Abstract
Introduction
This study reviewed the economic evidence of rapid HIV testing versus conventional HIV testing in low-prevalence high-income countries; evaluated the methodological quality of existing economic evaluations of HIV testing studies; and made recommendations on future economic evaluation directions of HIV testing approaches.
Methods
A systematic search of selected databases for relevant English language studies published between Jan 1, 2001, and Jan 30, 2019, was conducted. The methodological design quality was assessed using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) and the Drummond tool. We reported the systematic review according to the PRISMA guidelines.
Results
Five economic evaluations met the eligibility criteria but varied in comparators, evaluation type, perspective, and design. The methodologic quality of the included studies ranged from medium to high. We found evidence to support the cost-effectiveness of rapid HIV testing approaches in low-prevalence high-income countries. Rapid HIV testing was associated with cost per adjusted life year (QALY), ranging from $42,768 to $90,498. Additionally, regardless of HIV prevalence, rapid HIV testing approaches were the most cost-effective option.
Conclusions
There is evidence for the cost-effectiveness of rapid HIV testing, including the use of saliva-based testing compared to usual care or hospital-based serum testing. Further studies are needed to draw evidence on the relative cost-effectiveness of the distinct options and contexts of rapid HIV testing.
Similar content being viewed by others
Introduction
Human immunodeficiency virus (HIV) infection is an important contributor to disease’s global burden and a leading cause of death [1]. With the advent of antiretrovirals and treatment regimens, the disease, when diagnosed early, can be managed. As a result, HIV patients now have an improved quality of life and comparable life expectancies with persons uninfected with HIV [2]. The process of achieving this improved quality of life can be represented by the internationally recognized framework known as the cascade or continuum of care [2, 3]. This framework begins with disease diagnosis through HIV testing, linkage to care, retention in treatment programs, maintenance of treatment adherence and finally sustained viral suppression [4, 5].
HIV testing, early diagnosis and effective treatment improve outcomes significantly for infected individuals and the communities they live [6, 7]. This premise forms the foundation for the highly effective “treatment as prevention” approach [2, 8]. Getting people aware of their HIV status has been the focus of HIV control agencies. However, recent UNAIDS data shows that about 50% of people living with HIV are unaware of their diagnosis; for example, Canada, France, Spain and the United States report a substantial proportion of undiagnosed HIV cases [9,10,11,12,13]. Rates of undiagnosed HIV tend to be higher among men who have sex with men, youth and minority population groups [11].
There are currently several HIV testing approaches, including serum and saliva-based screening tests [14,15,16,17,18,19]. Serum-based testing can be categorized based on the duration to receipt of the test result. In conventional HIV testing, the serum-based results are usually available within a week; however, this may require the client to return to the facility to receive the result. In contrast, rapid testing approaches provide results within 24 h and do not require clients to return for results notification.
There have been clinical effectiveness studies of the various HIV testing approaches; however, economic evaluations of the different approaches from non-American perspectives are lacking. Economic studies have been conducted in high prevalence low-income African countries [20] and in high prevalence communities in the United States and Europe [21,22,23,24,25]. Individual studies and systematic reviews considered the effectiveness of rapid HIV testing [17, 26,27,28] and cost-effectiveness studies of rapid HIV testing options [29]. Still, there is no review focused on rapid HIV testing’s economic evidence compared to conventional HIV testing in low-prevalence, high-income countries. We focus on these countries because they tend to have similar epidemiology and comparable health care systems.
We seek to address this specific HIV testing evidence gap given the potential to increase access to HIV care and treatment programs to following the United Nations 90–90-90 goals. In particular, the first goal seeks to have 90% of all people living with HIV know their HIV status by 2020 [1]. This systematic review focuses on North America, Australia and Western Europe, areas with low HIV prevalence and high incomes with similar HIV epidemiology. These jurisdictions would benefit from economic evaluation of HIV testing to make informed decisions about cost-effective HIV screening programming and judicious use of health care resources.
The specific aims of this systematic review are to (i) search, select, appraise and synthesize published economic evaluations of HIV testing options; (ii) evaluate the methodological quality of the economic evaluations of HIV testing; (iii) make recommendations regarding future directions for economic evaluation of HIV testing approaches. This review also focuses on the strength and quality of evidence addressing the cost-effectiveness of rapid HIV testing approaches versus conventional testing approaches in managing HIV infection.
Methods
This work is a systematic review of available literature on any rapid HIV testing approach’s economic evaluations versus conventional serum-based HIV testing. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement in this article’s reporting [30].
Search strategy/process
We searched the medical literature in Medline (indexed, in-process and other non-), Embase, NHS EED and Tufts Cost-effectiveness analysis (CEA) registry to retrieve all relevant literature. Text words used in the search include ‘economic evaluation’, ‘cost’, ‘cost-effectiveness’, ‘cost-benefit’ or ‘cost-utility’, ‘rapid HIV testing, and ‘HIV testing’. Due to the review team’s limited language competency/expertise and resources, only English language studies were included in the search strategy. The review team also considered studies conducted between Jan 1, 2001, and Jan 30, 2019, in North America, Australia or Western Europe.
Inclusion criteria
For this review, the inclusion criteria were as follows: an economic evaluation study design that was either an economic evaluation, a clinical trial or model-based evaluation conducted in North America, Australia or Western Europe, involving adult patients aged 16 years and older tested for HIV using at least two of the following four HIV testing approaches (i) whole blood/serum-based hospital-based testing (also referred to as conventional HIV testing approaches); (ii) rapid hospital-based testing; (iii) rapid location-based testing; and (iv) rapid mobile testing.
This review excluded saliva-based testing due to concerns about test performance. Specifically, saliva-based testing options were associated with lower specificity when self-administered than healthcare provider administered tests [31, 32].
Rapid HIV testing has the following three components: (i) voluntary enrolment, (ii) rapid testing with results available within 24 h and (iii) provision of counselling at the delivery of results and treatment options.
The economic evaluations considered in this review included cost-effectiveness, cost-utility or cost-benefit analysis. These would include any of the following outcomes: (i) cost per quality-adjusted life years (QALY); (ii) cost per HIV test; (iii) cost per HIV transmission prevented; or (iv) total cost of HIV testing program. We excluded studies if they considered only one testing approach with no comparator. Additionally, we excluded cost minimization studies because these are not formal economic evaluations and usually are costing exercises where there is no difference in the effect of the comparators [33,34,35].
Finally, due to the difference in HIV epidemiology and characteristics of the health care systems, economic evaluations from Africa, Eastern Europe and Asia were excluded from this review.
Data abstraction
Two independent reviewers (OM and AL) selected eligible publications initially based on titles and abstracts. Potentially relevant articles were abstracted using standardized data abstraction form. Disagreement between reviewers was resolved by a third reviewer (DC).
Descriptive data was collected for each economic evaluation, including study objectives, perspective, analysis type and study design, sample size and population studied. Other considerations include comparator(s), intervention, results, and conclusions. Costs have been adjusted to reflect 2018 United States dollars using international exchange rates and the United States Bureau of Labour Statistics inflation calculator for medical costs [36].
Quality assessment
The methodological quality of HIV testing economic evaluations was assessed using two tools: Drummond’s ten-point criteria for economic evaluations and the 24-item Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist [37,38,39]. For both lists, each item was scored as ‘Yes’ (met the quality criterion), or ‘No’ (did not meet the quality criterion), or ‘Can’t tell’ where there was insufficient evidence to make a decision. A numeric score was not calculated for each study.
For the CHEERS criteria, the “Yes” responses were weighed against the total number of criteria for a percentage. This approach has been used in recently published systematic reviews of economic evaluations [40,41,42,43].
The two checklists used had slightly different focus but were nonetheless complimentary. While the Drummond checklist assesses appropriate methodology in the economic evaluation and evaluates the results’ validity, the CHEERS checklist focuses on reporting issues. Using the CHEERS checklist, studies were assessed into three categories: high if they satisfied greater than 75% of the criteria, average (50–75%) and low quality when less than 50% of the criteria was satisfied.
Results
Literature search and screening
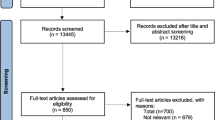
The initial search resulted in 1524 records. Five studies met our inclusion criteria for this systematic review (See Fig. 1). Studies were excluded because of a lack of comparators in the economic evaluation resulting in the studies being categorized as costing studies. Other reasons for exclusion included studies conducted in jurisdictions outside of specified geographic location, evaluations of hospitals or organization-specific testing programs that were not explicitly evaluations of HIV testing approaches.
Study and patient characteristics
Five primary articles met the inclusion criteria and were considered for data analysis and synthesis [44,45,46,47,48]. An overview of the study and methodological characteristics, study populations, interventions, and outcomes of the five economic evaluations included in the review are provided in Table 1. The earliest economic evaluations in this review were published in 2005 [47, 49], and the remaining three were published between 2010 and 2012 [44,45,46]. All the included studies in this review were conducted in the United States.
All the included studies were model-based economic evaluations comprised of two cost-effectiveness studies [45, 46] and three cost-utility studies [44, 47, 48].
Sanders et al. [46] was conducted from the healthcare insurer’s perspective, and the remaining four [44, 45, 47, 48] were from a societal perspective. Four studies [45,46,47,48] considered a lifetime horizon in the evaluation, and one study [32] considered a 20-year time horizon.
All studies included populations considered at high risk for HIV (prevalence higher than 1%), such as injection drug users and inner-city US populations as well as members of the general population with assumed prevalence higher than 1%, while the general population prevalence was approximately 0.1% [44,45,46,47,48].
Comparative interventions
Comparisons considered in the included studies include one-time and repeat interval rapid screening, rapid emergency department (ED) testing versus usual care, various rapid testing approaches [44,45,46, 48]. While varied, these approaches have the common theme of a rapid HIV testing arm compared to the usual standard of care or other rapid HIV testing approaches.
All five studies reported outcomes as cost per quality-adjusted life-years and found that rapid HIV testing approaches were cost-effective. Sanders et al., [46] found nurse-initiated routine screening with rapid HIV testing and streamlined counselling was more cost-effective at $42,769/QALY, while Dowdy et al., [45] found targeted HIV screening in emergency departments cost-effective at $90,498.34 per QALY. Furthermore, at a willingness to pay a threshold of $100,000 per QALY gained, this option was cost-effective in 89% of simulated scenarios [45, 46].
The comparisons considered in the included studies include one-time and repeat interval rapid screening [44] and various rapid testing approaches, including oral testing [45,46,47].
When varying prevalence of HIV was considered, rapid HIV screening was found to range in cost-effectiveness from $50,429/QALY in settings with at least 1% HIV prevalence to $91,171/QALY in settings with 0.1% HIV prevalence [48]. Another study by Paltiel et al., [47] found that one-time screening costs $51,284/QALY among high-risk populations. The study further found that testing every 5 years costs $71,227/QALY, and by reducing the frequency of testing to every 3 years, it cost $89,746/QALY [47].
Study quality assessment
Table 2 presents the distribution of scores across each of the 10-item Drummond checklist according to whether or not each study fulfilled the criterion (or was not applicable) in terms of study design, execution and reporting of relevant information on methods used in the study [50]. Across included studies, we noticed variability in methodological quality.
Specific concerns on the Drummond checklist showed that most studies did not accurately measure the costs and consequences or justify that the valuation costs and consequences were credible (See Table 2). Paltiel [47] and Walensky [48] did not adequately report five of the ten criteria identified on the Drummond checklist. These studies were considered lower quality relative to the other studies.
We present a summary of the included studies’ quality evaluation based on the CHEERS checklist in Table 3. Based on this assessment, all included studies were assessed as high quality.
Our review found that most criteria were adequately reported. We note that some criteria were generally underreported. For example, the abstract usually did not have enough information about the base case and the outcome’s uncertainty. We also identify that none of the studies adequately reported the populations and methods used to elicit outcome preferences. There were also concerns about how the studies reported the analytic methods used in the evaluations.
All included studies [44,45,46,47,48] performed a sensitivity analysis and provided varying depths of reporting about the sensitivity designs used (Table 4). Sanders et al. [46] conducted a probabilistic sensitivity analysis (PSA), Paltiel et al. [47] conducted both univariate and multivariate deterministic analysis. The remaining three evaluations conducted only univariate deterministic sensitivity analysis [44, 45, 48].
Table 4 shows the modelling approaches used by the various evaluations included in the analysis. One study [45] was a decision analysis model, three studies [46,47,48] were transition simulation models, and the last evaluation used a dynamic compartmental model [44]. Two [47, 48] of the transition model evaluations used four-state transition using Cost-Effectiveness of Preventing AIDS Complications (CEPAC) data [51]. The last model-based study used a seven-state model [46].
Discussion
Economic analysis is imperative to assist with rational decision-making about the allocation of limited health resources. It seeks to provide information about the value of competing health interventions. Our review found five studies that reported economic evaluations of rapid HIV testing approaches in North America. While our inclusion criteria expanded to studies conducted in Western Europe and Australia, we could not find any such evaluations from these countries. For this review, we have adjusted all cost figures to 2018 United States dollars. Economic evaluations reported a wide variation in comparators’ use, evaluation type, perspective, and design; thus, the estimates’ statistical pooling was not feasible.
The studies included in this analysis show that rapid HIV testing approaches, including saliva-based screening tests, may be a cost-effective option compared to usual care or hospital-based serum testing options. The conclusion from the highest methodological quality studies (studies that satisfied most of the conditions identified on the Drummond checklist) showed that rapid testing approaches were cost-effective compared to conventional hospital-based serum HIV testing with an ICER between $36,081 per QALY and $39,376 per QALY [44].
Lower-quality studies also showed that rapid HIV testing approaches were cost-effective at an estimated $51,284 per QALY. We found an increase in the cost per QALY when the test was used in populations with a lower prevalence of HIV increasing to about $91,171 per QALY [47].
Our study considered economic evaluations from high-income countries in North America, Europe, and Australia, where disease pattern is similar. We did not find studies from any country other than the United States; therefore, the estimates provided reflect more of the American health care system. While these ICERs are for the most above the $50,000 per QALY threshold, they are below the higher limits of the $100,000 threshold considered acceptable in some higher-income countries [52,53,54,55,56,57].
These higher ICER thresholds may potentially reflect an overestimation of the ICER because of the nature of the healthcare system that has shown a trend towards a willingness to pay threshold of US$ 150,000 per QALY gained [58]. These ICER values would be considered acceptable in most of the target countries and jurisdictions. In Canada, for example, the ICER values would be considered acceptable because they are below the maximum of the commonly used Canadian threshold of between $20,000 - $100,000 per QALY gained. In some circumstances, when considering high prevalence populations, the ICER value is lower than $50,000 per QALY gained threshold [34, 53]. While Australia and the United Kingdom do not have a fixed threshold value for ICER given the recommended ICER thresholds, some of the ICER amounts would be considered not acceptable [52, 57].
Two of our included studies [47, 48] used the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) model [51], a mathematical simulation of the detection, natural history and treatment of HIV disease in the US and it is thus expected that the findings would be consistent. With the variations in the settings and populations of interest, rapid HIV testing approaches remained cost-effective compared to conventional approaches.
Most of the studies considered rapid HIV testing as an approach resulting in early detection of disease with subsequent connection to care and treatment shown to result in improved outcomes and the prevention of new cases of HIV. The outcome measures included cost per QALY gained, cost per HIV test and cost per test notification. These outcomes are important because early HIV diagnosis benefits extend beyond potential immediate improvements to individual client health outcomes. Beyond the individual, it includes other considerations such as preventing new HIV transmission. These outcomes are however not adequately reported.
Finally, it is essential to highlight that none of the included studies explicitly considered equity factors, including place of residence, race/ethnicity/culture/language, occupation, gender/sex, religion, education, socioeconomic status, and social capital [59]. The non-inclusion of equity factors is likely because included studies considered the traditional approach of economic evaluation that ‘a QALY is a QALY’ that assumes all outcomes should be weighted equally, regardless of the characteristics of people receiving [33]. There is a school of thought that considers this a value judgment that is questionable when applied to public health and suggest that equity considerations should be incorporated in economic evaluations [60,61,62].
Limitations
We identified a few limitations. All studies were conducted in the United States, and published between 2005 and 2012. None of the studies was published since the recent HIV programming management changes, such as the 90–90-90 strategy [1]. The 90–90-90 strategy requires a scaling up of HIV testing and treatment. It aims to have 90% of persons HIV infected tested and aware of their status, 90% of infected persons on antiretroviral treatment and viral suppression in 90% of persons on antiretroviral drugs [1]. Second, we did not find any studies from other high-income economies with similar HIV prevalence. Generalizing these high-income countries may be difficult. Also, this review did not include studies published in languages other than English.
None of the studies considered the cost of rapid HIV testing per new HIV transmission prevented, an outcome that would be significant in advancing an economic argument for the use of rapid HIV testing approaches as an integral population strategy in HIV programming.
In this review, we also note that only one study conducted a probabilistic sensitivity analysis that is considered the only approach that can potentially address uncertainties in all inputs rather than confining this to a subset as is usual in the univariate and multivariate deterministic analysis [63].
The Consumer Price Index (CPI) used to adjust the costs indicates changes in population consumer prices. The CPI is obtained by comparing the cost of a fixed basket of goods and services purchased by consumers [64,65,66]. This approach is limited because it does not account for other options that may not be included in the fixed basket used in the assessment and likely ignore the cost savings from less costly alternatives [66].
While appropriate for assessing study inclusion criteria, we also identify that the Drummond checklist may not adequately address contextual and health system factors related to rapid HIV testing. We found that none of the five articles met Drummond’s entire 10-item checklist (Table 2); we had no study that satisfied all ten criteria using the checklist. Other limitations of the checklists were the lack of a developed scoring algorithm. Using the CHEERS criteria, each ‘Yes’ response was weighted against the total number of criteria for an aggregate score.
Finally, we cannot exclude publication bias as almost all the economic evaluations showed the cost-effectiveness of rapid HIV testing.
Conclusion
In conclusion, evidence exists from the United States that supports rapid HIV testing approaches compared to conventional HIV testing approaches. The evidence from this review is from a single low HIV prevalence high-income country. It does not specifically account for the difference in healthcare system characteristics and population contexts, making it difficult to generalize the evidence to other high-income, low HIV prevalence countries. The costs and outcomes associated with rapid HIV testing approaches suggest a cost-effective approach for population HIV screening, particularly among higher prevalence communities. However, there is inconsistent evidence of the use of rapid HIV testing approaches in lower prevalence settings. It would be of significant benefit to obtain estimates from other contexts and other countries besides the United States to account for the differences in healthcare system characteristics and enable more reliable generalization to these settings.
Availability of data and materials
The studies included in the review are publicly available.
Abbreviations
- CADTH:
-
Canadian Agency for Drug Technologies and Health
- CEA:
-
Cost-Effectiveness Analysis
- CEPAC:
-
Cost-Effectiveness of Preventing AIDS Complications
- ED:
-
Emergency Department
- HIV:
-
Human Immunodeficiency Virus
- QALY:
-
Quality-Adjusted Life Years
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
References
UNAIDS. 90–90-90: An ambitious treatment target to help end the AIDS epidemic. United Nations. 2014.
Montaner JSG. Treatment as prevention: toward an AIDS-free generation. Top Antivir Med. 2004;21(3):110–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23981598.
Nosyk B, Montaner JSG, Colley G, Lima VD, Keith C, Heath K, et al. The cascade of HIV care in British Ccolumbia, Canada, 1996-2011: a population-based retrospective cohort study. Lancet Infect Dis. 2014;14(1):40–9. https://doi.org/10.1016/S1473-3099(13)70254-8.
Kay ES, Batey DS, Mugavero MJ. The HIV treatment cascade and care continuum: updates, goals, and recommendations for the future. AIDS Res Ther. 2016;13(1):35. Available from: http://aidsrestherapy.biomedcentral.com/articles/10.1186/s12981-016-0120-0
Nosyk B, Montaner JSG, Colley G, Lima VDVD, Chan K, Heath K, et al. The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. Lancet Infect Dis. 2014;14(1):40–9 Available from: https://doi.org/10.1016/S1473-3099(13)70254-8.
Gianella S, von Wyl V, Fischer M, Niederoest B, Battegay M, Bernasconi E, et al. Effect of early antiretroviral therapy during primary HIV-1 infection on cell-associated HIV-1 DNA and plasma HIV-1 RNA. Antivir Ther. 2011;16(4):535–45 [cited 2013 Mar 20], Available from: http://www.ncbi.nlm.nih.gov/pubmed/21685541.
Bärnighausen T, Salomon JA, Sangrujee N. HIV treatment as prevention: issues in economic evaluation. PLoS Med. 2012;9(7):e1001263. [cited 2012 Nov 19] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3393650&tool=pmcentrez&rendertype=abstract
Wood E, Milloy MJ, Montaner JSG. HIV treatment as prevention among injection drug users. Curr Opin HIV AIDS. 2012;7(2):151–6. [cited 2014 Oct 19] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22227587.
Marty L, Cazein F, Panjo H, Pillonel J, Costagliola D, Supervie V, et al. Revealing geographical and population heterogeneity in HIV incidence, undiagnosed HIV prevalence and time to diagnosis to improve prevention and care: estimates for France. J Int AIDS Soc. 2018;21:1–11.
Reyes-Urueña JM, Campbell CNJ, Vives N, Esteve A, Ambrosioni J, Tural C, et al. Estimating the HIV undiagnosed population in Catalonia, Spain: descriptive and comparative data analysis to identify differences in MSM stratified by migrant and Spanish-born population. BMJ Open. 2018;8(2):e018533 Available from: http://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2017-018533.
Singh S, Song R, Johnson AS, McCray E, Hall HI. HIV incidence, prevalence, and undiagnosed infections in u.S. men who have sex with men. Ann Intern Med. 2018;168(10):684–94.
Wilton J. The state of HIV testing in Canada: a systematic review. Toronto, ON; 2015. Available from: http://www.catie.ca/en/pif/spring-2015/state-hiv-testing-canada-systematic-review
Public Health Agency of Canada. Summary: Estimates of HIV Incidence, Prevalence and Proportion Undiagnosed in Canada, 2014. 2015;9. Available from: http://www.phac-aspc.gc.ca/aids-sida/publication/survreport/estimat2011-eng.php
Sharma A, Stephenson RB, White D, Sullivan PS. Acceptability and intended usage preferences for six HIV testing options among internet-using men who have sex with men. Springerplus. 2014;3(109):1–10 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3942559&tool=pmcentrez&rendertype=abstract.
Myers JE, El-Sadr WM, Zerbe A, Branson BM. Rapid HIV Self-testing: long in coming but opportunities beckon. AIDS. 2013;27(February):1687–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23807269.
Galadanci HS, Iliyasu Z, Tukur J, Muktar-Yola M, Adeleke SI. Uptake of voluntary counselling and testing for HIV by pregnant women in a prevention-of-mother-to-child-transmission programme at Aminu Kano teaching hospital, Nigeria. African J AIDS Res. 2008;7(1):143–8. https://doi.org/10.2989/AJAR.2008.7.1.14.442.
Pottie K, Medu O, Welch V, Dahal GP, Tyndall M, Rader T, et al. Effect of rapid HIV testing on HIV incidence and services in populations at high risk for HIV exposure: an equity-focused systematic review. BMJ Open. 2014;4(12):e006859 Available from: http://bmjopen.bmj.com/cgi/doi/10.1136/bmjopen-2014-006859.
Martin EG, Salaru G, Paul SM, Cadoff EM. Use of a rapid HIV testing algorithm to improve linkage to care. J Clin Virol. 2011;52:S11–5. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1386653211003775. https://doi.org/10.1016/j.jcv.2011.09.014.
Lippman SA, Sullivan PS. Acceptability of self-conducted home-based HIV testing among men who have sex with men in Brazil: data from an on-line survey. Cad Saude Publica. 2014;30(4):724–34. https://doi.org/10.1590/0102-311X00008913.
Menzies N, Abang B, Wanyenze R, Nuwaha F, Mugisha B, Coutinho A, et al. The costs and effectiveness of four HIV counseling and testing strategies in Uganda. AIDS. 2009;23(3):395–401. [cited 2012 Nov 9] Available from: http://www.ncbi.nlm.nih.gov/pubmed/19114865.
Martin LJ, Houston S, Yasui Y, Wild TC, Saunders LD. Rates of initial virological suppression and subsequent virological failure after initiating highly active antiretroviral therapy: the impact of aboriginal ethnicity and injection drug use. Curr HIV Res. 2010;8(8):649–58. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21187007.
Yazdanpanah Y, Sloan CE, Charlois-Ou CC, Le Vu SS, Semaille C, Costagliola D, et al. Routine HIV screening in France: clinical impact and cost-effectiveness. PLoS One. 2010;5(10):1–9.
Schackman BR, Metsch LR, Colfax GN, Leff JA, Wong A, Scott CA, et al. The cost-effectiveness of rapid HIV testing in substance abuse treatment: results of a randomized trial. Drug Alcohol Depend. 2013;128(1–2):90–7 Available from: https://doi.org/10.1016/j.drugalcdep.2012.08.009.
Walensky RP, Freedberg KA, Weinstein MC, Paltiel AD. Cost-effectiveness of HIV testing and treatment in the United States. Clin Infect Dis. 2007;45 Suppl 4(November 2006):S248–54. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2365915&tool=pmcentrez&rendertype=abstract
Gidwani R, Goetz MB, Kominski G, Asch S, Mattocks K, Samet JH, et al. A Budget Impact Analysis of Rapid Human Immunodeficiency Virus Screening in Veterans Administration Emergency Departments. J Emerg Med. 2012;42(6):719–26. Available from: http://sfx.scholarsportal.info/ottawa?sid=OVID:medline&id=pmid:21277144&id=doi:10.1016/j.jemermed.2010.11.038&issn=0736-4679&isbn=&volume=42&issue=6&spage=719&pages=719-26&date=2012&title=Journal+of+Emergency+Medicine&atitle=A+budget+impact+analysis+of+ra
Read TRH, Hocking JS, Bradshaw CS, Morrow A, Grulich AE, Fairley CK, et al. Provision of rapid HIV tests within a health service and frequency of HIV testing among men who have sex with men: randomised controlled trial. BMJ. 2013;347(September):f5086. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3762440&tool=pmcentrez&rendertype=abstract. https://doi.org/10.1136/bmj.f5086.
Bateganya M, Abdulwadud O, Kiene S. Home-based HIV voluntary counselling and testing ( VCT ) for improving uptake of HIV testing (Review ). Cochrane Collab 2010;(7).
Dewsnap CH, Mcowan A. A review of HIV point-of-care tests. Int J STD AIDS. 2006;17(6):357–9. https://doi.org/10.1258/095646206777323418.
Shrestha RK, Clark HA, Sansom SL, Song B, Buckendahl H, Calhoun CB, et al. Cost-Effectiveness of Finding New HIV Diagnoses Using Rapid HIV Testing in Community-Based Organizations. Public Health Rep. 2008;12(Supplement 3):94–100. Available from: http://www.jstor.org/stable/25682059%5Cnhttp://www.jstor.org/stable/25682059%5Cnhttp://www.jstor.org/stable/25682059
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. https://doi.org/10.1016/j.jclinepi.2009.06.006.
Human Immunodeficiency Virus - HIV Screening and Testing Guide - Canada.ca. [cited 2019 May 20]. Available from: https://www.canada.ca/en/public-health/services/hiv-aids/hiv-screening-testing-guide.html
Association of Public Health Laboratories. Testing Oral Fluid for the Presence of HIV Antibodies: 2013 Status Report 2013.
Canadian Agency for Drugs and Technologies in Health. CADTH Methods and Guidelines: Guidelines for the Economic Evaluation of Health Technologies. Ottawa: CADTH; 2017. p. 1–76.
Cape JD, Beca JM, Hoch JS. Introduction to cost-effectiveness analysis for clinicians. Univ Tor Med J. 2013;90(3):103–5.
Kymes SM. An introduction to decision analysis in the economic evaluation of the prevention and treatment of vision-related diseases. Ophthalmic Epidemiol. 2008 ;15(2):76–83. [cited 2013 Mar 20] Available from: http://www.ncbi.nlm.nih.gov/pubmed/18432490.
Consumer Price Indexes Overview. Consumer Price Index. 2016 [cited 2017 Jan 17]. Available from: https://www.bls.gov/cpi/cpiovrvw.htm#item6
Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic. Br Med J. 1996;313(August):275–83. Available from: http://www.bmj.com/content/313/7052/275. https://doi.org/10.1136/bmj.313.7052.275.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. BMJ. 2013;346:1–6.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS)—explanation and elaboration: a report of the ISPOR health economic evaluations publication guidelines good reporting practices task force. Value Heal. 2013;16(2):231–50. https://doi.org/10.1016/j.jval.2013.02.002.
van Katwyk S, Coyle D, Cooper C, Pussegoda K, Cameron C, Skidmore B, et al. Transient elastography for the diagnosis of liver fibrosis: a systematic review of economic evaluations. Liver Int. 2016;(June):1–11. Available from: http://doi.wiley.com/10.1111/liv.13260
Rogers HJ, Rodd HD, Vermaire JH, Stevens K, Knapp R, El Yousfi S, et al. A systematic review of the quality and scope of economic evaluations in child oral health research. BMC Oral Health. 2019;19(1):1–15.
Costa S, Cary M, Helling DK, Pereira J, Mateus C. An overview of systematic reviews of economic evaluations of pharmacy-based public health interventions: addressing methodological challenges. Syst Rev. 2019;8(1):272. Published 2019 Nov 11. https://doi.org/10.1186/s13643-019-1177-3.
Korsholm M, Sørensen J, Mogensen O, Wu C, Karlsen K, Jensen PT. A systematic review about costing methodology in robotic surgery: evidence for low quality in most of the studies. Health Econ Rev. 2018;8(1):21. Published 2018 Sep 7. https://doi.org/10.1186/s13561-018-0207-5.
Cipriano LE, Zaric GS, Holodniy M, Bendavid E, Owens DK, Brandeau ML. Cost effectiveness of screening strategies for early identification of HIV and HCV infection in injection drug users. PLoS One. 2012;7(9):e45176.
Dowdy DW, Rodriguez RM, Bradley Hare C, Kaplan B. Cost-effectiveness of targeted human immunodeficiency virus screening in an urban emergency department. Acad Emerg Med. 2011;18(7):745–53. https://doi.org/10.1111/j.1553-2712.2011.01110.x.
Sanders GD, Anaya HD, Asch S, Hoang T, Golden JF, Bayoumi AM, et al. Cost-effectiveness of strategies to improve HIV testing and receipt of results: economic analysis of a randomized controlled trial. J Gen Intern Med. 2010;25(6):556–63. Jun [cited 2012 Dec 15] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2869414&tool=pmcentrez&rendertype=abstract
Paltiel DA, Weinstein MC, Kimmel AD, Seage GR, Losina E, Zhang H, et al. Expanded screening for HIV in the United States - an analysis of cost-effectiveness. N Engl J Med. 2005;352(6):586–95 Available from: http://www.ncbi.nlm.nih.gov/pubmed/15703423.
Walensky RP, Weinstein MC, Kimmel AD, Seage GR, Losina E, Sax PE, et al. Routine human immunodeficiency virus testing: an economic evaluation of current guidelines. Am J Med. 2005;118(3):292–300. https://doi.org/10.1016/j.amjmed.2004.07.055.
Walensky RP, Weinstein MC, Smith HE, Freedberg KA, Paltiel AD. Optimal allocation of testing dollars: the example of HIV counseling, testing, and referral. Med Decis Mak. 2005;25(3):321–9 Available from: http://mdm.sagepub.com/cgi/doi/10.1177/0272989X05276955.
Drummond M, Sculpher M. Common Methodological Flaws in Economic Evaluations 2005;43(7):5–14.
CEPAC. Cost-Effectiveness of Preventing AIDS Complications (CEPAC) Program User Guide; 2014. p. 1–72.
McCabe C, Claxton K, Culyer AJ. The NICE cost effectiveness threshold: what it is and what it means. Pharmacoeconomics. 2008;2(9):733–44.
Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. Can Med Assoc J. 1992;146(4):473–81.
Jaswal A. Valuing health in Canada. Canada 2020 Anal. Comment. 2013;8(3):1–24. Available at http://canada2020backup.see-design.com/wpcontent/uploads/2013/06/Canada-2020-Analytical-Commentary-No-3-Valuing-Health-in-Canada-FINAL.pdf.
Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness — the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–7 Available from: http://www.nejm.org/doi/abs/10.1056/NEJMp1405158.
Goeree R, Diaby V. Introduction to health economics and decision-making: is economics relevant for the frontline clinician? Best Pract Res Clin Gastroenterol. 2013;27(6):831–44 Available from: https://doi.org/10.1016/j.bpg.2013.08.016.
Wang S, Gum D, Merlin T. Comparing the ICERs in medicine reimbursement submissions to NICE and PBAC—does the presence of an explicit threshold affect the ICER proposed? Value Heal. 2018;21(8):938–43 Available from: https://doi.org/10.1016/j.jval.2018.01.017.
Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost – effectiveness of interventions : alternative approaches. Bull World Health Organ. 2015;93(October 2014):118–24. https://doi.org/10.2471/BLT.14.138206.
O’Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol. 2014;67(1):56–64 Available from: https://doi.org/10.1016/j.jclinepi.2013.08.005.
Asaria M, Griffin S, Cookson R. Measuring health inequality in the context of cost-effectiveness analysis. Res Econ Inequal. 2013;21(2013):491–507 Available from: https://doi.org/10.1108/S1049-2585(2013)0000021024%5Cnhttp://www.emeraldinsight.com/books.htm?chapterid=17103220.
Cookson R, Drummond M, Weatherly H. Explicit incorporation of equity considerations into economic evaluation of public health interventions. Health Econ Policy Law. 2009 ;4(Pt 2):231–45. [cited 2014 Aug 13] Available from: http://www.ncbi.nlm.nih.gov/pubmed/19216834.
Asaria M, Griffin S, Cookson R, Whyte S, Tappenden P. Distributional Cost-Effectiveness Analysis of Health Care Programs. Vol. 91, CHE Research Paper 56. 2013.
Adalsteinsson E, Toumi M. Benefits of probabilistic sensitivity analysis – a review of NICE decisions. J Mark Access Heal Policy. 2013;1(1):21240. https://doi.org/10.3402/jmahp.v1i0.21240.
El Khoury AC, Klimack WK, Wallace C, Razavi H. Economic burden of hepatitis C-associated diseases in the United States. J Viral Hepat. 2012;19(3):153–60. https://doi.org/10.1111/j.1365-2893.2011.01563.x.
Consumer Price Index, 2000 to Present. 2016 [cited 2017 Jan 17]. Available from: http://www.bankofcanada.ca/rates/price-indexes/cpi/
Sabourin P. Measurement Bias in the Canadian consumer Price Index : an update. Bank Canada Rev 2012;(May 2011):1–11.
Acknowledgements
Not applicable.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
Study conception and design: OM, DC, KP. Design of data extraction tool: OM, DC. Review of studies for inclusion in the analysis: OM, AKL, DC. Oversight of the analysis plan and: OM, KP. Initial draft of manuscript: OM. Critical revision of manuscript: OM, AKL, DC, KP. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
The review used data from published studies.
Competing interests
The authors do not have any disclosures to report.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Medu, O., Lawal, A., Coyle, D. et al. Economic evaluation of HIV testing options for low-prevalence high-income countries: a systematic review. Health Econ Rev 11, 19 (2021). https://doi.org/10.1186/s13561-021-00318-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13561-021-00318-y