Abstract
Background
There is sparse evidence on the impact of health information on mental health as well as on the mechanisms governing this relationship. We estimate the causal impact of health information on mental health via the effect of a diabetes diagnosis on depression.
Methods
We employ a fuzzy regression discontinuity design (RDD) exploiting the exogenous cut-off value of a biomarker used to diagnose type-2 diabetes (glycated haemoglobin, HbA1c) and information on psycometrically validated measures of diagnosed clinical depression drawn from rich administrative longitudinal individual-level data from a large municipality in Spain. This approach allows estimating the causal impact of a type-2 diabetes diagnosis on clinica ldepression.
Results
We find that overall a type-2 diabetes diagnosis increases the probability of becoming depressed, however this effect appears to be driven mostly by women, and in particular those who are relatively younger and obese. Results also appear to differ by changes in lifestyle induced by the diabetes diagnosis: while women who did not lose weight are more likely to develop depression, men who did lose weight present a reduced probability of being depressed. Results are robust to alternative parametric and non-parametric specifications and placebo tests.
Conclusions
The study provides novel empirical evidence on the causal impact of health information on mental health, shedding light on gender-based differences in such effects and potential mechanisms through changes in lifestyle behaviours.
Similar content being viewed by others
Background
An increasing body of evidence suggests the relevance of health information in influencing key health-behaviours. For instance, the medical literature finds that the information provided by portable devices [1, 2] or the diagnosis of specific types of cancer [3, 4] might trigger behavioural changes and ultimately affect health outcomes. More recently, the economics literature has started exploring the role of health information by focusing on the impact of the diagnosis of chronic conditions, including hypertension and diabetes [5,6,7,8,9]. While these recent economics studies often employ causal inference methods and are thus capable of identifying causal effects, they mostly focus on changes in health-behaviours while ignoring other relevant health outcomes such as mental health.
Mental health and depression have been consistently found to be linked with health-behaviours. A series of studies show that individuals with healthier patterns of lifestyle behaviours present lower levels of psychological distress [10,11,12,13,14,15,16]. In addition, the relationship between chronic conditions and mental health is well-documented with several papers suggesting strong correlations between major chronic conditions such as diabetes and mental health. More specifically, a large body of evidence finds strong and sizeable correlations between a diabetes diagnosis and several mental health outcomes such as clinical depression, consumption of antidepressants, and measures of quality of life and social interactions [17,18,19,20,21,22]. Equally, another stream of studies shows significant associations between mental ill-health and the risk of developing T2DM [17, 22,23,24]. However, the existing literature does not appear to have comprehensively investigated the role of health information in causally influencing mental health.
The main objective of this paper is to identify the causal impact of health information on mental health via the impact of a diagnosis of type-2 diabetes (T2DM) on clinical depression using a regression discontinuity design (RDD). More specifically, we exploit the discontinuity offered by the exogenous cut-off of a biomarker commonly used for the diagnosis of T2DM (i.e. glycated haemoglobin, HbA1c) to estimate the impact of a T2DM diagnosis on diagnosed clinical depression using rich longitudinal administrative data from Spain.
This paper offers several contributions to the literature. First, we provide novel causal evidence of the impact of health information on depression, one of the most widespread mental disorders affecting around 280 million individuals globally [25]. While the growing literature on the role of health information has mainly focused on its effect on health-behaviours especially among individuals with chronic conditions, to the best of our knowledge no previous studies have attempted to identify the causal impact of health information on diagnosed clinical depression. Second, our analysis suggests that the effect of a type-2 diabetes diagnosis on depression might vary by gender, age and BMI level as well as by the behavioural changes induced by it specifically weight loss. Hence, this contributes directly to the literature on the relevance of health information and the mechanisms through which it might affect health outcomes. From a policy perspective this might be also potentially useful as it highlights that the provision of health information (in the form of a diabetes diagnosis) could positively affect both lifestyle hehaviours (weight losses in particular) and mental health. Third, our empirical analysis also contributes to the large strand of the literature concerned with the determinants of mental health among individuals with major chronic conditions, including obesity and type-2 diabetes. This is also likely to be relevant policy-wise as type-2 diabetes is currently affecting 537 million individuals, and its burden of disease is projected to increase in both developing and developed countries [26]. Finally, differently from the majority of previous studies on the impact of a T2DM diagnosis employing a sharp RDD, the fuzzy RDD approach used here allows accounting for the possibility that a T2DM diagnosis may not be exclusively based on the cut-off of a single biomarker, but on a wider set of information including family history and the presence of comorbidities.
More generally, estimating the causal impact of a T2DM diagnosis on depression might be relevant from an economic perspective as these are both highly prevalent conditions with significant impacts on the quality of life and productivity of individuals. Indeed, both T2DM and mental ill-health greatly affect an individual’s ability to work leading to reduced labour force participation and increased absenteeism [27,28,29,30]. Hence, establishing and quantifying a causal relationship between these two widespread conditions, might provide valuable information to develop more targeted interventions. These should be aimed at reducing the combined economic burden of T2DM and depression, including screening and early detection programmes for patients with diabetes and appropriate treatment strategies more explicitly accounting for the potential risk of clinical depression.
Previous literature
Several recent studies in the field of medicine have explored the effects of health information either via the diagnosis of specific types of cancers or by portable devices on clinical outcomes as well as risky health-behaviours [1,2,3,4]. However, these findings are mostly based on standard statistical associations and are either mixed or observed only among specific sub-groups of individuals.
The economics literature has also started exploring the role of health information in influencing health and health-behaviours. This is highly relevant as standard economic models assume that individuals have complete knowledge about their health, and they can perfectly and rationally process it when making health investment decisions [31, 32]. However, this assumption has been recently re-assessed by empirical and experimental studies [33,34,35]. Early economic studies focus on the effects of public health information campaigns [36,37,38,39] or nutritional labels [40, 41] while more recent contributions attempt to identify the causal impact of health information via the diagnosis of chronic conditions, including T2DM, on lifestyle behaviours [5,6,7,8,9] and cardiovascular risk factors [42]. The latter studies tend to increasingly find significant causal impacts of a T2DM diagnosis mostly on weight loss or fat intake.
Moreover, the dual relationship between diabetes and mental health has been investigated, mainly in the medical literature. Several of these studies have shown a significant association between the diagnosis of T2DM and the deterioration of mental health [17,18,19,20] with negative effects on quality of life, social contacts [20, 22], and increases in the consumption of antidepressants [18]. In addition, a consistent finding in the medical literature is that major depressive disorders increase the risk of developing T2DM and subsequent complications [17, 23, 24].
Importantly, most medical evidence tends to rely on self-reported information of key variables of interest and only identifies standard statistical correlations, while overlooking potential endogeneity issues. Gaggero [7] appears to be among the very few economics studies attempting to identify the effect of a diabetes diagnosis on a measure of mental health. However, he only employs self-reported information on mental health together with a less reliable biomarker, i.e. Fasting Plasma Glucose, [43] to detect diabetes on a sample of older individuals in England, finding no statistically significant effects of a T2DM diagnosis. As a result, the literature has not yet established whether health information may have a causal impact on developing a diagnosed mental health condition and, more specifically, whether a T2DM diagnosis might causally affect clinical depression.
Methods
Data
We employ administrative data of patients followed over a seven-year period (2004–2010) drawn from six GP practices and two hospitals located in the city of Badalona (nort-east of Barcelona), Spain, an EU country with a universal health care system free at the point of delivery [44]. The initial sample includes patients aged 16 + who had at least one contact with those hospitals and centres between 1 January 2004 and 31 December 2010.Footnote 1
The dataset contains detailed information about patients’ clinical measurements of height and weight and any diagnosed health condition, including clinical or major depression.Footnote 2 More specifically, clinical depression is identified by a binary variable taking value 1 if the patient is diagnosed with clinical/major depression, corresponding to the code/registry P76 of the International Classification of Primary Care, second edition (ICPC-2), 0 otherwise. The information used by physicians to diagnose depression is based on a series of psychometrically validated measures of clinical depression, including the Golberg Anxiety and Depression Scale (GADS), the Hamilton Rating Scale for Depression (HRSD) as well as the Geriatric Depression Rating Scale (GDRS) [45,46,47,48]. All three measures are collected by physicians through interviews with patients on the basis of a series of items identifying several symptoms of depression experienced by the patients during the previous week (18 in the GADS; 17 in the HRSD; 15 items in the short form of the GDRS index or 35 in the longer version, respectively). While the GADS and the HRSD can be used to detect depression in the general population, the GDRS is specifically designed to diagnose depression among older patients. The latter measure might be particularly useful in this case given the average age of individuals in our sample. The standard recommended thresholds to diagnose clinical depression for each validated measure were used.Footnote 3
Other key variables and descriptive statistics
Our medical records also include data on glycated haemoglobin (henceforth, HbA1c), a biomarker providing a measure of a patient’s average blood sugar level in the previous 8–12 weeks that is commonly used to diagnose T2DM [52]. In our setting, physicians follow standard national and international medical guidelines for patients with T2DM and use the threshold value of HbA1c ≥ 6.5 percent to diagnose T2DM [53].Footnote 4 This test is administered as part of routine health checks to all individuals presenting relevant risk factors or symptoms of hyperglycaemia (high blood sugar levels).
HbA1c measurements are endorsed by the International Expert Committee (IEC) and the American Diabetes Association (ADA) as they are more reliable if compared to other measures of blood sugar such as the Fasting Plasma Glucose (FPG).Footnote 5 The latter appears to have a substantially shorter time validity; to be sensitive to short-term lifestyle changes and stress; and tend to systematically underestimate the prevalence of diabetes [43, 52, 58].Footnote 6 Relevant to this study, upon a T2DM diagnosis, patients of the Spanish health care system are normally recommended to follow a non-pharmacological treatment consisting of educational training sessions for diabetes self-management aimed at improving their lifestyle though dietary changes and regular exercise.
In addition, the dataset includes a rich set of demographic and socioeconomic characteristics such as age; gender; employment status (active/retired); marital status (married/cohabiting vs living alone); immigration status (EU vs non-EU), that we use as control variables. For the purpose of our analysis, we include in our estimating sample individuals with at least one biomarker measurement per year. This effectively includes any individual either diagnosed with diabetes; at risk of diabetes (including pre-diabetics, i.e. patients with a HbA1c value between 5.7–6.4 percent); or any other individual with relevant risk factors or symptoms that may lead to high blood sugar levels. This leads to a sample of 13,971 individuals (39,994 obs.).Footnote 7
Table 1 reports the summary statistics of the main variables of interest. The Table shows that around 18 percent of the patients are diagnosed with clinical depression.Footnote 8 We next report statistics on individuals diagnosed with T2DM via the corresponding ICPC-2 code informed by the HbA1c values. The average HbA1c for the patients in our sample is around 6.6 percent and on average patients have been diagnosed for a little over 3 years (see the variable labelled “onset”). With respect to other demographic variables, the average age of the sample is around 65, and the sample is almost evenly split by gender. Furthermore, 87, 27 and 2 percent of the sample are, respectively, living with a partner; active in the labour market; and were born outside the EU. Finally, the Table also reports that 59 and 53 percent of the patients are also diagnosed with hypertension and dyslipidaemia (the presence of high amounts of lipids, including cholesterol, in blood), respectively; while 4, 7 and 5 percent of the patients are affected by asthma, neoplasms/cancers and chronic obstructive pulmonary disease (COPD) respectively. Tables S1-S3 in the Additional file 1 show differences in individuals’ characteristics by treatment status, gender and bodyweight change.
Empirical approach
We employ a fuzzy regression discontinuity design (RDD) exploiting the discontinuity offered by the exogenous cut-off value of 6.5 of the biomarker (HbA1c) used to diagnose T2DM. We choose to follow a fuzzy design as physicians may not base their diagnosis solely on HbA1c values. For instance, they could potentially look at further patients’ characteristics as well as family history around T2DM or whether individuals may suffer from other metabolic conditions, such as hypertension or dyslipidaemia. For instance, it might be the case that some physicians may diagnose with T2DM individuals with several metabolic conditions and a value of HbA1c just below 6.5 percent. Our fuzzy RDD approach would allow accounting for such cases.Footnote 9
A fuzzy RDD estimation is akin to a two-stage least squares instrumental variable (2SLS-IV) specification [60]. Accordingly, the first stage equation can be represented as follows:
where \({D}_{i,t}\) is a dummy indicator for whether individual \(i\) was diagnosed with T2DM at time \(t\) (i.e., year).\(Abov{e}_{it}\) is a binary variable that takes the value of 1 when the HbA1c value of an individual is larger than or equal to the predetermined cut-off value of 6.5, defining treatment assignment, and acts as the instrument for the T2DM diagnosis. The covariate \(\left({HbA1c}_{i,t}-6.5\right)\) is the centred or normalised HbA1c.Footnote 10 There are two main alternative ways for selecting the functional form to estimate the magnitude of the discontinuity in the outcome of interest at the cut-off point within a RDD setting: the parametric approach and the non-parametric approach. While the parametric approach focuses on the optimal functional form to fit the full data, the nonparametric approach focuses on an arbitrarily small neighbourhood sample around the cut-off. To make full use of our estimating sample, and following Gelman and ImbensFootnote 11 [61], our main specification considers a linear polynomial of the running variable. However, we examine the robustness of our results to higher order polynomials and non-parametric estimations based on a local randomization approach [62]. All estimations control for a vector of covariates \({{\varvec{X}}}_{{\varvec{i}},{\varvec{t}}}^{\boldsymbol{^{\prime}}}\) including sociodemographic characteristics (age, gender, employment, marital and immigrant status). We also control for several pre-diagnosed conditions, including hypertension, dyslipidaemia, asthma, neoplasms/cancers, and COPD as well as time elapsed from the diagnosis. Additionally, our econometric specifications include time-, health area- and GP fixed-effects (FE). This allows controlling for any systematic (time-invariant) differences across physicians that might affect the diagnoses of T2DM and major depression.
The second stage equation can be written as:
\({Y}_{i,t+1}\) denotes the outcome of interest and measures whether individual \(i\) is diagnosed with clinical depression at time \(t+1\), that is after the T2DM diagnosis, conditional on not having been diagnosed at time t. As above, it is assumed a linear function (although other polynomials are further examined) of the normalised running variable, and this is allowed to vary around the cut-off. \({{\varvec{X}}}_{{\varvec{i}}{\varvec{t}}}^{\boldsymbol{^{\prime}}}\) is the same vector of covariates discussed above. Finally, \({\varepsilon }_{i,t}\) is a random error term. We cluster standard errors on the running variable based on the recommendation of Lee and Card [63].Footnote 12 The main term of interest is \(\beta\) as it measures the change in the probability of being diagnosed with depression following a T2DM diagnosis. This coefficient captures the local average treatment effect (LATE) of a diabetes diagnosis among the group of compliers around the cut-off [65]. To make full use of the sample size, we estimate Eqs. (1) and (2) parametrically. However, we further explore the robustness of our results using the non-parametric approach mentioned above as part of our sensitivity analysis.
RDD validity
In order to test whether the average outcome of those just below the cut-off can be used as the counterfactual for those above the cut-off, we provide two indirect tests suggesting the overall credibility of our RDD application [66].
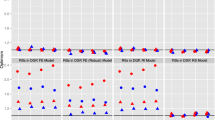
First, we examine whether there are any significant differences in pre-determined characteristics at the cut-off point. Ideally, we should find null effects of the diagnosis on these characteristics. The results of this exercise are presented in Fig. 1. Each graph presents the local polynomial smoothing (LPS) for pre-diagnosis major depression and covariates as a function of the HbA1c. This confirms the validity of our design by revealing non-significant jumps at the cut-off for any of these variables.
Second, for the RDD to be valid it is also critical that individuals would not be capable of manipulating their diabetes diagnosis [67]. In our case, it seems highly unlikely that patients could manipulate their HbA1c scores, as this measure is based on a blood test administered by physicians and refers to the average glucose concentration over the previous 8–12 weeks. Yet, in order to rule this out, in Fig. 2 we show the distribution of the density function of the normalised HbA1c around the cut-off, suggesting the absence of any discontinuity, as expected.Footnote 13
Results
Main results
Figure 3a-b examine the impact of having a HbA1c level above 6.5 percent. Specifically, Fig. 3a shows a sizeable discontinuity in the probability of being diagnosed with T2DM around the HbA1c cut-off as for our first stage. Similarly, the plot in Fig. 3b implies that patients with normalised HbA1c just above the cut-off are more likely to be diagnosed with depression than their counterparts. We next test the relevance of these findings in a regression framework while controlling for potential confounding factors.
RD Graphical Evidence. Note: A shows local polynomial estimates of the probability of being diagnosed with T2DM as a function of the (normalised) HbA1c, our rst stage. Similarly, b shows local polynomial estimates of the probability of being diagnosed with depression as a function of the (normalised) HbA1c
Table 2 reports the RDD estimates of a T2DM diagnosis, as outlined in Eq. (1). Specifically, column (1) includes the basic estimates with no covariates; column (2) adds a set of socioeconomic characteristics; column (3) further accounts for dummies indicating the presence of pre-existing medical conditions; column (4) includes time and area FE; and column (5) finally adds GP FE. Estimates appear statistically significant across all specifications, although as expected, the inclusion of pre-existing conditions appear to recude the size of the effects. According to the most comprehensive specification in column (5), the estimated coefficient implies that patients with an HbA1c above the 6.5 percent cut-off are around 9 percentage points more likely to be diagnosed with T2DM than their counterparts.
Table 3 reports fuzzy RDD estimates of a T2DM diagnosis of being diagnosed with major or clinical depression. Similarly to Table 2, in columns (1)-(5) we report findings of different specifications including an incremental number of covariates. Here, we observe positive and statistically significant effect of a T2DM diagnosis on clinical depression. In particular, according to our preferred model (column 5), receiving a T2DM diagnosis raises by 1.6 percent the probability of being diagnosed with depression.
Heterogeneity and potential mechanisms
Table 4 presents the results of the heterogeneity analysis splitting the data by gender aimed at discerning some potential mediating mechanisms. Panel A shows results for the full sample, whereas Panels B to H present the impacts induced by the T2DM diagnosis stratifying the sample by whether individuals lose weight following the diagnosis, as this is often one of the main lifestyle changes recommended by physicians according to the recent literature [6, 8, 69], by BMI categories (“healthy weight”: 18 < = BMI < 25; “overweight”: 25 < = BMI < 30; “obesity”: BMI > = 30) and also by age (> 60 years and < = 60 years-old). Columns (1)-(3) report RDD estimates for both genders, and for males and females, respectively.
The estimates in Panel A clearly suggest a gender pattern so that the increase in the probability of developing clinical depression appears to be driven by women: women present an increase of around 3.2 percentage points in the probability of being diagnosed with clinical depression following a diabetes diagnosis, while the corresponding estimate for men is not statistically significant. This appears to be in line with previous findings indicating that male patients with diabetes present higher levels of subjective well-being [70, 71].
We also explore whether losing weight after a T2DM diagnosis might also play a role in explaining the effects on clinical depression. Panels B and C in Table 4 present results for individuals who did versus who did not lose weight, respectively. Importantly, size and statistical significance of these estimates suggest that the observed increase in depression is mostly concentrated among female patients who did not lose weight following a diabetes diagnosis (5.8 percentage points). Yet, men who lost weight after the T2DM diagnosis, are less likely to be diagnosed with major depression (8.3 percentage points). Overall, this appears to suggest the presence of a potentially relevant “protective” effect with respect to the probability of being diagnosed with clinical depression but only among male individuals.Footnote 14
Panels D to F further investigate whether the impact of a T2DM diagnosis on depression might vary by BMI levels. Our estimates show that the increase in depression following a T2DM diagnosis appears to be mainly driven by women in the highest BMI category (4.1 percentage points). Finally, Panels G and H present estimates by two broad age groups (younger and older than 60 years-old) to explore whether age may affect the relationship between a T2DM diagnosis and mental health. Here our findings indicate that the positive and statistically significant impact of a T2DM diagnosis on clinical depression is stronger among the relatively younger age group and also appears to be driven by women. More specifically, women younger than 60 years-old present an increase of 5.5 percentage points in the probability of being diagnosed with clinical depression, while this is lower (2.5 percentage points) among older women.Footnote 15
Sensitivity analysis
In order to check the robustness of our main results, we also present a series of sensitivity tests. Table S4 shows RDD estimates based on a placebo test consisting of alternative cut-off values of the biomarker. This test should not produce a statistically significant effect for our outcome of interest (clinical depression) at values below the 6.5 percent of the biomarker. Indeed, our placebo test using 5.5, 5 and 4.5 percent cut-off values confirm that the impact on major depression is not statistically different from zero. At the 6 percent value of the biomarker we find statistically significant effects, although smaller in magnitude. Yet, this is expected as prediabetic patients (HbA1c ranging from 5.7–6.4 percent) are normally recommended similar non-pharmacological treatments (dietary changes and regular exercise) [53]. At the 7 percent theshold we find slightly larger effects of a T2DM diagnosis on mental health, and this is also in line with the lifestyle changes recommended by doctors to patients with uncontrolled diabetes.
Table S5 further shows the robustness of our results by estimating the fuzzy RDD parametrically by means of different polynomial orders. Importantly, the corresponding estimates obtained are qualitatively similar.
Finally, Table S6 reports RDD estimates produced using a non-parametric local randomization approach focusing on observations within a small neighbourhood around the cut-off [62]. Columns (2)-(6) show non-parametric RDD estimates based on different choices of bandwidth, around the threshold of 6.5 percent. Results confirm that all findings are consistent, similar in magnitude to our baseline estimates, and still statistically significant.
Discussion and conclusions
We contribute to the literature by identifying the causal impact of health information on mental health via the effect of a type-2 diabetes diagnosis on clinical depression. We exploit the exogenous cut-off value in the diagnosis of type-2 diabetes provided by a well-established biomarker (glycated haemoglobin) and information on diagnosed clinical depression drawn from rich administrative longitudinal data from Spain by employing a fuzzy regression discontinuity design. In addition, we explore heterogeneity in the effects on mental health by gender, age, bodyweight as well as the role played by weight losses as a potential mechanism leading to changes in the probability of developing clinical depression, following a diabetes diagnosis.
Our results suggest a statically significant impact of health information on diagnosed clinical depression. However, this appears to be driven by gender as well as weight losses eventually occurring after the diabetes diagnosis. More specifically, the overall increase in the risk of developing clinical depression following a diabetes diagnosis appears to be mostly influenced by women. Moreover, the occurrence of weight losses could be one of the possible mechanisms governing the relationship between a diabetes diagnosis and depression. Here differences by gender are also present: whereas a diabetes diagnosis increases the probability of depression among women who did not lose weight, it substantially decreases the risk of clinical depression among men who lost weight. Interestingly, this may suggest a somewhat protective effect of health information via weight losses for male patients with diabetes. In addition, increases in depression appear to be mainly driven by women in the highest category of BMI (> = 30, corresponding to obesity) and are also larger among relatively younger women as well.
In general, the finding that individuals with healthier behavioural patterns present lower levels of mental disorders is also supported by the medical literature [10,11,12,13,14,15,16]. As for the different effects by gender, these could be explained on several grounds. For instance, the literature suggests that women might have a higher propensity to clinical depression driven by biological factors [71]. Second, evidence also suggests that depression tend to be underdiagnosed among men [71]. Third, the mental health of individuals, especially that of women, with a high baseline weight may not be significantly affected by weight losses [72]. This might be the case in our data as well, where women present higher rates of obesity as well as a higher average baseline (i.e. before a diabetes diagnosis) BMI if compared to male patients (30.9 vs 29.35, respectively, with the difference being statistically significant as suggested by a pairwise t-test). However, further evidence might be needed to firmly establish the reasons behind heterogeneity in the effects by gender.
Importantly, our results differ from the ones of Gaggero [7], to the best of our knowledge the only paper providing causal evidence on diabetes and mental health, who finds no effects on self-reported mental health in a sample of older individuals. The divergence in results might be due to differences in reliability and precision of key variables including our running variable (glycated haemoglobin coupled with a physician’s diagnosis vs Fasting Plasma Glucose) and outcome variable (diagnosed vs self-reported depression) together with differences in the data employed (as our dataset includes health care records based on actual health care use of a large population of individuals followed over 10 years).
Our results might suggest two possible policy implications. A first recomendation might be that individuals at risk of diabetes should be closely monitored not just to avoid the expected physical health complications associated with T2DM, but also to prevent the onset of other relevant and costly mental health conditions such as clinical depression. In addition, our findings seem to suggest that monitoring should be specifically targeted at women, especially those who did not lose weight following a diagnosis and those who are obese. This may also imply that interventions aimed at reducing psycholigical distress among severely overweight women also include the promotion of healthy behaviours, including following an exercise regimen and a low-carbohydrate diet to decrease bodyweight and this could also help control T2DM. Ultimately, this could be linked to the larger debate around the relevance of changes in health behaviours in influencing mental health, showing that healthier habits such as regular physical activity, healthy eating and adequate sleep can result into improved mental health for people with other chronic conditions beyond T2DM [73,74,75,76]. As usual, this study may have some potential limitations. First, as our dataset does not include comprehensive information on the medications prescribed by physicians, we might not be able to account for those pharmaceuticals potentially affecting mental health. Second, the administrative records used here only includes a relatively limited number of variables proxying socioeconomic status. While this might not necessarily be a major issue given that the Spanish health care system is universal and free at the point of delivery, we might not be able to identify potentially informative socioeconomic gradients. Despite such limitations, our study provides novel empirical evidence on the causal impact of health information on mental health, shedding light on gender-based differences in such effects and potential mechanisms through changes in lifestyle behaviours.
Availability of data and materials
The data that support the findings of this study are available from BSA (Badalona Serveis Assistencials) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Notes
Data from both the Catalan and the Spanish National Statistical Institutes for the year 2010 (the year where the last wave of the data used here was collected) show that sociodemographic characteristics of individuals residing in the city of Badalona seem to match very closely national averages concerning age and gender with small differences in employment/activity (65.5% vs 60.2% in Badalona and Spain, respectively) and share of immigrants (around 15% vs 12.2%). Our sample also appears to closelyresemble main observables of the city of Badalona, with the exception of immigrant status (9.5% vs. 15% in our sample vs the city of Badalona) and employment rate (71% vs. 65.5%). As for all routinely collected administrative datasets, it should be noted that the data used here are drawn from administrative records of all individuals who used health care services in the province of Badalona during the period 2004–2010. As such, these are not meant to be statistically representative of the city of Badalona or the Spanish population and some differences might be expected.
These two terms are synonymous and can be used interchangeably.
That is, at least 8 over 17 symptoms need to be present to diagnose depression using the Hamilton scale, HRSD; 7 over 18 symptoms using the Golderbg scale, GADS (including at least 5 anxiety symptoms together with 2 depression symptoms); and 20 over 35 symptoms for the 35-item Geriatric scale and at least 5 over 15 for its short-form version, GDRS. Note that although we know that, according to the guidelines of the corresponding local health authority, these three psycometrically validated measures were used by physicians to diagnose clinical depression, our data just include a dummy variable defining a patient’s final diagnosis of clinical depression based on such measures, i.e. our data do not include the specific items/symptoms used within each measure. Yet, a detailed description of each item/symptom can be found [46, 49, 50]. A comprehensive discussion of the differences between depression scales can be found in Edelstein et al. [51].
While HbA1c was universally considered the main tool to diagnose diabetes only from 2009 [52], the Spanish health care system (as many other health care systems) was routinely using it to diagnose diabetes during the years covered by this analysis.
A series of papers suggest that HbA1c tests administered on previously undiagnosed individuals have generally a very high specificity (i.e., the ability to correctly identify individuals who do not have diabetes) normally around 97% and a more variable sensitivity (i.e., the ability to correctly identify individuals who have diabetes) ranging between around 50% to 83%, depending on the populations considered [54, 55]. Because of the latter, the IEC also recommends that a T2DM diagnosis should be confirmed by a repeated test, unless the diagnosis is clear on clinical grounds [52]. The current practice in Spain [56] is to retest the HbA1c level when the level is close to 6.5% after 3 to 6 months. According to recent evidence, this practice would help identifying more true-positives, potentially raising the sensitivity of the test [57]. Here we employ within-year mean value of HbA1c tests, exploiting the presence of repeated measurements.
Note that in our dataset patients usually present multiple HbA1c measurements per year. In order to account for this, our models employ the within-year mean value of HbA1c. Note that results are similar when using the last HbA1c measurement of the corresponding year. In addition, given the differences between type-1 and type-2 diabetes and related treatments, we dropped all individuals with type-1 diabetes.
The initial sample includes 123,453 individuals. Since our analysis relies on the information provided by the biomarkers, we include in our sample those individuals with at least one measurement per year, resulting into 13,971 individuals (corresponding to 39,994 obs.). Given that almost all individuals in the sample who are at risk of or have been diagnosed with T2DM present at least one biomarker measurement per year, the exclusion of individuals with no biomarker measurements is unlikely to bias the results of our empirical analysis.
The relatively high clinical depression rate might be in part due to the fact that our sample includes older individuals most of whom are diagnosed with T2DM.
In this analysis, once individuals are diagnosed with type-2 diabetes, they are also considered diabetics in the subsequent waves. This appears to be a realistic assumption in this case as T2DM is a chronic condition and individuals might eventually achieve what is now defined as “remission” only after drastic lifestyle changes (also note that there still appears to be a debate around the actual definition of a T2DM remission [59].
In practice, we allow the function form to vary on either side of the cut-off by including an interaction term between the binary variable \(Abov{e}_{it}\) and the normalised running variable.
In a recent paper Gelman and Imbens [61] advice against the use of polynomial orders higher than 2, given their poor properties (they appear to be more sensitive to outcome values of far distant observations from the cut-off point and yield inaccurate confidence intervals). Accordingly we use a simple linear parametric approach as our baseline estimates. However, we also present estimates based on polynomials 2–4 as a robustness check.
We also produced heteroskedasticity-robust standard errors according to Kolesár and Rothe [64] as these are often recommended when the number of support points around the cut-off is sufficiently large and are based on a smaller bandwidth. Results are similar and available upon request.
We also estimated the McCrary test obtaining an estimated log discontinuity in the density of \(\widehat{\theta }\)= – 0.071 (s.e. 0.0157), failing to reject the null hypothesis of no discontinuity. Similarly, following Cattaneo et al. [68] we obtained a manipulation test score of T =—0.4614 (p-value of 0.6445), also suggesting no evidence of systematic manipulation of the running variable.
Due to the lack of observations and corresponding limited sample, we could not explore whether the impact of a T2DM on depression might vary by smoking or drinking cessation.
We find similar results when considering 65 years-old (these are available upon request).
Abbreviations
- ADA:
-
American Diabetes Association
- COPD:
-
Chronic obstructive pulmonary disease
- EU:
-
European Union
- FPG:
-
Fasting Plasma Glucose
- GADS:
-
Golberg Anxiety and Depression Scale
- GP:
-
General practitioner
- HRSD:
-
Hamilton Rating Scale for Depression
- HbA1c:
-
Glycated hemoglobin
- ICPC:
-
International Classification of Primary Care
- IEC:
-
International Expert Committee
- RDD:
-
Regression discontinuity design
- T2DM:
-
Type 2 diabetes mellitus
- 2SLS:
-
Two-stage least squares
References
Patel MS, Asch DA, Volpp KG. Wearable devices as facilitators, not drivers, of health behavior change. J Amer Med Assoc. 2015;313(5):459–60. https://doi.org/10.1001/jama.2014.14781.
Jo A, Coronel BD, Coakes CE, Mainous AG III. Is there a benefit to patients using wearable devices such as Fitbit or Health Apps on Mobiles? A systematic review. Am J Med. 2019;132(12):1394–400.
Jazieh AR, Foraida M, Ghouse M, Khalil MM, Kopp M, Savidge M. The impact of cancer diagnosis on the lifestyle and habits of patients served at a Veterans Administration Hospital. J Cancer Educ. 2006;21(3):147–50.
Burris JL, Studts JL, DeRosa AP, Ostroff JS. Systematic review of tobacco use after lung or head/neck cancer diagnosis: results and recommendations for future research. Cancer Epidem Biomar. 2015;24(10):1450–61.
Zhao M, Konishi Y, Glewwe P. Does information on health status lead to a healthier lifestyle? Evidence from China on the effect of hypertension diagnosis on food consumption. J Health Econ. 2013;32(2):367–85.
Kim HB, Lee SA, Lim W. Knowing is not half the battle: impacts of information from the National health screening program in Korea. J Health Econ. 2019;65:1–14.
Gaggero A. The effect of type 2 diabetes diagnosis in the elderly. Econ Hum Biol. 2020;37:1–23.
Gaggero A, Gil J, Jiménez-Rubio D, Zucchelli E. Does health information affect lifestyle behaviours? The impact of a diabetes diagnosis. Soc Sci Med. 2022;31(4):115420.
Iizuka T, Nishiyama T, Chen B, Eggleston K. False alarm? Estimating the marginal value of health signals. J Public Econ. 2021;195: 104368.
Conry MC, Morgan K, Curry P, McGee H, Harrington J, Ward M, Shelley E. The clustering of health behaviours in Ireland and their relationship with mental health, self-rated health and quality of life. BMC Public Health. 2011;11(1):1–10.
Conway KP, Swendsen J, Husky MM, He JP, Merikangas KR. Association of lifetime mental disorders and subsequent alcohol and illicit drug use: results from the National Comorbidity Survey-Adolescent Supplement. J Am Acad Child Psy. 2016;55(4):280–8.
Scott D, Happell B. The high prevalence of poor physical health and unhealthy lifestyle behaviours in individuals with severe mental illness. Issues Ment Health N. 2011;32(9):589–97.
Jacka FN, Pasco JA, Mykletun A, Williams LJ, Hodge AM, O’Reilly SL, Nicholson GC, Kotowicz MA, Berk M. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry. 2010;167(3):305–11. https://doi.org/10.1176/appi.ajp.2009.09060881.
Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiat. 2010;67(3):220–9.
Stathopoulou G, Powers MB, Berry AC, Smits JA, Otto MW. Exercise interventions for mental health: a quantitative and qualitative review. Clin Psychol-Sci Pr. 2006;13(2):179.
Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. J Amer Med Assoc. 2000;284(20):2606–10.
Pan A, Lucas M, Sun Q, van Dam RM, Franco OH, Manson JE, Willet WC, Ascherio A, Hu FB. Bidirectional association between depression and type 2 diabetes mellitus in women. Arch Intern Med. 2010;170(21):1884–91. https://doi.org/10.1001/archinternmed.2010.356.
Mezuk B, Johnson-Lawrence V, Lee H, Rafferty JA, Abdou CM, Uzogara EE, Jackson JS. Is ignorance bliss? Depression, antidepressants, and the diagnosis of prediabetes and type 2 diabetes. Health Psychol. 2013;32(3):254.
Deschênes SS, Burns RJ, Schmitz N. Associations between diabetes, major depressive disorder and generalized anxiety disorder comorbidity, and disability: Findings from the 2012 Canadian Community Health Survey—Mental Health (CCHS-MH). J Psychosomatic Res. 2015;78(2):137–42.
Feng X, Astell-Burt T. Impact of a type 2 diabetes diagnosis on mental health, quality of life, and social contacts: a longitudinal study. BMJ Open Diabetes Res Care. 2017;5(1):e000198. https://doi.org/10.1136/bmjdrc-2016-000198.
Robinson DJ, Coons M, Haensel H, Vallis M, Yale JF. Diabetes and mental health. Can J Diabetes. 2018;42(Suppl. 1):S130–41.
Saito I, Inami F, Ikebe T, Moriwaki C, Tsubakimoto A, Yonemasu K, Ozawa H. Impact of diabetes on health-related quality of life in a population study in Japan. Diabetes Res Clin Pr. 2006;73(1):51–7. https://doi.org/10.1016/j.diabres.2005.11.015.
Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes: A prospective population-based study. Diabetes Care. 1996;19(10):1097–102. https://doi.org/10.2337/diacare.19.10.1097.
Estrodi E, Kenardy J. Psychosocial and non-psychosocial risk factors for the new diagnosis of diabetes in elderly women. Diabetes Res Clin Pr. 2006;74(1):57–65. https://doi.org/10.1016/j.diabres.2006.02.011.
World Health Organization. Depressive disorder (depression). 2023. Retrieved from Depressive disorder (depression) (who.int).
International Diabetes Federation. IDF Diabetes Atlas, 10th edn. Brussels, Belgium: International Diabetes Federation, 2021.
Kahn ME. Health and labor market performance: the case of diabetes. J Labor Econ. 1998;16(4):878–99.
Zhang X, Zhao X, Harris A. Chronic diseases and labour force participation in Australia. J Health Econ. 2009;28(1):91–108.
Frijters P, Johnston DW, Shields MA. The effect of mental health on employment: evidence from Australian-30 panel data. Health Econ. 2014;23(9):1058–71.
Bubonya M, Cobb-Clark DA, Wooden M. Mental health and productivity at work: Does what you do matter? Labor Econ. 2017;46:150–65.
Grossman M. On the concept of health capital and the demand for health. J Polit Econ. 1972;80(2):223–55.
Cawley J, Ruhm CJ. The economics of risky health behaviors. In: Pauly M, McGuire TG, Barros PP, editors. Handbook of Health Economics. Elsevier; 2011. p. 95–199.
Bhargava S, Loewenstein G, Sydnor J. Choose to lose: health plan choices from a menu with dominated option. Q J Econ. 2017;132(3):1319–72.
Kettlewell N. Policy choice and product bundling in a complicated health insurance market: do people get it right? J Hum Resour. 2020;55(2):566–610.
Arni P, Dragone D, Goette L, Ziebarth NR. Biased health perceptions and risky health behaviors—Theory and evidence. J Health Econ. 2021;21(76):102415. https://doi.org/10.1016/j.jhealeco.2021.10245.
Brown DJ, Schrader LF. Cholesterol information and shell egg consumption. Amer J Agr Econ. 1990;72(3):548–55.
Chern WS, Loehman ET, Yen ST. Information, health risk beliefs and the demand for fats and oils. Rev Econ Stat. 1995;77(3):555–64. https://doi.org/10.2307/2109915.
Kim SR, Chern WS. Alternative measures of health information and demand for fats and oils in Japan. J Consum Aff. 1999;33:92–109.
Roosen J, Marette S, Blanchemanche S, Verge P. Does health information matter for modifying consumption? A field experiment measuring the impact of risk information on fish consumption. Rev Agr Econ. 2009;31(1):2–20.
Allais O, Etilé F, Lecocq S. Mandatory labels, taxes and market forces: An empirical evaluation of fat policies. J Health Econ. 2015;43:27–44. https://doi.org/10.1016/j.jhealeco.2015.06.003.
Fichera E, von Hinke S. The response to nutritional labels: Evidence from a quasi-experiment. J Health Econ. 2020;72.
Fukuma S, Iizuka T, Ikenoue T, Tsugawa Y. Association of the National Health Guidance Intervention for obesity and cardiovascular risks with health outcomes among Japanese men. J Amer Med Assoc Internal Med. 2020;180(12):1630–7.
American Diabetes Association. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43(Suppl. 1):S14–S31.
Bernal-Delgado E, García-Armesto S, Oliva J, Martínez FI, Repullo JR, Peña-Longobardo LM, Ridao-López M, Hernández-Quevedo C. Spain-Health System Review 2018. Health Systems in Transition, Vol. 20(2). European Observatory on Health Systems and Policies. Spain: health system review 2018 (who.int)
Hamilton M. Development of a rating scale for primary depressive illness. Brit J Clin Psychol. 1967;6:278–96.
Goldberg D, Bridges K, Duncan-Jones P, Grayson D. Detecting anxiety and depression in general medical settings. Brit Med J. 1988;297(6653):897–9.
Goldberg IK. Questions and answers about depression and its treatment: a consultation with a leading psychiatrist. Charles Press Publishers; 1993. ISBN-13: 978-0914783688.
Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. https://doi.org/10.1016/0022-3956(82)90033-4.
Herrmann N, Mittmann N, Silver IL, Shulman KI, Busto UA, Shear NH, Naranjo CA. A validation study of the Geriatric Depression Scale short form. Int J Geriatr Psych. 1996;11(5):457–60.
Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton depression rating scale. J Affect Disorders. 2013;150(2):384–8.
Edelstein BA, Bamonti PM, Gregg JJ, Gerolimatos LA. Depression in later life. Encyclopedia Appl Psychol. 2004: 593–599.
International Expert Committee. International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care. 2009;32(7):1327–34.
Mata M, Cos FX, Morros R, Diego L, Barrot J, Berengué M. et al.. Abordatge de la Diabetes Mellitus Tipus 2. 2a. Edició. Institut Català de la Salut 2013 (accessible at Abordatge de la diabetis mellitus tipus 2: guies de pràctica clínica).
Kaur G, Lakshmi PVM, Rastogi A, Bhansali A, Jalin S, et al. Diagnostic accuracy of tests for type 2 diabetes and prediabetes; A systematic review and meta-analysis. PLoS ONE. 2020;15(11): e0242415.
Rohlfing CL, Little RR, Wiedmeyer HM, England JD, Madsen R, Harris MI, Flegal KM, Eberthardt MS, Goldstein DE. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the US population. Diabetes Care. 2000;23(2):187–91.
Fernández SCM, Gamarra OJ. Manejo práctico de la diabetes mellitus tipo 2. Grupo Diabetes SEMG. España. 2013;36.
Oke JL, Stevens RJ, Gaitskell K, Farmer AJ. Establishing an evidence base for frequency of monitoring glycated haemoglobin levels in patients with Type 2 diabetes: projections of effectiveness from a regression model. Diabet Med. 2012;29(2):266–71.
Ho-Pham LT, Nguyen UD, Tran TX, Nguyen TV. Discordance in the diagnosis of diabetes: Comparison between HbA1c and fasting plasma glucose. PLoS One. 2017;12(8):e0182192. https://doi.org/10.1371/journal.pone.0182192.
Holst JJ, Madsbad S. What is diabetes remission? Diabetes Ther. 2021;12(3):641–6. https://doi.org/10.1007/s13300-021-01032-y.
Hahn J, Todd P, van der Klaauw W. Identification and estimation of treatment effects with regression-discontinuity design. Econometrica. 2001;69(1):201–9.
Gelman A, Imbens G. Why high-order polynomials should not be used in regression discontinuity designs. J Bus Econ Stat. 2019;37(3):447–56. https://doi.org/10.1080/07350015.2017.1366909.
Cattaneo MD, Idrobo N, Titiunik R. A Practical Introduction to Regression Discontinuity Designs (Elements in Quantitative and Computational Methods for the Social Sciences). Cambridge University Press; 2020.
Lee DS, Card D. Regression discontinuity inference with specification error. J Econometrics. 2008;142(2):655–74. https://doi.org/10.1016/j.jeconom.2007.05.003.
Kolesár M, Rothe C. Inference in regression discontinuity designs with a discrete running variable. Am Econ Rev. 2018;108(8):2277–304.
Angrist JD, Imbens GW, Rubin DB. Identification of Causal Effects Using Instrumental Variables. J Am Stat Assoc. 1996;91(434):444–55.
Lee DS, Lemieux T. Regression discontinuity designs in economics. J Econ Lit. 2010;48(2):281–355. https://doi.org/10.1257/jel.48.2.281.
McCrary J. Manipulation of the Running Variable in the Regression Discontinuity Design: A Density Test. J Econometrics. 2008;142(2):698–714.
Cattaneo MD, Jansson M, Ma X. Manipulation testing based on density discontinuity. Stand Genomic Sci. 2018;18(1):234–61.
Seuring T, Serneels P, Suhrcke M, Bachmann M. Diabetes, employment and behavioural risk factors in China: Marginal structural models versus fixed effects models. Econ Hum Biol. 2020;39:100925. https://doi.org/10.1016/j.ehb.2020.100925.
Siddiqui MA, Khan MF, Carline TE. Gender differences in living with diabetes mellitus. Materia Socio Medica. 2013;25(2):140–2.
Deischinger C, Dervic E, Leutner M, Kosi-Trebotic L, Klimek P, Kautzky A, Kautzky-Willer A. Diabetes mellitus is associated with a higher risk for major depressive disorder in women than in men. BMJ Open Diabetes Res Care. 2020;8(1): e001430.
Simon GE, Rohde P, Ludman EJ, Jeffery RW, Linde JA, Operskalski BH, Arterburn D. Association between change in depression and change in weight among women enrolled in weight loss treatment. Gen Hosp Psychiatry. 2010;32(6):583–9.
Dale H, Brassington L, King K. The impact of healthy lifestyle interventions on mental health and wellbeing: a systematic review. Ment Health Rev J. 2014;19(1):1–26.
Marquez DX, Aguiñaga S, Vásquez PM, Conroy DE, Erickson KI, Hillman C, Stillman CM, Ballard RM, Sheppard BB, Petruzzello SJ, King AC. A systematic review of physical activity and quality of life and well-being. Transl Behav Med. 2020;10(5):1098–109.
Li C, Ford ES, Mokdad AH, Jiles R, Giles WH. Clustering of multiple healthy lifestyle habits and health-related quality of life among US adults with diabetes. Diabetes Care. 2007;30(7):1770–6.
Campbell HM, Khan N, Cone C, Raisch DW. Relationship between diet, exercise habits, and health status among patients with diabetes. Res Social Adm Pharm. 2011;7(2):151–61.
Acknowledgements
We would like to thank participants to the EvaluAES session of the Conference of the Spanish Health Economics Association (Zaragoza, June 2022) for their useful suggestions and two anonymous referees for their highly valuable comments. We gratefully acknowledge financial support from the Spanish Ministry of Science, Innovation and Universities (grant number PID2019-105688RB-I00). Eugenio Zucchelli also acknowledges support from the Tomás y Valiente Fellowship funded by the Madrid Institute for Advanced Study (MIAS), Universidad Autónoma de Madrid (UAM), and grants H2019/HUM-5793 and PID2019-111765GB-I00 funded by the Regional Government of Madrid and the Spanish Ministry of Science, Innovation and Universities, respectively.
Funding
The study benefited from the financial aid received from the Spanish Ministry of Science, Innovation and Universities (grant number PID2019-105688RB-I00); the Tomás y Valiente Fellowship, Madrid Institute for Advanced Study (MIAS), Universidad Autónoma de Madrid (UAM), the Regional Government of Madrid (grant number H2019/HUM-5793) and the Spanish Ministry of Science, Innovation and Universities (grant number PID2019-111765 GB-I00).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the design of the study, econometric analysis and drafting of the manuscript, and all of them reviewed and agreed on the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Summary Statistics - By T2DM Diagnosis Status. Table S2. Summary Statistics - By Gender. Table S3. Summary Statistics - By Weight Loss Status. Table S4. RDD Estimates of the Impact of a T2DM Diagnosis on Depression - Different Cut-offs. Table S5. RDD Estimates of the Impact of a T2DM Diagnosis on Depression – Different Polynomials. Table S6. Fuzzy RDD Estimates - Non-Parametric Approach.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gaggero, A., Gil, J., Jiménez-Rubio, D. et al. Sick and depressed? The causal impact of a diabetes diagnosis on depression. Health Econ Rev 13, 38 (2023). https://doi.org/10.1186/s13561-023-00451-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13561-023-00451-w