Abstract
Lyophilized Streptococcus spp. isolates (n = 50) from animal samples submitted to the diagnostic laboratory at the University of Connecticut in the 1940s were revivified to investigate the genetic characteristics using whole-genome sequencing (WGS). The Streptococcus spp. isolates were identified as follows; S. agalactiae (n = 14), S. dysgalactiae subsp. dysgalactiae (n = 10), S. dysgalactiae subsp. equisimils (n = 5), S. uberis (n = 8), S. pyogenes (n = 7), S. equi subsp. zooepidemicus (n = 4), S. oralis (n = 1), and S. pseudoporcinus (n = 1). We identified sequence types (ST) of S. agalactiae, S. dysgalactiae, S. uberis, S. pyogenes, and S. equi subsp. zooepidemicus and reported ten novel sequence types of those species. WGS analysis revealed that none of Streptococcus spp. carried antibiotic resistance genes. However, tetracycline resistance was observed in four out of 15 S. dysgalactiae isolates and in one out of four S. equi subsp. zooepidemicus isolate. This data highlights that antimicrobial resistance is pre-existed in nature before the use of antibiotics. The draft genome sequences of isolates from this study and 426 complete genome sequences of Streptococcus spp. downloaded from BV-BRC and NCBI GenBank database were analyzed for virulence gene profiles and phylogenetic relationships. Different Streptococcus species demonstrated distinct virulence gene profiles, with no time-related variations observed. Phylogenetic analysis revealed high genetic diversity of Streptococcus spp. isolates from the 1940s, and no clear spatio-temporal clustering patterns were observed among Streptococcus spp. analyzed in this study. This study provides an invaluable resource for studying the evolutionary aspects of antibiotic resistance acquisition and virulence in Streptococcus spp.
Similar content being viewed by others
Introduction
Streptococci are Gram-positive bacteria that can be classified into the Lancefield group taxonomic system based on colony morphology, hemolysis, and serological specificity [1]. Many streptococci are non-pathogenic and belong to the commensal microbiota of humans and animals; however, some can cause severe diseases and health issues [1]. Several Streptococcus species can cause bovine mastitis (e.g., S. uberis, S. agalactiae, S. dysgalactiae subsp. dysgalactiae, and S. canis) and are responsible for major economic losses in the dairy industry [2, 3]. Various species of Streptococcus, such as S. equi, S. suis, S. porcinus, S. oralis, and S. iniae are associated with infections in pigs, horses, sheep, birds, aquatic mammals, and fish [4].
Streptococcus spp. is typically sensitive to penicillin, which has been used as the drug of choice to combat gram-positive mastitis-causing organisms since 1945 [5, 6]. However, Streptococcus spp. quickly developed resistance to antibiotics, and the limited efficacy of mastitis control through the treatment of clinical cases was first noted by Murphy et al. in 1956 [7]. As a result, conventional antibiotic therapy often proves ineffective [5].
To improve our understanding of the emergence of antimicrobial resistance (AMR) and the evolution of bacterial pathogens, the AMR and genetic characteristics of historical isolates from before the widespread clinical use of antimicrobials, that is, the “pre-antibiotic” era, have been analyzed in previous studies, including those on Klebsiella pneumoniae [8], methicillin-resistant Staphylococcus aureus [9], Salmonella enterica serotype Typhimurium [10], Neisseria gonorrhoeae [11], Vibrio cholerae [12], and Enterobacteriaceae [13]. These studies demonstrated that a significant proportion of isolates from the pre-antibiotic era were resistant to antibiotics before their routine use, and the association between antibiotic use and selection of resistance determinants was not as direct as often presumed.
In this study, we sequenced the whole genomes of 50 lyophilized Streptococcus spp. isolates from clinical animal samples submitted to the diagnostic laboratory at the University of Connecticut in the pre-antibiotic era (1940s). We analyzed the phenotypic AMR, presence of AMR genes in the draft genomes, the sequence type (ST) using multi-locus sequence typing (MLST), phylogenetic relationships, and virulence gene profiles to examine the genetic characteristics of these historical isolates.
Materials and methods
Reviving lyophilized Streptococcus spp. isolates
A total of 50 lyophilized Streptococcus spp. cultures isolated from animal samples and stored by the Connecticut Veterinary Medical Diagnostic Laboratory (CVMDL), Department of Pathobiology and Veterinary Science, University of Connecticut, between 1941 and 1947 were revivified according to the Reviving Freeze-Dried Microorganisms Instructional Guide method published by the American Type Culture Collection (ATCC). Information on the isolates includes the isolation year and bacterial species which were indicated in the stock list but lacks other metadata such as host and disease information.
Briefly, lyophilized bacterial stocks were rehydrated and cultured in Tryptic Soy Broth medium (TSB) (Becton Dickinson, Franklin Lakes, NJ) for 24 h at 37 ℃. The cultured broth was streaked on blood agar plates, followed by incubation for an additional 24 h at 37 ℃. Next, colonies from blood agar plates (a colony from each plate) were cultured in TSB for 24 h at 37 ℃. The cultures were stored in the Cryocare Bead Storage system (Key Scientific Product, Stamford, Texas) at −80 ℃ until ready for analysis.
Antimicrobial susceptibility testing
The antimicrobial susceptibility of Streptococcus spp. isolates was determined using a Sensititre™ Streptococcus STP6F AST Plate (Thermo Fisher Scientific, Waltham, MA) which is a colorimetric microdilution test consisting of the following 20 antimicrobials: moxifloxacin, levofloxacin, tetracycline, cefuroxime, ceftriaxone, cefotaxime, daptomycin, chloramphenicol, penicillin, meropenem, ertapenem, amoxicillin/clavulanic acid 2:1 ratio, linezolid, clindamycin, cefepime, tigecycline, azithromycin, erythromycin, trimethoprim/sulfamethoxazole, and vancomycin. Briefly, bacterial colonies were suspended in sterile distilled water to approximate the 0.5 McFarland turbidity standard. Next, 100 µL of the bacterial suspension was transferred into 5 mL of Sensititre™ Mueller Hinton broth with lysed horse blood, and 100 µL of the inoculum was inoculated into each well of a Sensititre™ Streptococcus species STP6F susceptibility plate. After 24 h of incubation at 37 ℃, the minimum inhibitory concentration (MIC) was determined using a BIOMIC V3 Microbiology system (Giles Scientific Inc., Santa Barbara, CA) according to the manufacturer’s instructions. The AMR of the isolates was determined according to the concentrations of each drug range and interpretive criteria in the instruction of M100 of the Clinical and Laboratory Standards Institute (CLSI) [14].
Whole genome sequencing (WGS)
For WGS, genomic DNA was extracted from pure cultures of Streptococcus spp. using the DNeasy Blood and Tissue kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Sample DNA concentrations were determined using a Qubit dsDNA HS assay kit (Invitrogen, Carlsbad, CA, USA), and DNA samples were diluted to 0.2 ng/µL. Sample libraries were prepared using the Illumina Nextera XT DNA Library Prep Kit (Illumina, San Diego, CA, USA), followed by dilution to a concentration of 2 nM; the concentration of libraries was measured using the Qubit dsDNA HS assay kit. Samples were sequenced using a MiSeq Reagent Kit V2 (500 cycle) cartridge (Illumina) after loading 600 µL of the 10 pM pooled libraries.
Species identification
The BIOLOG MicroLog3 Microbial Identification System (Biolog, Hayward, CA, USA) was used to identify the species of all isolates. For the confirmation of bacterial species identification, we analyzed the genome sequences of 16S rRNA region. The 16S rRNA region in the assembled contigs of the isolates was predicted using bacterial ribosomal RNA predictor barnap (Galaxy Version 1.2.1) and extracted manually. For each 16S rRNA sequence, the nearest-neighbor species with > 99% identity were searched using the Basic Local Alignment Search Tool (BLAST) on the National Center for Biotechnology Information (NCBI) database to identify the species of each isolate with the default parameters.
Genomic characterization
Raw reads were de novo assembled using the SPAdes algorithm [15] at the BV-BRC online server. The assembled contigs with a coverage of less than 5 × and sizes below 300 bases were removed. The presence of acquired antimicrobial resistance genes was determined using ResFinder 3.2 with settings for other species, a threshold of 90%, and a minimum length of 60% with raw sequencing reads [16]. Plasmids were detected using PlasmidFinder 2.1 [17], a web-based tool for in silico detection and characterization of plasmid sequences based on BLAST searches against plasmid replicon genes with the assembled contigs of gram-positive bacteria. MLST 2.0 (Multi-Locus Sequence Typing) [18] was used to determine the STs of the predicted species using the assembled contigs. The virulence genes of the isolates used for the phylogenetic analysis including our isolates (Additional file 1) were analyzed using ABRicate (Version 1.0.1) against virulence factor database (VFDB) [19], with a 80% sequence identity and a 80% coverage.
Phylogenetic analysis
All available complete genome sequences of S. agalactiae (n = 185), S. dysgalactiae (n = 23), S. equi subsp. zooepidemicus (n = 25), and S. pyogenes (n = 193) with collection year information were downloaded from BV-BRC and NCBI GenBank database to investigate the genetic relationships between the isolates. The species which the number of complete genomes in the NCBI GenBank database was less than 20 as of February 21, 2024, were excluded from the phylogenetic analysis; S. oralis (n = 19), S. uberis (n = 4), and S. pseudoporcinus (n = 3). The information of the genome sequences used for the analysis is listed in Additional file 1. Whole genome SNPs were identified using kSNP4 [20] which employs an alignment-free approach for SNP identification. The SNPs-based ML tree was generated using FastTree [21], which was automatically applied in the kSNP4 pipeline.
Results
Genomic characteristics
The de novo assembly results and genomic characteristics of the isolates are shown in Additional file 2. The average depth of coverage ranged from 40.8 to 187.1, the number of contigs from 12 to 167, and the N50 from 32 972 to 1 034 038. The average number of protein coding sequences (CDS) of each species ranged from 1795 (S. pyogenes) to 2301 (S. equi subsp. Zooepidemicus), the rRNA was from 4 to 5, and the tRNA was from 39 (S. pyogenes) to 51 (S. uberis) (Additional file 2).
In this study, the average GC content for each species was 35% for the S. agalactiae isolates, 39% for the S. dysgalactiae isolates, 36% for the S. uberis isolates, 38% for the S. pyogenes isolates, 41% for the S. equi subsp. zooepidemicus isolates, 40.9% for the S. oralis isolate, and 37.3% for the S. pseudoporcinus isolate (Additional file 2).
Species identification, sequence type and plasmid of Streptococcus spp.
The Streptococcus spp. isolates (n = 50) included 14 S. agalactiae, 10 S. dysgalactiae subsp. dysgalactiae, five S. dysgalactiae subsp. equisimils, eight S. uberis, seven S. pyogenes, four S. equi subsp. zooepidemicus, one S. oralis, and one S. pseudoporcinus (Table 1).
We identified STs of S. agalactiae, S. dysgalactiae, S. uberis, S. pyogenes, and S. equi subsp. zooepidemicus, which are available in MLST 2.0. (Table 1). The most frequent STs were ST 61 in S. agalactiae isolates (5 out of 14), ST 531 in S. dysgalactiae isolates (5 out of 15), and ST28 in S. pyogenes isolates (3 out of 7). In this study, we reported the novel sequence types of one S. agalactiae isolates (ST 2225), one S. dysgalactiae isolates (ST 723), seven S. uberis isolates (ST 1801, 1802, 1804, 1815, 1817, and 1818), and two S. equi subsp. zooepidemicus (ST 529 and 530) isolates (Table 1 and Additional file 3).
Plasmid detection via the PlasmidFinder 2.1 revealed that among the 50 Streptococcus spp. isolates, two S. agalactiae (G2 and G19) carried two plasmids, pA996 and pSSU1, and one S. uberis isolate carried the pA996 plasmid (Table 1). It should be noted that plasmid fragments without replicons may have been missed in this analysis since the PlasmidFinder 2.1 identifies plasmids based on replicon sequences.
Antibiotic resistance of Streptococcus spp.
The presence of antimicrobial resistance genes and phenotypic antimicrobial susceptibility testing of Streptococcus spp. isolates are shown in Table 1. Antimicrobial resistance genes were not found in the Streptococcus spp.isolates. All of S. uberi, S. pyogenes, S. oralis, and S. pseudoporcinus isolates were susceptible to all antibiotics tested. However, phenotypic resistance to tetracycline was observed in three out of ten S. dysgalactiae subsp. dysgalactiae isolates, one out of five S. dysgalactiae subsp. equimilis isolate, and one out of four S. equi subsp. zooepidemicus isolate. Four of ten S. dysgalactiae subsp. dysgalactiae isolates and two out of four S. equi subsp. zooepidemicus isolates showed intermediate resistance to tetracycline. In addition, three out of four S. equi subsp. zooepidemicus isolates showed intermediate resistance to clindamycin.
Virulence gene profile
The virulence gene profiles of 50 Streptococcus spp. isolates sequenced in this study were analyzed and compared with those of 426 complete genome sequences of Streptococcus spp. downloaded from BV-BRC and NCBI GenBank database (Tables 2, 3, 4, 5 and Additional file 4).
The virulence genes were classified into eight categories based on their function: adherence, anti-proteolysis, antiphagocytosis, exoenzymes, immune evasion, manganese uptake, stress proteins, and toxins (Additional file 4). Different Streptococcus species demonstrated distinct virulence gene profiles, and no time-related variations were observed in the virulence gene profile across all analyzed Streptococcus species (Additional file 4). All 14 S. agalactiae isolates sequenced in this study carried the genes related to antiphagocytosis (cpsA-F, cpsL, and neuA-D) and toxins (cfa/cfb) (Table 2). Among these genes, cfa/cfb, cpsL, and neuB-D genes were detected in all S. agalactiae complete genome sequences analyzed in this study (Additional file 4). All 38 genome sequences of S. dysgalactiae including isolates from this study harbored fbp54 (adherence) and hasC genes (anti-proteolysis) (Table 3 and Additional file 4). All 200 S. pyogenes genome sequences carried lmb (adherence), ideS/mac (antiphagocytosis), fbp54 (toxin), ska (toxin), and slo (toxin) (Additional file 4), and all our S. pyogenes isolates (n = 7) additionally harbored scpA (immune invasion) and SmeZ (toxin) (Table 4). Two virulence genes encoding exozyme, hylP and mf2, were observed in all analyzed S. equi subsp. zooepidemicus, and two of four S. equi subsp. zooepidemicus isolates from this study harbored only mf2 gene (Table 5 and Additional file 4). S. pseudoporcinus isolates carried speB genes encoding exozyme, while S. oralis isolates harbored the pavA (adherence) and psaA (magnese uptake) genes (Table 5). Lastly, no virulence gene in VFDB was detected in our S. uberis isolates (Additional file 4).
Phylogenetic analysis
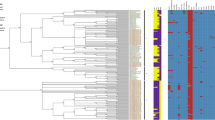
The genome sequences of S. agalactiae (n = 199), S. dysgalactiae (n = 38), S. pyogenes (n = 200), and S. equi subsp. zooepidemicus (n = 29) including our isolates were analyzed to investigate the genetic relationships using SNP analysis. The phylogenetic trees and the heatmaps of four different species are shown in Additional files 5, 6, 7 and 8.
The host species of 185 S. agalactiae sequences downloaded from databases were humans (n = 81), aquatic animals (n = 81), cows (n = 22), a dog (n = 1), and unknown (n = 11). S. agalactiae from aquatic animals formed two distinct clusters which are compressed in phylogeny (Additional file 5). The phylogenetic analysis revealed that our isolates were divided into four groups (Additional file 5). S. agalactiae N49 and H36B were grouped with isolates from cows in 1970 and 1954, respectively, exhibiting high sequence identity (82.6% and 87.2%, respectively). Five isolates (S101, S104, B090R, B2142, and B2151) demonstrated an average identity of 89.6% with two isolates, one from a cow in 1977 and another from a human in 2011. Seven isolates (B1006, G42, G19, G2, S102, 39, and 16) were grouped with two isolates from cows in 1954 and 1964 showing average 68.2% identity.
The sequences of S. dysgalactiae were divided into two subspecies, S. dysgalactiae subsp. equisimillis and S. dysgalactiae subsp. dysgalactiae (Additional file 6). Four S. dysgalactiae subsp. equisimillis isolates from this study (30, 29, 19, and 18) clustered with isolates from humans between 1953 and 2018, and three of them showed an average 78.3% identity with an isolate from a human in 1953. One isolate (34) was grouped with two isolates from a cow and a rhinoceros, exhibiting an average 58.8% identity. All S. dysgalactiae subsp. dysgalactiae isolates from this study clustered with an isolate from a cow in 2020, exhibited an average 76.0% identity.
S. pyogenes sequences available in the databases were from human isolates (n = 184) and unknown (n = 9). The S. pyogenes isolates of this study were grouped into five clusters (Additional file 7). The isolates (6, 14, and 9) were grouped with isolates from humans in 1950–2019, exhibiting an average 95.3% identity. S. pyogenes isolates 12 and 5 showed high identity with historical strains isolated in 1927 (90.6%) and 1950 (98.3%), respectively. S. pyogenes isolate 15 also exhibited high identity with human strains isolated between 1997 and 2016 (97.4%), and S. pyogenes isolate 7 were clustered with human strains isolated between 2009 and 2015 (84.6%). For S. equi subsp. zooepidemicus, four isolates of this study clustered into three different groups (Additional file 8). The clusters containing S. equi subsp. zooepidemicus isolates 37 and 38 exhibited average 52.1% and 54.0% identity and S. equi subsp. zooepidemicus isolates 40 and 24 clustered with an isolate from a cow with an average identity of 45.5%.
Discussion
Streptococcus spp. have been identified using classical phenotypic microbiological procedures [22]. However, previous studies have reported the limited discriminatory power of these methods for Streptococcus spp. [22,23,24,25], and the 16S rRNA sequence started to be used as a reference for species identification [3, 22, 25]. Therefore, in the present study, we determined the species using a BLASTn search of the 16S rRNA sequences. One of the limitations of this study is the lack of information regarding the isolates, such as host and disease. Therefore, we assumed the host of each isolate based on the prevalence of Streptococcus spp. in different animals as reported in the previous studies. S. agalactiae, S. dysgalactiae, and S. uberis are the main species involved in clinical and subclinical bovine mastitis [1, 3, 5, 26]. A few bovine mastitis cases caused by S. pyogenes were reported between 1930 and 1940 [27, 28]. S. equi subsp. zooepidemicus is an opportunistic pathogen in both humans and a broad range of animal species, including horses, dogs, and pigs [29]. S. oralis, a member of the mitis group of streptococci, has been isolated from milk samples from women [30] and lactating cows [31]. S. pseudoporcinus was initially thought to be S. porcinus, which was first isolated frompigs in 1937 [32].
All S. agalactiae isolates of this study carried the mre(A) gene (data not shown), which is known to probably reside in S. agalactiae and may encode a metabolic function [33]. The mre(A) gene, which encodes a flavokinase, was discovered in a unique strain of S. agalactiae COH31 γ/δ as a macrolide efflux gene by Clancy et al. [34], and cumulative data suggested that the mreA gene was located on the chromosome of S. agalactiae COH31 γ/δ [33]. This is supported by our finding that all S. agalactiae isolates in this study carried the mre(A) gene with an erythromycin-sensitive phenotype. The mre(A) gene was found in all S. agalactiae isolates analyzed with either erythromycin-resistant or erythromycin-sensitive phenotypes in previous studies [33, 35, 36], indicating its ubiquity in this bacterial species. In this study, phenotypic resistance to tetracycline and intermediate resistance to clindamycin were observed in the S. dysgalactiae subsp. dysgalactiae isolates, S. dysgalactiae subsp. equimilis isolate, and S. equi subsp. zooepidemicus isolate, while resistance genes were not found in the Streptococcus spp. isolates. Tetracycline resistance in S. agalactiae, S. dysgalactiae, and S. equi subsp. zooepidemicus has been detected by several resistance monitoring programs in previous studies [29, 37], and AMR in Streptococcus spp. varies greatly depending on the streptococcal species, geographical location, study design (sampling size, scheme, and method for resistance determination), and literature source [1]. A poor correlation between tetracycline-resistant phenotypes and resistance genes has been reported previously [38,39,40]. In the previous study [40], six of 18 tetracycline resistant S. dysgalactiae subsp. dysgalactiae isolates did not carry the tet genes. In addition, in Tian et al.’s study on 64 Streptococcus isolates from mastitic milk samples in China [38], the average consistency between resistant phenotypes and resistance genes was 35.87%, and the consistency rate for tetracycline was 50%.
The phenotypic and genotypic AMR of bacterial pathogens from the pre-antibiotic era have been reported in previous studies, such as the Murray Collection of the pre-antibiotic era Enterobacteriaceae strains carrying antibiotic resistance genes [13], Proteus spp. resistant to tetracycline [41], Klebsiella resistant to ampicillin [8], Escherichia spp. resistant to both ampicillin and kanamycin [42], and Vibrio cholerae strains harboring functional β-lactamase antibiotic resistance genes [12]. In addition, metagenomic studies on ancient human guts from the pre-antibiotic era have been reported [43,44,45]. These investigations on the gut microbiome of pre-Columbian Andean [44], pre-Inca/Inca, and Italian nobility mummies [43, 45] revealed the presence of genes associated with beta-lactamases, penicillin-binding proteins, resistance to fosfomycin, chloramphenicol, aminoglycosides, macrolides, sulfa, quinolones, tetracycline, and vancomycin, as well as multi-drug transporters. This suggests that resistance may not necessarily be associated solely with the selective pressure of antibiotics.
Moreover, the studies propose that antibiotic resistance might have an environmental origin, indicating that a higher exposure to the environment could lead to a greater acquisition of antibiotic-resistance genes [43, 45]. Additionally, it has been hypothesized that antibiotic resistance in pathogens likely originated in non-pathogenic bacteria, possibly those originating from the soil [43, 45]. Contrastingly, our study has revealed that Streptococcus spp. isolates from animal origins during the pre-antibiotic era did not carry antibiotic resistance genes. This disparity in findings appears to be attributed to the differing origins (animal vs. human) and pathogenicity of the bacteria.
Several studies where PCR was used to screen for virulence genes have reported differences in the detection of the virulence factors of Streptococcus spp. from different sources, such as S. agalactiae strains from human and bovine sources [46, 47]. However, literature reports on the virulence gene profiles of Streptococcus spp. isolates from animals using WGS are scarce [39, 48]. In this study, we compared the virulence gene profiles of Streptococcus spp. isolates from 1940s with those of 426 complete genome sequences of Streptococcus spp. obtained from diverse hosts and different years to investigate potential time-related variations and evolutionary trends. The results revealed conserved virulence gene profiles among different Streptococcus species and no time-related variations in the virulence gene profile in analyzed Streptococcus species. This comprehensive approach provides insight into the diverse virulence gene pattern shaping the framework of Streptococcus spp. pathogenesis. A discrepancy in reporting of virulence gene prevalence was observed among different previous studies, which can be explained by the difference in the origin of the isolates as well as other factors [1, 5, 38, 46,47,48,49,50]. In addition, a limitation of VFDB is its primary focus on data from human pathogens, potentially overlooking virulence genes for animal pathogens. This raises concerns regarding the understanding of virulence gene datasets of Streptococcus spp. infecting animals, such as S. oralis, S. uberis, and S. pseudoporcinus. Therefore, further WGS analysis of Streptococcus spp. isolates from animals is essential to update the database and better understand the evolution of virulence genes in Streptococcus spp. from diverse host species.
In this study, all available complete genome sequences of S. agalactiae, S. dysgalactiae, S. equi subsp. zooepidemicus, and S. pyogenes with collection year information were downloaded from databases to investigate the genetic relationships with our isolates using SNP analysis. Phylogenetic analysis revealed high genetic diversity of Streptococcus spp. isolates from the 1940s, and no clear spatio-temporal clustering patterns were observed among Streptococcus spp. analyzed in this study. S. agalactiae isolates and S. dysgalactiae subsp. dysgalactiae isolates of this study exhibited high genetic similarity with the isolates from cows, suggesting a potential host-specific association. S. dysgalactiae subsp. equismillis and S. pyogenes displayed high genetic identity (> 90%) with both historical and contemporary human isolates, suggesting their persistence and adaptability within human populations overtime. However, the scarcity of sequence data for these species from animals constrained genetic analysis with animal isolates in this study. Further research with a wider range of animal isolates is needed to better understand genetic diversity and evolution of these subspecies.
This study reports on the antibiotic resistance, sequence type, phylogenetic relationships, and virulence gene profiles of lyophilized Streptococcus spp. isolated from animals in the 1940s, the pre-antibiotic era, using WGS analysis. This study provides an invaluable resource for further investigation of the evolutionary aspects of antibiotic resistance acquisition and adaptation of bacterial strains.
Availability of data and materials
Paired-end reads of the Streptococcus spp. isolates in this study were deposited in the National Center for Biotechnology Information (NCBI) under the Bioproject accession number PRJNA887842.
References
Kabelitz T, Aubry E, van Vorst K, Amon T, Fulde M (2021) The role of Streptococcus spp. in bovine mastitis. Microorganisms 9:1497
Zadoks RN, Middleton JR, McDougall S, Katholm J, Schukken YH (2011) Molecular epidemiology of mastitis pathogens of dairy cattle and comparative relevance to humans. J Mammary Gland Biol Neoplasia 16:357–372
Richards VP, Palmer SR, Pavinski Bitar PD, Qin X, Weinstock GM, Highlander SK, Town CD, Burne RA, Stanhope MJ (2014) Phylogenomics and the dynamic genome evolution of the genus Streptococcus. Genome Biol Evol 6:741–753
Lal D, Verma M, Lal R (2011) Exploring internal features of 16S rRNA gene for identification of clinically relevant species of the genus Streptococcus. Ann Clin Microbiol Antimicrob 10:28
Abril AG, Carrera M, Bohme K, Barros-Velazquez J, Rama JR, Calo-Mata P, Sanchez-Perez A, Villa TG (2020) Proteomic characterization of antibiotic resistance, and production of antimicrobial and virulence factors in Streptococcus species associated with bovine mastitis. Could Enzybiotics represent novel therapeutic agents against these pathogens? Antibiotics 9:302
Seeley HW, Anderson EO, Plastridge WN (1945) Action of penicillin against mastitis organisms in milk. J Dairy Sci 28:887–891
Murphy JM (1956) Mastitis—the struggle for understanding. J Dairy Sci 39:1768–1773
Wand ME, Baker KS, Benthall G, McGregor H, McCowen JW, Deheer-Graham A, Sutton JM (2015) Characterization of pre-antibiotic era Klebsiella pneumoniae isolates with respect to antibiotic/disinfectant susceptibility and virulence in Galleria mellonella. Antimicrob Agents Chemother 59:3966–3972
Harkins CP, Pichon B, Doumith M, Parkhill J, Westh H, Tomasz A, de Lencastre H, Bentley SD, Kearns AM, Holden MTG (2017) Methicillin-resistant Staphylococcus aureus emerged long before the introduction of methicillin into clinical practice. Genome Biol 18:130
Tran-Dien A, Le Hello S, Bouchier C, Weill FX (2018) Early transmissible ampicillin resistance in zoonotic Salmonella enterica serotype Typhimurium in the late 1950s: a retrospective, whole-genome sequencing study. Lancet Infect Dis 18:207–214
Golparian D, Harris SR, Sanchez-Buso L, Hoffmann S, Shafer WM, Bentley SD, Jensen JS, Unemo M (2020) Genomic evolution of Neisseria gonorrhoeae since the preantibiotic era (1928–2013): antimicrobial use/misuse selects for resistance and drives evolution. BMC Genom 21:116
Dorman MJ, Kane L, Domman D, Turnbull JD, Cormie C, Fazal MA, Goulding DA, Russell JE, Alexander S, Thomson NR (2019) The history, genome and biology of NCTC 30: a non-pandemic Vibrio cholerae isolate from World War One. Proc Biol Sci 286:20182025
Baker KS, Burnett E, McGregor H, Deheer-Graham A, Boinett C, Langridge GC, Wailan AM, Cain AK, Thomson NR, Russell JE, Parkhill J (2015) The Murray collection of pre-antibiotic era Enterobacteriacae: a unique research resource. Genome Med 7:97
Institute CLS (2020) M100 Performance standards for antimicrobial susceptibility testing. Clinical Laboratory Standads Institute (CLSI), Wayne
Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA (2012) SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477
Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV (2012) Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644
Carattoli A, Zankari E, Garcia-Fernandez A, Voldby Larsen M, Lund O, Villa L, Moller Aarestrup F, Hasman H (2014) In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903
Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, Jelsbak L, Sicheritz-Ponten T, Ussery DW, Aarestrup FM, Lund O (2012) Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 50:1355–1361
Liu B, Zheng D, Zhou S, Chen L, Yang J (2022) VFDB 2022: a general classification scheme for bacterial virulence factors. Nucleic Acids Res 50:D912–D917
Hall BG, Nisbet J (2023) Building phylogenetic trees from genome sequences With kSNP4. Mol Biol Evol 40:msad235
Price MN, Dehal PS, Arkin AP (2010) FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490
Raemy A, Meylan M, Casati S, Gaia V, Berchtold B, Boss R, Wyder A, Graber HU (2013) Phenotypic and genotypic identification of streptococci and related bacteria isolated from bovine intramammary infections. Acta Vet Scand 55:53
Gillespie BE, Oliver SP (2005) Simultaneous detection of mastitis pathogens, Staphylococcus aureus, Streptococcus uberis, and Streptococcus agalactiae by multiplex real-time polymerase chain reaction. J Dairy Sci 88:3510–3518
Odierno L, Calvinho L, Traverssa P, Lasagno M, Bogni C, Reinoso E (2006) Conventional identification of Streptococcus uberis isolated from bovine mastitis in Argentinean dairy herds. J Dairy Sci 89:3886–3890
Khan IU, Hassan AA, Abdulmawjood A, Lammler C, Wolter W, Zschock M (2003) Identification and epidemiological characterization of Streptococcus uberis isolated from bovine mastitis using conventional and molecular methods. J Vet Sci 4:213–224
Lindeman CJ, Portis E, Johansen L, Mullins LM, Stoltman GA (2013) Susceptibility to antimicrobial agents among bovine mastitis pathogens isolated from North American dairy cattle, 2002–2010. J Vet Diagn Invest 25:581–591
Bendixen HC, Minett FC (1938) Excretion of Streptococcus pyogenes in the milk of naturally infected cows. J Hygiene 38:374–383
Pullin JW (1947) Mastitis caused by S. Pyogenes. Can J Comp Med Vet Sci 11:45–46
Fonseca JD, Mavrides DE, Morgan AL, Na JG, Graham PA, McHugh TD (2020) Antibiotic resistance in bacteria associated with equine respiratory disease in the United Kingdom. Vet Rec 187:189
Marin M, Arroyo R, Espinosa-Martos I, Fernandez L, Rodriguez JM (2017) Identification of emerging human mastitis pathogens by MALDI-TOF and assessment of their antibiotic resistance patterns. Front Microbiol 8:1258
Nam HM, Lim SK, Kang HM, Kim JM, Moon JS, Jang KC, Joo YS, Kang MI, Jung SC (2009) Antimicrobial resistance of streptococci isolated from mastitic bovine milk samples in Korea. J Vet Diagn Invest 21:698–701
Gaudreau C, Simoneau E, Labrecque O, Laurence RA, Laferriere C, Miller M, Raynal L, Rallu F (2007) Epidemiological, biochemical and antimicrobial susceptibility characteristics of Streptococcus pseudoporcinus isolated in Quebec, Canada, from 1997 to 2006. J Med Microbiol 56:1620–1624
Clarebout G, Villers C, Leclercq R (2001) Macrolide resistance gene mreA of Streptococcus agalactiae encodes a flavokinase. Antimicrob Agents Chemother 45:2280–2286
Clancy J, Dib-Hajj F, Petitpas JW, Yuan W (1997) Cloning and characterization of a novel macrolide efflux gene, mreA, from Streptococcus agalactiae. Antimicrob Agents Chemother 41:2719–2723
Koide S, Nagano Y, Takizawa S, Sakaguchi K, Soga E, Hayashi W, Tanabe M, Denda T, Kimura K, Arakawa Y, Nagano N (2022) Genomic traits associated with virulence and antimicrobial resistance of invasive group B Streptococcus isolates with reduced penicillin susceptibility from elderly adults. Microbiol Spectr 10:e0056822
Portillo A, Lantero M, Olarte I, Ruiz-Larrea F, Torres C (2001) MLS resistance phenotypes and mechanisms in beta-haemolytic group B, C and G Streptococcus isolates in La Rioja, Spain. J Antimicrob Chemother 47:115–116
Haenni M, Lupo A, Madec JY (2018) Antimicrobial resistance in Streptococcus spp. Microbiol Spectr 6:ARBA-0008-2017
Tian XY, Zheng N, Han RW, Ho H, Wang J, Wang YT, Wang SQ, Li HG, Liu HW, Yu ZN (2019) Antimicrobial resistance and virulence genes of Streptococcus isolated from dairy cows with mastitis in China. Microb Pathog 131:33–39
Velez JR, Cameron M, Rodriguez-Lecompte JC, Xia F, Heider LC, Saab M, McClure JT, Sanchez J (2017) Whole-genome sequence analysis of antimicrobial resistance genes in Streptococcus uberis and Streptococcus dysgalactiae isolates from canadian dairy herds. Front Vet Sci 4:63
Rato MG, Bexiga R, Florindo C, Cavaco LM, Vilela CL, Santos-Sanches I (2013) Antimicrobial resistance and molecular epidemiology of streptococci from bovine mastitis. Vet Microbiol 161:286–294
Hughes VM, Datta N (1983) Conjugative plasmids in bacteria of the “pre-antibiotic” era. Nature 302:725–726
Houndt T, Ochman H (2000) Long-term shifts in patterns of antibiotic resistance in enteric bacteria. Appl Environ Microbiol 66:5406–5409
Santiago-Rodriguez TM, Fornaciari G, Luciani S, Toranzos GA, Marota I, Giuffra V, Sangwan N, Cano RJ (2018) Tetracycline-like resistome of ancient human guts. Human Microbiome J 10:21–26
Santiago-Rodriguez TM, Fornaciari G, Luciani S, Dowd SE, Toranzos GA, Marota I, Cano RJ (2015) Gut microbiome of an 11th century A.D. pre-columbian andean mummy. PLoS One 10:e0138135
Santiago-Rodriguez TM, Fornaciari G, Luciani S, Toranzos GA, Marota I, Giuffra V, Cano RJ (2017) Gut Microbiome and putative resistome of Inca and Italian nobility mummies. Genes 8:310
Emaneini M, Khoramian B, Jabalameli F, Abani S, Dabiri H, Beigverdi R (2016) Comparison of virulence factors and capsular types of Streptococcus agalactiae isolated from human and bovine infections. Microb Pathog 91:1–4
Duarte RS, Bellei BC, Miranda OP, Brito MA, Teixeira LM (2005) Distribution of antimicrobial resistance and virulence-related genes among Brazilian group B Streptococci recovered from bovine and human sources. Antimicrob Agents Chemother 49:97–103
Vezina B, Al-Harbi H, Ramay HR, Soust M, Moore RJ, Olchowy TWJ, Alawneh JI (2021) Sequence characterisation and novel insights into bovine mastitis-associated Streptococcus uberis in dairy herds. Sci Rep 11:3046
Lin L, Huang X, Yang H, He Y, He X, Huang J, Li S, Wang X, Tang S, Liu G, Pan Z (2021) Molecular epidemiology, antimicrobial activity, and virulence gene clustering of Streptococcus agalactiae isolated from dairy cattle with mastitis in China. J Dairy Sci 104:4893–4903
Hossain M, Egan SA, Coffey T, Ward PN, Wilson R, Leigh JA, Emes RD (2015) Virulence related sequences; insights provided by comparative genomics of Streptococcus uberis of differing virulence. BMC Genomics 16:334
Acknowledgements
We thank the staff and faculty from the Connecticut Veterinary Medical Diagnostic Laboratory, Department of Pathobiology and Veterinary Science, University of Connecticut for their unconditional support. This paper was supported by the KU Research Professor Program of Konkuk University.
Funding
This work was partially supported by the University of Connecticut SPS #180229 and University of Connecticut SPS # 181033.
Author information
Authors and Affiliations
Contributions
J-YH performed revivification of lyophilized Streptococcus spp. isolates, conducted antibiotic resistance tests and WGS, contributed to WGS data analysis, and drafted the manuscript. JK and DHC. Participated in revivification of lyophilized Streptococcus spp. isolates, conducted antibiotic resistance tests and WGS. ZHH and RP performed antibiotic resistance tests. D-HL participated in study design, contributed to the WGS data analysis and manuscript editing. GRR contributed to the study design and manuscript editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Handling editor: Freddy Haesebrouck
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
The information of the complete genomes of S. agalactiae (n = 185), S. dysgalactiae (n = 23), S. pyogenes (n = 193), and S. equi subsp. zooepidemicus (n = 25), used for phylogenetic analysis (n = 427).
Additional file 2.
Genome characteristics and the de novo assembly results for the 50 Streptococcus spp. sequenced in this study.
Additional file 3.
The MLST results of the Streptococcus spp. isolates with the novel STs.
Additional file 4.
Virulence gene profiles the complete genome sequences of Streptococcus spp. used for phylogenetic analysis and 50 Streptococcus spp. isolates sequenced in this study (highlighted in red).
Additional file 5.
Phylogenetic analysis of S. agalactiae (n = 199) including our isolates (highlighted in red) using SNP analysis. The phylogeny was rooted at midpoint. The scale bars show the number of substitutions per site. The numerical values represent 1000 bootstrap replicate values expressed as a percentage. Subtrees including the S. agalactiae from aquatic animals were compressed to better visualize the genetic relationships. The colors in the heat map represent the levels of identity (%) between isolates, with white indicating the lowest and green indicating the highest.
Additional file 6.
Phylogenetic analysis of S. dysgalactiae (n = 38) including our isolates (highlighted in red) using SNP analysis. The phylogeny was rooted at midpoint. The scale bars show the number of substitutions per site. The numerical values represent 1000 bootstrap replicate values expressed as a percentage. The colors in the heat map represent the levels of identity (%) between isolates, with white indicating the lowest and green indicating the highest.
Additional file 7.
Phylogenetic analysis of S. pyogenes (n = 76) including our isolates (highlighted in red) using SNP analysis. The phylogeny was rooted at midpoint. The scale bars show the number of substitutions per site. The numerical values represent 1000 bootstrap replicate values expressed as a percentage. Subtrees including the S. pyogenes sequences which showed low sequence identity with our isolates were compressed to better visualize the genetic relationships. The colors in the heat map represent the levels of identity (%) between isolates, with white indicating the lowest and green indicating the highest.
Additional file 8.
Phylogenetic analysis of S. equi subsp. zooepidemicus (n = 29) from the United States including our isolates (highlighted in red) using SNP analysis. The phylogeny was rooted at midpoint. The scale bars show the number of substitutions per site. The numerical values represent 1000 bootstrap replicate values expressed as a percentage. The colors in the heat map represent the levels of identity (%) between isolates, with white indicating the lowest and green indicating the highest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hyeon, JY., Kim, J., Chung, D.H. et al. Genome analysis of Streptococcus spp. isolates from animals in pre-antibiotic era with respect to antibiotic susceptibility and virulence gene profiles. Vet Res 55, 51 (2024). https://doi.org/10.1186/s13567-024-01302-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-024-01302-0