Abstract
The failure of remodeling process that constantly regenerates effete, aged bone is highly associated with bone nonunion and degenerative bone diseases. Numerous studies have demonstrated that aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) activate cytokines and mediators on osteoclasts, osteoblasts and their constituent progenitor cells located around the remodeling area. These cells contribute to a complex metabolic scenario, resulting in degradative or synthetic functions for bone mineral tissues. The spatiotemporal effects of aspirin and NSAIDs in the bone remodeling are controversial according the specific therapeutic doses used for different clinical conditions. Herein, we review in vitro, in vivo, and clinical studies on the dose-dependent roles of aspirin and NSAIDs in bone remodeling. Our results show that low-dose aspirin (< 100 μg/mL), which is widely recommended for prevention of thrombosis, is very likely to be benefit for maintaining bone mass and qualities by activation of osteoblastic bone formation and inhibition of osteoclast activities via cyclooxygenase-independent manner. While, the roles of high-dose aspirin (150–300 μg/mL) and other NSAIDs in bone self-regeneration and fracture-healing process are difficult to elucidate owing to their dual effects on osteoclast activity and bone formation of osteoblast. In conclusion, this study highlighted the potential clinical applications of low-dose aspirin in abnormal bone remodeling as well as the risks of high-dose aspirin and other NSAIDs for relieving pain and anti-inflammation in fractures and orthopedic operations.
Similar content being viewed by others
Background
Bone remodeling is a constant process that contributes to renewal of effete, aged bone and repair of the micro-damage of bone architectural integrity throughout life [1]. It couples the destructive process of bone resorption by teams of osteoclasts with bone synthesis by osteoblasts [2], and a period called reversal phase that separates bone-resorbing process from bone formation for several weeks [3]. Osteoclasts are derived from hematopoietic stem cells (HSCs), are unique for resorbing bone matrices, and share precursors with macrophages in the present knowledge [4]. In contrast, osteoblasts and reversal cells proven identical with osteoprogenitors (precursors of osteoblastic cells) [5] are of mesenchymal stem cell (MSC) origin [6]. The reversal cells are sort of mononucleated cells that colonize 80% of eroded bone surfaces undergoing remodeling [7]. It has been identified that the reversal cells are specific osteoblastic lineage cells, which play pivotal roles in coupling bone-resorption propitious to bone formation during a reversal phase [3, 8]. The organized functions of these cells is basic for keeping up physiological bone remodeling and advancing skeletal regeneration, which is strictly controlled by molecules or regulators such as the receptor activator of nuclear factor-κB ligand (RANKL), macrophage-colony stimulating factor [9], and activated T lymphocytes. Failure of bone remodeling in conditions with increased osteoclast activity or downregulated generation of the osteoblast lineage [10] leads to degenerative bone diseases such as osteoporosis, as well as increase the risk for delayed healing or nonunion of fractures. Previous epidemiological studies have demonstrated that taking of aspirin regularly is associated with changes in bone mineral density (BMD) and the fracture-healing processes. Further in vitro and in vivo investigations found that the aspirin participates in the regulation of bone remodeling when applied at the therapeutic doses.
Aspirin, also called acetylsalicylic acid, is known as a group of medications that belongs to nonsteroidal anti-inflammatory drugs (NSAIDs) and inhibits cyclooxygenase-1 (COX-1) and COX-2 enzymes in an irreversible manner [11]. COX-1 participates in physiological functions constitutively, whereas in pathophysiological processes such as pain, inflammation, and fever, COX-2 is inductively expressed and initial enzymatic activity converting arachidonic acid into prostaglandin E2 (PGE2) [12], which play pivotal roles in the promotion of pain and damage [13,14,15]. The high-dose aspirin (> 1000 mg per day) inhibits COX-2 more potently than COX-1 [16], and generally used for alleviation of pain and inflammatory response [17]. While low-dose aspirin (75–100 mg per day) inhibits COX-1 isozyme more strongly than COX-2 [11]. Such low doses could achieve persistent inhibition of platelet COX-1, preventing the formation of PGH2, and therefore thromboxane A2 (TXA2). It is widely recommended in prevention of acute coronary syndromes (ACS) and stroke in patients at high risk of developing blood clots [18, 19]. Low dose of aspirin is also considered to be alternative to other thrombosis phylactic agents following orthopedic operations [20]. The aspirin concentration in plasma usually ranges from 150 to 300 μg/mL in patients taking regular, high doses of aspirin and < 100 μg/mL after intake of therapeutic low doses [21].
Prior studies have shown that high-dose aspirin is related to the independent stimulation of osteoclast and osteoblast activity to destroy and generate bone tissues [22]. In addition, low-dose aspirin was shown to be associated with regulation of bone cells. Although its dose-dependent roles are conflicting and the detailed functional mechanisms of its regulation have not been fully elucidated, aspirin may exert multiple biological effects on bone remodeling [23]. In contrast to aspirin, other NSAIDs inhibit the COX enzymes reversibly [16], which leads to a modest increase in BMD of the hip and lumbar spine [23]. However, the conventional taking of NSAIDs as an analgesic seems to be negatively associated with union of long-bone fracture and the spinal-fusion rate [24]. Evidence of the relationship between NSAIDs use and bone remodeling is inconclusive. Here, we comprehensively reviewed the multiple roles of aspirin in bone remodeling and skeletal regeneration and the mechanism of actions by which aspirin may affect bone cells, especially in varying dose-dependent manners. In addition, we discuss the functions of different kinds of NSAIDs in bone remodeling and fracture healing, particularly their potential therapeutic effects or side effects during clinical applications in bone disorders.
Low-dose aspirin may benefit for bone remodeling and skeletal regeneration
Mesenchymal stem cell-based intervention with low-dose aspirin (< 100 μg/mL) may benefit osteoporosis treatment by inhibition of osteoclast differentiation and activities [25]. Aspirin at a dose of 50 μg/mL can partially block the formation of osteoclasts induced by RANKL and led to a significantly decreased number of tartrate-resistant acid phosphatase (TRAP)-positive osteoclasts. When low-dose aspirin was continuously administered to ovariectomized (OVX) mice for 3 months, their femurs showed a higher level of BMD than the control group at 4 weeks of observation [26]. In addition, a markedly number decrease of the TRAP-positive cells in the trabecular bone of OVX mice and the serum levels of RANKL were detected [25]. Further examination of osteoclast activity showed that several serum markers were systemically changed by long-term taking aspirin at a low dosage, RANKL levels reduced, and osteoprotegerin (OPG) levels increased in OVX mice, which are known to be crucial for osteoclast differentiation [25]. In terms of stable ligand-activation of TXA2 receptors can induce osteoclast-like cell formation in murine bone marrow cultures [27], inhibition of TXA2 synthesis by low dose of aspirin might decrease osteoclastogenesis and bone resorption. These findings all indicated that low-dose aspirin can inhibit osteoclast differentiation and activity.
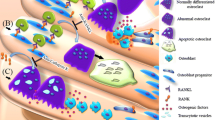
Additionally, low-dose aspirin was demonstrated to rescue impaired bone marrow MSC (BMMSCs) function by increasing the number of colony-forming unit fibroblasts and osteogenic capacities. Cultured BMMSCs treated with 50 μg/mL aspirin showed improvement of anti-apoptotic capacity and elevation of mineralized tissue formation in vitro and in vivo [26]. Aspirin at a dose of 50 μg/mL could also inhibit Fas/Fas ligand mediated apoptosis of BMMSCs via decrease viability of T-lymphocyte activation [25]. Moreover, in vitro low dose of aspirin significantly improved stem cell functions and prevented replicative senescence to improve bone formation [28]. Furthermore, aspirin at a dose of 75 μg/mL has been reported to enhance the immunomodulatory capacity of MSCs [29] and improve MSC-based tissue regeneration [30] by significantly reducing the expression of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) [31]. The study elucidated that the use of low-dose aspirin slightly upregulates telomerase activity in BMMSCs and elongates their telomere lengths to avoid replicative senescence and improve stem cell functions [28], and has the capability to increase bone formation [28]. The underlying mechanisms of aspirin seem to have an association with increased expression of certain osteogenic genes such as Runx2, a master gene for osteogenic differentiation. Additionally, in vitro aspirin treatment accelerated degradation of phospho-β-catenin, leading to increased Wnt signaling, which is involved in osteogenesis [32]. In addition, aspirin at a low dose is likely to acetylate histones H3 in addition to the COX isoenzymes [33]. Acetylation of histones H3 and H4 is functionally coupled with chromatin-remodeling events that mediate the developmental induction of osteocalcin gene during osteoblast differentiation [34]. In contrast, the aspirin (< 100 μg/mL) could reverse the down-regulated histone deacetylases activity and induce inhibition of BMMSCs adipogenesis [35]. Moreover, low-dose aspirin exhibited excellent chemotactic effects in vitro [36]. The study of Tang et al. [37] demonstrated that both 50 μg/mL and 100 μg/mL aspirin significantly increased transforming growth factor β-1 (TGF-β1) production of human BMMSCs, then induces migration of MSCs to the bone remodeling sites [38]. In the latest studies of Sien et al., the OVX rats orally administered with low dose of aspirin (9 mg/kg/day, equivalent to 100 mg/day of human dose) showed less bone loss by using Micro-CT and histomorphometry. However, their in vitro results indicated that aspirin at low dose may increase the mineral component (calcium) of bone but be unfavorable for the synthesis of organic component (collagen), which result in a disorder in composition of bone, then exhibited no distinct tendency for improvement in bone mechanical properties [39]. These findings indicate that low-dose of aspirin can enhance the osteogenic capacities of MSCs and may rescue the bone loss from abnormal bone remodeling, while its mechanical properties need to be further detected. In general, Fig. 1 presents a schematic diagram of the major roles of low-dose aspirin in regulating the balance of bone remodeling to the direction of osteogenesis.
The roles of low-dose aspirin in the regulation of bone remodeling. Aspirin at low dosage might suppress the differentiation of osteoclasts and promotes the bone formation via osteoblastic cells. The solid red arrows indicate the promotion of cellular processes, and the solid green lines indicate inhibition of cellular processes. The dotted lines indicate that the mechanism has not been fully elucidated. HSC hematopoietic stem cells, MSC mesenchymal stem cells, T cell T lymphocytes, Pre-OC precursors of osteoclasts, Pre-OB precursors of osteoblasts, OPG osteoprotegerin
Dual effects of high-dose aspirin on osteoclasts and osteoblasts activities
In contrast to low-dose aspirin, high doses of aspirin acts COX-2-dependent inhibition, or through mechanisms such as formation of nitric oxide (NO) radicals [23], modulation of nuclear factor (NF)-κB, and electron transport chain pathways, which are involved in bone remodeling [40]. Consistent with these findings, in vitro and in vivo studies (Fig. 2) have confirmed that regular, high doses of aspirin have multiple effects on both osteoclasts and osteoblasts activities [41]. COX-2 is an essential player in both intramembranous and endochondral osteogenesis. The skeletal repair was significantly delayed in COX-2 knockout mice compared with COX-1-knockout and wildtype mice. When used at high doses for anti-inflammatory purposes, aspirin may have strong effects on bone remodeling, because of that the production of PGs is primarily mediated by COX-2 in osteoblasts [42]. PGs including PGE2, PGD2, and PGF2α belong to a group of lipid mediators that perform different functions in the regulation of homeostasis and inflammation. PGs act by activating the prostanoid receptor subfamily, which consists of eight members: the PGE receptors EP1, EP2, EP3, and EP4; the PGD receptor DP1; the PGI receptor (IP); the PGF receptor; and the thromboxane receptor [43]. PGs have been proved to active osteoblasts and osteoclasts directly in bone healing process [44]. In a rabbit ulnar osteotomy model, aspirin was demonstrated that delayed bone union with a threshold equivalent to a human dose of 325 mg [45].
Dual effects of high-dose aspirin on osteoclasts and osteoblasts activities. Aspirin at high dosage regulates osteoclast-mediated bone resorption and osteoblastic bone formation by activating or inhibiting molecules and target cells. High-dose aspirin has multiple roles in the regulation of osteoclasts and osteoblasts. The solid red arrows indicate the promotion of cellular processes, and the solid green lines indicate inhibition of cellular processes. HSC hematopoietic stem cells, MSC mesenchymal stem cells, T cell T lymphocytes, Pre-OC precursors of osteoclasts, Pre-OB precursors of osteoblasts
PGE2 has direct, stable effects on long-term repopulating HSCs and promotes HSC implantation by up-regulating the chemokine receptor CXCR4 [46]. PGE2-induced Wnt/β-catenin signaling contributes to HSC development and regeneration [47]. Additionally, PGE2 enhances expression of survivin, an anti-apoptotic protein in the HSC niche [48]. With regard to the osteoblast/osteoclast differentiation and activities, studies have shown that PGE2 and PGF2α play a stimulating and inhibiting role in bone remodeling in an autocrine and/or paracrine manner [49, 50]. Aspirin at high concentration (> 200 μg/mL) has been demonstrated to have anti-proliferative effect on BMMSCs [37]. Chikazu et al. [51] demonstrated that COX-2 is involved in bone morphogenetic protein 2 (BMP-2)-induced osteoblast differentiation during ectopic bone formation. Correspondingly, Zhang et al. [52] discovered that bone marrow cell cultures from COX-2-knockout mice formed less osteoblasts than wild-type mice, while PGE2 and BMP-2 treatment could reverse this phenotype. Further study demonstrated that PGE2 may cooperate with BMPs to increase RUNX2 and SP7, two essential transcription factors required for bone formation [52]. Minamizaki et al. [53] indicated that the anabolic effect of PGE2 on osteogenesis are mediated partly by activation of specific EP4 receptors, which have been identified in human osteoblastic lineage [54]. In addition, the other studies suggested that selective EP4 receptor agonists could accelerate BMP-induced mineral nodule formation by stimulating the commitment and differentiation of osteoblast cell lines [55], within the involvement of protein kinase A (PKA) signaling pathway [56]. However, COX-2 deficient mice have satisfactory post-natal skeletal development, suggesting that the loss of COX-2 activity may not prevent stem cells from differentiating into osteoblasts. PGE2 can act as an immune suppressor of T-lymphocytes in a dose-dependent manner that involves EP4 receptors, and this immunosuppressive effect of PGE2 is correlated with IL-2 and IFN-γ inhibition [57]. Early in vitro studies have shown that PGF2α enhances the expression of IL-6 in osteoblasts [58]. Suda et al. have also reported that osteoblast formation was stimulated by IL-6 with induction of RANKL expression via a PGE2-related mechanism [59]. Previous studies have shown that PGF2α acts as a strong mitogenic and survival agent on osteoblasts, and these effects are, at least in part, mediated by fibroblast growth factor 2 (FGF-2), an anabolic bone agent that enhances bone formation by exerting an stimulation of the proliferation and differentiation of MSCs [60]. COX-2 inhibition transfers arachidonic acid to the 5-lipoxygenase (5-LO) pathway [61]. 5-LO is already known to be key factors in the enzymatic production of leukotrienes from arachidonic acid [62]. Leukotrienes can reduce the proliferation and activity of osteoblast in vitro while stimulating the formation and activity of osteoclast [63]. Consistent with the arachidonic acid-shunting mechanism, the levels of leukotriene B4 were found to be higher in fracture callus of COX-2 null mice than wide-type group. Together, inhibition of COX-2 activity by high-dose aspirin might decrease osteoblast differentiation or stimulate osteoclast activity [64, 65].
More recent data suggest that high-dose aspirin modulates signaling by suppressing NF-κB, a transcription factor complex, playing a pivotal role in many biological processes [66]. The NIK and RelB-dependent the receptor activator of nuclear factor-κB (RANK)-activated alternative NF-κB pathway controls osteoclast differentiation, mitochondrial biogenesis, and the respiratory chain [67], through independent pathways [68]. Oxidative phosphorylation in mitochondria seems to be the main bioenergetic source for osteoclast differentiation [69]. In addition, aspirin (oral dose of 200 mg/kg in mice) induces the formation of NO radicals, which independently reduces inflammation [70]. NO activates soluble guanylate cyclase to generate cGMP, which, in turn, stimulates production of protein kinase G (PKG). It also mediates the pro-survival effects of estrogens and mechanical stimulation in osteoblasts via cGMP/PKG signaling [71]. In murine primary osteoblasts, NO increases intracellular cGMP, Wnt/β-catenin signaling, and osteoblastic gene expression and protects cells from apoptosis [72]. NO-induced autophagy functions as a survival mechanism via adenosine monophosphate-activated protein kinase (AMPK) activation against apoptosis in the MC3T3-E1 (pre-osteoblast) cells [73]. A previous study showed that high-dose aspirin activates AMPK, a central regulator of cell growth and metabolism [74], which may inhibit the RANK/RANKL signaling, thereby explaining the mechanism of AMPK-mediated inhibition of osteoclastogenesis [75]. However, another study demonstrated that high-dose aspirin reduced osteoblast growth via cell cycle arrest and apoptosis induction, whereas low-dose aspirin had no effect on osteoblast growth [76]. Therefore, the underlying dose-dependent mechanism of aspirin might be responsible for this difference in its effects on bone cells [41].
Differences and similarities between the roles of high-dose aspirin and other NSAIDs in in bone remodeling and fracture healing
The mechanism of action of other NSAIDs differs from that of high-dose aspirin [11]: NSAIDs inhibit the COX enzymes in a reversible manner, whereas high-dose aspirin inhibits the COX enzymes irreversibly [16]. NSAIDs are classified according to their mechanism of action or chemical structure, such as selective COX-2 inhibitors and acetic acid, propionic acid, and enolic acid (Oxicam) derivatives. These NSAIDs tend to have similar characteristics and tolerability within a group. Short-term use of therapeutic doses of NSAIDs regulated the differentiation activity of osteoblast-like cells and reduced the synthesis of basic phosphates and matrix mineralization [77]. Be similar to high-dose aspirin, NSAIDs affect osteoblasts by reducing the synthesis of PGs, resulting in inhibition of COX-2 enzymes [78]. However, the deleterious impact of some kinds of NSAIDs on bone remodeling and fracture healing remains controversial [79]. Therapeutic doses of diclofenac, indomethacin or ketorolac would result in cell death during the osteoblast cultures; they may suppress bone formation and impair bone remodeling by causing cell cycle arrest in G0/G1 phase [80, 81]. In contrast, Arpornmaeklong et al. [82] found that indomethacin and celecoxib inhibit cell growth, but how alkaline phosphatase and osteocalcin synthesis were determined by them is unclear [76]. Studies of animal models indicated that celecoxib inhibits fracture consolidation at doses of 2–8 mg/kg/day, whereas other studies did not report any effects of celecoxib on fracture healing at doses of 1, 3, 10 and 50 mg/kg/day. Acetic acid and propionic acid [83] NSAIDs are known to play negative roles in bone healing; in contrast, naproxen, a propionic acid NSAID, inhibited osteoclastic activity and bone resorption and prevented transient loss of bone mass and structural deterioration. Numbers of research has reported that NSAIDs treatment can reduce bone generation to decrease the incidence of heterotopic bone formation following hip and femoral neck fractures [84]. NSAIDs are considered inhibitors of bone formation in experimental models of bone ingrowth into implants [85]. Although little is known about the mechanism underlying NSAID-induced reduction in bone formation, research in the latest decade continues to be in agreement with the results of earlier studies and strongly emphasizes the negative roles of traditional NSAIDs on bone healing [24]. Table 1 presents a list of animal studies regarding the functions of NSAIDs in bone remodeling and fracture healing. Several studies have shown that increased BMD indicates low bone-resorption rates in patients receiving NSAIDs, as measured by relevant biochemical markers [86]. The other study found modest beneficial effect on BMD, but no protective function on subsequent risk of fractures by taking NSAIDs 5–7 times/week [87]. However, another studies showed that NSAIDs have no effect on bone remodeling by regular and incidental use [88]. The contrasting findings may result from factors including treatment duration, dosage, and experimental species. For example, celecoxib, a COX-2 specific inhibitor, was found to impairing fracture healing in dose of 2 or 4 mg/kg/day [89]. The propionic acid NSAIDs, such as flurbiprofen, increased bone resorption [90] and aggravated the bone loss at the high dose of 1200 or 2400 mg/day [91, 92]. In contrast, Morton et al. [93] was unable to detect any effect of BMD in patients treated with acetic acid NSAIDs. Table 2 presents a review of clinical effects of aspirin and NSAIDs on BMD and fractures healing.
Discussion
Aspirin administration has controversial spatiotemporal effects in the skeletal system according to therapeutic doses applied and the clinical conditions of patients. Although several theories about the role of high-dose aspirin in inhibiting COX-2 and PGs in bone remodeling and destructive bone diseases has been reported, many basic questions remain unclear. For example, it is still unknown in which cells and when COX-2 is expressed during bone remodeling and fracture healing. Furthermore, the repertoire of PGs and other eicosanoids produced in bone remodeling is unknown, and it is unclear whether loss of activity in one of the arachidonic acid-metabolizing enzymes would affect the function of the remaining enzymes. Owing to involvement of inhibition in COX-1, COX-2, and PG pathways, the exact mechanisms of aspirin in bone remodeling are less conclusive. The COX- and PGs-independent pathways involved in the bone remodeling appear to either inhibit osteoclast differentiation or promote bone formation. Although we cannot overlook the negative effects of high-dose aspirin treatment on BMD and bone regeneration in human studies, there is no conclusive evidence to deny patients the analgesic benefits of these drugs for managing fractures. Owing to its anti-platelet effect, which prevents heart attacks and strokes, aspirin at a dose of 75 or 81 mg/day is commonly used in elderly patients requiring long-term treatment. In vitro studies and animal studies have indicated that low-dose aspirin could increase osteoblast formation and decrease osteoclast differentiation, thereby increasing mineralized tissue formation [26].
The clinical effects of regular use of aspirin on BMD and skeletal regeneration in elderly patients remain conflicting conclusions of previous epidemiological studies [26]. Bauer et al. found that daily aspirin use (5–7 days/week, at least 1 year) in white women aged > 65 years was associated with an increase in axial BMD as compared to non-users. However, this increase in BMD was not accompanied with a clinical protective effect on the fracture risk [87]. In another study including African–American ethnicity and men, current users of aspirin had higher BMD of the total body than nonusers, but data on fracture risk were not presented [11]. However, these studies did not demonstrate the differences and similarities in the effects of different doses of aspirin on BMD and fracture risk. High-dose aspirin is effective for patients with similar conditions such as degenerative bone and joint diseases and for those taking many medications. Vestergaard et al. [94] reported that the overall fracture risk is slightly reduced in OA patients with recent use of high-dose aspirin (≥ 500 mg/day) for the treatment of pain and inflammation. On the other hand, low-dose aspirin is commonly used for prevention of thromboembolic events in elderly people. Vestergaard et al. [41] found that the use of low-dose aspirin increased risks of overall fracture risk and hip fracture, although the effect size was only marginal. A recent study of Bonten et al. [95] demonstrated that the regular use of low-dose aspirin is not associated with lower BMD in general population. These findings between aspirin use and increased fracture risk is in contrast to the previous in vitro and in vivo findings. The disease itself and the medications used to treat it might confound the results of epidemiological studies on aspirin use. Furthermore, there are no conclusive criteria to define the precise doses of aspirin for in vitro investigations that correspond to the doses in human or animal studies. Therefore, it is difficult to elucidate the roles of aspirin in human studies. Anna et al. initiated a well-designed randomized controlled trial to assess whether daily using low-dose aspirin can benefit for bone health and prevent the fractures in elderly people [96]. Their research conclusions are expected to be a strong evidence for clinical usage of low-dose aspirin for skeletal regeneration.
NSAIDs are involved in bone remodeling and affect fracture healing [97], however, there is no clinically relevant result to prove the association between NSAIDs use and bone destructive diseases. Although all NSAIDs share a common mechanism of action, they vary widely in their clinical outcome because of their physicochemical differences [78]. This disparity could be due to several reasons. First, the differential effects of COX inhibition by different doses of NSAIDs on bone are not completely understood. Second, epidemiologic studies on the use of aspirin or NSAIDs may be confounded by age, sex, obesity and ethnicity, and involve inconformity of BMD, which may be a source of bias. Finally, patient compliance to the duration and frequency of taking medication should be considered.
Conclusions and perspective
In summary, low-dose aspirin might be a promising drug for relieving bone diseases that are related to abnormal bone remodeling, such as osteoporosis. Additionally, it is considered a suitable thrombosis phylactic agents following orthopedic operations. In contrast, the role of high-dose aspirin in bone remodeling is difficult to elucidate owing to its dual effects on osteoclast activity and osteoblast formation. Other NSAIDs have similar problems as high-dose aspirin. However, according to the promotional functions of COX-2-dependent PGs in angiogenesis [64, 65], COX-2 inhibitors and high-dose aspirin might be associated with a delay or nonunion of fracture healing, although the evidences for this finding are conflicting. Therefore, the clinical application of high-dose aspirin and NSAIDs for relieving pain in fractures should be more carefully and strictly controlled, particularly in the early stage of angiogenesis and bone repair. Low-dose aspirin used for the prevention of ACS might benefit bone health by protecting against the destruction of bone tissues and decreasing the risk of fractures. Further investigations are needed to fully illuminate the potential modulatory roles of aspirin in bone remodeling, especially with different doses, and determine the functions of aspirin and NSAIDs in relation with BMD and orthopedic operations.
Availability of data and materials
Not applicable.
Abbreviations
- NSAIDs:
-
non-steroidal anti-inflammatory drugs
- HSCs:
-
hematopoietic stem cells
- MSC:
-
mesenchymal stem cell
- RANKL:
-
receptor activator of nuclear factor-κB ligand
- BMD:
-
bone mineral density
- COX-1:
-
cyclooxygenase-1
- COX-2:
-
cyclooxygenase-2
- PGE2:
-
prostaglandin E2
- TXA2:
-
thromboxane A2
- ACS:
-
acute coronary syndromes
- TRAP:
-
tartrate-resistant acid phosphatase
- OVX:
-
ovariectomized
- OPG:
-
osteoprotegerin
- IFN-γ:
-
interferon-γ
- TNF-α:
-
tumor necrosis factor-α
- TGF-β1:
-
transforming growth factor β-1
- NO:
-
nitric oxide
- BMP-2:
-
bone morphogenetic protein 2
- FGF-2:
-
fibroblast growth factor 2
- 5-LO:
-
5-lipoxygenase
- RANK:
-
receptor activator of nuclear factor-κB
- PKG:
-
protein kinase G
- PKA:
-
protein kinase A
- AMPK:
-
adenosine monophosphate-activated protein kinase
References
Henriksen K, Karsdal MA, Martin TJ. Osteoclast-derived coupling factors in bone remodeling. Calcif Tissue Int. 2014;94(1):88–97.
Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008;473(2):201–9.
Delaisse JM. The reversal phase of the bone-remodeling cycle: cellular prerequisites for coupling resorption and formation. BoneKEy Rep. 2014;3:561.
Kikuta J, Ishii M. Osteoclast migration, differentiation and function: novel therapeutic targets for rheumatic diseases. Rheumatology. 2013;52(2):226–34.
Lassen NE, Andersen TL, Ploen GG, Soe K, Hauge EM, Harving S, Eschen GET, Delaisse JM. Coupling of bone resorption and formation in real time: new knowledge gained from human Haversian BMUs. J Bone Miner Res. 2017;32(7):1395–405.
Frost HM. The pathomechanics of osteoporoses. Clin Orthop Relat Res. 1985;(200):198-225.
Abdelgawad ME, Delaisse JM, Hinge M, Jensen PR, Alnaimi RW, Rolighed L, Engelholm LH, Marcussen N, Andersen TL. Early reversal cells in adult human bone remodeling: osteoblastic nature, catabolic functions and interactions with osteoclasts. Histochem Cell Biol. 2016;145(6):603–15.
Andersen TL, Abdelgawad ME, Kristensen HB, Hauge EM, Rolighed L, Bollerslev J, Kjaersgaard-Andersen P, Delaisse JM. Understanding coupling between bone resorption and formation: are reversal cells the missing link? Am J Pathol. 2013;183(1):235–46.
Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40(2):251–64.
Sucur A, Katavic V, Kelava T, Jajic Z, Kovacic N, Grcevic D. Induction of osteoclast progenitors in inflammatory conditions: key to bone destruction in arthritis. Int Orthop. 2014;38(9):1893–903.
Carbone LD, Tylavsky FA, Cauley JA, Harris TB, Lang TF, Bauer DC, Barrow KD, Kritchevsky SB. Association between bone mineral density and the use of nonsteroidal anti-inflammatory drugs and aspirin: impact of cyclooxygenase selectivity. J Bone Miner Res. 2003;18(10):1795–802.
Nakata K, Hanai T, Take Y, Osada T, Tsuchiya T, Shima D, Fujimoto Y. Disease-modifying effects of COX-2 selective inhibitors and non-selective NSAIDs in osteoarthritis: a systematic review. Osteoarthr Cartil. 2018;26:1263–73.
Iniguez MA, Pablos JL, Carreira PE, Cabre F, Gomez-Reino JJ. Detection of COX-1 and COX-2 isoforms in synovial fluid cells from inflammatory joint diseases. Br J Rheumatol. 1998;37(7):773–8.
Jee WS, Ma YF. The in vivo anabolic actions of prostaglandins in bone. Bone. 1997;21(4):297–304.
Woodiel FN, Fall PM, Raisz LG. Anabolic effects of prostaglandins in cultured fetal rat calvariae: structure–activity relations and signal transduction pathway. J Bone Miner Res. 1996;11(9):1249–55.
Abate A, Yang G, Dennery PA, Oberle S, Schroder H. Synergistic inhibition of cyclooxygenase-2 expression by vitamin E and aspirin. Free Radic Biol Med. 2000;29(11):1135–42.
Forder S, Voelker M, Lanas A. Gastrointestinal safety of aspirin for a high-dose, multiple-day treatment regimen: a meta-analysis of three randomized controlled trials. Drugs R D. 2016;16:263–9.
Nelson S, Cloonan L, Kanakis AS, Fitzpatrick KM, Shideler KI, Perilla AS, Furie KL, Rost NS. Antecedent aspirin use is associated with less severe symptoms on admission for ischemic stroke. J Stroke Cerebrovasc Dis. 2016;25:2519–25.
Schmidt M, Lamberts M, Olsen AM, Fosboll E, Niessner A, Tamargo J, Rosano G, Agewall S, Kaski JC, Kjeldsen K, Lewis BS, Torp-Pedersen C. Cardiovascular safety of non-aspirin non-steroidal anti-inflammatory drugs: review and position paper by the working group for Cardiovascular Pharmacotherapy of the European Society of Cardiology. Eur Heart J. 2016;37(13):1015–23.
Wilson DG, Poole WE, Chauhan SK, Rogers BA. Systematic review of aspirin for thromboprophylaxis in modern elective total hip and knee arthroplasty. Bone Joint J. 2016;98-b(8):1056–61.
Wood DM, Dargan PI, Jones AL. Measuring plasma salicylate concentrations in all patients with drug overdose or altered consciousness: is it necessary? Emerg Med J. 2005;22(6):401–3.
Radi ZA, Khan NK. Effects of cyclooxygenase inhibition on bone, tendon, and ligament healing. Inflamm Res. 2005;54(9):358–66.
Lane NE, Bauer DC, Nevitt MC, Pressman AR, Cummings SR. Aspirin and nonsteroidal antiinflammatory drug use in elderly women: effects on a marker of bone resorption. The study of osteoporotic fractures research group. J Rheumatol. 1997;24(6):1132–6.
Cottrell J, O’Connor JP. Effect of non-steroidal anti-inflammatory drugs on bone healing. Pharmaceuticals. 2010;3(5):1668–93.
Yamaza T, Miura Y, Bi Y, Liu Y, Akiyama K, Sonoyama W, Patel V, Gutkind S, Young M, Gronthos S, Le A, Wang CY, Chen W, Shi S. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS ONE. 2008;3(7):e2615.
Shi S, Yamaza T, Akiyama K. Is aspirin treatment an appropriate intervention to osteoporosis? Fut Rheumatol. 2008;3(6):499–502.
Gruber R, Schofnagl M, Karreth F, Fischer MB, Watzek G. The stable analog carbocyclic TXA2 but not platelet-released TXA2 induces osteoclast-like cell formation. Prostaglandins Leukotrienes Essent Fatty Acids. 2003;68(4):267–72.
Liu Y, Chen C, Liu S, Liu D, Xu X, Chen X, Shi S. Acetylsalicylic acid treatment improves differentiation and immunomodulation of SHED. J Dent Res. 2015;94(1):209–18.
Chen C, Akiyama K, Yamaza T, You YO, Xu X, Li B, Zhao Y, Shi S. Telomerase governs immunomodulatory properties of mesenchymal stem cells by regulating FAS ligand expression. EMBO Mol Med. 2014;6(3):322–34.
Cao Y, Xiong J, Mei S, Wang F, Zhao Z, Wang S, Liu Y. Aspirin promotes bone marrow mesenchymal stem cell-based calvarial bone regeneration in mini swine. Stem Cell Res Ther. 2015;6:210.
Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-gamma and TNF-alpha. Nat Med. 2011;17(12):1594–601.
Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG, Wang CY. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20(6):587–91.
Alfonso LF, Srivenugopal KS, Bhat GJ. Does aspirin acetylate multiple cellular proteins? (Review). Mol Med Rep. 2009;2(4):533–7.
Shen J, Hovhannisyan H, Lian JB, Montecino MA, Stein GS, Stein JL, Van Wijnen AJ. Transcriptional induction of the osteocalcin gene during osteoblast differentiation involves acetylation of histones h3 and h4. Mol Endocrinol. 2003;17(4):743–56.
Zhan Y, He Z, Liu X, Miao N, Lin F, Xu W, Mu S, Mu H, Yuan M, Cao X, Jin H, Liu Z, Li Y, Zhang B. Aspirin-induced attenuation of adipogenic differentiation of bone marrow mesenchymal stem cells is accompanied by the disturbed epigenetic modification. Int J Biochem Cell Biol. 2018;98:29–42.
Liu H, Li W, Liu Y, Zhang X, Zhou Y. Co-administration of aspirin and allogeneic adipose-derived stromal cells attenuates bone loss in ovariectomized rats through the anti-inflammatory and chemotactic abilities of aspirin. Stem Cell Res Ther. 2015;6:200.
Tang J, Xiong J, Wu T, Tang Z, Ding G, Zhang C, Wang S, Liu Y. Aspirin treatment improved mesenchymal stem cell immunomodulatory properties via the 15d-PGJ2/PPARgamma/TGF-beta1 pathway. Stem Cells Dev. 2014;23(17):2093–103.
Xu X, Zheng L, Yuan Q, Zhen G, Crane JL, Zhou X, Cao X. Transforming growth factor-beta in stem cells and tissue homeostasis. Bone Res. 2018;6:2.
Lin S, Lee WY, Huang M, Fu Z, Liang Y, Wu H, Xu L, Suen CW, Huang J, Wu T, Cui L, Li G. Aspirin prevents bone loss with little mechanical improvement in high-fat-fed ovariectomized rats. Eur J Pharmacol. 2016;791:331–8.
Chow JW, Fox SW, Lean JM, Chambers TJ. Role of nitric oxide and prostaglandins in mechanically induced bone formation. J Bone Miner Res. 1998;13(6):1039–44.
Vestergaard P, Steinberg TH, Schwarz P, Jorgensen NR. Use of the oral platelet inhibitors dipyridamole and acetylsalicylic acid is associated with increased risk of fracture. Int J Cardiol. 2012;160(1):36–40.
Cryer B, Feldman M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104(5):413–21.
Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001;108(1):25–30.
Chen MR, Dragoo JL. The effect of nonsteroidal anti-inflammatory drugs on tissue healing. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):540–9.
Lack WD, Fredericks D, Petersen E, Donovan M, George M, Nepola J, Smucker J, Femino JE. Effect of aspirin on bone healing in a rabbit ulnar osteotomy model. J Bone Joint Surg Am. 2013;95(6):488–96.
Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113(22):5444–55.
Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT, Zon LI. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136(6):1136–47.
Agas D, Marchetti L, Capitani M, Sabbieti MG. The dual face of parathyroid hormone and prostaglandins in the osteoimmune system. Am J Physiol Endocrinol Metab. 2013;305(10):E1185–94.
Agas D, Marchetti L, Hurley MM, Sabbieti MG. Prostaglandin F2alpha: a bone remodeling mediator. J Cell Physiol. 2013;228(1):25–9.
Raisz LG, Fall PM, Petersen DN, Lichtler A, Kream BE. Prostaglandin E2 inhibits alpha 1(I)procollagen gene transcription and promoter activity in the immortalized rat osteoblastic clonal cell line Py1a. Mol Endocrinol. 1993;7(1):17–22.
Chikazu D, Li X, Kawaguchi H, Sakuma Y, Voznesensky OS, Adams DJ, Xu M, Hoshio K, Katavic V, Herschman HR, Raisz LG, Pilbeam CC. Bone morphogenetic protein 2 induces cyclo-oxygenase 2 in osteoblasts via a Cbfal binding site: role in effects of bone morphogenetic protein 2 in vitro and in vivo. J Bone Miner Res. 2002;17(8):1430–40.
Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O’Keefe RJ. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109(11):1405–15.
Minamizaki T, Yoshiko Y, Kozai K, Aubin JE, Maeda N. EP2 and EP4 receptors differentially mediate MAPK pathways underlying anabolic actions of prostaglandin E2 on bone formation in rat calvaria cell cultures. Bone. 2009;44(6):1177–85.
Machwate M, Harada S, Leu CT, Seedor G, Labelle M, Gallant M, Hutchins S, Lachance N, Sawyer N, Slipetz D, Metters KM, Rodan SB, Young R, Rodan GA. Prostaglandin receptor EP(4) mediates the bone anabolic effects of PGE(2). Mol Pharmacol. 2001;60(1):36–41.
Graham S, Gamie Z, Polyzois I, Narvani AA, Tzafetta K, Tsiridis E, Helioti M, Mantalaris A, Tsiridis E. Prostaglandin EP2 and EP4 receptor agonists in bone formation and bone healing: in vivo and in vitro evidence. Expert Opin Investig Drugs. 2009;18(6):746–66.
Nakagawa K, Imai Y, Ohta Y, Takaoka K. Prostaglandin E2 EP4 agonist (ONO-4819) accelerates BMP-induced osteoblastic differentiation. Bone. 2007;41(4):543–8.
Snijdewint FG, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol (Baltimore, Md.: 1950). 1993;150(12):5321–9.
Tokuda H, Kozawa O, Uematsu T. Interleukin (IL)-17 enhances prostaglandin F(2 alpha)-stimulated IL-6 synthesis in osteoblasts. Prostaglandins Leukotrienes Essent Fatty Acids. 2002;66(4):427–33.
Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20(3):345–57.
Wang JS. Basic fibroblast growth factor for stimulation of bone formation in osteoinductive or conductive implants. Acta Orthop Scand. 1996;269:1–33.
Xie C, Liang B, Xue M, Lin AS, Loiselle A, Schwarz EM, Guldberg RE, O’Keefe RJ, Zhang X. Rescue of impaired fracture healing in COX-2−/− mice via activation of prostaglandin E2 receptor subtype 4. Am J Pathol. 2009;175(2):772–85.
Cottrell JA, O’Connor JP. Pharmacological inhibition of 5-lipoxygenase accelerates and enhances fracture-healing. J Bone Joint Surg Am. 2009;91(11):2653–65.
Maxis K, Delalandre A, Martel-Pelletier J, Pelletier JP, Duval N, Lajeunesse D. The shunt from the cyclooxygenase to lipoxygenase pathway in human osteoarthritic subchondral osteoblasts is linked with a variable expression of the 5-lipoxygenase-activating protein. Arthritis Res Ther. 2006;8(6):R181.
Einhorn TA, Majeska RJ, Rush EB, Levine PM, Horowitz MC. The expression of cytokine activity by fracture callus. J Bone Miner Res. 1995;10(8):1272–81.
Hausman MR, Schaffler MB, Majeska RJ. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone. 2001;29(6):560–4.
McCarty MF, Block KI. Preadministration of high-dose salicylates, suppressors of NF-kappaB activation, may increase the chemosensitivity of many cancers: an example of proapoptotic signal modulation therapy. Integr Cancer Ther. 2006;5(3):252–68.
Somasundaram S, Sigthorsson G, Simpson RJ, Watts J, Jacob M, Tavares IA, Rafi S, Roseth A, Foster R, Price AB, Wrigglesworth JM, Bjarnason I. Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat. Aliment Pharmacol Ther. 2000;14(5):639–50.
Zeng R, Faccio R, Novack DV. Alternative NF-kappaB regulates RANKL-induced osteoclast differentiation and mitochondrial biogenesis via independent mechanisms. J Bone Miner Res. 2015;30(12):2287–99.
Lemma S, Sboarina M, Porporato PE, Zini N, Sonveaux P, Di Pompo G, Baldini N, Avnet S. Energy metabolism in osteoclast formation and activity. Int J Biochem Cell Biol. 2016;79:168–80.
Paul-Clark MJ, Van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med. 2004;200(1):69–78.
Rangaswami H, Schwappacher R, Marathe N, Zhuang S, Casteel DE, Haas B, Chen Y, Pfeifer A, Kato H, Shattil S, Boss GR, Pilz RB. Cyclic GMP and protein kinase G control a Src-containing mechanosome in osteoblasts. Sci Signal. 2010;3(153):ra91.
Kalyanaraman H, Ramdani G, Joshua J, Schall N, Boss GR, Cory E, Sah RL, Casteel DE, Pilz RB. A novel, direct NO donor regulates osteoblast and osteoclast functions and increases bone mass in ovariectomized mice. J Bone Miner Res. 2016;32:46–59.
Yang JY, Park MY, Park SY, Yoo HI, Kim MS, Kim JH, Kim WJ, Jung JY. Nitric oxide-induced autophagy in MC3T3-E1 cells is associated with cytoprotection via AMPK activation. Korean J Physiol Pharmacol. 2015;19(6):507–14.
Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green KA, Mustard KJ, Kemp BE, Sakamoto K, Steinberg GR, Hardie DG. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336(6083):918–22.
Kang H, Viollet B, Wu D. Genetic deletion of catalytic subunits of AMP-activated protein kinase increases osteoclasts and reduces bone mass in young adult mice. J Biol Chem. 2013;288(17):12187–96.
De Luna-Bertos E, Ramos-Torrecillas J, Garcia-Martinez O, Diaz-Rodriguez L, Ruiz C. Effect of aspirin on cell growth of human MG-63 osteosarcoma line. Sci World J. 2012;2012:834246.
De Luna-Bertos E, Ramos-Torrecillas J, Garcia-Martinez O, Guildford A, Santin M, Ruiz C. Therapeutic doses of nonsteroidal anti-inflammatory drugs inhibit osteosarcoma MG-63 osteoblast-like cells maturation, viability, and biomineralization potential. Sci World J. 2013;2013:809891.
Salari P, Abdollahi M. Controversial effects of non-steroidal anti-inflammatory drugs on bone: a review. Inflamm Allergy Drug Targets. 2009;8(3):169–75.
Abukawa H, Phelps M, Jackson P, Smith RM, Vacanti JP, Kaban LB, Troulis MJ. Effect of ibuprofen on osteoblast differentiation of porcine bone marrow-derived progenitor cells. J Oral Maxillofac Surg. 2009;67(11):2412–7.
Chang JK, Wang GJ, Tsai ST, Ho ML. Nonsteroidal anti-inflammatory drug effects on osteoblastic cell cycle, cytotoxicity, and cell death. Connect Tissue Res. 2005;46(4–5):200–10.
Chang JK, Li CJ, Liao HJ, Wang CK, Wang GJ, Ho ML. Anti-inflammatory drugs suppress proliferation and induce apoptosis through altering expressions of cell cycle regulators and pro-apoptotic factors in cultured human osteoblasts. Toxicology. 2009;258(2–3):148–56.
Arpornmaeklong P, Akarawatcharangura B, Pripatnanont P. Factors influencing effects of specific COX-2 inhibitor NSAIDs on growth and differentiation of mouse osteoblasts on titanium surfaces. Int J Oral Maxillofac Implants. 2008;23(6):1071–81.
Tornkvist H, Lindholm TS, Netz P, Stromberg L, Lindholm TC. Effect of ibuprofen and indomethacin on bone metabolism reflected in bone strength. Clin Orthop Relat Res. 1984;187:255–9.
Burd TA, Lowry KJ, Anglen JO. Indomethacin compared with localized irradiation for the prevention of heterotopic ossification following surgical treatment of acetabular fractures. J Bone Joint Surg Am. 2001;83-A(12):1783–8.
O’Connor JP, Lysz T. Celecoxib, NSAIDs and the skeleton. Drugs Today (Barcelona, Spain: 1998). 2008;44(9):693–709.
Konstantinidis I, Papageorgiou SN, Kyrgidis A, Tzellos TG, Kouvelas D. Effect of non-steroidal anti-inflammatory drugs on bone turnover: an evidence-based review. Rev Recent Clin Trials. 2013;8(1):48–60.
Bauer DC, Orwoll ES, Fox KM, Vogt TM, Lane NE, Hochberg MC, Stone K, Nevitt MC. Aspirin and NSAID use in older women: effect on bone mineral density and fracture risk. Study of osteoporotic fractures research group. J Bone Miner Res. 1996;11(1):29–35.
van Staa TP, Leufkens HG, Cooper C. Use of nonsteroidal anti-inflammatory drugs and risk of fractures. Bone. 2000;27(4):563–8.
Simon AM, O’Connor JP. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J Bone Joint Surg Am. 2007;89(3):500–11.
Head JE, Bryant BJ, Grills BL, Ebeling PR. Effects of short-term use of ibuprofen or acetaminophen on bone resorption in healthy men: a double-blind, placebo-controlled pilot study. Bone. 2001;29(5):437–41.
Alissa R, Sakka S, Oliver R, Horner K, Esposito M, Worthington HV, Coulthard P. Influence of ibuprofen on bone healing around dental implants: a randomised double-blind placebo-controlled clinical study. Eur J Oral Implantol. 2009;2(3):185–99.
Gineyts E, Mo JA, Ko A, Henriksen DB, Curtis SP, Gertz BJ, Garnero P, Delmas PD. Effects of ibuprofen on molecular markers of cartilage and synovium turnover in patients with knee osteoarthritis. Ann Rheum Dis. 2004;63(7):857–61.
Morton DJ, Barrett-Connor EL, Schneider DL. Nonsteroidal anti-inflammatory drugs and bone mineral density in older women: the Rancho Bernardo study. J Bone Miner Res. 1998;13(12):1924–31.
Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with use of nonsteroidal anti-inflammatory drugs, acetylsalicylic acid, and acetaminophen and the effects of rheumatoid arthritis and osteoarthritis. Calcif Tissue Int. 2006;79(2):84–94.
Bonten TN, de Mutsert R, Rosendaal FR, Jukema JW, van der Bom JG, de Jongh RT, den Heijer M. Chronic use of low-dose aspirin is not associated with lower bone mineral density in the general population. Int J Cardiol. 2017;244:298–302.
Barker AL, McNeil JJ, Seeman E, Ward SA, Sanders KM, Khosla S, Cumming RG, Pasco JA, Bohensky MA, Ebeling PR, Woods RL, Lockery JE, Wolfe R, Talevski J. A randomised controlled trial of low-dose aspirin for the prevention of fractures in healthy older people: protocol for the ASPREE-Fracture substudy. Inj Prev. 2016;22(4):297–301.
Yates JE, Hadi Shah S, Blackwell JC. Clinical inquiries: do NSAIDs impede fracture healing? J Fam Pract. 2011;60(1):41–2.
Long J, Lewis S, Kuklo T, Zhu Y, Riew KD. The effect of cyclooxygenase-2 inhibitors on spinal fusion. J Bone Joint Surg Am. 2002;84-A(10):1763–8.
Tiseo BC, Namur GN, de Paula EJ, Junior RM, de Oliveira CR. Experimental study of the action of COX-2 selective nonsteroidal anti-inflammatory drugs and traditional anti-inflammatory drugs in bone regeneration. Clinics. 2006;61(3):223–30.
Akman S, Gogus A, Sener N, Bilgic B, Aksoy B, Seckin F. Effect of diclofenac sodium on union of tibial fractures in rats. Adv Ther. 2002;19(3):119–25.
Sudmann E, Dregelid E, Bessesen A, Morland J. Inhibition of fracture healing by indomethacin in rats. Eur J Clin Invest. 1979;9(5):333–9.
Hogevold HE, Grogaard B, Reikeras O. Effects of short-term treatment with corticosteroids and indomethacin on bone healing. A mechanical study of osteotomies in rats. Acta Orthop Scand. 1992;63(6):607–11.
Mullis BH, Copland ST, Weinhold PS, Miclau T, Lester GE, Bos GD. Effect of COX-2 inhibitors and non-steroidal anti-inflammatory drugs on a mouse fracture model. Injury. 2006;37(9):827–37.
Huo MH, Troiano NW, Pelker RR, Gundberg CM, Friedlaender GE. The influence of ibuprofen on fracture repair: biomechanical, biochemical, histologic, and histomorphometric parameters in rats. J Orthop Res. 1991;9(3):383–90.
Altman RD, Latta LL, Keer R, Renfree K, Hornicek FJ, Banovac K. Effect of nonsteroidal antiinflammatory drugs on fracture healing: a laboratory study in rats. J Orthop Trauma. 1995;9(5):392–400.
Lane N, Coble T, Kimmel DB. Effect of naproxen on cancellous bone in ovariectomized rats. J Bone Miner Res. 1990;5(10):1029–35.
Lane N, Maeda H, Cullen DM, Kimmel DB. Cancellous bone behavior in hindlimb immobilized rats during and after naproxen treatment. Bone Miner. 1994;26(1):43–59.
Jiang Y, Zhao J, Genant HK, Dequeker J, Geusens P. Bone mineral density and biomechanical properties of spine and femur of ovariectomized rats treated with naproxen. Bone. 1998;22(5):509–14.
Wanders A, Heijde D, Landewe R, Behier JM, Calin A, Olivieri I, Zeidler H, Dougados M. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005;52(6):1756–65.
Lionberger DR, Noble PC. Celecoxib does not affect osteointegration of cementless total hip stems. J Arthroplast. 2005;20(7 Suppl 3):115–22.
Bell NH, Hollis BW, Shary JR, Eyre DR, Eastell R, Colwell A, Russell RG. Diclofenac sodium inhibits bone resorption in postmenopausal women. Am J Med. 1994;96(4):349–53.
Moore KD, Goss K, Anglen JO. Indomethacin versus radiation therapy for prophylaxis against heterotopic ossification in acetabular fractures: a randomised, prospective study. J Bone Joint Surg Br. 1998;80(2):259–63.
Davis TR, Ackroyd CE. Non-steroidal anti-inflammatory agents in the management of Colles’ fractures. Br J Clin Pract. 1988;42(5):184–9.
Bichara J, Greenwell H, Drisko C, Wittwer JW, Vest TM, Yancey J, Goldsmith J, Rebitski G. The effect of postsurgical naproxen and a bioabsorbable membrane on osseous healing in intrabony defects. J Periodontol. 1999;70(8):869–77.
Jeffcoat MK, Williams RC, Reddy MS, English R, Goldhaber P. Flurbiprofen treatment of human periodontitis: effect on alveolar bone height and metabolism. J Periodontal Res. 1988;23(6):381–5.
Adolphson P, Abbaszadegan H, Jonsson U, Dalen N, Sjoberg HE, Kalen S. No effects of piroxicam on osteopenia and recovery after Colles’ fracture. A randomized, double-blind, placebo-controlled, prospective trial. Arch Orthop Trauma Surg. 1993;112(3):127–30.
Acknowledgements
Y.X., L.Z. and P.T. are supported by the National Natural Science Foundation of China (81772369) and Beijing Nova Program (Z171100001117110). M.P., Y.G. and W.G. is supported by CAMS Innovation Fund for Medical Sciences (CIFMS, 2017-I2M-3-001).
Funding
Y.X., L.Z. and P.T. are supported by the National Natural Science Foundation of China (81772369) and Beijing Nova Program (Z171100001117110). M.P., Y.G. and W.G. is supported by CAMS Innovation Fund for Medical Sciences (CIFMS, 2017-I2M-3-001).
Author information
Authors and Affiliations
Contributions
PT and WG designed the review and developed the theoretical framework. YX and MP wrote the manuscript and completed the figures. LZ and YG completed the tables. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agree to the publication of this manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xie, Y., Pan, M., Gao, Y. et al. Dose-dependent roles of aspirin and other non-steroidal anti-inflammatory drugs in abnormal bone remodeling and skeletal regeneration. Cell Biosci 9, 103 (2019). https://doi.org/10.1186/s13578-019-0369-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13578-019-0369-9