Abstract
Infectious bronchitis virus (IBV) is a pathogenic coronavirus with high morbidity and mortality in chicken breeding. Macrophages with normal biofunctions are essential for host immune responses. In this study, the HD11 chicken macrophage cell line and chicken peripheral blood mononuclear cell-derived macrophages (PBMCs-Mφ) were infected with IBV at multiplicity of infection (MOI) of 10. The dynamic changes of their biofunctions, including cell viability, pathogen elimination function, phagocytic ability, and gene expressions of related proteins/mediators in innate and acquired immunity, inflammation, autophagy and apoptosis were analyzed. Results showed that IBV infection decreased chicken macrophage viability and phagocytic ability, and increased pathogen elimination function. Moreover, IBV augmented the gene expressions of most related proteins in macrophages involved in multiple host bioprocesses, and the dynamic changes of gene expressions had a close relationship with virus replication. Among them, MHCII, Fc receptor, TLR3, IFN-α, CCL4, MIF, IL-1β, IL-6, and iNOS showed significantly higher expressions in IBV-infected cells. However, TLR7, MyD88, MDA5, IFN-γ, MHCII, Fc receptor, MARCO, CD36, MIF, XCL1, CXCL12, TNF-α, iNOS, and IL-10 showed early decreased expressions. Overall, chicken macrophages play an important role in host innate and acquired immune responses to resist IBV infection, despite early damage or suppression. Moreover, the IBV-induced autophagy and apoptosis might participate in the virus-host cell interaction which is attributed to the biological process.
Similar content being viewed by others
Introduction
Infectious bronchitis (IB) is a highly contagious respiratory disease in chickens with worldwide distribution and economic significance [1]. IB is caused by infectious bronchitis virus (IBV) which is a single-stranded, positive-sense enveloped RNA virus of the Coronavirus family [2]. IBV was first described in the 1930s in the USA [3]. The IBV Massachusetts 41 (M41) strain was subsequently isolated and assigned to the Massachusetts serotype [4, 5], which is frequently used in experimental and clinical research [6, 7]. IBV is liable to mutate and recombine, which gives rise to multiple serotypes [2, 8]. Generally, the available commercial IB vaccines may not trigger powerful immune responses, provide reciprocal protection among different serotypes of IBV infections, and lead to huge economic losses [9]. Therefore, it is necessary to understand the mechanism of host immune responses to IBV infection.
Macrophages are important innate immune effectors against microbial infections and participate in innate immune responses and the subsequent acquired immunity [10]. Pattern recognition receptors (PRRs) in macrophages can recognize the pathogen-associated molecular pattern (PAMP) [11]. Various intracellular signals are triggered subsequently to promote the production of immunomodulation molecules [12, 13] and activate the processing pathways of antigen presentation in macrophages to switch on acquired immune responses [14]. Also, macrophage-programmed cell death-related genes changed in defense against pathogens [6, 15]. It has been shown that IBV could significantly increase the number of macrophages in chicken respiratory tracts [16]. However, the immune function and immune regulation of macrophages in IBV infection remain mostly unclear. In this study, HD11 chicken macrophage cells and chicken peripheral blood mononuclear cell-derived macrophages (PBMCs-Mφ) were infected with IBV M41 strain, and the dynamic changes of macrophage functions and gene expressions of related proteins/mediators in innate and acquired immunity, inflammation, autophagy and apoptosis were systematically analyzed.
Materials and methods
Cells and virus
HD11 cells were kindly provided by Dr. Yulong Gao (Harbin Veterinary Research Institute, Chinese Academy of Agricultural Science). HD11 cells were cultured in RPMI 1640 complete medium at 1.5 × 105 cells/mL and were kept at 41 °C in 5% CO2. PBMCs-Mφ were separated from chicken blood based on a previous method with minor modifications [17]. In brief, pooled whole blood in 10 U/mL heparins (Ncbiotech Co., Ltd, Harbin, China) was collected from 3-month-old specific-pathogen-free (SPF) chickens by heart punctures. PBMCs were separated by Ficoll-Hypaque (Tianjin Hao Yang Biological Manufacture Co., Ltd, Tianjin, China) density gradient centrifugation according to the manufacturer’s instructions. After being cleaned with 1 × PBS (pH 7.2), PBMCs were seeded in 2 × 107 cells/mL in RPMI 1640 complete medium for 24 h and incubated at 41 °C in 5% CO2. Finally, nonadherent cells in the supernatant were removed. The procedures of the experiment complied with the Northeast Agriculture University Health Guidelines for the Care and Use of Laboratory Animals.
IBV strain M41 (Accession number: DQ834384.1) was preserved in the Veterinary Pathology Laboratory, College of Veterinary Medicine in Northeast Agricultural University. The virus was propagated in 9–11-day-old SPF chicken embryos.
KUL01 + cell assay
The purity of HD11 cells and PBMCs-Mφ were evaluated with flow cytometry via staining of chicken macrophage marker, KUL01 [18]. PBMCs-Mφ and HD11 cells were dispersed with 1% trypsin into a single-cell solution and fixed with 1% paraformaldehyde for 30 min. The cells were incubated with mouse anti-chicken monocyte/macrophage antibodies KUL01 (1: 50, Bio-Rad, USA) for 30 min. After being washed 3 times with PBS, FITC labeled goat anti-mouse IgG (1: 50, ZSGB-Biotechnology Co., Ltd, Beijing, China) was added and incubated in the dark for 30 min. Washed cells were assayed by flow cytometry (BD FACSAria II, USA).
IBV infection in macrophages
HD11 cells and PBMCs-Mφ were seeded in 6-well plates and grown to 80% confluence. Macrophages were infected with IBV at a multiplicity of infection (MOI) of 10. After being incubated for 2 h at 41 °C, the virus was replaced with RPMI 1640 supplemented with 2% FBS. Cytopathic effects (CPE) were observed daily. Virus titers were determined by endpoint dilutions as 50% tissue culture infective dose (TCID50) at 6, 12, 18, 24, 30, 36, 42 and 48 h post-infection (hpi) by the Reed-Muench method, as described previously [19].
Quantitative real-time polymerase chain reaction (qRT-PCR) was also carried out to detect IBV. Total RNA was extracted at 12, 24, 36, 48 hpi using TRIzol reagent (Invitrogen, Shanghai, China) according to the manufacturer’s instructions. Total RNA of 1 μg in each sample was reverse transcribed into cDNA using PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time) (Takara Biomedical Technology Co., Ltd, Beijing, China). IBV copies were measured by IBV N gene copies using fluorescence quantitative PCR reagent (Bioteke Corporation, Beijing, China). The primers were designed according to the IBV N gene (Accession number: FJ904723.1) and are shown in Table 1. The steps for thermal cycling were as follows: 94 °C, 2 min for denaturation and 40 cycles of PCR (94 °C, 15 s; 60 °C, 30 s).
Cell viability assay
A CCK-8 reagent (MCE, USA) was used to detect cell viability. UV-IBV was prepared by inactivating IBV M41 with UV germicidal light for 30 min. Cells were seeded into 96-well plates and infected with 10 MOI IBV, or incubated with UV-IBV (same amount of virion to 10 MOI IBV), or PBS (Mock cells) in RPMI 1640 supplemented with 2% FBS. CCK-8 reagent was added to the cells at 12, 24, 36, and 48 hpi, and they were incubated for 2.5 h at 41 °C. The optical density (OD) values were measured with a microplate reader (ELx808, BioTek, USA) at 450 nm, as previously described [20]. Cell viability was calculated according to the formula in the manufacturer’s instructions: Cell viability (%) = [optical density (OD) of Mock or IBV-infected or UV-IBV-treated cells-OD of medium]/[OD of Mock cells-OD of medium] × 100.
Nitric oxide assay
Cells were seeded into 6-well plates. Supernatants of Mock cells, IBV-infected cells, and UV-IBV-treated cells were collected at 12, 24, 36, and 48 hpi and centrifuged at 1000 g for 30 min at 4 °C. Nitric oxide (NO) content in the supernatant was detected by a NO assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), as previously described [21].
Macrophage phagocytic ability assay
Phagocytic functions of macrophages were assayed according to the method of Lee et al. [17]. Briefly, yellow-green fluorescent latex beads (50 beads/cell, Sigma, USA) were used to incubate with Mock cells, UV-IBV-treated cells and IBV-infected cells at 24 hpi at 41 °C for 2 h. Cells were then separated by 1% trypsin for macrophage phagocytic ability assay using flow cytometry. At the meantime, cells in these 3 groups were fixed with methanol for 10 min, and stained with 0.01 mg/mL propidium iodide solution for 20 min for observation of phagocytic function under fluorescence microscopy (Nikon, Japan).
QRT-PCR assay of gene expression
Total RNA of cells was extracted and reverse transcribed into cDNA using PrimeScript™ RT reagent Kit with gDNA Eraser at 12, 24, 36, and 48 hpi. QRT-PCR reagents were used to detect the relative gene expressions of related factors in innate immunity (TLR3, TLR7, MyD88, MDA5, IFN-α, IFN-β, IFN-γ), acquired immunity (MHCI, MHCII, Fc receptor, MARCO, CD36), chemokines (MIF, CCL4, K60, XCL1, CXCL12), inflammation (IL-1β, IL-6, TNF-α, NF-κB, iNOS, IL-10, PPAR-γ), autophagy (LC3II, mTOR, Beclin-1) and apoptosis (Caspase-3, Bax, Bcl-2). The primers are listed in Table 1. β-actin was chosen as a reference gene. The steps for thermal cycling were as follows: 94 °C, 2 min for denaturation and 40 cycles of PCR (94 °C, 15 s; 60 °C, 30 s). The expression fold changes were calculated using the 2−△△CT method [22].
Statistical analyses
Each treatment was analyzed in triplicate, values were expressed as mean ± SD and statistical significances were assessed by Duncan multiple range test of SPSS 19.0 software using the one-way ANOVA method. Probabilities of p < 0.05 and p < 0.01 were preset for statistical significance. Flow cytometry analyses were performed on FlowJo software (version 10.0.6). Figures were created with Image J and GraphPad Prism (version 5.0) software.
Results
KUL01 + cell percentages of HD11 cells and PBMCs-Mφ
Both HD11 cells and PBMCs-Mφ showed high positive fluorescent signals (Figure 1A). The percentages of KUL01 + cells in HD11 cells and PBMCs-Mφ were 99.8 ± 0.1% and 91.3 ± 1.2%, respectively (Figure 1B), indicating these two kinds of macrophage satisfied the needs of the experiments.
IBV M41 infection in macrophages
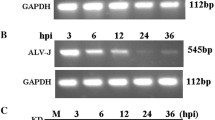
HD11 cells and PBMCs-Mφ were infected with IBV at an MOI of 10. CPE, qRT-PCR and virus titer assays were carried out. After 5 times of adaptive cell culture of IBV M41 strain in both kinds of cell, the typical CPE appeared in macrophages at 24 hpi, and peaked at 36 hpi (Figure 2A). QRT-PCR and TCID50 results showed IBV replication increased in a time-dependent manner, while there was a decrease at 48 hpi (Figures 2B, C). The results indicate IBV M41 could infect HD11 cells and PBMCs-Mφ.
IBV M41 infection in macrophages. Macrophages infected with IBV at MOI of 10. A CPE observation. CPE results of IBV-infected HD11 cells and PBMCs-Mφ were recorded at 0, 12, 24, 36, 48 hpi. B IBV growth curves. Virus titers in IBV-infected HD11 cells and PBMCs-Mφ from 6 to 48 hpi were tested by TCID50 method. C IBV copies. Virus copies of IBV-infected HD11 cells and PBMCs-Mφ from 12 to 48 hpi were tested by qRT-PCR method. IBV mRNA copies were calculated by the formula: y = − 2.8207x + 40.827 (R2 = 0.991). Data presented as means ± SD (n = 3).
IBV M41 decreased macrophage viability
Cell viability was tested. CCK-8 assay results showed that cell viability of IBV-infected macrophages decreased in a time-dependent manner (p < 0.01), while there were no significant changes in Mock cells and UV-IBV-treated cells (Figure 3A).
Macrophages activity assays. A CCK-8 assay. CCK-8 was used to detect the viability of HD11 cells and PBMCs-Mφ infected with IBV or treated with UV-IBV or PBS (Mock cells) from 12 to 48 hpi. B NO content assay. NO content of culture supernatants of IBV-infected, UV-IBV-treated or Mock cells were measured from 12 to 48 hpi. C, D Macrophage phagocytic function assay by flow cytometry. Phagocytic functions of chicken macrophages were detected at 24 hpi by phagocytosis of yellow-green fluorescent beads. Flow cytometry histogram shows fluorescent intensity of cells containing yellow-green fluorescent beads (green arrow) and cells not containing yellow-green fluorescent beads in three groups (C). The mean percentages of positive cells post-infection are shown in histograms (D). Data presented as means ± SD (n = 3). E Macrophage phagocytic function observation under fluorescent microscopy. Beads (green arrow) and nucleus (red) were observed under the inverted fluorescence microscope. Data presented as mean ± SD (n = 3). * means the significance of between IBV-infected or UV-IBV-treated cells with Mock cells. * means p < 0.05, ** means p < 0.01.
IBV M41 activated macrophage pathogen elimination function
NO content was examined to determine the pathogen elimination function of macrophages. NO secreted by macrophages is a key aspect of the antimicrobial response [23]. NO content increased significantly in IBV-infected (HD11/PBMCs-Mφ: p < 0.01) and UV-IBV-treated (HD11: p < 0.01, PBMCs-Mφ: p < 0.05) cell supernatants at 36 hpi compared with Mock cells (Figure 3B). The up-regulated NO content of UV-IBV-treated cells was lower than that of IBV-infected cells, indicating that IBV replication stimulates NO production in chicken macrophages.
IBV M41 affected macrophage phagocytic function
Phagocytic function of macrophages was determined by detecting the percentage of macrophages phagocytizing yellow-green fluorescent latex beads by flow cytometry and fluorescence microscopy. Flow cytometry results showed less yellow-green fluorescence in IBV-infected cells compared with Mock cells at 24 hpi, HD11 cells (Mock: 38.8%, IBV: 30.0%; p < 0.01) and PBMCs-Mφ (Mock: 71.5%, IBV: 38.4%; p < 0.01). There were no significant changes in UV-IBV-treated cells (Figure 3C, D). Fluorescence microscopy results were consistent with those of the flow cytometry assays (Figure 3E). These indicate that IBV infection could significantly damage the phagocytic functions of macrophages.
IBV M41 activated macrophage innate immunity
Toll-like receptors (TLRs) are PRRs that detect characteristic microbial motifs to signal the presence of invading microbial organisms [24]. Among them, TLR3 and TLR7 recognize viral or non-viral RNA [25]. Myeloid differentiation factor 88 (MyD88) is a key linker molecule in the TLRs signaling pathway [26]. As is known, TLR3/7-MyD88 are classic pathways involved in host recognition of viruses and innate immunity. In IBV-infected HD11 cells, the gene expressions of TLR7 and MyD88 both decreased at 12 hpi (p < 0.01). As the virus replicated, the gene expressions of TLR3/7 and MyD88 showed obvious upward trends (p < 0.01) and peaked at 36 hpi. The general expression trends of TLR3/7 and MyD88 in PBMCs-Mφ were consistent with those of HD11 cells. In UV-IBV-treated HD11 cells, the gene expression of TLR3 decreased significantly at 12 hpi (p < 0.01), and MyD88 at 36 and 48 hpi (p < 0.01). In UV-IBV-treated PBMCs-Mφ, TLR7 and MyD88 gene expressions decreased at 12 hpi (p < 0.01). TLR3 expression decreased at 24 and 48 hpi (p < 0.01), and increased at 36 hpi (p < 0.01). However, the TLR3 up-regulation in UV-IBV-treated PBMCs-Mφ was lower than that of IBV-infected cells (Figure 4A). These results indicate that viral replication is involved in IBV-induced TLR3/7-MyD88 modulation. By comparing the increasing mRNA expressions of TLR3 (HD11: sevenfold, PBMCs-Mφ: 33-fold) and TLR7 (HD11: fourfold, PBMCs-Mφ: 22-fold) at 36 hpi in IBV-infected cells, it can be concluded that IBV infection activates the TLR3/7 signaling pathways, especially the TLR3 signaling pathway.
Antiviral innate and acquired immunity-related gene expressions. A Antiviral innate immunity-related factors mRNA expressions. Relative mRNA expressions of TLRs (TLR3, TLR7), MyD88, MDA5, IFNs (IFN-α, IFN-β, IFN-γ) in IBV-infected, UV-IBV-treated cells and Mock cells were detected by qRT-PCR method. β-actin acted as a reference gene. B Acquired immunity-related gene mRNA expressions. Relative mRNA expressions of MHC molecules (MHCI, MHCII), Fc receptor, MARCO, CD36 in IBV-infected, UV-IBV-treated cells and Mock cells were detected by qRT-PCR method. β-actin acted as a reference gene. Data presented as mean ± SD (n = 3). * means the significance of between IBV-infected or UV-IBV-treated cells with Mock cells. * means p < 0.05, ** means p < 0.01.
Cytoplasmic retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) identify viral RNAs and initiate innate immune responses [27]. Chickens do not have RIG-I [28]. Therefore, the other RLR member, melanoma differentiation associated protein 5 (MDA5), plays an important role in recognizing intracellular viruses in chickens [29]. In IBV-infected HD11 cells, MDA5 expression decreased at 12 hpi (p < 0.01), then increased at 24, 36 and 48 hpi (24 and 36 hpi: p < 0.01, 48 hpi: p < 0.05). The trend of MDA5 expression in PBMCs-Mφ was consistent with that of HD11 cells, which increased at 12, 24 and 36 hpi (p < 0.01), and peaked at 24 hpi. In UV-IBV-treated HD11 cells and PBMCs-Mφ, the gene expression of MDA5 decreased at 12 and 24 hpi (p < 0.01, Figure 4A).
These results suggest that IBV activates antiviral innate immunity pathways in macrophages. However, immunosuppression might occur at the early stage of IBV infection.
IBV M41 upregulated IFN expressions in macrophages
IFNs, including type I (IFN-α and IFN-β) and type II (IFN-γ), play important roles in antiviral defense, inhibit viral replication and work as immune effectors/modulators [30, 31]. In IBV-infected HD11 cells, the expressions of IFN-α and IFN-β increased at 12 hpi (p < 0.01), while the expression of IFN-γ decreased (p < 0.01). As the virus replicated, IFNs expressions showed upward trends, increasing at 24, 36, and 48 hpi (p < 0.01), and peaking at 36 hpi. The IFNs expression trends of PBMCs-Mφ were consistent with those of HD11 cells. In UV-IBV-treated HD11 cells, the expression of IFN-α decreased at 36 and 48 hpi (p < 0.01), IFN-β decreased at 12, 24 and 36 hpi (p < 0.01), and IFN-γ decreased at 24 hpi (p < 0.01). In UV-IBV-treated PBMCs-Mφ, IFN-α and IFN-γ expressions decreased at 36 and 24 hpi, respectively (p < 0.01). However, IFN-β expression increased at 36 hpi (p < 0.01, Figure 4A).
These results indicate that IBV could activate IFNs production in macrophages, which has a relationship with viral replication. Comparing the increasing expressions of IFNs, IFN-α up-regulations (HD11: 490-fold, PBMCs-Mφ: 49-fold) were the highest.
IBV M41 activated macrophage acquired immunity
Major histocompatibility complex (MHC) is a family of cell surface molecules, including MHC class I and II, which is involved in antigen presentation and activation of T cells [32]. Fc receptor is involved in receptor-mediated antibody-dependent internalization of immune complexes destined for intracellular degradation [33]. In IBV-infected HD11 cells, MHCII expression decreased at 12 hpi (p < 0.05). As the virus replicated, elevated expressions of MHC molecules and Fc receptors appeared at 24 and 36 hpi (p < 0.01), and peaked at 36 hpi. The expression trends of MHC molecules and Fc receptor in PBMC-Mφ were consistent with those of HD11 cells. In IBV-infected PBMCs-Mφ, MHC molecules and Fc receptor expressions were up-regulated, but MHCII expression decreased at 24 hpi (p < 0.01). In the UV-IBV-treated HD11 cells, only MHCII expression increased at 12 hpi (p < 0.01, Figure 4B).
Scavenger receptors and mannose receptors, parts of PRRs, are also involved in pathogen elimination. CD36 is a scavenger receptor involved in immunity, metabolism and angiogenesis [34]. Mannose receptor, MARCO, plays a role in pathogen clearance and inflammatory ligand recognition [35]. In IBV-infected HD11 cells, the expressions of MARCO decreased at 12 hpi significantly (p < 0.01), and CD36 at 12, and 24 hpi (p < 0.01). Increased expressions of both genes appeared and peaked at 36 hpi (p < 0.01). The expression trends of MARCO and CD36 in PBMC-Mφ were similar to those in HD11 cells. Both MARCO and CD36 expressions down-regulated at 12 hpi (p < 0.01), and then increased and peaked at 36 hpi (p < 0.01). For the UV-IBV-treated HD11 cells, there were no distinct changes in the two kinds of cell (Figure 4B).
These results indicate that IBV inhibits the ability of macrophages to eliminate pathogen and antigen presentation at the early stage of viral infection, but do not affect the latter activation of acquired immunity. Among these genes, the expression of MHCII was the highest (23-fold) compared with other genes in IBV-infected HD11 cells, and MHCII (26-fold) and Fc receptor (67-fold) in IBV-infected PBMCs-Mφ, showing that IBV infection mainly activates MHCII and Fc receptors.
IBV M41 activated macrophage chemokine expressions
Chemokines are involved in host immune responses and inflammatory processes [36, 37]. Macrophage migration inhibitory factor (MIF), CCL4 (also known as macrophage inflammatory protein-1β) and CXC K60, that can activate macrophages and T cells, are involved in cell migration, and promote cell maturation [38,39,40]. In IBV-infected HD11 cells, the gene expressions of MIF, CCL4 and CXC K60 up-regulated significantly (p < 0.01), and peaked at 24 hpi, but there was a marked decrease of MIF expression at 12 hpi (p < 0.01). The expression trends in PBMCs-Mφ were similar to those in HD11 cells. In UV-IBV-treated HD11 cells, CCL4 expression decreased at 12 hpi (p < 0.01) and CXC K60 decreased at 12 (p < 0.01) and 36 hpi (p < 0.05). In UV-IBV-treated PBMCs-Mφ, the decreased expression of MIF showed at 36 hpi (p < 0.01), CCL4 decreased at 24 and 36 hpi (p < 0.01), and CXCK60 decreased at 36 hpi (p < 0.01, Figure 5A).
Chemokines and inflammatory factors gene expressions. A Chemokine mRNA expressions. Relative mRNA expressions of MIF, CCL4, CXC K60, XCL-1, CXCL12 in IBV-infected, UV-IBV-treated cells and Mock cells were detected by qRT-PCR method. β-actin acted as a reference gene. B Inflammatory factor mRNA expressions. Relative mRNA expressions of IL-1β, IL-6, NF-κB, TNF-α, iNOS, IL-10 and PPAR-γ in IBV-infected, UV-IBV-treated cells and Mock cells were detected by qRT-PCR method. β-actin acted as a reference gene. Data presented as mean ± SD (n = 3). * means the significance of between IBV-infected or UV-IBV-treated cells with Mock cells. * means p < 0.05, ** means p < 0.01.
XCL1 (also known as lymphotactin) and CXCL12 (also known as stromal cell derived factor-1) have a strong chemotactic effect on lymphocytes, involved in inflammatory responses [41, 42]. In IBV-infected HD11 cells, there was an abrupt increase in XCL1 and CXCL12 expressions at 24 hpi (p < 0.01). However, XCL1 and CXCL12 expressions decreased significantly at 12 and 48 hpi, respectively (p < 0.01). The XCL1 and CXCL12 expression trends in PBMCs-Mφ were similar to those in HD11 cells (p < 0.01), but there was a gradual decrease of XCL1 and CXCL12 expressions at 36 hpi (p < 0.01). In the UV-IBV-treatment experiment, there were only increased expressions of CXCL12 in HD11 cells (p < 0.05) and of XCL1 in PBMCs-Mφ at 12 hpi (p < 0.01, Figure 5A).
These results suggest that IBV could activate the chemotaxis in macrophages with temporary suppression at the early stage of infection. The mRNA expressions of CCL4 (29-fold) and MIF (24-fold) were the highest in IBV-infected HD11 cells compared with other chemokines, indicating that IBV-infected macrophages mainly secrete CCL4 and MIF.
IBV M41 up-regulated inflammatory factor expressions in macrophages
Interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α are classic pro-inflammatory cytokines and activate a variety of stress responses [43,44,45]. Transcription factor Nuclear factor-κB (NF-κB) is a redox-sensitive transcription factor and modulates ILs production [46]. Inducible nitric oxide synthase (iNOS) is involved in NO synthesis and the pro-inflammatory responses of macrophages [47]. IL-10 is an anti-inflammatory cytokine which controls antigen presentation function and the ability to synthesize cytokines [48]. Peroxisome proliferator-activated receptor (PPAR)-γ is related to inflammation inhibition [49, 50]. In IBV-infected HD11 cells, pro-inflammatory cytokines showed upward trends from 12 to 24 hpi. IL-1β, IL-6, TNF-α, iNOS peaked at 24 hpi (p < 0.01), and NF-κB peaked at 36 hpi (p < 0.01). The expression trends in PBMCs-Mφ were similar to those in HD11 cells. For anti-inflammatory cytokines, IL-10 decreased at 12 hpi in IBV-infected HD11 cells (p < 0.01), and PPAR-γ was up-regulated at 24, 36, and 48 hpi (p < 0.01). However, in PBMCs-Mφ, there was an obvious increase in IL-10 expression at 24, 36, and 48 hpi (p < 0.01), and in PPAR-γ at 24 hpi (p < 0.01), respectively (Figure 5B).
These results indicate that IBV induces macrophage pro-inflammatory and anti-inflammatory responses. In IBV infection, there were increased expressions of IL-6 mRNA (27-fold) in HD11 cells. The relative mRNA expression of IL-1β (246-fold), IL-6 (1560-fold), iNOS mRNA (118-fold) in PBMCs-Mφ had higher expressions compared with other cytokines.
IBV M41 induced autophagy in macrophages
Microtubule-associated protein 1 light chain 3 II (LC3II) is an autophagic target protein involved in the formation of autophagosomes [51]. Mammalian target of rapamycin (mTOR) and Beclin-1 are key proteins involved in the autophagic pathways [52, 53]. LC3II expression peaked at 36 hpi in IBV-infected HD11 cells and PBMCs-Mφ (p < 0.01). mTOR and Beclin-1 increased to a peak at 24 or 36 hpi (p < 0.01), and subsequently decreased rapidly in IBV-infected HD11 cells and PBMCs-Mφ, respectively (Figure 6A). This indicates that IBV infection could induce autophagy at the middle term of viral replication and participate in the disease pathogenic process.
Gene expressions of related proteins in apoptosis and autophagy. A Autophagy-related gene mRNA expressions. Relative mRNA expressions of LC3II, mTOR and Beclin-1 in IBV-infected, UV-IBV-treated cells and Mock cells were detected by qRT-PCR method. β-actin acted as a reference gene. B Apoptosis-related gene mRNA expressions. Relative mRNA expressions of Caspase-3, Bax, Bcl-2 in IBV-infected, UV-IBV-treated cells and Mock cells were detected by qRT-PCR method. β-actin acted as a reference gene. Data presented as mean ± SD (n = 3). * means the significance of between IBV-infected or UV-IBV-treated cells with Mock cells. * means p < 0.05, ** means p < 0.01.
IBV M41 induced apoptosis in macrophages
Caspase-3 is an executive factor of apoptosis [54]. Bcl-2-associated X (Bax) is a classic pro-apoptotic factor [55]. Bcl-2 is an inhibitor of apoptosis [56]. Caspase-3 expression peaked at 36 hpi in IBV-infected HD11 cells and PBMCs-Mφ (p < 0.01). However, Caspase-3 expression was down-regulated at 12 and 48 hpi in IBV-infected HD11 cells (p < 0.01), and at 24 hpi in PBMCs-Mφ (p < 0.01), respectively. In IBV-infected HD11 cells, the expression of Bax decreased at 12 hpi (p < 0.05), and 24 hpi in PBMCs-Mφ (p < 0.01). As the virus replicated, the expression of Bax showed an increase trend, and peaked at 48 hpi (p < 0.01). Remarkably, decreased expressions of Bcl-2 appeared in both HD11 cells and PBMCs-Mφ at 48 hpi (p < 0.01). UV-IBV treatment did not change the mRNA expressions of Caspase-3, Bax and Bcl-2 in macrophages (Figure 6B). These results indicate that viral replication provokes apoptosis in macrophages at the late stage of infection.
Discussion
Macrophages are an important part of innate immunity [57]. Macrophages respond to external stimuli with rapid changes in their expressions of various related genes. In this study, we established an experimental model of IBV infection of macrophages. The high-purity HD11 cells (99.8%) and PBMCs-Mφ (91.3%) could exhibit typical CPE at 36 h post-IBV M41 strain infection, and the virus could replicate on them. These results demonstrated that both kinds of cell were suitable for studying the interaction of IBV and macrophages and the underlying mechanisms. Macrophage viability and abilities were checked. IBV could affect macrophage viability and damage their phagocytic functions, which are related to viral replication. Han et al. [58] found that IBV Beaudette strain could propagate stably in HD11 cells and the virus titer reached the high level of 106.85 TCID50/mL, typical CPE appeared at 36 hpi, and cell viability decreased obviously at the same time. In other words, IBV could impair the viability of chicken macrophages and their biological functions, which was consistent with our results.
Host immune response is divided into innate immunity and acquired immunity [59]. TLRs and RLRs are two types of PRR in the innate immune system [60]. TLR3 and TLR7 are both involved in IBV infection, which is consistent with previous studies [16, 61], and TLR3 may play a key role in IBV-induced viral immune mechanisms. Furthermore, IBV infection activates MDA5 expression [16, 62]. The expressions of the key antiviral molecules, IFNs and other acquired immunity-related genes, which are involved in antigen presentation and pathogen clearance, increased significantly with virus replication. This indicates that IBV activates the innate immune response of macrophages. Interestingly, the gene expressions of certain signal proteins, such as TLR7, MyD88, MDA5, MHCII, and Fc receptor, decreased in HD11 cells and PBMCs-Mφ at 12 hpi, respectively, indicating that IBV could inhibit the immune regulatory function of macrophages at the early stage of its infection.
Macrophages secrete chemokines post-IBV infection, which attract immune competent cells to the sites of infection and inflammation [63]. Meanwhile, cytokines are activated to participate in information transmission, immune regulation and effector functions [43, 44]. Similar to other research, we found that IBV activated the gene expressions of most chemokines and inflammatory cytokines [16, 61, 64], demonstrating a promotion of inflammatory response. Among them, CCL4, IL-1β, IL-6, and iNOS mainly activated and participated in innate immunity of IBV infection. The expressions of MIF, XCL1 and CXCL12 decreased at the early stage of infection, indicating IBV affects macrophage chemotaxis. Moreover, the up-regulation of inflammatory cytokines in IBV infection was higher in percentage terms than in anti-inflammatory cytokines at 12 and 24 hpi, indicating that a pro-inflammatory response is activated at the early stage of IBV infection. From 24 to 48 hpi, the expressions of cytokines gradually decreased, indicating that at the late stage of IBV infection, an anti-inflammatory response is dominant in the macrophages.
Finally, the gene expressions of related proteins involved in apoptosis and autophagy were investigated. There have been reports that IBV could induce autophagy and apoptosis in both chicken and mammalian cells [58, 65, 66]. In this study, there occurred autophagy and apoptosis in HD11 cells and PBMCs-Mφ post-IBV infection. In addition, we found that autophagy appeared and peaked at the period of extensive viral replication, and apoptosis occurred obviously at the later stage of stable virus replication. With virus replication in macrophages, there appeared autophagy firstly and apoptosis subsequently. Furthermore, the gene expression levels of related proteins enhanced post-IBV infection, while there were no obvious changes post-UV-IBV treatment. This further supports the fact that the up-regulation of gene expressions has a close relationship with virus replication.
In conclusion, IBV decreased macrophage phagocytic functions and viability, but strengthened pathogen elimination functions. IBV promoted nearly all the gene expressions of related proteins in macrophages—except some degree of suppression at the earlier stage—to exert its biofunctions in multiple host responses, and the dynamic changes of gene expression had a close relationship with virus replication. This might provide some insight into understanding the immunopathogenesis mechanism of IBV infection.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- IBV:
-
Infectious bronchitis virus
- UV-IBV:
-
UV-inactivated IBV
- PAMP:
-
Pathogen-associated molecular pattern
- PRRs:
-
Pattern recognition receptors
- PBMCs-Mφ:
-
Peripheral blood mononuclear cells-derived macrophages
- SPF:
-
Specific-pathogen-free
- MOI:
-
Multiplicity of infection
- CPE:
-
Cytopathic effects
- TCID50 :
-
50% Tissue culture infective dose
- NO:
-
Nitric oxide
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- hpi:
-
Hours post-infection
- MHC:
-
Major histocompatibility complex
- TLRs:
-
Toll-like receptors
- MyD88:
-
Myeloid differentiation factor 88
- MDA5:
-
Melanoma differentiation associated protein 5
- IFNs:
-
Interferons
- MIF:
-
Macrophage migration inhibitory factor
- iNOS:
-
Inducible nitric oxide synthase
- IL:
-
Interleukin
- NF-κB:
-
Nuclear factor κB
- TNF-α:
-
Tumor necrosis factor-α
- PPAR-γ:
-
Proliferator-activated receptor γ
- mTOR:
-
Mammalian target of rapamycin
- LC3II:
-
Microtubules associated protein 1 light chain 3 II
- Bax:
-
Bcl-2-associated X
References
Cavanagh D (2007) Coronavirus avian infectious bronchitis virus. Vet Res 38:281–297. https://doi.org/10.1051/vetres:2006055
Brierley I, Boursnell ME, Binns MM, Bilimoria B, Blok VC, Brown TD, Inglis SC (1987) An efficient ribosomal frame-shifting signal in the polymerase-encoding region of the coronavirus IBV. EMBO J 6:3779–3785
Cook JKA, Jackwood M, Jones RC (2012) The long view: 40 years of infectious bronchitis research. Avian Pathol 41:239–250
Cavanagh D, Davis PJ, Cook JKA (1992) Infectious bronchitis virus: evidence for recombination within the Massachusetts serotype. Avian Pathol 21:401–408. https://doi.org/10.1080/03079459208418858
Cavanagh D, Davis PJ, Mockett APA (1988) Amino acids within hypervariable region 1 of avian coronavirus IBV (Massachusetts serotype) spike glycoprotein are associated with neutralization epitopes. Virus Res 11:141–150. https://doi.org/10.1016/0168-1702(88)90039-1
de Wit JJS, Malo A, Cook JKA (2019) Induction of IBV strain-specific neutralizing antibodies and broad spectrum protection in layer pullets primed with IBV Massachusetts (Mass) and 793B vaccines prior to injection of inactivated vaccine containing Mass antigen. Avian Pathol 48:135–147. https://doi.org/10.1080/03079457.2018.1556778
Parsons LM, Bouwman KM, Azurmendi H, de Vries RP, Cipollo JF, Verheije MH (2019) Glycosylation of the viral attachment protein of avian coronavirus is essential for host cell and receptor binding. J Biol Chem 294:7797–7809. https://doi.org/10.1074/jbc.RA119.007532
Kusters JG, Niesters HG, Lenstra JA, Horzinek MC, van der Zeijst BA (1989) Phylogeny of antigenic variants of avian coronavirus IBV. Virology 169:217–221. https://doi.org/10.1016/0042-6822(89)90058-5
Dolz R, Pujols J, Ordonez G, Porta R, Majo N (2008) Molecular epidemiology and evolution of avian infectious bronchitis virus in Spain over a fourteen-year period. Virology 374:50–59. https://doi.org/10.1016/j.virol.2007.12.020
Chen R, Zeng L, Zhu S, Liu J, Zeh HJ, Kroemer G, Wang H, Billiar TR, Jiang J, Tang D, Kang R (2019) cAMP metabolism controls caspase-11 inflammasome activation and pyroptosis in sepsis. Sci Adv. https://doi.org/10.1126/sciadv.aav5562
Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S (2005) Macrophage receptors and immune recognition. Annu Rev Immunol 23:901–944. https://doi.org/10.1146/annurev.immunol.23.021704.115816
Wadsworth SA, Cavender DE, Beers SA, Lalan P, Schafer PH, Malloy EA, Wu W, Fahmy B, Olini GC, Davis JE, Pellegrino-Gensey JL, Wachter MP, Siekierka JJ (1999) RWJ 67657, a potent, orally active inhibitor of p38 mitogen-activated protein kinase. J Pharmacol Exp Ther 291:680–687. https://doi.org/10.1016/j.ejphar.2010.01.011
Lee JW, Kim NH, Kim JY, Park JH, Shin SY, Kwon YS, Lee HJ, Kim SS, Chun W (2013) Aromadendrin inhibits lipopolysaccharide-induced nuclear translocation of NF-kappaB and phosphorylation of JNK in RAW 264.7 Macrophage Cells. Biomol Ther (Seoul) 21:216–221. https://doi.org/10.4062/biomolther.2013.023
Majewska M, Szczepanik M (2006) The role of Toll-like receptors (TLR) in innate and adaptive immune responses and their function in immune response regulation. Postepy Hig Med Dosw 60:52–63. https://doi.org/10.4049/jimmunol.1101640
Xaus J, Cardo M, Valledor AF, Soler C, Lloberas J, Celada A (1999) Interferon gamma induces the expression of p21waf-1 and arrests macrophage cell cycle, preventing induction of apoptosis. Immunity 11:103–113. https://doi.org/10.1016/s1074-7613(00)80085-0
Kameka AM, Haddadi S, Kim DS, Cork SC, Abdul-Careem MF (2014) Induction of innate immune response following infectious bronchitis corona virus infection in the respiratory tract of chickens. Virology 450–451:114–121. https://doi.org/10.1016/j.virol.2013.12.001
Lee KW, Li G, Lillehoj HS, Lee SH, Jang SI, Babu US, Lillehoj EP, Neumann AP, Siragusa GR (2011) Bacillus subtilis-based direct-fed microbials augment macrophage function in broiler chickens. Res Vet Sci 91:e87-91. https://doi.org/10.1016/j.rvsc.2011.01.018
Guabiraba R, Garrido D, Bailleul G, Trotereau A, Pinaud M, Lalmanach AC, Chanteloup NK, Schouler C (2017) Unveiling the participation of avian kinin ornithokinin and its receptors in the chicken inflammatory response. Vet Immunol Immunopathol 188:34–47. https://doi.org/10.1016/j.vetimm.2017.04.005
Wang X, Zhao J, Tang S, Ye Z, Hewlett I (2010) Viremia associated with fatal outcomes in ferrets infected with avian H5N1 influenza virus. PLoS ONE 5:e12099. https://doi.org/10.1371/journal.pone.0012099
Cao J, Chen X, Jiang L, Lu B, Ying M (2020) DJ-1 suppresses ferroptosis through preserving the activity of S-adenosyl homocysteine hydrolase. Nat Commun 11:1251. https://doi.org/10.1038/s41467-020-15109-y
Yuan G, Zhou X, Gong Z, Zhang P, Sun X, Zheng S (2006) Expression and activity of inducible nitric oxide synthase and endothelial nitric oxide synthase correlate with ethanolinduced liver injury. World J Gastroenterol 12:2375–2381. https://doi.org/10.3748/wjg.v12.i15.2375
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
MacMicking J, Xie QW, Nathan C (1997) Nitric oxide and macrophage function. Annu Rev Immunol 15:323–350. https://doi.org/10.1146/annurev.immunol.15.1.323
Fonceca AM, Zosky GR, Bozanich EM, Sutanto EN, Kicic A, McNamara PS, Knight DA, Sly PD, Turner DJ, Stick SM (2018) Accumulation mode particles and LPS exposure induce TLR-4 dependent and independent inflammatory responses in the lung. Respir Res 19:15. https://doi.org/10.1186/s12931-017-0701-z
Lin Y, Ren L, Wang W, Di J, Zeng S, Saito S (2009) Effect of TLR3 and TLR7 activation in uterine NK cells from non-obese diabetic (NOD) mice. J Reprod Immunol 82:12–23. https://doi.org/10.1016/j.jri.2009.03.004
Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S (2003) TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol 4:1144–1150. https://doi.org/10.1038/ni986
Mukherjee K, Korithoski B, Kolaczkowski B (2014) Ancient origins of vertebrate-specific innate antiviral immunity. Mol Biol Evol 31:140–153. https://doi.org/10.1093/molbev/mst184
Lee C-C, Tung C-Y, Wu CC, Lin TL (2019) Avian innate immunity with an emphasis on chicken melanoma differentiation-associated gene 5 (MDA5). Taiwan Veterinary J 45:43–55. https://doi.org/10.1142/S1682648519300016
Lazear HM, Pinto AK, Ramos HJ, Vick SC, Shrestha B, Suthar MS, Gale M Jr, Diamond MS (2013) Pattern recognition receptor MDA5 modulates CD8+ T cell-dependent clearance of West Nile virus from the central nervous system. J Virol 87:11401–11415. https://doi.org/10.1128/JVI.01403-13
Echebli N, Tchitchek N, Dupuy S, Bruel T, Peireira Bittencourt Passaes C, Bosquet N, Le Grand R, Bourgeois C, Favier B, Cheynier R, Lambotte O, Vaslin B (2018) Stage-specific IFN-induced and IFN gene expression reveal convergence of type I and type II IFN and highlight their role in both acute and chronic stage of pathogenic SIV infection. PLoS ONE 13:e0190334. https://doi.org/10.1371/journal.pone.0190334
Ivashkiv LB (2018) IFNgamma: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat Rev Immunol 18:545–558. https://doi.org/10.1038/s41577-018-0029-z
Roche PA, Furuta K (2015) The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol 15:203–216. https://doi.org/10.1038/nri3818
Sapinoro R, Volcy K, Rodrigo WW, Schlesinger JJ, Dewhurst S (2008) Fc receptor-mediated, antibody-dependent enhancement of bacteriophage lambda-mediated gene transfer in mammalian cells. Virology 373:274–286. https://doi.org/10.1016/j.virol.2007.12.013
Febbraio M, Hajjar DP, Silverstein RL (2001) CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest 108:785–791. https://doi.org/10.1172/JCI14006
Mukhopadhyay S, Varin A, Chen Y, Liu B, Tryggvason K, Gordon S (2011) SR-A/MARCO-mediated ligand delivery enhances intracellular TLR and NLR function, but ligand scavenging from cell surface limits TLR4 response to pathogens. Blood 117:1319–1328. https://doi.org/10.1182/blood-2010-03-276733
Heydtmann M, Shields P, McCaughan G, Adams D (2001) Cytokines and chemokines in the immune response to hepatitis C infection. Curr Opin Infect Dis 14:279–287. https://doi.org/10.1097/00001432-200106000-00006
Us D (2003) Chemokine receptors: Their roles in the pathogenesis of human immunodeficiency virus (HIV) and resistance to HIV infection. Mikrobiyol Bul 37:75–87
Flores M, Saavedra R, Bautista R, Viedma R, Tenorio EP, Leng L, Sanchez Y, Juarez I, Satoskar AA, Shenoy AS, Terrazas LI, Bucala R, Barbi J, Satoskar AR, Rodriguez-Sosa M (2008) Macrophage migration inhibitory factor (MIF) is critical for the host resistance against Toxoplasma gondii. FASEB J 22:3661–3671. https://doi.org/10.1096/fj.08-111666
Hughes S, Bumstead N (2000) The gene encoding the chicken chemokine K60 maps to chromosome 4. Anim Genet 31:418–419. https://doi.org/10.1046/j.1365-2052.2000.00697.x
Sun B, Lei Y, Cao Z, Zhou Y, Sun Y, Wu Y, Wang S, Guo W, Liu C (2019) TroCCL4, a CC chemokine of Trachinotus ovatus, is involved in the antimicrobial immune response. Fish Shellfish Immunol 86:525–535. https://doi.org/10.1016/j.fsi.2018.11.080
Razmkhah M, Talei AR, Doroudchi M, Khalili-Azad T, Ghaderi A (2005) Stromal cell-derived factor-1 (SDF-1) alleles and susceptibility to breast carcinoma. Cancer Lett 225:261–266. https://doi.org/10.1016/j.canlet.2004.10.039
Guzzo C, Fox J, Lin Y, Miao H, Cimbro R, Volkman BF, Fauci AS, Lusso P (2013) The CD8-derived chemokine XCL1/lymphotactin is a conformation-dependent, broad-spectrum inhibitor of HIV-1. PLoS Pathog 9:e1003852. https://doi.org/10.1371/journal.ppat.1003852
Woolley DE, Tetlow LC (2000) Mast cell activation and its relation to proinflammatory cytokine production in the rheumatoid lesion. Arthritis Res 2:65–74. https://doi.org/10.1186/ar70
Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S (2011) The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813:878–888. https://doi.org/10.1016/j.bbamcr.2011.01.034
Feldmann M, Maini RN (2001) Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol 19:163–196. https://doi.org/10.1146/annurev.immunol.19.1.163
Karin M, Ben-Neriah Y (2000) Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol 18:621–663. https://doi.org/10.1146/annurev.immunol.18.1.621
Aktan F (2004) iNOS-mediated nitric oxide production and its regulation. Life Sci 75:639–653. https://doi.org/10.1016/j.lfs.2003.10.042
Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, Morris PJ, Powrie F, Wood KJ (2001) IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol 166:3789–3796. https://doi.org/10.4049/jimmunol.166.6.3789
Zhang P, Ding Z, Liu X, Chen Y, Li J, Tao Z, Fei Y, Xue C, Qian J, Wang X, Li Q, Stoeger T, Chen J, Bi Y, Yin R (2018) Enhanced replication of virulent Newcastle disease virus in chicken macrophages is due to polarized activation of cells by inhibition of TLR7. Front Immunol 9:366. https://doi.org/10.3389/fimmu.2018.00366
Chawla A (2010) Control of macrophage activation and function by PPARs. Circ Res 106:1559–1569. https://doi.org/10.1161/CIRCRESAHA.110.216523
Bansal M, Moharir SC, Swarup G (2018) Autophagy receptor optineurin promotes autophagosome formation by potentiating LC3-II production and phagophore maturation. Commun Integr Biol 11:1–4. https://doi.org/10.1080/19420889.2018.1467189
Wang Y, Liu J, Zhou JS, Huang HQ, Li ZY, Xu XC, Lai TW, Hu Y, Zhou HB, Chen HP, Ying SM, Li W, Shen HH, Chen ZH (2018) MTOR suppresses cigarette smoke-induced epithelial cell death and airway inflammation in chronic obstructive pulmonary disease. J Immunol 200:2571–2580. https://doi.org/10.4049/jimmunol.1701681
Qian X, Li X, Cai Q, Zhang C, Yu Q, Jiang Y, Lee JH, Hawke D, Wang Y, Xia Y, Zheng Y, Jiang BH, Liu DX, Jiang T, Lu Z (2017) Phosphoglycerate kinase 1 phosphorylates Beclin1 to induce autophagy. Mol Cell 65:917-931.e916. https://doi.org/10.1016/j.molcel.2017.01.027
Park HJ, Kim MJ, Ha E, Chung JH (2008) Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells, SNU-C4. Phytomedicine 15:147–151. https://doi.org/10.1016/j.phymed.2007.07.061
Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH (2000) Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ 7:1166–1173. https://doi.org/10.1038/sj.cdd.4400783
Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O’Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH (2005) An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435:677–681. https://doi.org/10.1038/nature03579
Starkey Lewis PJ, Moroni F, Forbes SJ (2019) Macrophages as a cell-based therapy for liver disease. Semin Liver Dis 39:442–451. https://doi.org/10.1055/s-0039-1688502
Han X, Tian Y, Guan R, Gao W, Yang X, Zhou L, Wang H (2017) Infectious bronchitis virus infection induces apoptosis during replication in chicken macrophage HD11 cells. Viruses 9:198. https://doi.org/10.3390/v9080198
Sonoda KH (2008) Association of ocular inflammation and innate immune response. Nippon Ganka Gakkai Zasshi 112:279–297 (in Japanese)
Sokolova TM, Poloskov VV, Shuvalov AN, Burova OS, Sokolova ZA (2019) Signaling TLR/RLR-mechanisms of immunomodulating action of ingavirin and thymogen preparations. Russ J Biother 18:60–66. https://doi.org/10.17650/1726-9784-2019-18-1-60-66
Chhabra R, Ball C, Chantrey J, Ganapathy K (2018) Differential innate immune responses induced by classical and variant infectious bronchitis viruses in specific pathogen free chicks. Dev Comp Immunol 87:16–23. https://doi.org/10.1016/j.dci.2018.04.026
Yu L, Zhang X, Wu T, Su J, Wang Y, Wang Y, Ruan B, Niu X, Wu Y (2017) Avian infectious bronchitis virus disrupts the melanoma differentiation associated gene 5 (MDA5) signaling pathway by cleavage of the adaptor protein MAVS. BMC Vet Res 13:332. https://doi.org/10.1186/s12917-017-1253-7
Sallusto F, Baggiolini M (2008) Chemokines and leukocyte traffic. Nat Immunol 9:949–952. https://doi.org/10.1038/ni.f.214
Dar A, Tikoo S, Potter A, Babiuk LA, Townsend H, Gerdts V, Mutwiri G (2014) CpG-ODNs induced changes in cytokine/chemokines genes expression associated with suppression of infectious bronchitis virus replication in chicken lungs. Vet Immunol Immunopathol 160:209–217. https://doi.org/10.1016/j.vetimm.2014.05.004
Cottam EM, Maier HJ, Manifava M, Vaux LC, Chandra-Schoenfelder P, Gerner W, Britton P, Ktistakis NT, Wileman T (2011) Coronavirus nsp6 proteins generate autophagosomes from the endoplasmic reticulum via an omegasome intermediate. Autophagy 7:1335–1347. https://doi.org/10.4161/auto.7.11.16642
Fung TS, Liu DX (2017) Activation of the c-Jun NH2-terminal kinase pathway by coronavirus infectious bronchitis virus promotes apoptosis independently of c-Jun. Cell Death Dis 8:3215. https://doi.org/10.1038/s41419-017-0053-0
Acknowledgements
The first author Xiaoqi Sun thanks Dr Yicong Chang of the College of Veterinary Medicine (Northeast Agricultural University, Harbin, China) for his support and assistance during her study and life.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Numbers 31172295, 31272569).
Author information
Authors and Affiliations
Contributions
GL supervised the whole experiments. XS performed the practical work and completed the experiments, ZW, CS, JY, HL, HC, LL, YR, XH and RZ provided help during the experiments. XW helped to revise the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Animal Care and Use Committee of Northeast Agricultural University (Harbin, Heilongjiang Province) approved the experimental procedures in the present study (SYXK (Hei) 2012–2067).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, X., Wang, Z., Shao, C. et al. Analysis of chicken macrophage functions and gene expressions following infectious bronchitis virus M41 infection. Vet Res 52, 14 (2021). https://doi.org/10.1186/s13567-021-00896-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-021-00896-z