Abstract
The current study was aimed at investigating the prevalence of the mutations upstream of the oprD coding region and its promoters among imipenem-resistant and sensitive Pseudomonas aeruginosa isolated from educational hospitals in Yazd City, Iran. All isolates were identified by the conventional biochemical tests. Then, the antibiotic resistance of these isolates was determined using the disk diffusion method according to the CLSI guidelines. Also, the E.test was performed to determine the minimum inhibitory concentrations (MIC) of imipenem. The mutations of this gene were recognized by the amplification of this region and subsequently sequenced. Sequencing of the genomic region upstream of oprD these regions were done in the 29 clinical strains. Statistical analysis was done by the statistical software SPSS-18. Seventy (77.7%) of isolates had MIC ≥ 16 and were resistant to imipenem. Mutations of the upstream of the oprD gene and its promoters were seen in 25 (86.2%) isolates and 4 isolates had no mutation. One isolate had a base substitution A→Cat nt 25 in the coding region and this isolate had a point mutation leading to an amino acid change at positions 9 (I→L). Our study results indicated that none of the strains had mutation in Shine-Dalgarno and the point mutations were the most common mutations upstream of the oprD coding region among P. aeruginosa isolates. Mutations were observed in imipenem-resistant isolates and it seems this mechanism is effective in resistance of isolates to imipenem and this confirmed that the indiscriminate use of antibiotic should be controlled.
Similar content being viewed by others
Key points
-
1. Carbapenems, mainly imipenem and meropenem, are important and useful antibiotics for the treatment of infections due to multidrug-resistant Pseudomonas.
-
2. The Loss or mutations of outer membrane porin (OprD) and promoters of the oprD gene appears to be the most common mechanisms of intrinsic resistance to imipenem.
-
3. The antibiotic resistance of these isolates was determined using the disk diffusion method and E. test according to the CLSI guidelines. The mutations were recognized by the amplification of this region and subsequently sequenced.
-
4. All the imipenem-resistant isolates had mutations and the mutation was not seen in susceptible isolates
-
5. In Iran, there is little information about the contribution of different mechanisms to imipenem resistance in these isolates, especially about oprD mutations in the upstream region of gene and promoter in imipenem-resistance isolates.
Introduction
Pseudomonas aeruginosa is an opportunistic pathogen that causes a variety of infections in immunocompromised patients. In recent years, Antibiotic resistance of P. aeruginosa is increasing and the selection of suitable treatments has become difficult and is associated with increased morbidity and mortality (Riera et al. 2011; Yan et al. 2014).
Carbapenems, mainly imipenem and meropenem, are important and useful antibiotics for the treatment of infections due to multidrug-resistant Pseudomonas. Carbapenems are a class of β-lactam antibiotics with good antimicrobial activity against P. aeruginosa (Liste et al. 2009; Ocampo-Sosa et al. 2012). Carbapenem resistance of P. aeruginosa is mainly due to a combination of different factors, including low permeability of outer membrane porin and mutations in the gene encoding OprD, the production of the AmpC bate-lactamases, overproduction of efflux systems, and producing Carbapenemase (Hancock and Brinkman, 2002; Pirnay et al. 2002; Rostami et al. 2018). However, among these mechanisms, the Loss or mutation of outer membrane porin (OprD) and promoter of this gene appears to be the most common mechanisms of intrinsic resistance to imipenem and a lesser extent to meropenem. This mechanism causes blocking of the entrance of carbapenems particularly imipenem into a bacterium (Amin et al. 2005; Shen et al.2015).
OprD, an outer membrane porin is a semipermeable barrier and substrate-specific a penetrable protein consisting of 443 amino acids that allows the diffusion of sugars, small peptides, basic amino acids, and carbapenems typically imipenem into the cell (Cowan et al. 1992; Pirnay et al. 2002).
OprD mediated resistance occurs as a result of decreased transcriptional expression of oprD and imipenem resistance has been associated with (i) mutations that inactivate or destroy at least one of the oprD promoters, (ii) premature termination of oprD transcription, (iii) co-regulation with trace metal resistance mechanisms such as Zinc and copper, (iv) salicylate-mediated reduction, and (v) decreased transcriptional expression via co-regulation with the multidrug efflux pump encoded by mexEF-oprN (Amin et al. 2005).
The typed of mutations in the oprD gene and upstream regions and promoters of this gene are various such as nucleotide deletions, insertions, and point mutations that have been recognized to be the major mechanisms leading to inactivation of the oprD gene and promoter in imipenem-resistant isolates of P.aeruginosa (Gutiérrez et al. 2007; Pirnay et al. 2002). Transcription of oprD in P. aeruginosa PAO1 initiates with equal frequencies from two start sites, located 23 bases (SS1) and 71 bases (SS2) upstream of the structural gene. In the previous investigation, two or three types of imipenem-resistance mutants in clinical isolates were observed. The major type involves deletion and point mutations (Lynch et al. 1987). These well-known alterations are commonly reported, include point mutations or insertion sequences (ISs) inactivating in the resistance to imipenem, especially in Iran. Therefore, this study aimed to evaluate the prevalence of mutations upstream of the oprD coding region and its promoters in imipenem-resistant and -sensitive Pseudomonas aeruginosa isolated from educational hospitals.
Materials and methods
Bacterial isolates
In a descriptive study, 90 isolates of P. aeruginosa were collected from June 2018 to April 2019 at the Teaching Hospitals of Shahid Sadoghi University of Medical Science, Yazd, Iran. These isolates were originated from different clinical specimens of hospitalized patients, including blood, burn wounds, urine, lungs, etc.
Antimicrobial susceptibility testing
After transferring the plate containing Gram-negative rod colonies to the Laboratory of Microbiology, suspected colonies were identified by Gram staining and conventional biochemical tests such as catalase, oxidase, growth in 42 °C, oxidative/fermentative test, and differential media such as TSI (Merck, Germany). Isolate identified as P. aeruginosa were stored at 7 °C in trypticase soy broth (Merck) supplemented with a 20 °C glycerol unit.
Minimum inhibitory concentration and phenotypic confirmatory tests
Antibiotic susceptibility testing of the isolates was performed using the disk diffusion method (Kirby-Bauer) according to Clinical and Laboratory Standard Institute guideline (CLSI, 2019) using Mueller-Hinton agar (Merck, Germany) and Imipenem, meropenem, ertapenem, ciprofloxacin, ceftazidime, Cefepime, ceftriaxone, gentamicin, and tobramycin (MAST, UK). P. aeruginosa ATCC27853 was used as quality control. The Minimum Inhibitory Concentration (MIC) of imipenem was performed by E. test strips (Liofilchem, Italy) as described in the manufacturer's instructions. MIC breakpoint was defined according to CLSI guidelines (CLSI, 2019).
DNA extraction
DNA extraction was performed using by salting out method and was stored at – 20 °C until further use (18).
PCR for detection of oprD gene
PCR technique was performed. Primers were developed for each gene using Primer 3. The primers used for DNA amplification, as follows: 5′-AGACATGCCGTGGATACAAA0-3′ for the forward and 5′- AGTGCTACCTGCGGAAACC -3′ for the reverse primers. The final optimized PCR reaction consisted of 0.5 μl MgCl2 (100 mM), 0.5 μl dNTP (10 mM), 0.2 µl (1 unit) Taq DNA polymerase (Cinnagen, Iran), 1 µl of each primer (10 pmol) (Alpha DNA, Canada), 2.5 µl PCR buffer (10 X), and 0.5 μl of DNA template (100 μg/ml) in a total volume of 25 μl with double distilled water. DNA amplification was carried out with a thermocycler (Quanta Biotech, England), PCR amplification was performed as follows: one cycle at 95 °C for 300 s, then 30 cycles at 95 °C for 45 s, 56 °C for 45 s, and 72 °C for 60 s and a final extension at 72 °C for 10 min using an initial denaturation step for 5 min at 94 °C (one cycle), followed by 35 cycles of 1 min at 94 °C, 1 min at 50 °C, and 1 min at 72 °C. The amplified products were analyzed by 1.5% (w/w) agarose gel electrophoresis and were visualized on an ultraviolet illumination after staining with ethidium bromide.
DNA sequencing and analyses of sequence data
According to imipenem MIC results, 29 isolates were selected randomly (due to Financial Limitations) for evaluation of the mutations. We amplified and sequenced the genomic region upstream of oprD genes in the imipenem-resistant (n = 25) and imipenem-sensitive (n = 4) bacteria. For DNA sequencing, upstream regions and fifty-four (54) primary nucleotides of the oprD gene were sequenced. The sequence results were aligned and analyzed using MEGA 6 software and CLUSTAL W2, Vector NTI Advance version9.0.0 software (InforMax; Invitrogen). Protein alignments were carried out using. ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/). A mutation in the promoter and the upstream coding region of the OprD gene (Table 3) was identified by DNA sequencing.
Statistical analysis
The data were analyzed using the Statistical Program for Social Sciences version 18. (SPSS Version. 18 IBM, Chicago, IL, USA). For the analysis of data, chi-square tests were employed to calculate the P-value. Statistical significance and levels were set at P < 0.05.
Results
Bacterial isolates
Of 90 P. aeruginosa isolates, 38.9%, 20%, and 13.3% of them were isolated from burn wounds, urine, and wound specimens respectively. The Sources of P. aeruginosa isolates according to the hospital ward include Burn (43.3%), ICU (22.2%), Internal (15.6%), Surgery (11.1 %), and other wards (7.7 %).
Antibiotic resistance patterns
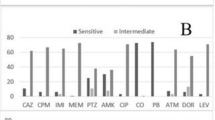
The frequency of resistance to carbapenems was as follows: imipenem 48.9%, meropenem 56.6%, and Ertapenem 52.5%. The results of antimicrobial susceptibility testing using the disk diffusion method are shown in Table 1. The results of the MIC of Imipenem by an E-test method are shown, 77.7% of isolates had MIC ≥ 16 and were resistant to imipenem and 22.2% of isolates had MIC ≤4 and Sensitive to imipenem.
PCR and sequencing
The oprD gene and genomic region upstream of oprD were amplified by PCR. The electrophoresis agarose gel was performing on PCR products that were shown in Fig. 1. The size of the amplified fragment is 570 base pairs. As shown in Fig. 1.
The oprD gene was sequenced, including the promoter and upstream regions including Shine-Dalgarno (GGAG; nucleotides − 12 to − 9), − 10 (TAAGTT; nucleotides − 84 to − 79), and − 35 (TCGCCA; nucleotides − 107 to − 102) sequences. Of 29 isolates selected for sequencing, 25 (86.2%) of isolates had mutations that all (100%) the isoltes were resistant to imipenem and four (13.7%) isolates had no mutations. Mutations’ percentages in resistant and isolates are shown in Table 2. There was a significant relationship (P < 0.05) between mutations upstream of the oprD coding regions and MIC of imipenem.
The frequency of mutations based on specimens was as follows: Burn 57.69%, Urine 19.23%, and other specimens 23.07%. Most mutations were seen in P. aeruginosa isolated from burn specimens and burn ward. The Statistical analysis found a significant correlation between the type of specimens and MIC (P ≤ 0.05). The Statistical analysis found a significant correlation between MIC and resistance to imipenem (P ≤ 0.05).
Based on the observed mutations, none of the strains had no mutation in Shine-Dalgarno (GGAG; nucleotides − 12 to − 9), − 10 (TAAGTT; nucleotides − 84 to − 79), and − 35 (TCGCCA; nucleotides − 107 to − 102) sequences. Six isolates have point mutations in the promoter, Five isolates had T→C base substitution at nt -90 and One isolate had a base substitution G→ Cat nt − 120. Also, One isolate had a base substitution A→ Cat n t 25 in the coding region, and this isolate had a point mutation leading to an amino acid change at positions 9(I→L). The insertion of one base was seen in five isolates and the insertion of tree nucleotide was observed in one isolate. The rest of the results sequencing of upstream regions and promoter regions are shown in Table 3.
Discussion
In medicine, the treatment of community-acquired infections and nosocomial infections caused by P. aeruginosa is important. Carbapenem is effective against infectious diseases caused by P. aeruginosa. However, carbapenem-resistant P. aeruginosa strains are emerging worldwide, and the rate of resistance in most countries ranges from 10 to 50 % (Huang, Jeanteur, Pattus, & Hancock, 1995). In the present study, the prevalence of imipenem resistance in bacteremic P. aeruginosa was 48.9 %, and the rate of resistance of P. aeruginosa to imipenem was 5.5 % to 62.5% in other studies(Dantas et al. 2017; Dubois et al. 2008; Gill et al. 2011; Hammami et al. 2009; Kohanteb et al. 2007; Lei et al. 2003; Levine et al. 1998; Sapino et al. 2012; Zarei-Yazdeli et al. 2014).
According to studies of antibiotic resistance in different parts of the world and the result of the present study; it can be concluded that resistance rates in P. aeruginosa isolates were higher than previous reports, which can be due to a combination of different factors such as the inconsiderate use or the previous use of antibiotics in prophylaxis, differences in the type of sample, and the geographical region and care of patients in hospitals and difference in the mechanism of resistance. Since the carbapenems are commonly used in the treatment and mutations in the genomic region upstream of oprD and promoter are the most current reason against resistance to these antibiotics, so identifying and assessing the prevalence of these mutations in the bacteria population can be very effective in controlling the resistance pattern. The mutational inactivation of the oprD gene and disruption in promoter represents the major cause of OprD loss in P. aeruginosa strains. In our study, alterations were observed in resistant isolates. Mutations of the upstream region oprD gene were seen in all (25) the imipenem-resistant isolates. Mutations in SS1 and SS2 were point mutations. One isolate had a base substitution A→ Cat n t 25 in the coding region and this isolate had a point mutation leading to an amino acid change at positions 9 (I→L). Also, the insertion of one base was seen in five isolates and the insertion of tree nucleotide were observed in one isolate.
A similar study was performed by Damien Fournier et al (Fournier et al. 2013). Mutations of the oprD gene were seen in 86.2% of imipenem-resistant isolates and Reported the lack of OprD was due to tot the disruption of the oprD promoter by ISPsy2 in one strain and the other strains had a mutation or gene disruption by different insertion sequences ISPa1635, ISPa1328, IS911, ISPs1, IS51, IS222, and ISPa41). In a study conducted by Alain A et al (Ocampo-Sosa et al. 2012). seventy-seven (77%) isolates had mutations and mutations were observed in both sensitive and resistant isolates. Most isolates showed point mutations and deletion mutations. In a study performed by Aki Hirabayashi et al (2017). Sequencing of oprD gene and the promoter and downstream regions were done and the results revealed that most of the resistant-isolates had insertion mutations in the oprD gene, also there was a direct relationship between the alteration or loss of oprD and the increase in MIC, for imipenem but not meropenem and other carbapenems (Cowan et al. 1992; Ocampo-Sosa et al. 2012; Shen et al. 2015; Zarei-Yazdeli et al. 2014). In a study conducted by Yumiko Sanbongi et al (2009). Most mutations were frame-shift mutations or deletion mutations. Gutiérrez et al (2007). Have reported different mutations in the oprD gene, the most frequent mutations were frameshift mutations produced by one nucleotide insertions or deletions and point mutations leading to the creation of a premature stop. In a study performed by EL Amin et al (2005). Sequence analysis revealed mutation of inactivation, including the insertion or deletion of one and two or more nucleotides and insertion sequences (IS). In investigating Performed by Wolter DJ et al (2004). PCR and sequence analysis revealed an interpolation of a large fragment in the oprD gene was known as IS elements that are not observed in this study. Jill Shen et al (2015). Reported 96.5% (136/141) of the resistant isolates had mutations. Ninety-six strains had a small deletion in the OprD gene or multi-site mutations and 34 strains had a large deletion in the OprD gene, 6 strains had IS, and 4 strains had no mutation and showed a normal OprD2 gene. In this study, the insertion of one base was seen in five strains. Twenty-five strains had point mutations and 4 strains had no mutation.
Yoneyama et al (1993). Reported a large deletion encompassing a region from upstream to downstream across the promoter region (from nucleotides 519-685) that prevented transcription of oprD and also deletion mutations were observed, including deletion an11 bp. Qinghui Sun et al (2016). Have reported an insertion sequence element (ISRP10) that causes disrupt of the oprD gene and is seen in 96% of imipenem-resistant P. aeruginosa isolates. In a study performed by Yingjun Yan et al (2014). The result of the analysis, indicatied that the 4-bp insertion in the oprD gene resulted in a frameshift in the OprD gene and imipenem resistance.
A different study conducted by Hussein Chalhoub (2016). DNA sequencing showed several mutations in the coding region oprD, but no mutation was observed in the promoter region of the gene. Reports had shown that mutation and inactivation or loss of an oprD gene, disruption in promoter and upstream region of oprD gene in P. aeruginosa strains are the major mechanisms that cause resistance to imipenem. This result was in accordance with the previous investigation of the clinical isolates of P. aeruginosa.
The results of this study show, increase in the resistance of P. aeruginosa to imipenem. Sequencing of the genomic region upstream of oprD in clinical strains revealed the point mutations in resistant strains. One isolate had a base substitution in the coding region oprD gene and this isolate had a point mutation leading to an amino acid change. All the imipenem-resistant isolates had mutations and Sensitive strains had no mutation. Judicious use of antimicrobials and controlled usage of imipenem may prevent P. aeruginosa from acquiring resistance to IPM. Neutralization of the mutation or the presence of a substance that can inactivate the mutation could lead to bacterial susceptibility to imipenem antibiotics.In our country, there is little information about the contribution of different mechanisms to imipenem resistance in these isolates, especially about oprD mutations in the upstream region of gene and promoter in imipenem-resistance isolates. Awareness of resistant mechanisms in P.aeruginosa isolates could help to regulate infection control strategies and to enhance the efficacy of imipenem for the treatment of infections due to these bacteria. Thus, there is a need to focus on intrinsic resistance mechanisms, especially Porin alteration which also confers significant imipenem resistance, it also suggests in the future other mechanisms such as gene expression and its relationship with the oprD mutations are evaluated and investigated in other isolates and other places.
Availability of data and materials
The data are available. All data generated or analyzed during this study are included in this study.
References
Amin NE, Giske CG, Jalal S, Keijser B, Kronvall G, Wretlind B (2005) Carbapenem resistance mechanisms in Pseudomonas aeruginosa: alterations of porin OprD and efflux proteins do not fully explain resistance patterns observed in clinical isolates. Apmis 113(3):187–196
Chalhoub H, Sáenz Y, Rodriguez-Villalobos H, Denis O, Kahl BC, Tulkens PM, Van Bambeke F (2016) High-level resistance to meropenem in clinical isolates of Pseudomonas aeruginosa in the absence of carbapenemases: role of active efflux and porin alterations. Int J Antimicrob Agents 48(6):740–743
Cowan S, Schirmer T, Rummel G, Steiert M, Ghosh R, Pauptit R, Jansonius JN, Rosenbusch J (1992) Crystal structures explain functional properties of two E. coli porins. Nature 358(6389):727
Dantas RC, Silva RT, Ferreira ML, Gonçalves IR, Araújo BF, Campos PA, Royer R, William D, Batistão F, Gontijo-Filho P, Ribas RM (2017) Molecular epidemiological survey of bacteremia by multidrug resistant Pseudomonas aeruginosa: the relevance of intrinsic resistance mechanisms. Plos One 12(5):e0176774
Dubois V, Arpin C, Dupart V, Scavelli A, Coulange L, André C, Fischer I, Grobost F, Brochet J, Dutilh B, Jullin J, Noury P, Larribet G, Quentin C (2008) β-Lactam and aminoglycoside resistance rates and mechanisms among Pseudomonas aeruginosa in French general practice (community and private healthcare centres). J Antimicrob Chemother 62(2):316–323
Fournier D, Richardot C, Müller E, Robert-Nicoud M, Llanes C, Plésiat P, Jeannot K (2013) Complexity of resistance mechanisms to imipenem in intensive care unit strains of Pseudomonas aeruginosa. J Antimicrob Chemother 68(8):1772–1780
Gill MM, Usman J, Kaleem F, Hassan A, Khalid A, Anjum R, Fahim Q (2011) Frequency and antibiogram of multi-drug resistant Pseudomonas aeruginosa. J Coll Physicians Surg Pak 21(9):531–534
Gutiérrez O, Juan C, Cercenado E, Navarro F, Bouza E, Coll P, J L, Pérez. Oliver, A. (2007) Molecular epidemiology and mechanisms of carbapenem resistance in Pseudomonas aeruginosa isolates from Spanish hospitals. Antimicrob Agents Chemother 51(12):4329–4335
Hammami S, Ghozzi R, Burghoffer B, Arlet G, Redjeb S (2009) Mechanisms of carbapenem resistance in non-metallo-β-lactamase-producing clinical isolates of Pseudomonas aeruginosa from a Tunisian hospital. Pathologie Biologie 57(7–8):530–535
Hancock RE, Brinkman FS (2002) Function of Pseudomonas porins in uptake and efflux. Ann Rev Microbiol 56(1):17–38
Hirabayashi A, Kato D, Tomita Y, Iguchi M, Yamada K, Kouyama Y, Morioka H, Tetsuka N, Yagi T (2017) Risk factors for and role of OprD protein in increasing minimal inhibitory concentrations of carbapenems in clinical isolates of Pseudomonas aeruginosa. J Med Microbiol 66(11):1562–1572
Huang H, Jeanteur D, Pattus F, Hancock RE (1995) Membrane topology and site-specific mutagenesis of Pseudomonas aeruginosa porin OprD. Mol Microbiol 16(5):931–941
Kohanteb J, Dayaghi M, Motazedian M, Ghayumi M-A (2007) Comparison of biotyping and antibiotyping of Pseudomonas aeruginosa isolated from patients with burn wound infection and nosocomial pneumonia in Shiraz Iran. PJBS 10(11):1817–1822
Lei Y, Wang H, Sun Z, Shen Z (2003) Susceptibility of 570 Pseudomonas aeruginosa strains to 11 antimicrobial agents and the mechanism of its resistance to fluoroquinolones. Zhonghua yi xue za zhi 83(5):403–407
Levine C, Hiasa H, Marians KJ (1998) DNA gyrase and topoisomerase IV: biochemical activities, physiological roles during chromosome replication, and drug sensitivities. Biochimica et Biophysica Acta 1400(1–3):29–43
Lister PD, Wolter DJ, Hanson ND (2009) Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 22(4):582–610
Lynch M, Drusano G, Mobley H (1987) Emergence of resistance to imipenem in Pseudomonas aeruginosa. Antimicrob Agents Chemother 31(12):1892–1896
Ocampo-Sosa AA, Cabot G, Rodríguez C, Roman E, Tubau F, Macia MD, Zamorano Moya B, L, Suárez C, Peña, C. (2012) Alterations of OprD in carbapenem-intermediate and-susceptible strains of Pseudomonas aeruginosa isolated from bacteremia in a Spanish multicenter study. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.05451-11
Pirnay JP, Vos DD, Mossialos D, Vanderkelen A, Cornelis P, Zizi M (2002) Analysis of the Pseudomonas aeruginosa oprD gene from clinical and environmental isolates. Environ Microbiol 4(12):872–882
Riera E, Cabot G, Mulet X, García-Castillo M, del Campo R, Juan R, Cantón. Oliver, A. (2011) Pseudomonas aeruginosa carbapenem resistance mechanisms in Spain: impact on the activity of imipenem, meropenem and doripenem. J Antimicrob Chemother 66(9):2022–2027
Rostami S, Sheikh AF, Shoja S, Farahani A, Tabatabaiefar MA, Jolodar A, Sheikhi R (2018) Investigating of four main carbapenem-resistance mechanisms in high-level carbapenem resistant Pseudomonas aeruginosa isolated from burn patients. J Chin Med Assoc 81(2):127–132
Sanbongi Y, Shimizu A, Suzuki T, Nagaso H, Ida T, Maebashi K, Gotoh N (2009) Classification of OprD sequence and correlation with antimicrobial activity of carbapenem agents in Pseudomonas aeruginosa clinical isolates collected in Japan. Microbiol Immunol 53(7):361–367
Sapino B, Mazzucato S, Solinas M, Gion M, Grandesso S (2012) Comparison of different methods for determining beta-lactam susceptibility in Pseudomonas aeruginosa. Microbiologica 35(4):491
Shen J, Pan Y, Fang Y (2015) Role of the outer membrane protein OprD2 in carbapenem-resistance mechanisms of Pseudomonas aeruginosa. Plos One 10(10):e0139995
Sun Q, Ba Z, Wu G, Wang W, Lin S, Yang H (2016) Insertion sequence ISRP10 inactivation of the oprD gene in imipenem-resistant Pseudomonas aeruginosa clinical isolates. Int Antimicrob Agents 47(5):375–379
Wolter DJ, Hanson ND, Lister PD (2004) Insertional inactivation of oprD in clinical isolates of Pseudomonas aeruginosa leading to carbapenem resistance. FEMS Microbiol Lett 236(1):137–143
Yan Y, Yao X, Li H, Zhou Z, Huang W, Stratton CW, Lu C-D, Tang Y-W (2014) A novel oprD-mutated Pseudomonas aeruginosa strain in relation to a nosocomial respiratory infection outbreak in an intensive care unit. J Clin Microbiol. https://doi.org/10.1128/JCM.02782-14
Yoneyama H, Nakae T (1993) Mechanism of efficient elimination of protein D2 in outer membrane of imipenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 37(11):2385–2390
Zarei-Yazdeli M, Eslami G, Zandi H, Mousavi SM, Kosha H, Akhavan F, Kiani M (2014) Relationship between antimicrobial resistance and class I integron in Pseudomonas aeruginosa isolated from clinical specimens in Yazd during 2012–2013. Feyz J Kashan Univ Med Sci 18(1):60–67
Acknowledgements
This research project was financially supported by Shahid Sadoughi University of Medical Sciences. The authors wish to thank Shahid Sadoughi University of Medical Sciences for providing this grant to finance this research.
Funding
This research project was financially supported by Shahid Sadoughi University of Medical Sciences. The authors wish to thank Shahid Sadoughi University of Medical Sciences for providing this grant to finance this research.
Author information
Authors and Affiliations
Contributions
HZ: project development, management and manuscript writing. MK: project development; management and manuscript writing. AA: data collection and manuscript writing. HA: data collection and data analysis. AP: manuscript writing and data analysis. MS: manuscript writing, manuscript editing and data analysis. SR: manuscript writing and data analysis. MZ: data collection and manuscript writing. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by Shahid Sadoughi University of Medical Sciences, Yazd, Iran and ethical code was IR.SSU.REC.1391.24.In this study, the patient and human samples were not examined directly and the bacterial samples were collected from the Teaching Hospitals of Shahid Sadoghi University of Medical Science, Yazd, Iran. Informed consent was obtained from all individual participants included in the study.
Consent for publication
The participant has consented to the submission of the case report to the journal.
Competing interests
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kiani, M., Astani, A., Eslami, G. et al. Upstream region of OprD mutations in imipenem-resistant and imipenem-sensitive Pseudomonas isolates. AMB Expr 11, 82 (2021). https://doi.org/10.1186/s13568-021-01243-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-021-01243-3