Abstract
Antibacterial coating of medical devices is a promising approach to reduce the risk of infection but has not yet been achieved on wear surfaces, e.g. polyethylene (PE). We quantitatively determined the antimicrobial potency of different PE surfaces, which had been conversed to diamond-like carbon (DLC-PE) and doped with silver ions (Ag-DLC-PE). Bacterial adhesion and planktonic growth of various strains of S. epidermidis on Ag-DLC-PE were compared to untreated PE by quantification of colony forming units on the adherent surface and in the growth medium as well as semiquantitatively by determining the grade of biofilm formation by scanning electron microscopy. (1) A significant (p < 0.05) antimicrobial effect could be found for Ag-DLC-PE. (2) The antimicrobial effect was positively correlated with the applied fluences of Ag (fivefold reduced bacterial surface growth and fourfold reduced bacterial concentration in the surrounding medium with fluences of 1 × 1017 vs. 1 × 1016 cm−2 under implantation energy of 10 keV). (3) A low depth of Ag penetration using low ion energies (10 or 20 vs. 100 keV) led to evident antimicrobial effects (fourfold reduced bacterial surface growth and twofold reduced bacterial concentration in the surrounding medium with 10 or 20 keV and 1 × 1017 cm−2 vs. no reduction of growth with 100 keV and 1 × 1017 cm−2). (4) Biofilm formation was decreased by Ag-DLC-PE surfaces. The results obtained in this study suggest that PE-surfaces can be equipped with antibacterial effects and may provide a promising platform to finally add antibacterial coatings on wear surfaces of joint prostheses.
Similar content being viewed by others
Introduction
The great success of surgically-implanted biomaterials may be compromised in every case by the challenging complication of bacterial periimplant infection (Liu et al. 2012; Zimmerli and Ochsner 2003). Approximately 2.6 million orthopedic biomaterials are implanted annually in the USA, hence the incidence of implant-associated infections is also increasing (Kurtz et al. 2008). Most important in the pathogenesis of infection is the colonization of the device surface and the consecutive formation of a biofilm (Zimmerli and Moser 2012; Gosheger et al. 2004), in which Staphylococcus aureus and koagulase-negative Staphylococci are most frequently implicated as the etiologic agents (Zimmerli and Ochsner 2003; Hunter and Dandy 1977). Prevention of these infections has an important impact not only on patient’s morbidity but also on the cost effectiveness of hospital care (Gosheger et al. 2004). Systemic antibiotic prophylaxis and various local antibiotic delivery techniques have been proven to reduce the rate of infection (Gollwitzer et al. 2003; Schmidmaier et al. 2006). Hereby locally applied antibiotics are advantageous in delivering high drug concentrations to the required site without producing systemic toxicity (Zhang et al. 2014). Because pathogens involved in implant associated infections are diverse and bacteria in biofilms are protected from antibiotics (Ceri et al. 1999), the restricted activity of these substances limits their clinical effectiveness, especially in infections involving antibiotic-resistant bacterial strains (e.g. MRSA) (Liu et al. 2012). Therefore, alternatives to local antibiotic delivery systems are highly favored. In this context employment of implant materials or coatings that control infection and biofilm formation would be particularly advantageous (Schmidmaier et al. 2006). This led to the development of antiadhesive and non-antibiotic antibacterial surfaces. The first mentioned coatings (e.g. polyethylene glycol, polyethylene oxide brushes) reduce bacterial adhesion by altering the physicochemical properties of the substrate. Thus, formation of protein surface layers (conditioning films) on the implant and bacteria–substrate interactions are hindered (Hetrick and Schoenfisch 2006). This mode of action is referred to as ‘‘passive’’. However the effectiveness of these coatings for reducing bacterial adhesion is very limited and varies greatly depending on bacterial species. Additionally, osseointegration is poor. In sum, the importance of these antiadhesive coatings in orthopedic surgery is limited. In contrast non-antibiotic “active” antibacterial coatings release antibacterial agents, e.g. silver ions (Ag+), copper ions (Cu++), nitric oxide, chlorhexidine/chloroxylenol or chitosan (Kumar and Munstedt 2005; Hardes et al. 2007; Gosheger et al. 2004; Shirai et al. 2009). Compared to antibiotics these agents act more broadly against a wide range of bacteria. In addition, at least proven for the use of Ag+, microbes without intrinsic resistance cannot gain resistance (Kumar and Munstedt 2005; Lee et al. 2005).
So far, these antibacterial coatings have not been applied on soft wear surfaces, e.g. polyethylene (PE). In total knee replacement roughly half of the surface is exposed to synovial fluid and in main parts tribologically active. Therefore in septic revision surgery major portions of the susceptible prosthesis are not protected against bacterial reinfection. Antibacterial-agent-enriched diamond-like carbon (DLC) surfaces may solve this dilemma. By release of Ag+ these surfaces could act as local antibacterial agents (Cloutier et al. 2014; Katsikogianni et al. 2006). At the same time appropriate DLC surfaces can exhibit excellent tribological features as already shown for hip or knee arthroplasty (Saikko et al. 2001; Dearnaley 1993; Oliveira et al. 2014).
In this study the antimicrobial effects of silver (Ag) incorporated DLC surfaces on PE (Ag-DLC-PE) are investigated. PE was chosen due to its outstanding importance in orthopedic surgery as a wear surface. This study provides valuable information for determining the suitability of Ag-DLC-PE for septic revision surgery.
Materials and methods
Study substrates and surface conversion
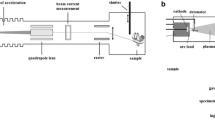
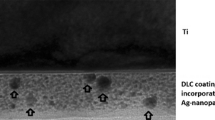
Study objects were cylindrical substrates (diameter: 10 mm, height: 2 mm; Goodfellow GmbH, Nauheim, Germany) of PE (ultrahigh molecular weight PE, UHMWPE). DLC-processing of the plates was performed at the Department of Experimental Physics IV, University of Augsburg (Germany). The samples were treated according to a modified technique of ion irradiation of polymers, in which DLC-processing was achieved by direct ion bombardment of Ag+ or nitrogen (Bertóti et al. 2007). In contrast to common DLC techniques, the PE surface is not coated with DLC but rather modified by silver ion implantation. Due to the kinetic energy of the implanted Ag+, the polymer surface is modified from crystalline PE to amorphous DLC, while the metal ions agglomerate to Ag nano-particles directly under the surface. In this way, the implantation of silver ions leads to a wear-resistant, silver-containing modified PE surface reducing the risk of detachment compared to surface coatings (Fig. 1) (Schwarz and Stritzker 2010).
These surface conversed DLC-PE samples were investigated in three groups with modified parameters of Ag+-implantation: firstly, to determine the influence of different ion energies (first group) and secondly, to determine the influence of different fluences (second group). In order to elucidate which of these factors (ion energy vs. fluence) has a major impact on microbes a third testing group was conducted. All sample features and testing groups are given in Table 1.
In the first group three Ag-doped samples with constant fluences (1 × 1017 cm−2) and different ion energies (60, 80, 100 keV) were assembled. DLC processing was carried out via direct ion implantation of Ag+. Based on the findings of the first group the second group was performed with three different fluences of Ag+ (1 × 1016, 5 × 1016 and 1 × 1017 cm−2) and constant low implantation energy (10 keV). In the third group samples were subjected to ion bombardment of Ag+ with different fluences and low ion energies (20 keV: 5 × 1016 and 1 × 1017 cm−2; 10 keV: 5 × 1016 and 1 × 1017 cm−2). Non-modified PE samples served as a control.
After sample preparation incubation for 24 h with Staphylococcus epidermidis (ATCC35984) was carried out. Thereafter, antimicrobial effects on the sample’s surface (i.e. bacterial sessile growth) and the surrounding fluid medium (i.e. bacterial planktonic growth) were investigated.
Sterilization of samples and sealing of surfaces with paraffin wax
Samples were rinsed with distilled water for 10 min, air-dried in a laminar flow cabinet and thereafter sterilized with gamma-beam with the dose of 26.5 kGy (Isotron Deutschland GmbH, Allershausen, Germany). All manipulations of the samples were conducted by holding the lower surface. As a consequence these parts of the samples were not surface treated and needed protection from the testing environment. Hence, paraffin wax was first autoclaved in a glass container with 120 °C for 20 min (Varioklav®, H + P Labortechnik, H + P Labortechnik AG, Oberschleißheim, Germany), the samples’ lower surfaces were then dip-coated in the solvent paraffin wax so that a thin protection layer was formed. Specimens were then placed in 24-well culture plates (Fig. 2a, b). Pretesting with paraffin wax revealed no intrinsic antimicrobial potential and was therefore appropriate as a mechanical sample stabilizer.
Bacterial sample preparation
The bacterial strains used in the present study were S. epidermidis (ATCC 35984; LGC Standards GmbH, Wesel, Germany) for determination of surface and planktonic growth and a strong biofilm-forming variant of S. epidermidis (RP62a; LGC Standards GmbH, Wesel, Germany) for scanning electron microscopy (SEM-) evaluation of biofilm formation on the samples. These strains are known for their outstanding significance in implant-associated infections (Zimmerli and Ochsner 2003; Darouiche 2004). Test strains were routinely cultured in Columbia Agar with 5 % sheep blood (S. epidermidis, ATCC 35984) or Trypticase™ Soy Agar (S. epidermidis, RP62a) (Becton–Dickinson, Heidelberg, Germany) at 37 °C overnight before testing. Bacteria were then harvested by centrifugation, rinsed, suspended, diluted in sterile phosphate buffered saline (PBS) and adjusted by densitometry to a MacFarland 0.5 standard (MacFarland Densimat™, BioMérieux, Marcy l’Etoile, France). To control bacterial concentration, 100 μl of each suspension was again cultured for 24 h at 37 °C. After 24 h serial dilutions of this suspension were plated on Colombia-Agar. The colonies were counted and colony numbers calculated accordingly. For the study every suspension with its known bacterial concentration was diluted with DMEM + 10 % FCS to reach the targeted value for bacterial concentration (105 CFU/ml). Sample plates with paraffin-coated lower surfaces were placed in 24-well culture plates and 1 ml of 105 CFU/ml bacterial suspensions were added. Incubation of the well plates was conducted for 24 h at 37 °C.
Analysis
Bacterial surface adhesion was evaluated by determining bacterial concentration on the specimen. Bacterial planktonic growth was measured in the growth medium. For every group four independent testing runs with four different samples were conducted. Therefore, altogether 16 samples were tested for every group.
Determination of bacterial growth on sample surfaces
Colonized sample plates were removed from the wells with a sterile forceps, carefully rinsed twice with sterile PBS, transferred to vials containing 3 ml of sterile PBS and sonicated for 7 min (Elmasonic S60H, Elma, Singen, Germany) to remove adhering bacteria. 100 μl of the fluid were aspirated, plated on Colombia Agar at 37 °C for 24 h and quantified after incubation (CFU/ml).
Scanning electron microscopy-analysis was conducted semiquantitatively to evaluate inhibition of biofilm formation. SEM-images were compiled of native DLC coated PE samples and Ag-DLC-PE samples. Biofilm formation was quantified in five categories: (1) no biofilm formation, (2) biofilm covering less than 25 % of the surface, (3) biofilm covering between 25 and 75 % of the surface, (4) biofilm covering more than 75 % of the surface, (5) biofilm formation covering the entire surface.
Determination of bacterial planktonic growth
A 700-μl volume of each well was supplemented with 700 μl neutralizing solution as described by Tilton and Rosenberg (1978) (1.0 g sodium thioglycolate + 1.46 g sodium thiosulfate in 1.000 ml deionized water). The neutralizing solution acts as an inhibitor for reminiscent metal toxicity on bacteria. The suspension was plated on Columbia Agar after serial dilutions and incubated at 37 °C for 24 h. Thereafter, CFU were quantified and extrapolated to CFU/ml.
Statistics
All results are presented as mean ± standard deviation. Statistical significance was computed using non-parametric methods and the method of closed testing procedure (Kruskal–Wallis and Mann–Whitney U test). P < 0.05 was considered statistically significant. Statistical tests were performed with use of SPSS (version 20.0; Chicago, IL, USA). Statistical analysis was conducted per consultation with the Institute of Medical Statistics and Epidemiology (Klinikum rechts der Isar, Technische Universität München, Munich, Germany).
Results
Antimicrobial effect of Ag-DLC-PE with different high ion energies (60, 80, 100 keV) and equal fluences (1 × 1017 cm−2): testing group 1
Compared to non-treated PE samples a minimally increased bacterial surface adhesion was found on samples after DLC conversion and Ag+ implantation with 100 keV. A comparable finding was observed with samples treated with 80 keV. However, on Ag-DLC-PE samples treated with only 60 keV a significantly and clinically relevant decreased bacterial growth was evident (Table 1; Fig. 3). Analysis of planktonic growth in the supernatant growth medium showed significantly increased bacterial concentrations for samples treated with 100 and 80 keV compared to PE; samples conversed with 60 keV did not show a significant increase of bacterial growth (Table 1; Fig. 3).
Antimicrobial effect of Ag-DLC–PE with different fluences (1 × 1016, 5 × 1016 and 1 × 1017 cm−2) and constant low ion energy (10 keV): testing group 2
Ag-DLC-PE showed a decreased bacterial surface adhesion compared to PE by 0.03 log-levels (p > 0.05) for fluences of 1 × 1016 cm2, by 0.6 log-levels (p < 0.05) with fluences of 5 × 1016 cm−2 and by 1.5 log-levels (p < 0.05) on samples with the highest fluences (1 × 1017 cm−2). Analysis of planktonic growth showed minimally increased bacterial concentrations compared to untreated PE only in broths containing samples with fluences of 1 × 1016 cm−2. Surface conversion with Ag and fluences of 5 × 1016 and 1 × 1017 cm−2 resulted in a reduction of planktonic bacterial growth (Table 1; Fig. 4).
Low ion energy (10, 20 keV) vs. fluence (5 × 1016 and 1 × 1017 cm−2): comparison of these two features regarding the antimicrobial effect of Ag-DLC-PE: testing group 3
Analysis of surface adhesion on Ag-DLC-PE samples conversed with 10 keV ion energy implantation showed a significant reduction of bacterial growth on specimen treated with fluences of 5 × 1016 and 1 × 1017 cm−2. Similarly, samples treated with 20 keV ion energy implantation showed significantly decreased bacterial growth on samples with fluences of 5 × 1016 and 1 × 1017 cm−2 (Table 1; Fig. 5). Analysis of planktonic growth of samples treated with 20 keV ion energy implantation showed significantly decreased bacterial concentrations with fluences of 5 × 1016 and 1 × 1017 cm−2. Samples with 10 keV ion energy implantation and fluences of 5 × 1016 and 1 × 1017 cm−2 also showed a reduction of bacterial growth (Table 1; Fig. 5).
Surface biofilm formation in scanning electron micrographs
Biofilm formation was ubiquitous and graded type 5 on all pure PE samples without Ag incorporation covering the entire specimen surfaces with thick layers of S. epidermidis. Ag-DLC-PE samples on the other hand showed biofilm inhibiting effects with at the most rare spot-like biofilm formation graded type 3 (Fig. 6a, b).
Discussion
Recent strategies to lower periimplant infection rates are based on the primary prevention of bacterial adhesion by non-adhesive coatings (Groll et al. 2009; Harris et al. 2004) or impairment of bacterial survival and biofilm formation by surface coatings releasing non-antibiotic organic antimicrobial agents like chlorhexidine or chitosan (Bumgardner et al. 2003; Verraedt et al. 2011; Baffone et al. 2011) and inorganic antimicrobial agents like Ag+, Cu++ or nitric-oxide (Zhao et al. 2011; Fiedler et al. 2011; Holt et al. 2011; Kumar and Munstedt 2005). To our best knowledge, no attempt has been conducted so far to apply these coatings on soft wear surfaces, e.g. PE. This leads to a major unprotected surface area of joint prostheses favoring reinfection, especially in septic revision surgery. To solve this problem addition of bactericidal agents to DLC surface modifications could be promising, based on the finding that DLC applied at PE is known to exhibit excellent wear behavior (Saikko et al. 2001; Dearnaley 1993; Oliveira et al. 2014). Some studies investigated Ag doped DLC coatings on hard wear surfaces e.g. steel and found significant bactericidal effects (Soininen et al. 2011; Marciano et al. 2009; Katsikogianni et al. 2006; Kwok et al. 2007; Baba et al. 2013). To our knowledge, no report has been published so far describing antibacterial conversion of PE for antibacterial purposes.
Ag seems to be of outstanding value in the prevention and treatment of implant associated infections (Morones et al. 2005; Taglietti et al. 2012; Hardes et al. 2010). Ag acts by binding to membranes, enzymes and nucleic acids. Consequently the respiratory chain is inhibited and therefore the aerobe metabolism of microorganisms disturbed (Gosheger et al. 2004). Bacteria are quite susceptible to Ag with only negligible possibility of intrinsic resistance (Kumar and Munstedt 2005). Antibacterial effects have been reported to be directly proportional to Ag concentrations and therefore directly depend on Ag release into the surrounding environment (Schierholz et al. 1998; Morones et al. 2005). These findings were confirmed in the present study (Table 1). In this context, important properties of the tested coatings could be identified: it was found that antimicrobial efficacy on the surface of Ag-DLC-PE treated with high energies of ion implantation (60–100 keV) was only significant in samples treated with ion energies of 60 keV. No bactericidal effect in this setting was determined in the surrounding medium. From a physical point of view this is not surprising since high ion energies determine a rather deep implantation of Ag preventing the atoms from release into the surrounding medium. This “deep depositioning” effect of DLC surfaces on ions implanted with high energies has already been described in the literature in other materials than PE (Furno et al. 2004). Compared to native PE we found Ag-DLC-PE treated with high implantation energies (100 keV) to be even more susceptible for bacterial colonization. This finding is surprising, since DLC coatings of other materials than PE (e.g. steel, PVC) showed significant antibacterial potency in several investigations (Baba et al. 2013; Katsikogianni et al. 2006; Marciano et al. 2009). In consequence, low ion energies (10 keV) were used in the second testing group. The results showed clearly, that antibacterial potency increased with lower ion energies due to the deposition of Ag proximate to the surface and a therefore potentially higher concentration of released Ag+. Therefore an increased antimicrobial effect was determined not only on the surface but also in the surrounding medium. To identify which of the parameters (ion energy or fluence) might have major impact on Ag+ dissolution and consequently the antimicrobial effect of the coating the third testing group with rearranged sample features was conducted. We found a strong dependency of antibacterial activity and the fluence of Ag+ in the coatings. This led to the conclusion, that ion energy plays a minor role as long as low energies (e.g. 10 or 20 keV) are applied during Ag+ implantation.
Moreover, the conversion of the superficial PE by ion implantation might be beneficial with regard to mechanical properties compared to conventional surface coatings. Conversion of the superficial PE material to DLC-PE results in a gradient of conversed DLC, and thus reduces the risk of abrasive wear observed with certain DLC coatings on various metallic biomaterials.
This study involves several limitations. Ion concentrations in the surrounding medium were not measured. Thus, Ag+ release could not be quantified though antibacterial effectiveness of the surface modification was proven. A significant antibacterial effect of DLC-PE without integrated Ag, on the other hand could be ruled out in the present study (Table 1) and in our previous experiments (data not shown). Another limitation is that only two bacterial strains were used in this study. Although the investigated strains are of major importance in periprosthetic joint infections, antibacterial effect against other bacteria has to be investigated in future studies. In fact, several studies confirmed even higher bactericidal potency of Ag+ against Gram-negative compared to Gram-positive bacteria (Flores et al. 2013; Kim et al. 2007). Additionally, no influence of Ag-DLC on osseointegration was investigated. A negative effect on eukaryotic cells in this context could be of major interest in the clinical use of this antibacterial coating even though PE is not used in direct bone contact. However, this was not the scope of this proof of principle investigation. Further investigations are needed in order to clear whether the concentration and duration of delivery of the released Ag+ of Ag-DLC-PE is sufficient to avoid implant infection in vivo and how they interact with bony tissue.
Taken together, our findings strongly support further investigation of Ag-DLC conversion of PE for prophylaxis of implant-associated infections. Antibacterial effectiveness of Ag-DLC-PE has been demonstrated. The suitability of this surface modification for biomedical applications will be confirmed by wear tests and in vitro biocompatibility assessments.
Abbreviations
- PJI:
-
periprosthetic joint infections
- Ag:
-
silver
- Ag+ :
-
silver ion
- DLC:
-
diamond-like carbon
- PE:
-
polyethylene
- DLC-PE:
-
diamond-like carbon coating on polyethylene
- Ag-DLC:
-
silver incorporated diamond-like carbon coating
- Ag-DLC-PE:
-
silver incorporated diamond-like carbon coating on polyethylene
- CFU:
-
colony forming units
- SD:
-
standard deviation
References
Baba K, Hatada R, Flege S, Ensinger W, Shibata Y, Nakashima J, Sawase T, Morimura T (2013) Preparation and antibacterial properties of Ag-containing diamond-like carbon films prepared by a combination of magnetron sputtering and plasma source ion implantation. Vacuum 89(89):179–184
Baffone W, Sorgente G, Campana R, Patrone V, Sisti D, Falcioni T (2011) Comparative effect of chlorhexidine and some mouthrinses on bacterial biofilm formation on titanium surface. Curr Microbiol 62(2):445–451. doi:10.1007/s00284-010-9727-x
Bertóti I, Mohai M, Tóth A, Ujvári T (2007) Nitrogen-PBII modification of ultra-high molecular weight polyethylene: composition, structure and nanomechanical properties. Surf Coat Technol 201:6839–6842
Bumgardner JD, Wiser R, Elder SH, Jouett R, Yang Y, Ong JL (2003) Contact angle, protein adsorption and osteoblast precursor cell attachment to chitosan coatings bonded to titanium. J Biomater Sci Polym Ed 14(12):1401–1409
Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A (1999) The calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol 37(6):1771–1776
Cloutier M, Tolouei R, Lesage O, Levesque L, Turgeon S, Tatoulian M, Mantovani D (2014) On the long term antibacterial features of silver-doped diamond like carbon coatings deposited via a hybrid plasma process. Biointerphases 9(2):029013. doi:10.1116/1.4871435
Darouiche RO (2004) Treatment of infections associated with surgical implants. N Engl J Med 350(14):1422–1429. doi:10.1056/NEJMra035415
Dearnaley G (1993) Diamond-like carbon: a potential means of reducing wear in total joint replacements. Clin Mater 12(4):237–244
Fiedler J, Kolitsch A, Kleffner B, Henke D, Stenger S, Brenner RE (2011) Copper and silver ion implantation of aluminium oxide-blasted titanium surfaces: proliferative response of osteoblasts and antibacterial effects. Int J Artif Organs 34(9):882–888. doi:10.5301/ijao.5000022
Flores CY, Minan AG, Grillo CA, Salvarezza RC, Vericat C, Schilardi PL (2013) Citrate-capped silver nanoparticles showing good bactericidal effect against both planktonic and sessile bacteria and a low cytotoxicity to osteoblastic cells. ACS Appl Mater Interfaces 5(8):3149–3159. doi:10.1021/am400044e
Furno F, Morley KS, Wong B, Sharp BL, Arnold PL, Howdle SM, Bayston R, Brown PD, Winship PD, Reid HJ (2004) Silver nanoparticles and polymeric medical devices: a new approach to prevention of infection? J Antimicrob Chemother 54(6):1019–1024. doi:10.1093/jac/dkh478
Gollwitzer H, Ibrahim K, Meyer H, Mittelmeier W, Busch R, Stemberger A (2003) Antibacterial poly(d, l-lactic acid) coating of medical implants using a biodegradable drug delivery technology. J Antimicrob Chemother 51(3):585–591
Gosheger G, Hardes J, Ahrens H, Streitburger A, Buerger H, Erren M, Gunsel A, Kemper FH, Winkelmann W, Von Eiff C (2004) Silver-coated megaendoprostheses in a rabbit model—an analysis of the infection rate and toxicological side effects. Biomaterials 25(24):5547–5556. doi:10.1016/j.biomaterials.2004.01.008
Groll J, Fiedler J, Bruellhoff K, Moeller M, Brenner RE (2009) Novel surface coatings modulating eukaryotic cell adhesion and preventing implant infection. Int J Artif Organs 32(9):655–662
Hardes J, Ahrens H, Gebert C, Streitbuerger A, Buerger H, Erren M, Gunsel A, Wedemeyer C, Saxler G, Winkelmann W, Gosheger G (2007) Lack of toxicological side-effects in silver-coated megaprostheses in humans. Biomaterials 28(18):2869–2875. doi:10.1016/j.biomaterials.2007.02.033
Hardes J, von Eiff C, Streitbuerger A, Balke M, Budny T, Henrichs MP, Hauschild G, Ahrens H (2010) Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol 101(5):389–395. doi:10.1002/jso.21498
Harris LG, Tosatti S, Wieland M, Textor M, Richards RG (2004) Staphylococcus aureus adhesion to titanium oxide surfaces coated with non-functionalized and peptide-functionalized poly(l-lysine)-grafted-poly(ethylene glycol) copolymers. Biomaterials 25(18):4135–4148. doi:10.1016/j.biomaterials.2003.11.033
Hetrick EM, Schoenfisch MH (2006) Reducing implant-related infections: active release strategies. Chem Soc Rev 35(9):780–789. doi:10.1039/b515219b
Holt J, Hertzberg B, Weinhold P, Storm W, Schoenfisch M, Dahners L (2011) Decreasing bacterial colonization of external fixation pins through nitric oxide release coatings. J Orthop Trauma 25(7):432–437. doi:10.1097/BOT.0b013e3181f9ac8a
Hunter G, Dandy D (1977) The natural history of the patient with an infected total hip replacement. J Bone Joint Surg Br 59(3):293–297
Katsikogianni M, Spiliopoulou I, Dowling DP, Missirlis YF (2006) Adhesion of slime producing Staphylococcus epidermidis strains to PVC and diamond-like carbon/silver/fluorinated coatings. J Mater Sci Mater Med 17(8):679–689. doi:10.1007/s10856-006-9678-8
Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH (2007) Antimicrobial effects of silver nanoparticles. Nanomedicine 3(1):95–101. doi:10.1016/j.nano.2006.12.001
Kumar R, Munstedt H (2005) Silver ion release from antimicrobial polyamide/silver composites. Biomaterials 26(14):2081–2088. doi:10.1016/j.biomaterials.2004.05.030
Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J (2008) Infection burden for hip and knee arthroplasty in the United States. J Arthroplast 23(7):984–991. doi:10.1016/j.arth.2007.10.017
Kwok SCH, Zhang W, Wan GJ, McKenzie DR, Bilek MMM, Chu PK (2007) Hemocompatibility and anti-bacterial properties of silver doped diamond-like carbon prepared by pulsed filtered cathodic vacuum arc deposition. Diam Relat Mater 16:1353–1360
Lee D, Cohen RE, Rubner MF (2005) Antibacterial properties of Ag nanoparticle loaded multilayers and formation of magnetically directed antibacterial microparticles. Langmuir 21(21):9651–9659. doi:10.1021/la0513306
Liu Y, Zheng Z, Zara JN, Hsu C, Soofer DE, Lee KS, Siu RK, Miller LS, Zhang X, Carpenter D, Wang C, Ting K, Soo C (2012) The antimicrobial and osteoinductive properties of silver nanoparticle/poly (dl-lactic-co-glycolic acid)-coated stainless steel. Biomaterials 33(34):8745–8756. doi:10.1016/j.biomaterials.2012.08.010
Marciano FR, Bonetti LF, Santos LV, Da-Silva NS, Corat EJ, Trava-Airoldi VJ (2009) Antibacterial activity of DLC and Ag, ÄìDLC films produced by PECVD technique. Diam Relat Mater 18:1010–1014
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16(10):2346–2353. doi:10.1088/0957-4484/16/10/059
Oliveira LY, Kuromoto NK, Siqueira CJ (2014) Treating orthopedic prosthesis with diamond-like carbon: minimizing debris in Ti6Al4V. J Mater Sci Mater Med 25(10):2347–2355. doi:10.1007/s10856-014-5252-y
Saikko V, Ahlroos T, Calonius O, Keranen J (2001) Wear simulation of total hip prostheses with polyethylene against CoCr, alumina and diamond-like carbon. Biomaterials 22(12):1507–1514
Schierholz JM, Lucas LJ, Rump A, Pulverer G (1998) Efficacy of silver-coated medical devices. J Hosp Infect 40(4):257–262
Schmidmaier G, Lucke M, Wildemann B, Haas NP, Raschke M (2006) Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: a review. Injury 37(Suppl 2):S105–S112. doi:10.1016/j.injury.2006.04.016
Schwarz FS, Stritzker B (2010) Plasma immersion ion implantation of polymers and silver–polymer nano composites. Surf Coat Technol 204:1875–1879
Shirai T, Tsuchiya H, Shimizu T, Ohtani K, Zen Y, Tomita K (2009) Prevention of pin tract infection with titanium–copper alloys. J Biomed Mater Res B Appl Biomater 91(1):373–380. doi:10.1002/jbm.b.31412
Soininen A, Levon J, Katsikogianni M, Myllymaa K, Lappalainen R, Konttinen YT, Kinnari TJ, Tiainen VM, Missirlis Y (2011) In vitro adhesion of staphylococci to diamond-like carbon polymer hybrids under dynamic flow conditions. J Mater Sci Mater Med 22(3):629–636. doi:10.1007/s10856-011-4231-9
Taglietti A, Diaz Fernandez YA, Amato E, Cucca L, Dacarro G, Grisoli P, Necchi V, Pallavicini P, Pasotti L, Patrini M (2012) Antibacterial activity of glutathione-coated silver nanoparticles against Gram positive and Gram negative bacteria. Langmuir 28(21):8140–8148. doi:10.1021/la3003838
Tilton RC, Rosenberg B (1978) Reversal of the silver inhibition of microorganisms by agar. Appl Environ Microbiol 35(6):1116–1120
Verraedt E, Braem A, Chaudhari A, Thevissen K, Adams E, Van Mellaert L, Cammue BP, Duyck J, Anne J, Vleugels J, Martens JA (2011) Controlled release of chlorhexidine antiseptic from microporous amorphous silica applied in open porosity of an implant surface. Int J Pharm 419(1–2):28–32. doi:10.1016/j.ijpharm.2011.06.053
Zhang L, Yan J, Yin Z, Tang C, Guo Y, Li D, Wei B, Xu Y, Gu Q, Wang L (2014) Electrospun vancomycin-loaded coating on titanium implants for the prevention of implant-associated infections. Int J Nanomed 9:3027–3036. doi:10.2147/IJN.S63991
Zhao L, Wang H, Huo K, Cui L, Zhang W, Ni H, Zhang Y, Wu Z, Chu PK (2011) Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 32(24):5706–5716. doi:10.1016/j.biomaterials.2011.04.040
Zimmerli W, Moser C (2012) Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol 65(2):158–168. doi:10.1111/j.1574-695X.2012.00938.x
Zimmerli W, Ochsner PE (2003) Management of infection associated with prosthetic joints. Infection 31(2):99–108. doi:10.1007/s15010-002-3079-9
Authors’ contributions
NH, SJ carried out the microbiological testing and drafted the manuscript. RK and BS provided DLC-processing of samples. HG, RB, RvER and IB conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank PD Dr. Thomas Grupp for providing the PE discs and Jutta Tübel for excellent technical help and advice. This work was supported by the “Deutsche Forschungsgemeinschaft (DFG)” within the interdisciplinary project “Quantitative Evaluation der statischen und dynamischen Zelladhäsion und –aktivität an antibakteriellen DLC-Schichten für den biomedizinischen Einsatz” (BU 1154/2-1 and GO 1906/2-1, STR 361/18-1).
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Harrasser, N., Jüssen, S., Banke, I.J. et al. Antibacterial efficacy of ultrahigh molecular weight polyethylene with silver containing diamond-like surface layers. AMB Expr 5, 64 (2015). https://doi.org/10.1186/s13568-015-0148-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-015-0148-x