREADDs for neuroscientists. Neuron. 2016;89(4):683–94.
Atasoy D, Sternson SM. Chemogenetic tools for causal cellular and neuronal biology. Physiol Rev. 2018;98(1):391–418.
Ogrodnik M, Zhu Y, Langhi LGP, Tchkonia T, Kruger P, Fielder E, Victorelli S, Ruswhandi RA, Giorgadze N, Pirtskhalava T, et al. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 2019;29(5):1061-1077 e1068.
Huang Z, Liu L, Zhang J, Conde K, Phansalkar J, Li Z, Yao L, Xu Z, Wang W, Zhou J, et al. Glucose-sensing glucagon-like peptide-1 receptor neurons in the dorsomedial hypothalamus regulate glucose metabolism. Sci Adv. 2022;8(23):eabn5345.
Hsu TM, Hahn JD, Konanur VR, Lam A, Kanoski SE. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology. 2015;40(2):327–37.
Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci USA. 2009;106(4):1285–90.
Herkenham M. Mismatches between neurotransmitter and receptor localizations in brain: observations and implications. Neuroscience. 1987;23(1):1–38.
Tallent MK. Presynaptic inhibition of glutamate release by neuropeptides: use-dependent synaptic modification. Results Probl Cell Differ. 2008;44:177–200.
van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76(1):98–115.
Orskov C, Poulsen SS, Moller M, Holst JJ. Glucagon-like peptide I receptors in the subfornical organ and the area postrema are accessible to circulating glucagon-like peptide I. Diabetes. 1996;45(6):832–5.
Kim DW, Glendining KA, Grattan DR, Jasoni CL. Maternal obesity in the mouse compromises the blood-brain barrier in the arcuate nucleus of offspring. Endocrinology. 2016;157(6):2229–42.
Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, Luo M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci. 2013;33(8):3624–32.
Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155(6):1337–50.
Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y, Sternson SM. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521(7551):180–5.
Tamura H, Kamegai J, Shimizu T, Ishii S, Sugihara H, Oikawa S. Ghrelin stimulates GH but not food intake in arcuate nucleus ablated rats. Endocrinology. 2002;143(9):3268–75.
Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60(4):582–9.
Zhu C, Jiang Z, Xu Y, Cai ZL, Jiang Q, Xu Y, Xue M, Arenkiel BR, Wu Q, Shu G, et al. Profound and redundant functions of arcuate neurons in obesity development. Nat Metab. 2020;2(8):763–74.
Gabery S, Salinas CG, Paulsen SJ, Ahnfelt-Ronne J, Alanentalo T, Baquero AF, Buckley ST, Farkas E, Fekete C, Frederiksen KS, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020. https://doi.org/10.1172/jci.insight.133429.
Peterfi Z, Szilvasy-Szabo A, Farkas E, Ruska Y, Pyke C, Knudsen LB, Fekete C. Glucagon-like peptide-1 regulates the proopiomelanocortin neurons of the arcuate nucleus both directly and indirectly via presynaptic action. Neuroendocrinology. 2021;111(10):986–97.
Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57(8):2046–54.
Dirice E, Kahraman S, De Jesus DF, El Ouaamari A, Basile G, Baker RL, Yigit B, Piehowski PD, Kim M-J, Dwyer AJ, et al. Increased β-cell proliferation before immune cell invasion prevents progression of type 1 diabetes. Nat Metab. 2019;1(5):509–18.
Liu JJ, Tsien RW, Pang ZP. Hypothalamic melanin-concentrating hormone regulates hippocampus-dorsolateral septum activity. Nat Neurosci. 2022;25(1):61–71.
Zhang Z, Piro AL, Dai FF, Wheeler MB. Adaptive changes in glucose homeostasis and islet function during pregnancy: a targeted metabolomics study in mice. Front Endocrinol. 2022. https://doi.org/10.3389/fendo.2022.852149.
Acknowledgements
The authors would like to thank Dr. Ranjie Xu (Purdue University), Xiao Su (Rutgers), and Rohan Savani (Rutgers) for reviewing the manuscript.
Funding
This study was supported by grants from the Robert Wood Johnson Foundation to the Child Health Institute of New Jersey (RWJF Grant #74260), and the NIH NIDDK R01DK131452. M.W. is funded by a grant from the Banting and Best Diabetes Centre, University of Toronto. A.E. is funded by NIH NIDDK R01DK122167.
Author information
Authors and Affiliations
Contributions
ZPP and LW conceived the project and designed the experiments. IS and LW conducted all experiments and analyzed the data. BX performed GLP-1R-ires-Cre: Ai14 mice histology experiment. JL performed POMC-Cre mice recording experiment. AT and AEO helped with the blood glucose (GTT) experiment. IS, LW, MW, and ZPP wrote the paper. All authors provided intellectual contributions toward the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All studies and procedures involving mice were approved by the Rutgers University Institutional Animal Care and Use Committee (IACUC) and by the National Institute of Health (NIH) guidelines.
Consent for publication
The publication of this manuscript has been approved by all authors.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
GLP-1 receptor neuron distribution in the whole brain. Representative images of GLP-1R-ires-cre mouse crossed with Ai14 tdTomato reporter mice line.
Additional file 2: Figure S2.
GLP-1 receptor neuron distribution in the whole brain. Representative images of GLP-1R-ires-cre mouse crossed with Ai14 tdTomato reporter mice line.
Additional file 3: Figure S3.
NTS GLP-1 neuron projection mapping in the whole brain. Representative images of Gcg-cre BAC mouse injected with DIO-Chr2-EYFP.
Additional file 4: Figure S4.
NTS GLP-1 neuron projection mapping in the whole brain. Representative images of Gcg-cre BAC mouse injected with DIO-Chr2-EYFP.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Singh, I., Wang, L., Xia, B. et al. Activation of arcuate nucleus glucagon-like peptide-1 receptor-expressing neurons suppresses food intake. Cell Biosci 12, 178 (2022). https://doi.org/10.1186/s13578-022-00914-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13578-022-00914-3