Abstract
The spread of chronic wasting disease (CWD) during the last six decades has resulted in cervid populations of North America where CWD has become enzootic. This insidious disease has also been reported in wild and captive cervids from other continents, threatening ecosystems, livestock and public health. These CWD “hot zones” are particularly complex given the interplay between cervid PRNP genetics, the infection biology, the strain diversity of infectious prions and the long-term environmental persistence of infectivity, which hinder eradication efforts. Here, we review different aspects of CWD including transmission mechanisms, pathogenesis, epidemiology and assessment of interspecies infection. Further understanding of these aspects could help identify “control points” that could help reduce exposure for humans and livestock and decrease CWD spread between cervids.
Similar content being viewed by others
1 Introduction
Chronic wasting disease (CWD) is a highly prevalent prion disease affecting various species of the Cervidae family and has been described in North America, South Korea and Scandinavia [1, 2]. Prion diseases are fatal neurodegenerative disorders affecting numerous mammalian species. In addition to CWD, prion diseases include scrapie in sheep and goats, bovine spongiform encephalopathy (BSE), transmissible mink encephalopathy (TME), and Creutzfeldt-Jakob disease (CJD) in humans. CWD is the only prion disease affecting both wild and farmed animals and stands out for being highly contagious, widespread and persistent in the environment, which facilitates the transmission of the disease and hinders its control in deer populations [3,4,5,6].
Pathogenesis of CWD, as described for other prion diseases, occurs over extended asymptomatic periods and depends on the misfolding of the cellular prion protein (PrPC), encoded by the PRNP gene, into an infectious template-directing conformation (PrPSc) [7]. Following exposure to prions, the host’s susceptibility to develop disease, the clinical presentation, and the neuropathology are regulated by the interaction between the host PrPC primary structure and the invading prion agent or strain [8,9,10].
The neuropathology of affected cervids includes spongiform degeneration, neuronal loss, gliosis, and accumulation of PrPCWD (cervid PrPSc) in the form of aggregates [3, 4, 11]. Variation in the disease presentation between cervids, including survival period, distribution of brain lesions and PrPCWD properties occur in concert with different prion strains [12,13,14,15]. Prion strains are reproducible biological information encoded in specific PrPCWD conformers that are replicated by the templated-misfolding of the host PrPC [16,17,18]. While the transmission of a prion strain between hosts sharing similar PrPC is more efficient given the compatibility of selected strain-specific PrPSc conformers, transmission between hosts species expressing different PrPC primary structures is relatively inefficient and can introduce permanent conformational modifications resulting in the emergence of strains with novel properties [14, 19,20,21]. Alternatively, a strain can be transmitted back and forth between two species expressing various PrPC amino acid differences and remain unaltered informationally [22,23,24]. Strain selection from a host co-infected with multiple strains can also occur following transmission between species expressing different PrPC molecules [12, 25]. In addition, some tissues may show differential susceptibility for some strains compared to the brain (e.g. spleen) [26].
The transmission cycle of CWD in wild and captive cervids also involves the propagation of prion strains within and between various host species expressing distinct PrPC primary structures [12, 27, 28]. As CWD outbreaks become enzootic in cervid populations, circulating CWD strains must adapt to the shifting PrPC landscape that each new host provides which might result in novel strain emergence [13, 14, 21, 29]. These shifts in the prion replication substrate (PrPC) also occur at the population level, with allele frequencies of protective PrPC polymorphisms increasing in response to CWD in deer and elk [27, 30]. Here, we review the current understanding of the CWD transmission cycle, pathogenesis, infection biology and infer from numerous bioassay studies the potential for transmission to other species, including humans.
2 Transmission of CWD
The progression of CWD is less understood in wild free-ranging cervids given its direct relationship with the infectious dose, the route of exposure, the prion strain and the host PRNP genotype. Under controlled conditions, the incubation period (i.e., time to onset of clinical signs) of experimentally infected, orally dosed white-tailed deer, mule deer and reindeer expressing different PrPC ranged between 1.5 and 6 years post-exposure [13, 31,32,33]. Similarly in elk, differences in incubation period were observed to range between 1.8 to 5.2 years depending on the elk PRNP genotype [34,35,36]. During the asymptomatic period, both captive and wild infected cervids contribute to the spread of CWD as they accumulate considerable amounts of prion infectivity throughout the body, which is shed through secretions and excretions into the environment [6, 37, 38].
The age range documented for cervids infected with CWD in captivity is fairly similar to that described for free-ranging animals [39, 40]. However, depending on the historical CWD prevalence and given the social nature of cervids it is possible to find pre-clinical CWD positive fawns (< 1 year-old animals) and yearlings (1–2 year old) [41]. The youngest identified clinically-positive free-ranging deer and elk were 16 and 21 months of age, respectively [4, 42]. An age range of 2.5–7.5 years has been reported for free ranging clinical deer and 1.8 to 10.5 years for elk [4]. More recently, evaluation of the prevalence of CWD infection by age as determined by detection of abnormal prion protein in 28 954 deer collected over seven years of surveillance in Wisconsin and Illinois enzootic zones reached similar conclusions as in the previous studies; the risk of CWD infection increases with age in both male and female deer (prevalence in adults 1.93%, yearlings 0.89 and 0.45% in fawns) [43]. Previous analyses of prevalence in a sample of 4510 deer culled within Wisconsin eradication zones, where CWD incidence has historically been and remains the highest, confirmed CWD infection in 3 to 3.4% of yearling deer irrespective of sex [44]. These findings suggest the higher prevalence in younger animals (yearlings and fawns) could be related to the extent to which CWD has been historically prevalent within a particular cervid population. The detection of prions in fawns could as well represent mother to offspring transmission [45].
Differences in age at which clinical CWD is observed can vary between species and are likely to depend on the source or origin of the infection. The first cases of CWD Scandinavian cervids, were described in various 9–16 year old moose, a 16 year-old red deer and 3–4 year old reindeer [2, 46, 47].
The prevalence of CWD in wild cervids also varies by sex. In CWD enzootic areas the incidence of infection is much higher in males than females [44, 48, 49]. Considering that no differences in susceptibility have been detected between captive male and female deer [3, 39, 40, 50, 51], the higher prevalence in free ranging males may be attributable to differences in behavior, particularly during the breeding season when males roam widely and interact with other males more avidly, increase the risk of contact with contaminated environments and infected animals [52, 53].
The clinical progression and signs of CWD in both captive and experimentally infected cervids vary within and between species [3, 13, 31]. A brief summary is included in Table 1. Initial clinical features are often subtle and transitory [13]. The most prominent clinical features include behavioral alterations and progressive deterioration of body condition (i.e., weight loss) that worsen over the course of weeks to months [54]. Altered postures with lowered head and ears, arching of the back and ataxia can also be displayed [13, 31, 42]. Advanced clinical disease may involve odontoprisis, polydipsia, polyuria, difficulty swallowing, regurgitation of rumen contents and excessive salivation with drooling. Following recumbency, aspiration pneumonia, dehydration, or hypothermia during the winter season (in wild affected animals) are the most likely causes of death [42]. Compared to deer, CWD-affected elk can present with nervousness and hyperesthesia and are more likely to display motor disturbances but less likely to develop polydipsia [54].
Various factors contribute to the efficiency of the CWD transmission cycle. The primary mode of transmission between cervids, early in an outbreak, is likely by direct animal to animal interactions following exposure to an infected animal or environment [53, 55]. Although experimental infection in pregnant muntjac deer (Muntiacus reevesi) and detection of PrPCWD in tissues from wild pregnant elk dams provide evidence for in utero transmission [45, 56], other studies indicate it plays a minor role in epidemiology compared to deer-to-deer transmission [57]. Given the rapid pre-clinical accumulation of PrPCWD aggregates in lymphoid tissues associated with the alimentary and the intestinal mucosa, the oral route of infection is most likely [31, 58, 59]. However, inhalation of CWD-fomites has also been proposed as a mechanism of exposure [60]. The presence of CWD infectivity in antler velvet [61] leads to the question of whether prions can persist in the calcified antler and favor male to male intradermal inoculation during the rut, or represent a risk factor in terms of oral CWD transmission, as antler gnawing is common among cervids. Antler gnawing has been suggested as a factor involved in the transmission of CWD in the reindeer population from Nordfjella, Norway [62].
The spread of CWD into “naïve” cervids also occurs through exposure to contaminated environments previously inhabited by infected animals [38, 57, 63]. This mode of transmission becomes more relevant as the prevalence of CWD in affected cervid populations increases and the disease becomes enzootic. Secretions (saliva) and excretions (urine and feces) of CWD-infected cervids contain considerable CWD infectivity. The minimum infectious dose in saliva required for a deer to become infected, assuming a single oral exposure to CWD prions, is equivalent to the infectivity contained in 100–300 ng of brain (approximate equivalent of 30 mL of CWD-positive saliva) [6]. Secretions and excretions from CWD positive animals and the decomposition of diseased carcasses contaminate the environment, in which prions can persist in a bioavailable state for years [38, 64,65,66]. The physical association of prions with certain soil microparticles enhances transmissibility [67, 68]. Environmental persistence of CWD infectivity depends on the composition of minerals and organic constituents of soil, which vary between geographical areas [69]. Minerals such as montmorillonite, can enhance the experimental transmission of prions by the oral route [67]. Deer consume a significant amount of soil, especially in adjacent areas to mineral licks, which have tested positive for infectivity in CWD endemic regions [70]. Soils, depending on their composition, represent an important reservoir of CWD infectivity in the environment. Although determination of the degree of contamination of a particular soil surface becomes more difficult with time, the soil-bound prion infectivity is not significantly altered [66].
Plants can also represent an important reservoir for CWD contamination and transmission. CWD contaminated pastures can remain infectious for at least 2 years after prion exposure [65]. Regarding the uptake of prions by plants, results are more controversial. One study, using protein misfolding cyclic amplification (PMCA), an ultrasensitive technique for the detection of prions, demonstrated that grass plants exposed to brain or excretions from CWD-infected cervids can uptake prions from the soil and transport them to the aerial parts of the plant [71]. Another study, however, showed that wheat plants do not transport CWD prions from the roots to the stems [72].
3 Prion neuroinvasion and body distribution of infectivity
The pathological hallmarks of CWD in deer resemble those observed in sheep with scrapie and other prion diseases acquired by ingestion of contaminated material. In orally infected deer, PrPCWD crosses the intestinal epithelial barrier and can be detected, within the first 30–42 days post-exposure, in lymphoid tissues associated with the alimentary tract such as the gut-associated lymphoid tissue (GALT), the tonsils and the retropharyngeal lymph nodes [31, 59, 73, 74]. Modified enterocytes, called M cells, participate in the uptake of the prion, incorporating it into the subepithelial lymphoid tissue. The pathological prion protein then accumulates and replicates in follicular dendritic cells and tingible body macrophages [58, 75, 76]. PrPCWD cellular targeting during early pathogenesis suggests that prions are transported by dendritic cells and/or macrophages to Peyer’s Patches and regional mesenteric lymph nodes [58, 59].
Once infection has been established in the GALT, prion colonization of the nerve endings of the Enteric Nervous System (ENS) and leakage into the lymph and blood facilitates the spread to other organs [77, 78]. Prion infection of the ENS results in the spread of infectivity through sympathetic and parasympathetic nerves [75]. The initial site of PrPCWD detection within the deer brain is the dorsal motor nucleus of the vagus nerve (DMNV), suggesting this nerve as the major route for PrPCWD traffic from the alimentary tract to the brain [73].
CWD infectivity trafficked via the lymph and the blood can reach multiple organs, including the brain. Prion neuroinvasion by this route likely occurs via the circumventricular organs [79]. Consistent with this observation, considerable prion infectivity has been demonstrated in numerous blood cell types from CWD-infected deer, suggesting that the haematogenous dissemination of infection may be important during the pathogenesis of the disease [80].
Another major route of prion neuroinvasion involving the entry via ENS is by retrograde transport of prions through the splanchnic nerve circuitry. This is consistent with the presence of prion aggregates in the intermediolateral columns of the thoracic spinal cord during early stages of prion infection [81, 82]. This route is particularly important during neuroinvasion by BSE prions in cattle, however, analysis of CWD prion accumulation following oral infection did not detect prion deposits in the coeliac ganglion of deer. This suggests that the accumulation of PrPCWD in the intermediolateral column of orally infected cervids results from the centrifugal dissemination of prions replicated within the central nervous system (CNS) [73].
The early lymphoid replication phase is particularly important for the neuroinvasion of CWD prions in deer [73, 74]. Interestingly, North American elk as well as Scandinavian moose and red deer accumulate PrPCWD in the brainstem with little to no accumulation in lymphoid tissues [46, 47, 83, 84]. This could be explained by a predominantly neural route of neuroinvasion (as in BSE pathogenesis), sporadic misfolding of PrPC or differences in the route of exposure. Differences in pathogenesis and neuropathology of sheep inoculated via different routes were not seen [85]. Similarly, no significant differences have been detected in the peripheral burden of CWD prions from deer infected through different routes [64]. Once neuroinvasion has occurred, PrPCWD accumulates producing the characteristic lesions of prion diseases, including intraneuronal vacuolation, neuropil spongiosis, gliosis and formation of amyloid plaques [86].
In addition to lymphoid and brain tissues, CWD prions have been detected in nasal mucosa, salivary glands, urinary bladder, pancreas, kidney, intestine and reproductive tract of female and male deer [15, 31, 64, 73, 87, 88]. The accumulation of PrPCWD in some of these tissues is tightly associated with shedding of infectivity through secretions and excretions [64]. Similar to scrapie in sheep [89], PRNP genotype can influence CWD pathogenesis in deer affecting PrPCWD deposition in peripheral tissues [13, 15, 31, 74, 90].
4 CWD in cervids
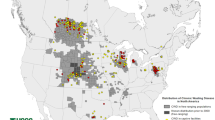
The origin of CWD remains unknown. In North America, epidemiological data suggests emergence occurred in Colorado and Wyoming [55], in the late 1960s, in captive mule deer (Odocoileus hemionus) and black-tailed deer (Odocoileus hemionus columbianus) at research facilities. These herds were captured cervids from different wild populations, including pregnant females that were released after parturition. Transfer of animals between facilities was a common practice [3]. CWD was subsequently detected in Rocky Mountain elk (Cervus elaphus nelsoni) at these facilities and, thereafter, in free-ranging populations of mule deer and elk in Wyoming and Colorado [3, 50, 91].
Cervid migration and commercial movement of preclinical animals contributed to the geographic expansion of CWD into free-ranging and captive populations of North America [42, 55]. To date, CWD occurs in at least 26 U.S. states and three Canadian provinces (Saskatchewan, Alberta and Québec). In Canada, CWD was first identified in farmed elk from Saskatchewan in 1996 [42]. In the following years, CWD was reported in farmed white-tailed deer in Alberta and in wild cervid populations from Saskatchewan and Alberta [92]. Epidemiological studies suggested that the infection was introduced into Saskatchewan farms via import of captive elk from a farm in South Dakota [42]. The origin of the CWD epidemic in wild cervids of Canada remains unknown. Transmission by contact exposure between wild deer and infected farmed elk is a possibility [92]. CWD was first detected in 2013 in wild moose from Alberta [93]. CWD has not, to date, been detected in the wild North American subspecies of caribou (Rangifer tarandus spp).
Outside of North America, CWD outbreaks in captive cervids in South Korean farms occurred following cohabitation with asymptomatic infected elk and deer imported between 1994 and 2003 from a farm in Saskatchewan (later determined to house CWD-infected animals [94]. A direct consequence was the transmission into South Korean captive red deer (Cervus elaphus), sika deer (Cervus nippon) and crosses of these two species [28]. Epidemiological studies of CWD in wild cervids from the Korean peninsula are lacking.
In 2016, CWD was identified in a free-ranging Norwegian reindeer (Rangifer tarandus tarandus), representing the first CWD case detected in Europe [2]. Since this event, thousands of cervids have been surveyed, leading to the detection of the CWD in 19 reindeer, 4 moose and one red deer in Norway, 3 moose in Sweden and one moose in Finland, suggesting that CWD has been quietly emerging in European cervids [46, 47, 95]. The origin of these cases is still unknown. Transmission of Norwegian CWD isolates into bank voles demonstrated the presence of strains different than those seen in North America, suggesting these epizootics are not epidemiologically linked [96]. In addition, importation of cervids to Norway is not allowed and, therefore, it is unlikely that these CWD infections emerged from imported positive animals as was the case for CWD in Korea [1].
The prevalence of CWD in North America has been increasing exponentially during the last 6 decades. In farmed herds the prevalence of CWD positive animals can be higher than 80% and higher than 45% in wild populations [41]. In areas where CWD has become enzootic, the CWD prevalence can be greater than 50% in adult males [97]. The latest Alberta CWD surveillance update (2019 fall hunting season) indicates that the prevalence of CWD continues to increase in all cervid species. Compared to the 2018 fall hunting season, the prevalence in the 2019 season saw an increase of 3.8% (from 7.4% in 2018 to 11.2% 2019 prevalence). Consistent with previous years, white-tailed and mule deer in Alberta and Saskatchewan show differences in prevalence between species and sexes. The prevalence rank among Alberta deer is mule deer males > mule deer females > white-tailed males > white-tailed females. The burden of CWD in Alberta wild elk has not been as extensive as in deer. In 2019, 1.3% of the tested elk resulted positive for CWD (0.8% in 2018). In addition, for the first time, CWD was detected in two hunter-harvested moose [98].
Population declines are observed in cervid herds with high CWD prevalence. CWD positive deer not only succumb to the disease but are also more prone to be killed by predators or hunters, and are more vulnerable to vehicle collisions [99]. Average declines in elk survival in Rocky Mountain National Park were attributed almost entirely to CWD [100] Mean annual survival rates of CWD-negative and CWD-positive deer were estimated as 76% and 32%, respectively, and CWD was considered a significant contributor to mule deer population decline [101]. Miller et al. also observed that the 2-year survival of infected and uninfected tagged wild mule deer was 47 and 82%, respectively [99].
5 Experimental CWD in cervid species
No natural cases of CWD have been described in some species of cervids, although they have proven to be susceptible to CWD following experimental exposure. These include the Asian muntjac (Muntiacus reevesi) [45] and fallow deer (Dama dama) [102]. Muntjac deer were successfully infected through oral and subcutaneous routes with CWD from white-tailed deer. Interestingly, PrPCWD was detected in fetuses from CWD-infected does, demonstrating vertical CWD transmission in this species [45]. Although fallow deer were suggested to show certain resistance to infection with CWD [103], Hamir et al. reported that this species is susceptible to the disease after intracerebral inoculation with elk and white-tailed deer prions [102]. The differences in these studies may have arisen because intracranial inoculation is more efficient to produce disease compared to environmental exposure, however, PrPC sequence and prion strain compatibility could also explain these differences [102, 103]. No natural cases of CWD have been reported in North American caribou, although these species are susceptible to experimental infection with CWD from mule deer, white-tailed deer and elk. Naive caribou can acquire the disease after oral infection [33] and environmental exposure [63]. A summary of this transmission experiments and the ones described below is included in Table 2.
6 Evaluating the potential transmission of CWD to non-cervid species
Most of the transmission studies of CWD into various animal species have been conducted with North American CWD isolates and have revealed different transmission patterns. The host range of European CWD isolates is still to be determined [95].
6.1 Livestock species
The interactions between different animal species in captivity is a known factor favoring the emergence of new pathogens with novel zoonotic properties, as has been recently proposed as the origin of BSE in cattle by contact with sheep infected with atypical/Nor98 scrapie [104]. The distribution of CWD in North America could favor the interspecies transmission of cervid prions into cattle (i.e., overlapping of these species is common in CWD enzootic areas of North America). The transmission of a prion disease to the cattle is cause for alarm due to the potential emergence of BSE-like zoonotic capacity. CWD from different species (white-tailed deer, mule deer and elk) has been successfully transmitted to domestic cattle after intracerebral challenge with different attack rates [105,106,107,108]. The neuropathological and biochemical characteristics of bovine CWD are, however, clearly distinct from BSE [105]. In addition, oral infections in cattle with mule deer prions have been unsuccessful, and no positive transmission has been detected in this species after 10 years of environmental exposure to mule deer and elk CWD [109]. This demonstrates that an important species barrier limits the oral transmission of CWD to cattle.
In 2006, Hamir et al. reported the transmission of mule deer CWD into sheep via the intracranial route [110]. Only 2 of 8 inoculated lambs developed lesions compatible with a prion disease, and they expressed different PRNP genotypes at codons 136, 154 and 171, which are known to determine sheep susceptibility to scrapie [111,112,113]. One animal expressed ARQ/ARQ (subclinical) and one ARQ/VRQ (clinical) sheep. ARQ/ARR sheep were completely resistant to CWD inoculation, suggesting that the transmission of CWD to small ruminants is strongly determined by the host genotype, as seen with scrapie [110]. Clinical disease was described, however, in ARQ/ARQ sheep inoculated with elk CWD prions, which suggests a different strain in the elk isolate. Transgenic mice overexpressing the ovine VRQ PrP allele (tg338 mice) do not accumulate prions in the brain after experimental infection with a number of different CWD isolates [26, 114, 115]. These tg mice, however, efficiently replicate CWD prions in the spleen, suggesting that the lymphoid tissue is more permissive than the brain for interspecies transmission [26].
The susceptibility of pigs to CWD has also been investigated. Moore et al. found that white-tailed deer CWD prions can be detected by real-time quaking-induced conversion (RT-QuIC) in some orally and intracranially inoculated pigs when euthanized at market weight (8 months age, 6 months after inoculation). One aged pig showed clinical signs and, in these aged animals, PrPCWD was detectable by immunohistochemistry and Western blotting in 4/10 intracranially inoculated and in 1/10 orally inoculated pigs. Passages in transgenic mice expressing the porcine PrP showed reduced attack rates. Therefore, they concluded that pigs could support a low-level propagation of CWD prions, albeit with a high species barrier. These results are not necessarily encouraging since it is possible that feral pigs, whose ranges are shared with CWD affected cervids, could act as a reservoir of CWD [116].
6.2 Other wildlife species
Comparison of the PRNP sequences of different species of ungulates that inhabit CWD endemic areas showed high sequence identity between bighorn sheep (Ovis canadensis), mountain goats (Oreamnos americanus) and domestic sheep suggesting that these species are potentially susceptible to CWD [117]. No experimental challenges of these wildlife species, sympatric to deer in CWD-endemic areas have been performed yet.
6.2.1 Rodents
Several different rodent species sympatric with deer in CWD endemic areas including meadow voles (Microtus pennsylvanicus), red-backed voles (Myodes gapperi), white-footed mice (Peromyscus leucopus) and deer mice (P. maniculatus) have proven to be susceptible to CWD after experimental inoculation [118]. Among these species, meadow voles showed to be the most susceptible, but incubation periods were shortened in all the rodent species upon second passage, indicating CWD adaptation to these hosts. House mice (Mus musculus) live in close proximity to humans, and their susceptibility to particular CWD strains has been demonstrated [29]. It is possible that wild rodents represent a reservoir for CWD in ecosystems considering that these animals are scavengers, and one of the main sources of food for predators. In addition, they can be accidentally consumed by deer or livestock since rodent carcasses contaminate pastures and forage [118].
CWD is also transmissible to other rodent species that do not cohabitate with deer in CWD-affected regions. These include Syrian golden hamsters (Mesocricetus auratus) [119] and European bank voles (Myodes glareolus) [120]. Curiously, the adaptation of CWD to the European bank vole resulted in the identification of a prion strain (CWD-vole strain) with the shortest incubation period observed to date [120].
6.2.2 Carnivores
Ferrets (Mustela putorius) are a valuable model for the study of prions, including CWD [121,122,123]. Mink (Mustela vison) can also be infected with CWD, but only by intracerebral inoculation. The disease characteristics differed from those of TME-affected mink, demonstrating different strains cause CWD and TME, and suggest that mink are unlikely involved in natural CWD transmission [124].
Oral and intracerebral inoculation of mule deer prions into domestic cats (Felis catus) resulted in no clinical disease or low attack rates, respectively, on first passage. A second passage of the prions from the intracerebrally inoculated cats resulted in 100% of the recipient cats presenting with clinical disease while the second oral passage resulted in a 50% attack rate demonstrating the adaptability of CWD prions to felines [125]. The PrPC sequence similarity between cats and mountain lions (Puma concolor) suggests that these wild carnivores would be susceptible to CWD infection [126]. As mountain lions selectively prey CWD-infected cervids, it would be of interest to test dead animals for CWD to evaluate for prion spillover [127].
CWD prions from white-tailed deer and elk transmitted with low attack rates (25%) following intracerebral challenge in raccoons (Procyon lotor) [128]. Accumulation of protease resistant prions in the cerebrum and obex differed depending on the inoculum. Interestingly, prions from mule deer did not transmit to raccoons after 6 years following intracerebral challenge [129, 130]. This further suggests strain differences in CWD prions from the various cervid species affected.
Among all mammals, canids are probably the most resistant to prion diseases, with the amino acid residue 163 of canine PrPC conferring protection [131,132,133]. Oral exposure of captive coyotes (Canis latrans) to elk prions demonstrated the presence of prions in the coyote fecal material during the first days after consumption [134]. However, even after a large volume of infectious brain homogenate was inoculated, only 50% of exposed coyotes had detectable infectivity in feces between 1- and 4-days post-exposure (dpe) as evaluated by bioassay in tg12 mice (expressing elk PrPC), while the other half lacked detectable prions or were only recovered in feces after 1 day. No evidence of CWD accumulation in the coyote lymph tissue was detected [134]. These results suggest that coyotes were capable of degrading the CWD infectivity. Consistent with this interpretation, the attack rates were incomplete in tg12 mice inoculated with feces collected at various times following exposure. When inoculated with brain homogenates from CWD-infected elk, this transgenic mouse line develops prion disease with full attack rates after incubation periods of < 150 days post-infection [135]. In addition, in the wild, coyotes will likely consume a smaller infective dose as CWD infectivity in muscle and fat is lower than in the brain [51, 136]. The role of canine predators in the control of CWD has been discussed previously, suggesting that the selective predation exerted by wolves (Canis lupus), which hunt weak and vulnerable cervids, could represent an important natural tool to limit CWD contamination of the environment [137]. The reintroduction and protection of wolves in CWD-affected areas, although controversial, could be very efficient for the natural control of the disease.
6.3 Humans
To date, there is no clear evidence that CWD can cross the transmission barrier and infect humans, as other animal prions such as BSE [138]. Several epidemiological studies have been developed to assess whether, statistically, there are more cases of prion diseases in population groups living in endemic areas for CWD. These studies mainly consider people exposed to CWD-infected cervids, such as consumers of deer meat and hunters. None of these studies have found a clear correlation between CWD exposure and an increase in human prion disease frequency [90, 139,140,141]. Evaluating the risk of humans to CWD through this type of studies is difficult due to the variety of strains present in the environment, the transport of hunted animals between long distances and the long incubation period of prion diseases in humans (even decades).The identification of the zoonotic ability of an agent requires an abnormally high number of human cases within a particular geographical location or period of time, which necessitates a large number of human exposures to the disease. Prevalence of CWD in several areas has increased exponentially in the last decade, therefore, there may not historically have been a sufficient level of exposure to the disease to detect a zoonotic transmission of CWD.
There are tools, however, to evaluate the susceptibility of humans to CWD. These include bioassays in non-human primates and transgenic mice expressing human PrPC and in vitro studies of the human transmission barrier to CWD.
Squirrel monkeys (Saimiri sciureus) are susceptible to CWD prions from mule deer, elk and white-tailed deer after oral and intracerebral challenge [142, 143]. Race et al. did not observe evidence for CWD transmission to macaques (Macaca fascicularis) at 13 years post-inoculation and using ultra-sensitive techniques for the detection of prions [144]. In an ongoing study, macaques were exposed to different sources of CWD through various routes. Analysis of the tissues identified PrP deposition in the dorsal horns of the spinal cord in a subset of the macaques [145]. Similar PrP immunopositive staining affecting the spinal cord was also reported by Race et al.; these deposits were found in both CWD-challenged and uninoculated, aged macaques, suggesting that this staining was likely due to cellular PrP [144]. Evaluating the zoonotic potential to humans through bioassays in non-human primates has, however, several drawbacks. The degree of sequence similarity between human PrP and the PrP from non-human primates varies between 92.2 and 99.7% [146]. Even species with high sequence homology, such as chimpanzees, express amino acid substitutions in key structural motifs of PrP that could alter the transmission barrier of prions [146, 147]. Although chimpanzees are more closely related to humans, the presence of 2 amino acid polymorphisms adjacent and within the α2-β2 loop, an important structural motif modulating interspecies transmission of some prion strains [148], undermines their utility in testing the species barrier. In particular, the residue E168 in humans (Q168 in chimpanzees) appears to be fundamental for human reduced susceptibility to CWD and other prion strains from ruminants [149].
Transgenic mice expressing different variants of human PrPC (MM129, MV129 and VV129) at 1–16-fold the levels expressed in the human brain were challenged with US and Canadian CWD isolates in seven different studies. Elegantly reviewed by Waddell et al., none of these studies found evidence of transmission to any of the transgenic mice (reviewed by [141]). Studies in chimeric mice suggested that the α2–β2 loop of the prion protein is the key to the transmission barrier of humans to CWD [148]. However, a recent study found low levels of RT-QuIC seeding activity in four mice overexpressing human prion protein (MM129) inoculated with elk and white-tailed deer isolates (2 mice per inoculation group). These results need to be interpreted with caution, as these reactions were inconsistently positive perhaps representing poorly adapted CWD prions into human PrP or alternatively, persistence of the inoculum in the brain of these mice since human prions can physically persist even in knock-out mice for extended periods post-inoculation [149]. In addition, these RT-QuIC positive mice (tg66) express the highest levels of human PrP tested in CWD transmission studies (8–16 × compared to human brain), which could facilitate the replication of CWD in human PrP [150]. In contrast, the same CWD isolates when inoculated into the tgRM (2–4×) resulted in less clinical suspects and no positive detection by RT-QuIC [150].
Finally, the transmission barrier of humans for CWD has been studied using ultra-sensitive in vitro techniques. The first in vitro study suggested a substantial molecular barrier limiting susceptibility of humans to CWD [151]. Davenport et al. demonstrated positive seeding activity when human recombinant PrP was seeded with CWD, but not when using BSE, contradicting results observed in vivo [152]. Successful conversion of human PrP using different CWD seeds in PMCA has been reported by Barria et al. Their studies suggest that CWD from MM132 elk and CWD from reindeer have the highest potential to convert human PrP, followed by white-tailed deer prions and, finally, mule deer CWD isolates, which require an intermediate step of in vitro conditioning to deer substrate [153,154,155]. This positive conversion was achieved, however, in PMCA reactions using a large CWD prions-to- human substrate ratio [154].
7 Conclusions
The prevalence and geographic spread of CWD continues to rise, expanding the likelihood of transmission to other species. Particularly of concern in North America is the risk to caribou, an endangered species. Although canids appear to have resistance to infection by CWD prions, other carnivores, i.e., the big felids, are predicted to be susceptible to infection. The zoonotic potential is still unclear but the increased prevalence of CWD in cervids will result in greater likelihood of human exposure.
Abbreviations
- BSE:
-
bovine spongiform encephalopathy
- CJD:
-
Creutzfeldt-Jakob disease
- CNS:
-
central nervous system
- CWD:
-
chronic wasting disease
- DMNV:
-
dorsal motor nucleus of the vagus nerve
- dpe:
-
days post-exposure
- ENS:
-
enteric nervous system
- GALT:
-
gut-associated lymphoid tissue
- PMCA:
-
protein misfolding cyclic amplification
- PrPC :
-
cellular prion protein
- PrPCWD :
-
cervid PrPSc
- PrPSc :
-
pathological prion protein
- RT-QuIC:
-
real-time quaking-induced conversion
- TME:
-
transmissible mink encephalopathy
References
Sohn HJ, Kim JH, Choi KS, Nah JJ, Joo YS, Jean YH, Ahn SW, Kim OK, Kim DY, Balachandran A (2002) A case of chronic wasting disease in an elk imported to Korea from Canada. J Vet Med Sci 64:855–858. https://doi.org/10.1292/jvms.64.855
Benestad SL, Mitchell G, Simmons M, Ytrehus B, Vikoren T (2016) First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer. Vet Res 47:88. https://doi.org/10.1186/s13567-016-0375-4
Williams ES, Young S (1980) Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis 16:89–98. https://doi.org/10.7589/0090-3558-16.1.89
Spraker TR, Miller MW, Williams ES, Getzy DM, Adrian WJ, Schoonveld GG, Spowart RA, O’Rourke KI, Miller JM, Merz PA (1997) Spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus) and Rocky Mountain elk (Cervus elaphus nelsoni) in northcentral Colorado. J Wildl Dis 33:1–6. https://doi.org/10.7589/0090-3558-33.1.1
Uehlinger FD, Johnston AC, Bollinger TK, Waldner CL (2016) Systematic review of management strategies to control chronic wasting disease in wild deer populations in North America. BMC Vet Res 12:173. https://doi.org/10.1186/s12917-016-0804-7
Denkers ND, Hoover CE, Davenport KA, Henderson DM, McNulty EE, Nalls AV, Mathiason CK, Hoover EA (2020) Very low oral exposure to prions of brain or saliva origin can transmit chronic wasting disease. PLoS One 15:e0237410. https://doi.org/10.1371/journal.pone.0237410
Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C (1993) Mice devoid of PrP are resistant to scrapie. Cell 73:1339–1347. https://doi.org/10.1016/0092-8674(93)90360-3
Race R, Raines A, Raymond GJ, Caughey B, Chesebro B (2001) Long-term subclinical carrier state precedes scrapie replication and adaptation in a resistant species: analogies to bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease in humans. J Virol 75:10106–10112. https://doi.org/10.1128/JVI.75.21.10106-10112.2001
Hill AF, Desbruslais M, Joiner S, Sidle KC, Gowland I, Collinge J, Doey LJ, Lantos P (1997) The same prion strain causes vCJD and BSE. Nature 389:448-450. https://doi.org/10.1038/38925
Wadsworth JD, Asante EA, Desbruslais M, Linehan JM, Joiner S, Gowland I, Welch J, Stone L, Lloyd SE, Hill AF, Brandner S, Collinge J (2004) Human prion protein with valine 129 prevents expression of variant CJD phenotype. Science 306:1793–1796. https://doi.org/10.1126/science.1103932
Spraker TR, O’Rourke KI, Balachandran A, Zink RR, Cummings BA, Miller MW, Powers BE (2002) Validation of monoclonal antibody F99/97.6.1 for immunohistochemical staining of brain and tonsil in mule deer (Odocoileus hemionus) with chronic wasting disease. J Vet Diagn Invest 14:3–7. https://doi.org/10.1177/104063870201400102
Angers RC, Kang HE, Napier D, Browning S, Seward T, Mathiason C, Balachandran A, McKenzie D, Castilla J, Soto C, Jewell J, Graham C, Hoover EA, Telling GC (2010) Prion strain mutation determined by prion protein conformational compatibility and primary structure. Science 328:1154–1158. https://doi.org/10.1126/science.1187107
Johnson CJ, Herbst A, Duque-Velasquez C, Vanderloo JP, Bochsler P, Chappell R, McKenzie D (2011) Prion protein polymorphisms affect chronic wasting disease progression. PLoS One 6:e17450. https://doi.org/10.1371/journal.pone.0017450
Duque Velasquez C, Kim C, Herbst A, Daude N, Garza MC, Wille H, Aiken J, McKenzie D (2015) Deer prion proteins modulate the emergence and adaptation of chronic wasting disease strains. J Virol 89:12362–12373. https://doi.org/10.1128/JVI.02010-15
Otero A, Duque Velasquez C, Johnson C, Herbst A, Bolea R, Badiola JJ, Aiken J, McKenzie D (2019) Prion protein polymorphisms associated with reduced CWD susceptibility limit peripheral PrPCWD deposition in orally infected white-tailed deer. BMC Vet Res 15:50. https://doi.org/10.1186/s12917-019-1794-z
Bessen RA, Marsh RF (1992) Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J Virol 66:2096–2101
Safar JG, Xiao X, Kabir ME, Chen S, Kim C, Haldiman T, Cohen Y, Chen W, Cohen ML, Surewicz WK (2015) Structural determinants of phenotypic diversity and replication rate of human prions. PLoS Pathog 11:e1004832. https://doi.org/10.1371/journal.ppat.1004832
Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB (1998) Eight prion strains have PrPSc molecules with different conformations. Nat Med 4:1157–1165. https://doi.org/10.1038/2654
Bessen RA, Marsh RF (1992) Identification of two biologically distinct strains of transmissible mink encephalopathy in hamsters. J Gen Virol 73:329–334. https://doi.org/10.1099/0022-1317-73-2-329
Bartz JC, Bessen RA, McKenzie D, Marsh RF, Aiken JM (2000) Adaptation and selection of prion protein strain conformations following interspecies transmission of transmissible mink encephalopathy. J Virol 74:5542–5547. https://doi.org/10.1128/jvi.74.12.5542-5547.2000
Duque Velasquez C, Kim C, Haldiman T, Kim C, Herbst A, Aiken J, Safar JG, McKenzie D (2020) Chronic wasting disease (CWD) prion strains evolve via adaptive diversification of conformers in hosts expressing prion protein polymorphisms. J Biol Chem 295:4985–5001. https://doi.org/10.1074/jbc.RA120.012546
Kimberlin RH, Cole S, Walker CA (1987) Temporary and permanent modifications to a single strain of mouse scrapie on transmission to rats and hamsters. J Gen Virol 68:1875–1881. https://doi.org/10.1099/0022-1317-68-7-1875
Bruce M, Chree A, McConnell I, Foster J, Pearson G, Fraser H (1994) Transmission of bovine spongiform encephalopathy and scrapie to mice: strain variation and the species barrier. Philos Trans R Soc Lond B Biol Sci 343:405–411. https://doi.org/10.1098/rstb.1994.0036
Bartz JC, McKenzie DI, Bessen RA, Marsh RF, Aiken JM (1994) Transmissible mink encephalopathy species barrier effect between ferret and mink: PrP gene and protein analysis. J Gen Virol 75:2947–2953. https://doi.org/10.1099/0022-1317-75-11-2947
Bruce ME (1993) Scrapie strain variation and mutation. Br Med Bull 49:822–838. https://doi.org/10.1093/oxfordjournals.bmb.a072649
Beringue V, Herzog L, Jaumain E, Reine F, Sibille P, Le Dur A, Vilotte JL, Laude H (2012) Facilitated cross-species transmission of prions in extraneural tissue. Science 335:472–475. https://doi.org/10.1126/science.1215659
Robinson SJ, Samuel MD, Johnson CJ, Adams M, McKenzie DI (2012) Emerging prion disease drives host selection in a wildlife population. Ecol Appl 22:1050–1059. https://doi.org/10.1890/11-0907.1
Lee YH, Sohn HJ, Kim MJ, Kim HJ, Lee WY, Yun EI, Tark DS, Cho IS, Balachandran A (2013) Strain characterization of the Korean CWD cases in 2001 and 2004. J Vet Med Sci 75:95–98. https://doi.org/10.1292/jvms.12-0077
Herbst A, Velasquez CD, Triscott E, Aiken JM, McKenzie D (2017) Chronic wasting disease prion strain emergence and host range expansion. Emerg Infect Dis 23:1598–1600. https://doi.org/10.3201/eid2309.161474
Monello RJ, Galloway NL, Powers JG, Madsen-Bouterse SA, Edwards WH, Wood ME, O’Rourke KI, Wild MA (2017) Pathogen-mediated selection in free-ranging elk populations infected by chronic wasting disease. Proc Natl Acad Sci U S A 114:12208–12212. https://doi.org/10.1073/pnas.1707807114
Fox KA, Jewell JE, Williams ES, Miller MW (2006) Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus). J Gen Virol 87:3451–3461. https://doi.org/10.1099/vir.0.81999-0
Miller MW, Wolfe LL, Sirochman TM, Sirochman MA, Jewell JE, Williams ES (2012) Survival patterns in white-tailed and mule deer after oral inoculation with a standardized, conspecific prion dose. J Wildl Dis 48:526–529. https://doi.org/10.7589/0090-3558-48.2.526
Mitchell GB, Sigurdson CJ, O’Rourke KI, Algire J, Harrington NP, Walther I, Spraker TR, Balachandran A (2012) Experimental oral transmission of chronic wasting disease to reindeer (Rangifer tarandus tarandus). PLoS One 7:e39055. https://doi.org/10.1371/journal.pone.0039055
Hamir AN, Gidlewski T, Spraker TR, Miller JM, Creekmore L, Crocheck M, Cline T, O’Rourke KI (2006) Preliminary observations of genetic susceptibility of elk (Cervus elaphus nelsoni) to chronic wasting disease by experimental oral inoculation. J Vet Diagn Invest 18:110–114. https://doi.org/10.1177/104063870601800118
O’Rourke KI, Spraker TR, Zhuang D, Greenlee JJ, Gidlewski TE, Hamir AN (2007) Elk with a long incubation prion disease phenotype have a unique PrPd profile. NeuroReport 18:1935–1938. https://doi.org/10.1097/WNR.0b013e3282f1ca2f
Moore SJ, Vrentas CE, Hwang S, West Greenlee MH, Nicholson EM, Greenlee JJ (2018) Pathologic and biochemical characterization of PrPSc from elk with PRNP polymorphisms at codon 132 after experimental infection with the chronic wasting disease agent. BMC Vet Res 14:80. https://doi.org/10.1186/s12917-018-1400-9
Henderson DM, Denkers ND, Hoover CE, Garbino N, Mathiason CK, Hoover EA (2015) Longitudinal detection of prion shedding in saliva and urine by chronic wasting disease-infected deer by real-time quaking-induced conversion. J Virol 89:9338–9347. https://doi.org/10.1128/JVI.01118-15
Mathiason CK, Hays SA, Powers J, Hayes-Klug J, Langenberg J, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hoover EA (2009) Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS One 4:e5916. https://doi.org/10.1371/journal.pone.0005916
Miller MW, Wild MA, Williams ES (1998) Epidemiology of chronic wasting disease in captive Rocky Mountain elk. J Wildl Dis 34:532–538. https://doi.org/10.7589/0090-3558-34.3.532
Miller MW, Wild MA (2004) Epidemiology of chronic wasting disease in captive white-tailed and mule deer. J Wildl Dis 40:320–327. https://doi.org/10.7589/0090-3558-40.2.320
Keane DP, Barr DJ, Bochsler PN, Hall SM, Gidlewski T, O’Rourke KI, Spraker TR, Samuel MD (2008) Chronic wasting disease in a Wisconsin white-tailed deer farm. J Vet Diagn Invest 20:698–703. https://doi.org/10.1177/104063870802000534
Williams ES, Miller MW (2002) Chronic wasting disease in deer and elk in North America. Rev Sci Tech 21:305–316. https://doi.org/10.20506/rst.21.2.1340
O’Hara Ruiz M, Kelly AC, Brown WM, Novakofski JE, Mateus-Pinilla NE (2013) Influence of landscape factors and management decisions on spatial and temporal patterns of the transmission of chronic wasting disease transmission in white-tailed deer. Geospat Health 8:215–227. https://doi.org/10.4081/gh.2013.68
Grear DA, Samuel MD, Langenberg JA, Keane D (2006) Demographic patterns and harvest vulnerability of chronic wasting disease infected white-tailed deer in Wisconsin. J Wild Manag 70:546–553
Nalls AV, McNulty E, Powers J, Seelig DM, Hoover C, Haley NJ, Hayes-Klug J, Anderson K, Stewart P, Goldmann W, Hoover EA, Mathiason CK (2013) Mother to offspring transmission of chronic wasting disease in reeves’ muntjac deer. PLoS One 8:e71844. https://doi.org/10.1371/journal.pone.0071844
Pirisinu L, Tran L, Chiappini B, Vanni I, Di Bari MA, Vaccari G, Vikoren T, Madslien KI, Vage J, Spraker T, Mitchell G, Balachandran A, Baron T, Casalone C, Rolandsen CM, Roed KH, Agrimi U, Nonno R, Benestad SL (2018) Novel type of chronic wasting disease detected in moose (Alces alces), Norway. Emerg Infect Dis 24:2210–2218. https://doi.org/10.3201/eid2412.180702
Vikøren T, Våge J, Madslien KI, Roed KH, Rolandsen CM, Tran L, Hopp P, Veiberg V, Heum M, Moldal T, Neves CGD, Handeland K, Ytrehus B, Kolbjornsen O, Wisloff H, Terland R, Saure B, Dessen KM, Svendsen SG, Nordvik BS, Benestad SL (2019) First detection of chronic wasting disease in a wild red deer (Cervus elaphus) in Europe. J Wildl Dis 55:970–972
Miller MW, Conner MM (2005) Epidemiology of chronic wasting disease in free-ranging mule deer: spatial, temporal, and demographic influences on observed prevalence patterns. J Wildl Dis 41:275–290. https://doi.org/10.7589/0090-3558-41.2.275
Dufford D, McDonald P (2018) Illinois Chronic Wasting Disease: 2017–2018 Surveillance and Management Report. Illinois Department of Natural Resources, Springfield, USA.
Williams ES, Young S (1992) Spongiform encephalopathies in Cervidae. Rev Sci Tech 11:551–567. https://doi.org/10.20506/rst.11.2.611
Angers RC, Browning SR, Seward TS, Sigurdson CJ, Miller MW, Hoover EA, Telling GC (2006) Prions in skeletal muscles of deer with chronic wasting disease. Science 311:1117. https://doi.org/10.1126/science.1122864
Koutnik DL (1981) Sex-related differences in the seasonality of agonistic behavior in mule deer. J Mammal 62:1–11
Miller MW, Williams ES (2003) Prion disease: horizontal prion transmission in mule deer. Nature 425:35–36. https://doi.org/10.1038/425035a
Williams ES (2005) Chronic wasting disease. Vet Pathol 42:530–549. https://doi.org/10.1354/vp.42-5-530
Miller MW, Williams ES, McCarty CW, Spraker TR, Kreeger TJ, Larsen CT, Thorne ET (2000) Epizootiology of chronic wasting disease in free-ranging cervids in Colorado and Wyoming. J Wildl Dis 36:676–690. https://doi.org/10.7589/0090-3558-36.4.676
Selariu A, Powers JG, Nalls A, Brandhuber M, Mayfield A, Fullaway S, Wyckoff CA, Goldmann W, Zabel MM, Wild MA, Hoover EA, Mathiason CK (2015) In utero transmission and tissue distribution of chronic wasting disease-associated prions in free-ranging Rocky Mountain elk. J Gen Virol 96:3444–3455. https://doi.org/10.1099/jgv.0.000281
Miller MW, Williams ES (2004) Chronic wasting disease of cervids. Curr Top Microbiol Immunol 284:193–214. https://doi.org/10.1007/978-3-662-08441-0_8
Sigurdson CJ, Barillas-Mury C, Miller MW, Oesch B, van Keulen LJM, Langeveld JPM, Hoover EA (2002) PrPCWD lymphoid cell targets in early and advanced chronic wasting disease of mule deer. J Gen Virol 83:2617–2628. https://doi.org/10.1099/0022-1317-83-10-2617
Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O’Rourke KI, Hoover EA (1999) Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). J Gen Virol 80:2757–2764. https://doi.org/10.1099/0022-1317-80-10-2757
Denkers ND, Hayes-Klug J, Anderson KR, Seelig DM, Haley NJ, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mathiason CK, Hoover EA (2013) Aerosol transmission of chronic wasting disease in white-tailed deer. J Virol 87:1890–1892. https://doi.org/10.1128/JVI.02852-12
Angers RC, Seward TS, Napier D, Green M, Hoover E, Spraker T, O’Rourke K, Balachandran A, Telling GC (2009) Chronic wasting disease prions in elk antler velvet. Emerg Infect Dis 15:696–703. https://doi.org/10.3201/eid1505.081458
Mysterud A, Ytrehus B, Tranulis MA, Rauset GR, Rolandsen CM, Strand O (2020) Antler cannibalism in reindeer. Sci Rep 10:22168. https://doi.org/10.1038/s41598-020-79050-2
Moore SJ, Kunkle R, Greenlee MH, Nicholson E, Richt J, Hamir A, Waters WR, Greenlee J (2016) Horizontal transmission of chronic wasting disease in reindeer. Emerg Infect Dis 22:2142–2145. https://doi.org/10.3201/eid2212.160635
Haley NJ, Mathiason CK, Carver S, Zabel M, Telling GC, Hoover EA (2011) Detection of chronic wasting disease prions in salivary, urinary, and intestinal tissues of deer: potential mechanisms of prion shedding and transmission. J Virol 85:6309–6318. https://doi.org/10.1128/JVI.00425-11
Miller MW, Williams ES, Hobbs NT, Wolfe LL (2004) Environmental sources of prion transmission in mule deer. Emerg Infect Dis 10:1003–1006. https://doi.org/10.3201/eid1006.040010
Kuznetsova A, McKenzie D, Cullingham C, Aiken JM (2020) Long-term incubation PrPCWD with soils affects prion recovery but not infectivity. Pathogens 9:311. https://doi.org/10.3390/pathogens9040311
Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D, Aiken JM (2007) Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathog 3:e93. https://doi.org/10.1371/journal.ppat.0030093
Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA (2006) Prions adhere to soil minerals and remain infectious. PLoS Pathog 2:e32. https://doi.org/10.1371/journal.ppat.0020032
Kuznetsova A, McKenzie D, Banser P, Siddique T, Aiken JM (2014) Potential role of soil properties in the spread of CWD in western Canada. Prion 8:92–99. https://doi.org/10.4161/pri.28467
Plummer IH, Johnson CJ, Chesney AR, Pedersen JA, Samuel MD (2018) Mineral licks as environmental reservoirs of chronic wasting disease prions. PLoS One 13:e0196745. https://doi.org/10.1371/journal.pone.0196745
Pritzkow S, Morales R, Moda F, Khan U, Telling GC, Hoover E, Soto C (2015) Grass plants bind, retain, uptake, and transport infectious prions. Cell Rep 11:1168–1175. https://doi.org/10.1016/j.celrep.2015.04.036
Rasmussen J, Gilroyed BH, Reuter T, Dudas S, Neumann NF, Balachandran A, Kav NN, Graham C, Czub S, McAllister TA (2014) Can plants serve as a vector for prions causing chronic wasting disease? Prion 8:136–142. https://doi.org/10.4161/pri.27963
Sigurdson CJ, Spraker TR, Miller MW, Oesch B, Hoover EA (2001) PrPCWD in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J Gen Virol 82:2327–2334. https://doi.org/10.1099/0022-1317-82-10-2327
Hoover CE, Davenport KA, Henderson DM, Denkers ND, Mathiason CK, Soto C, Zabel MD, Hoover EA (2017) Pathways of prion spread during early chronic wasting disease in deer. J Virol 91:e00077-17. https://doi.org/10.1128/JVI.00077-17
van Keulen LJ, Vromans ME, van Zijderveld FG (2002) Early and late pathogenesis of natural scrapie infection in sheep. APMIS 110:23–32. https://doi.org/10.1034/j.1600-0463.2002.100104.x
Mabbott NA, Mackay F, Minns F, Bruce ME (2000) Temporary inactivation of follicular dendritic cells delays neuroinvasion of scrapie. Nat Med 6:719–720. https://doi.org/10.1038/77401
Heggebo R, Gonzalez L, Press CM, Gunnes G, Espenes A, Jeffrey M (2003) Disease-associated PrP in the enteric nervous system of scrapie-affected Suffolk sheep. J Gen Virol 84:1327–1338. https://doi.org/10.1099/vir.0.18874-0
van Keulen LJ, Vromans ME, Dolstra CH, Bossers A, van Zijderveld FG (2008) Pathogenesis of bovine spongiform encephalopathy in sheep. Arch Virol 153:445–453. https://doi.org/10.1007/s00705-007-0007-4
Siso S, Gonzalez L, Jeffrey M (2010) Neuroinvasion in prion diseases: the roles of ascending neural infection and blood dissemination. Interdiscip Perspect Infect Dis 2010:747892. https://doi.org/10.1155/2010/747892
Mathiason CK, Hayes-Klug J, Hays SA, Powers J, Osborn DA, Dahmes SJ, Miller KV, Warren RJ, Mason GL, Telling GC, Young AJ, Hoover EA (2010) B cells and platelets harbor prion infectivity in the blood of deer infected with chronic wasting disease. J Virol 84:5097–5107. https://doi.org/10.1128/JVI.02169-09
McBride PA, Schulz-Schaeffer WJ, Donaldson M, Bruce M, Diringer H, Kretzschmar HA, Beekes M (2001) Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J Virol 75:9320–9327. https://doi.org/10.1128/JVI.75.19.9320-9327.2001
Kaatz M, Fast C, Ziegler U, Balkema-Buschmann A, Hammerschmidt B, Keller M, Oelschlegel A, McIntyre L, Groschup MH (2012) Spread of classic BSE prions from the gut via the peripheral nervous system to the brain. Am J Pathol 181:515–524. https://doi.org/10.1016/j.ajpath.2012.05.001
Spraker TR, Balachandran A, Zhuang D, O’Rourke KI (2004) Variable patterns of distribution of PrPCWD in the obex and cranial lymphoid tissues of Rocky Mountain elk (Cervus elaphus nelsoni) with subclinical chronic wasting disease. Vet Rec 155:295–302. https://doi.org/10.1136/vr.155.10.295
Race BL, Meade-White KD, Ward A, Jewell J, Miller MW, Williams ES, Chesebro B, Race RE (2007) Levels of abnormal prion protein in deer and elk with chronic wasting disease. Emerg Infect Dis 13:824–830. https://doi.org/10.3201/eid1306.070186
Gonzalez L, Pitarch JL, Martin S, Thurston L, Moore J, Acin C, Jeffrey M (2014) Identical pathogenesis and neuropathological phenotype of scrapie in valine, arginine, glutamine/valine, arginine, glutamine sheep infected experimentally by the oral and conjunctival routes. J Comp Pathol 150:47–56. https://doi.org/10.1016/j.jcpa.2013.06.006
Williams ES, Young S (1993) Neuropathology of chronic wasting disease of mule deer (Odocoileus hemionus) and elk (Cervus elaphus nelsoni). Vet Pathol 30:36–45. https://doi.org/10.1177/030098589303000105
Nalls AV, McNulty E, Hoover CE, Pulscher LA, Hoover EA, Mathiason CK (2017) Infectious prions in the pregnancy microenvironment of chronic wasting disease-infected Reeves’ muntjac deer. J Virol 91:e00501-17. https://doi.org/10.1128/JVI.00501-17
Kramm C, Gomez-Gutierrez R, Soto C, Telling G, Nichols T, Morales R (2019) In vitro detection of Chronic Wasting Disease (CWD) prions in semen and reproductive tissues of white tailed deer bucks (Odocoileus virginianus). PLoS One 14:e0226560. https://doi.org/10.1371/journal.pone.0226560
Andreoletti O, Berthon P, Marc D, Sarradin P, Grosclaude J, van Keulen L, Schelcher F, Elsen JM, Lantier F (2000) Early accumulation of PrPSc in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J Gen Virol 81:3115–3126. https://doi.org/10.1099/0022-1317-81-12-3115
Maddox RA, Person MK, Blevins JE, Abrams JY, Bryant BL, Appleby BS, Schonberger LB, Belay ED Prion disease incidence, United States, 2003–2016. In: Prion, 2019. Taylor & Francis INC 530 Walnut street, ste 850, Philadelphia, PA 19106 USA, pp 39–39
Williams ES, Young S (1982) Spongiform encephalopathy of Rocky Mountain elk. J Wildl Dis 18:465–471. https://doi.org/10.7589/0090-3558-18.4.465
Kahn S, Dube C, Bates L, Balachandran A (2004) Chronic wasting disease in Canada: Part 1. Can Vet J 45:397–404
Go A (2013) CWD in moose in Alberta info sheet. Wild Info Bull 8:1–2
Kim TY, Shon HJ, Joo YS, Mun UK, Kang KS, Lee YS (2005) Additional cases of Chronic Wasting Disease in imported deer in Korea. J Vet Med Sci 67:753–759. https://doi.org/10.1292/jvms.67.753
Hazards EPoB, Koutsoumanis K, Allende A, Alvarez-Ordonez A, Bolton D, Bover-Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Skandamis P, Suffredini E, Andreoletti O, Benestad SL, Comoy E, Nonno R, da Silva FT, Ortiz-Pelaez A, Simmons MM (2019) Update on chronic wasting disease (CWD) III. EFSA J 17:e05863. https://doi.org/10.2903/j.efsa.2019.5863
Nonno R, Di Bari MA, Pirisinu L, D’Agostino C, Vanni I, Chiappini B, Marcon S, Riccardi G, Tran L, Vikoren T, Vage J, Madslien K, Mitchell G, Telling GC, Benestad SL, Agrimi U (2020) Studies in bank voles reveal strain differences between chronic wasting disease prions from Norway and North America. Proc Natl Acad Sci U S A 117:31417–31426. https://doi.org/10.1073/pnas.2013237117
Resources WDoN (2019) CWD prevalence in Wisconsin. . https://dnr.wi.gov/topic/wildlifehabitat/prevalence.html#prevalence. Accessed 13/08/2020
Alberta Go (2020) Chronic Wasting Disease (CWD) surveillance update: May 5, 2020. https://www.alberta.ca/chronic-wasting-disease-updates.aspx. Accessed 13/08/2020
Miller MW, Swanson HM, Wolfe LL, Quartarone FG, Huwer SL, Southwick CH, Lukacs PM (2008) Lions and prions and deer demise. PLoS One 3:e4019. https://doi.org/10.1371/journal.pone.0004019
Monello RJ, Powers JG, Hobbs NT, Spraker TR, Watry MK, Wild MA (2014) Survival and population growth of a free-ranging elk population with a long history of exposure to chronic wasting disease. J Wild Manag 78:214–223
DeVivo MT, Edmunds DR, Kauffman MJ, Schumaker BA, Binfet J, Kreeger TJ, Richards BJ, Schatzl HM, Cornish TE (2017) Endemic chronic wasting disease causes mule deer population decline in Wyoming. PLoS One 12:e0186512. https://doi.org/10.1371/journal.pone.0186512
Hamir AN, Greenlee JJ, Nicholson EM, Kunkle RA, Richt JA, Miller JM, Hall M (2011) Experimental transmission of chronic wasting disease (CWD) from elk and white-tailed deer to fallow deer by intracerebral route: final report. Can J Vet Res 75:152–156
Rhyan JC, Miller MW, Spraker TR, McCollum M, Nol P, Wolfe LL, Davis TR, Creekmore L, O’Rourke KI (2011) Failure of fallow deer (Dama dama) to develop chronic wasting disease when exposed to a contaminated environment and infected mule deer (Odocoileus hemionus). J Wildl Dis 47:739–744. https://doi.org/10.7589/0090-3558-47.3.739
Huor A, Espinosa JC, Vidal E, Cassard H, Douet JY, Lugan S, Aron N, Marin-Moreno A, Lorenzo P, Aguilar-Calvo P, Badiola J, Bolea R, Pumarola M, Benestad SL, Orge L, Thackray AM, Bujdoso R, Torres JM, Andreoletti O (2019) The emergence of classical BSE from atypical/Nor98 scrapie. Proc Natl Acad Sci U S A 116:26853–26862. https://doi.org/10.1073/pnas.1915737116
Hamir AN, Kehrli ME Jr, Kunkle RA, Greenlee JJ, Nicholson EM, Richt JA, Miller JM, Cutlip RC (2011) Experimental interspecies transmission studies of the transmissible spongiform encephalopathies to cattle: comparison to bovine spongiform encephalopathy in cattle. J Vet Diagn Invest 23:407–420. https://doi.org/10.1177/1040638711403404
Hamir AN, Kunkle RA, Cutlip RC, Miller JM, O’Rourke KI, Williams ES, Miller MW, Stack MJ, Chaplin MJ, Richt JA (2005) Experimental transmission of chronic wasting disease agent from mule deer to cattle by the intracerebral route. J Vet Diagn Invest 17:276–281. https://doi.org/10.1177/104063870501700313
Hamir AN, Miller JM, Kunkle RA, Hall SM, Richt JA (2007) Susceptibility of cattle to first-passage intracerebral inoculation with chronic wasting disease agent from white-tailed deer. Vet Pathol 44:487–493. https://doi.org/10.1354/vp.44-4-487
Greenlee JJ, Nicholson EM, Smith JD, Kunkle RA, Hamir AN (2012) Susceptibility of cattle to the agent of chronic wasting disease from elk after intracranial inoculation. J Vet Diagn Invest 24:1087–1093. https://doi.org/10.1177/1040638712461249
Williams ES, O’Toole D, Miller MW, Kreeger TJ, Jewell JE (2018) Cattle (Bos Taurus) Resist chronic wasting disease following oral inoculation challenge or ten years’ natural exposure in contaminated environments. J Wildl Dis 54:460–470. https://doi.org/10.7589/2017-12-299
Hamir AN, Kunkle RA, Cutlip RC, Miller JM, Williams ES, Richt JA (2006) Transmission of chronic wasting disease of mule deer to Suffolk sheep following intracerebral inoculation. J Vet Diagn Invest 18:558–565. https://doi.org/10.1177/104063870601800606
Goldmann W, Hunter N, Foster JD, Salbaum JM, Beyreuther K, Hope J (1990) Two alleles of a neural protein gene linked to scrapie in sheep. Proc Natl Acad Sci U S A 87:2476–2480. https://doi.org/10.1073/pnas.87.7.2476
Westaway D, Zuliani V, Cooper CM, Da Costa M, Neuman S, Jenny AL, Detwiler L, Prusiner SB (1994) Homozygosity for prion protein alleles encoding glutamine-171 renders sheep susceptible to natural scrapie. Genes Dev 8:959–969. https://doi.org/10.1101/gad.8.8.959
Bossers A, Schreuder BE, Muileman IH, Belt PB, Smits MA (1996) PrP genotype contributes to determining survival times of sheep with natural scrapie. J Gen Virol 77:2669–2673. https://doi.org/10.1099/0022-1317-77-10-2669
Tamguney G, Giles K, Bouzamondo-Bernstein E, Bosque PJ, Miller MW, Safar J, DeArmond SJ, Prusiner SB (2006) Transmission of elk and deer prions to transgenic mice. J Virol 80:9104–9114. https://doi.org/10.1128/JVI.00098-06
Madsen-Bouterse SA, Schneider DA, Zhuang D, Dassanayake RP, Balachandran A, Mitchell GB, O’Rourke KI (2016) Primary transmission of chronic wasting disease versus scrapie prions from small ruminants to transgenic mice expressing ovine or cervid prion protein. J Gen Virol 97:2451–2460. https://doi.org/10.1099/jgv.0.000539
Moore SJ, West Greenlee MH, Kondru N, Manne S, Smith JD, Kunkle RA, Kanthasamy A, Greenlee JJ (2017) Experimental transmission of the chronic wasting disease agent to swine after oral or intracranial inoculation. J Virol 91:e00926-17. https://doi.org/10.1128/JVI.00926-17
Cullingham CI, Peery RM, Dao A, McKenzie DI, Coltman DW (2020) Predicting the spread-risk potential of chronic wasting disease to sympatric ungulate species. Prion 14:56–66. https://doi.org/10.1080/19336896.2020.1720486
Heisey DM, Mickelsen NA, Schneider JR, Johnson CJ, Johnson CJ, Langenberg JA, Bochsler PN, Keane DP, Barr DJ (2010) Chronic wasting disease (CWD) susceptibility of several North American rodents that are sympatric with cervid CWD epidemics. J Virol 84:210–215. https://doi.org/10.1128/JVI.00560-09
Raymond GJ, Raymond LD, Meade-White KD, Hughson AG, Favara C, Gardner D, Williams ES, Miller MW, Race RE, Caughey B (2007) Transmission and adaptation of chronic wasting disease to hamsters and transgenic mice: evidence for strains. J Virol 81:4305–4314. https://doi.org/10.1128/JVI.02474-06
Di Bari MA, Nonno R, Castilla J, D’Agostino C, Pirisinu L, Riccardi G, Conte M, Richt J, Kunkle R, Langeveld J, Vaccari G, Agrimi U (2013) Chronic wasting disease in bank voles: characterisation of the shortest incubation time model for prion diseases. PLoS Pathog 9:e1003219. https://doi.org/10.1371/journal.ppat.1003219
Bartz JC, Marsh RF, McKenzie DI, Aiken JM (1998) The host range of chronic wasting disease is altered on passage in ferrets. Virology 251:297–301. https://doi.org/10.1006/viro.1998.9427
Sigurdson CJ, Mathiason CK, Perrott MR, Eliason GA, Spraker TR, Glatzel M, Manco G, Bartz JC, Miller MW, Hoover EA (2008) Experimental chronic wasting disease (CWD) in the ferret. J Comp Pathol 138:189–196. https://doi.org/10.1016/j.jcpa.2008.01.004
Perrott MR, Sigurdson CJ, Mason GL, Hoover EA (2012) Evidence for distinct chronic wasting disease (CWD) strains in experimental CWD in ferrets. J Gen Virol 93:212–221. https://doi.org/10.1099/vir.0.035006-0
Harrington RD, Baszler TV, O’Rourke KI, Schneider DA, Spraker TR, Liggitt HD, Knowles DP (2008) A species barrier limits transmission of chronic wasting disease to mink (Mustela vison). J Gen Virol 89:1086–1096. https://doi.org/10.1099/vir.0.83422-0
Mathiason CK, Nalls AV, Seelig DM, Kraft SL, Carnes K, Anderson KR, Hayes-Klug J, Hoover EA (2013) Susceptibility of domestic cats to chronic wasting disease. J Virol 87:1947–1956. https://doi.org/10.1128/JVI.02592-12
Stewart P, Campbell L, Skogtvedt S, Griffin KA, Arnemo JM, Tryland M, Girling S, Miller MW, Tranulis MA, Goldmann W (2012) Genetic predictions of prion disease susceptibility in carnivore species based on variability of the prion gene coding region. PLoS One 7:e50623. https://doi.org/10.1371/journal.pone.0050623
Krumm CE, Conner MM, Hobbs NT, Hunter DO, Miller MW (2010) Mountain lions prey selectively on prion-infected mule deer. Biol Lett 6:209–211. https://doi.org/10.1098/rsbl.2009.0742
Moore SJ, Smith JD, Richt JA, Greenlee JJ (2019) Raccoons accumulate PrPSc after intracranial inoculation of the agents of chronic wasting disease or transmissible mink encephalopathy but not atypical scrapie. J Vet Diagn Invest 31:200–209. https://doi.org/10.1177/1040638718825290
Hamir AN, Miller JM, Cutlip RC, Stack MJ, Chaplin MJ, Jenny AL, Williams ES (2003) Experimental inoculation of scrapie and chronic wasting disease agents in raccoons (Procyon lotor). Vet Rec 153:121–123. https://doi.org/10.1136/vr.153.4.121
Hamir AN, Kunkle RA, Miller JM, Cutlip RC, Richt JA, Kehrli ME Jr, Williams ES (2007) Age-related lesions in laboratory-confined raccoons (Procyon lotor) inoculated with the agent of chronic wasting disease of mule deer. J Vet Diagn Invest 19:680–686. https://doi.org/10.1177/104063870701900610
Vidal E, Fernandez-Borges N, Erana H, Parra B, Pintado B, Sanchez-Martin MA, Charco JM, Ordonez M, Perez-Castro MA, Pumarola M, Mathiason CK, Mayoral T, Castilla J (2020) Dogs are resistant to prion infection, due to the presence of aspartic or glutamic acid at position 163 of their prion protein. FASEB J 34:3969–3982. https://doi.org/10.1096/fj.201902646R
Otero A, Bolea R, Hedman C, Fernandez-Borges N, Marin B, Lopez-Perez O, Barrio T, Erana H, Sanchez-Martin MA, Monzon M, Badiola JJ, Castilla J (2017) An amino acid substitution found in animals with low susceptibility to prion diseases confers a protective dominant-negative effect in prion-infected transgenic mice. Mol Neurobiol 55:6182–6192. https://doi.org/10.1007/s12035-017-0832-8
Otero A, Hedman C, Fernandez-Borges N, Erana H, Marin B, Monzon M, Sanchez-Martin MA, Nonno R, Badiola JJ, Bolea R, Castilla J (2019) A single amino acid substitution, found in mammals with low susceptibility to prion diseases, delays propagation of two prion strains in highly susceptible transgenic mouse models. Mol Neurobiol 56:6501–6511. https://doi.org/10.1007/s12035-019-1535-0
Nichols TA, Fischer JW, Spraker TR, Kong Q, VerCauteren KC (2015) CWD prions remain infectious after passage through the digestive system of coyotes (Canis latrans). Prion 9:367–375. https://doi.org/10.1080/19336896.2015.1086061
Kong Q, Huang S, Zou W, Vanegas D, Wang M, Wu D, Yuan J, Zheng M, Bai H, Deng H, Chen K, Jenny AL, O’Rourke K, Belay ED, Schonberger LB, Petersen RB, Sy MS, Chen SG, Gambetti P (2005) Chronic wasting disease of elk: transmissibility to humans examined by transgenic mouse models. J Neurosci 25:7944–7949. https://doi.org/10.1523/JNEUROSCI.2467-05.2005
Race B, Meade-White K, Race R, Chesebro B (2009) Prion infectivity in fat of deer with chronic wasting disease. J Virol 83:9608–9610. https://doi.org/10.1128/JVI.01127-09
Wild MA, Hobbs NT, Graham MS, Miller MW (2011) The role of predation in disease control: a comparison of selective and nonselective removal on prion disease dynamics in deer. J Wildl Dis 47:78–93. https://doi.org/10.7589/0090-3558-47.1.78
Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock CJ (1997) Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 389:498–501. https://doi.org/10.1038/39057
MaWhinney S, Pape WJ, Forster JE, Anderson CA, Bosque P, Miller MW (2006) Human prion disease and relative risk associated with chronic wasting disease. Emerg Infect Dis 12:1527–1535
Abrams J, Maddox R, Schonberger L, Person M, Appleby B, Belay E (2018) Human prion disease mortality rates by occurrence of chronic wasting disease in free-ranging cervids, United States. Prion 14:182–183
Waddell L, Greig J, Mascarenhas M, Otten A, Corrin T, Hierlihy K (2018) Current evidence on the transmissibility of chronic wasting disease prions to humans—a systematic review. Transbound Emerg Dis 65:37–49. https://doi.org/10.1111/tbed.12612
Marsh RF, Kincaid AE, Bessen RA, Bartz JC (2005) Interspecies transmission of chronic wasting disease prions to squirrel monkeys (Saimiri sciureus). J Virol 79:13794–13796. https://doi.org/10.1128/JVI.79.21.13794-13796.2005
Race B, Meade-White KD, Miller MW, Barbian KD, Rubenstein R, LaFauci G, Cervenakova L, Favara C, Gardner D, Long D, Parnell M, Striebel J, Priola SA, Ward A, Williams ES, Race R, Chesebro B (2009) Susceptibilities of nonhuman primates to chronic wasting disease. Emerg Infect Dis 15:1366–1376. https://doi.org/10.3201/eid1509.090253
Race B, Williams K, Orru CD, Hughson AG, Lubke L, Chesebro B (2018) Lack of transmission of chronic wasting disease to cynomolgus macaques. J Virol 92:e00550-18. https://doi.org/10.1128/JVI.00550-18
Czub S, Schulz-Schaeffer WJ, Stahl‐Hennig C, Beekes M, Schaetzl H, Motzkus D (2017) First evidence of intracranial and peroral transmission of Chronic Wasting Disease (CWD) into Cynomolgus macaques: a work in progress. Prion Conference Book of Abstracts. Paper presented at the PRION 2017, Edinburgh
Schatzl HM, Da Costa M, Taylor L, Cohen FE, Prusiner SB (1997) Prion protein gene variation among primates. J Mol Biol 265:257. https://doi.org/10.1006/jmbi.1996.0791
Kurt TD, Bett C, Fernandez-Borges N, Joshi-Barr S, Hornemann S, Rulicke T, Castilla J, Wuthrich K, Aguzzi A, Sigurdson CJ (2014) Prion transmission prevented by modifying the beta2-alpha2 loop structure of host PrPC. J Neurosci 34:1022–1027. https://doi.org/10.1523/JNEUROSCI.4636-13.2014
Kurt TD, Jiang L, Fernandez-Borges N, Bett C, Liu J, Yang T, Spraker TR, Castilla J, Eisenberg D, Kong Q, Sigurdson CJ (2015) Human prion protein sequence elements impede cross-species chronic wasting disease transmission. J Clin Invest 125:2548. https://doi.org/10.1172/JCI82647
Martin D, Reine F, Herzog L, Igel-Egalon A, Aron N, Michel C, Moudjou M, Fichet G, Quadrio I, Perret-Liaudet A, Andreoletti O, Rezaei H, Beringue V (2021) Prion potentiation after life-long dormancy in mice devoid of PrP. Brain Commun 3:fcab092. https://doi.org/10.1093/braincomms/fcab092
Race B, Williams K, Chesebro B (2019) Transmission studies of chronic wasting disease to transgenic mice overexpressing human prion protein using the RT-QuIC assay. Vet Res 50:6. https://doi.org/10.1186/s13567-019-0626-2
Raymond GJ, Bossers A, Raymond LD, O’Rourke KI, McHolland LE, Bryant PK 3rd, Miller MW, Williams ES, Smits M, Caughey B (2000) Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO J 19:4425–4430. https://doi.org/10.1093/emboj/19.17.4425
Davenport K, Henderson D, Mathiason C, Hoover E (2014) P. 28: modeling prion species barriers and the new host effect using RT-QuIC. Prion 8:36–37
Barria MA, Balachandran A, Morita M, Kitamoto T, Barron R, Manson J, Knight R, Ironside JW, Head MW (2014) Molecular barriers to zoonotic transmission of prions. Emerg Infect Dis 20:88–97. https://doi.org/10.3201/eid2001.130858
Barria MA, Libori A, Mitchell G, Head MW (2018) Susceptibility of human prion protein to conversion by chronic wasting disease prions. Emerg Infect Dis 24:1482–1489. https://doi.org/10.3201/eid2408.161888
Barria MA, Telling GC, Gambetti P, Mastrianni JA, Soto C (2011) Generation of a new form of human PrPSc in vitro by interspecies transmission from cervid prions. J Biol Chem 286:7490–7495. https://doi.org/10.1074/jbc.M110.198465
Schwabenlander MD, Culhane MR, Hall SM, Goyal SM, Anderson PL, Carstensen M, Wells SJ, Slade WB, Armien AG (2013) A case of chronic wasting disease in a captive red deer (Cervus elaphus). J Vet Diagn Invest 25:573–576. https://doi.org/10.1177/1040638713499914
Mitchell G, Yogasingam N, Walther I, Balachandran A (2015) Experimental transmission of chronic wasting disease to sheep and goats. In: Prion, 2015. Taylor & Francis INC 530 Walnut Street, Ste 850, Philadelphia, PA 19106 USA, pp S48–S48
Acknowledgements
We acknowledge funding for this research to Dr Aiken and Dr McKenzie from Genome Canada, Alberta Prion Research Institute and Alberta Agriculture and Forestry through Genome Alberta and the University of Alberta in support of the Systems Biology and Molecular Ecology of Chronic Wasting Disease project. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. We would also like to thank all members of the Aiken-McKenzie lab for their constructive comments about our manuscript.
Author information
Authors and Affiliations
Contributions
AO and CDV wrote the original draft. AO, CDV, JA and DM revised the manuscript and created the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Otero, A., Velásquez, C.D., Aiken, J. et al. Chronic wasting disease: a cervid prion infection looming to spillover. Vet Res 52, 115 (2021). https://doi.org/10.1186/s13567-021-00986-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-021-00986-y