Abstract
Background
In addition to social and cultural factors, sex differences in the central nervous system have a critical influence on behavior, although the neurobiology underlying these differences remains unclear. Interestingly, the Locus Coeruleus (LC), a noradrenergic nucleus that exhibits sexual dimorphism, integrates signals that are related to diverse activities, including emotions, cognition and pain. Therefore, we set-out to evaluate sex differences in behaviors related to LC nucleus, and subsequently, to assess the sex differences in LC morphology and function.
Methods
Female and male C57BL/6J mice were studied to explore the role of the LC in anxiety, depressive-like behavior, well-being, pain, and learning and memory. We also explored the number of noradrenergic LC cells, their somatodendritic volume, as well as the electrophysiological properties of LC neurons in each sex.
Results
While both male and female mice displayed similar depressive-like behavior, female mice exhibited more anxiety-related behaviors. Interestingly, females outperformed males in memory tasks that involved distinguishing objects with small differences and they also showed greater thermal pain sensitivity. Immunohistological analysis revealed that females had fewer noradrenergic cells yet they showed a larger dendritic volume than males. Patch clamp electrophysiology studies demonstrated that LC neurons in female mice had a lower capacitance and that they were more excitable than male LC neurons, albeit with similar action potential properties.
Conclusions
Overall, this study provides new insights into the sex differences related to LC nucleus and associated behaviors, which may explain the heightened emotional arousal response observed in females.
Plain Language Summary
Exploring sex differences in the brain is important to understand the impact of such differences in pathological conditions characterized by gender bias, as well as their therapeutic implications. In this manuscript, we examined sex differences in the mouse locus coeruleus (LC) and how this might affect related behaviours. The LC is a sexually dimorphic nucleus that integrates signals associated with attention, anxiety, stress, arousal, pain, memory and learning. Our findings reveal that female mice exhibit more intense anxiety-related behaviors but that they perform better than males in recognizing small differences between objects. Additionally, we found pronounced sex differences in the LC, which contained fewer noradrenergic cells in females, with a larger dendritic volume and displaying enhanced cell excitability. These differences in the LC, a nucleus that fulfils a pivotal role in stress and pain, could be important for understanding the higher prevalence of stress-related disorders in women, such as anxiety and depression, but also of chronic pain. Hence, it is clearly important to consider sex differences in both preclinical and clinical research studies that attempt to understand pathologies related to these phenomena.
Highlights
-
Female mice exhibit stronger anxiety-related behaviors than males, yet both show similar depressive-like behaviors.
-
The discrimination index to distinguish similar objects with small differences is higher in females than in males.
-
Females exhibit greater thermal pain sensitivity than males, yet both respond similarly to mechanical stimuli.
-
Females have fewer noradrenergic cells in the LC yet they show a larger dendritic volume than males.
-
LC neurons from female mice have a smaller cell capacitance but stronger excitability than those in males.
Similar content being viewed by others
Introduction
It is known that sex differences at the level of the central nervous system can affect many biological activities and behaviors, such as stress-related responses, cognitive performance and pain [1,2,3]. Exploring sexual dimorphism in the brain is important to understand the impact of these differences, as well as their therapeutic implications in neurological and psychiatric diseases, given that gender bias is evident in several mental illnesses. Indeed, stress-related disorders like anxiety and depression, and chronic pain, are more common in women than in men [4,5,6,7,8,9]. Symptoms are also more severe in women with a younger age at onset, with prolonged or recurrent symptomatic periods and worse quality of life. Conversely, neurodevelopmental disorders like autism spectrum and attention-deficit disorders are more prevalent in boys than girls [10,11,12]. However, dissecting out the role of the biological and environmental influences for each disorder is challenging in humans. Therefore, preclinical studies in rodents, where a larger number of variables can be controlled, are adequate tools to investigate sexual dimorphisms in the brain and brain diseases. Such studies would encourage more research in the field and leading to the development of sex-specific and personalized diagnosis and treatment.
In recent decades, sexual dimorphism has been reported in several brain structures, such as the hypothalamus, hippocampus and locus coeruleus (LC) [13,14,15,16,17]. The LC is a brainstem noradrenergic nucleus that projects to a variety of regions through ascending and descending projections, and reciprocally, LC neurons receive extensive inputs from different brain regions [18]. Through these circuits, the LC integrates signals related to a variety of activities, including attention, anxiety, stress response, arousal/sleep, learning and memory, sensory processing, pain and reward processing [19,20,21,22,23,24]. In terms of LC sex differences, rat studies reported that the dendritic morphology of LC neurons is more complex in females than in males [25], with dendrites more prominent in the peri-LC region, and that the nucleus receives more stress-related afferents (e.g., from the central amygdala, the bed nucleus of the stria terminalis, etc.) [26]. However, these results are not always consistent due to variations in the methods and protocols used, or in the rat strains studied. In recent decades, more studies have been carried out on mice since the activity of specific neuronal subpopulations in the LC can be manipulated genetically, allowing the neural circuits that control certain behaviors to be modulated. In fact, transcriptional profiling of more than 3000 genes from the LC has revealed sex differences in more than 100 of these at the transcript level and different sex-related behavioral responses could be generated [27]. However, studies into the morphological and functional characteristics of LC neurons in naïve mice are scarce. Therefore, studying the sex differences in the LC nucleus in naïve animals, and their possible implications in anxiety and depressive-like behaviors, as well as pain thresholds and cognition, will be crucial to understand brain activity in a sex-specific manner.
Thus, this study was designed to investigate the differences in the LC of naïve male and female mice, and the implications of these in a variety of behaviors related to LC function, including anxiety, depressive-like behavior, well-being, learning and memory, and pain. The number of noradrenergic LC cells, the somatodendritic volume occupied and the electrophysiological properties of LC neurons were also evaluated to obtain a more comprehensive understanding of their function in C57BL/6J mice.
Materials and methods
Animals and experimental design
Male and female adult wild-type C57BL/6J mice (8–11 weeks of age) were maintained in separate rooms according to their sex under standard laboratory conditions (22 °C, 12-h light–dark cycle, and ad libitum access to food and water). All procedures were approved by the Committee for Animal Experimentation at the University of Cadiz and the UPV/EHU (M20/2021/234, Spain), conforming to the European Commission’s Directive (2010/63/EU) and Spanish Law (RD 53/2013) regulating animal research. Firstly, a behavioral evaluation was performed on three different sets of animals with n = 7–10 mice of each sex per set at 22 ± 1 °C and ∼ 13 lx of light. All the behavioral devices were cleaned with 70% ethanol between the testing of each animal, especially when changing between sexes. After performing the elevated plus maze (EPM) test, the estrous cycle of the female mice was assessed by vaginal cytology using crystal violet staining (Additional file 1: Fig. S1a) [28]. Immunohistochemistry studies were carried out after the behavioral assays. For that, 5 animals per group were evaluated using DAB (3,3′-diaminobenzidine tetrahydrochloride), and another 5 animals per group were assessed by immunofluorescence, analyzing the mean of the left and right LC for each animal. Moreover, the electrophysiological properties of LC neurons were analyzed in a different set of male and female mice (n = 5 per group).
Emotional assessment
Elevated plus maze test
The EPM test was performed to evaluate anxiety-like behavior based on the tendency of rodents to explore novel environments, and their innate avoidance of unprotected and elevated places. An animal was placed in a gray cross-shaped maze (Panlab S.L., Barcelona, Spain) elevated 40 cm above the floor, with two open arms (29.5 cm × 6 cm) and two closed arms (29.5 cm × 6 cm × 15 cm-high walls), and with a central square (6 cm × 6 cm). The animal’s behavior was recorded over 5 min and the percentage of time spent in the open arms was determined using the SMART video-tracking software (Panlab, S.L., Barcelona, Spain), providing an estimate of anxiety-like behavior [29].
Light/dark test
The light/dark test evaluates anxiety-like behavior by assessing the animal’s displacement between two compartments of different sizes and colors, and with distinct illumination [30]. The apparatus consists of a small black compartment (25 cm × 16 cm) illuminated with a red bulb (~ 6 lx) and a larger white compartment (25 cm × 25 cm) lit with a white bulb (~ 1000 lx), the two separated by a connecting gate (7 cm × 7 cm) at floor level. Mice were placed into the dark compartment and allowed to move freely for 5 min. The latency to first enter the lit compartment, the total number of transitions (index of exploration) and the time spent in the lit compartment (reflection of aversion) was recorded automatically using weight transducer technology for animal detection, and with the PPCWIN software (Panlab, S.L., Barcelona, Spain).
Open field test
The open field test (OFT) was used to assess anxiety-like behavior as well as locomotor activity, as it is based on the rodent’s innate tendency to explore novel environments and avoid bright open spaces. The animal was placed in the center of a 45 cm × 45 cm square enclosure and allowed to move freely for 10 min while being recorded. The center was defined as a square area about 50% the size of the whole arena. The percentage of time spent in the central area and the total distance traveled were analyzed with the SMART video-tracking software (Panlab, S.L., Barcelona, Spain) [31].
Burrowing test
The burrowing test was performed to monitor animal well-being [32]. Mice were tested individually in a cage with a 154 mm long and 56 mm wide plastic tube filled with 140 g of food pellets. The burrow was located with the closed end against the back wall of the cage to provide sufficient distance for effective displacement of the burrowing material. Burrowing activity was calculated by subtracting the weight of the pellets present 1 h, 3 h, 6 h and 24 h after the start of the experiment from the original amount.
Sucrose splash test
The sucrose splash test consisted of spraying a 20% sucrose solution on the dorsal coat of the animal and recording its behavior was over the next 5 min. The grooming activity (licking, scratching and/or face-washing) was measured in seconds [33], whereby reduced grooming time indicated weaker motivational and self-care behavior.
Tail suspension test (TST)
The TST was used to evaluate depressive-like behavior. Mice were suspended from the distal end of the tail using adhesive tape and raised 20 cm above the floor during a 6 min recorded test session [34]. Their resulting behavior was analyzed to determine the time they spent immobile, defined as hanging by the tail without showing any active behavior. We also evaluated other behaviors [31], such as: (a) climbing—the mouse climbs up its tail; (b) swinging—the mouse moves its body from side to side; (c) curling—the mouse performs twisting body movements; and (d) clasping—the mouse retracts its hind limbs towards its abdomen.
Forced swimming test (FST)
The FST was used to evaluate depressive-like behavior [35, 36]. During a 6 min test, individual animals were placed in glass cylinders (10 cm diameter × 18 cm height) filled to a depth of 10 cm with water at 22 ± 1 °C. The sessions were recorded and the last 4 min were analyzed. Immobility behavior was determined when animals only undertook movements necessary to keep their head above water.
Cognitive assessment
Novel object recognition (NOR) test
The NOR test relies on the innate preference of rodents for novelty. We used different NOR protocols to assess short-term (STM) and long-term memory (LTM), as well as a STM protocol to test the ability to discriminate small differences between objects. Mice were tested in a 45 cm × 45 cm square enclosure using different plastic objects (shapes, colors and textures). The mice were placed into the arena for a 10 min habituation phase in the absence of any objects, and then two identical objects were placed in opposite corners of the arena during a 15 min training phase. Subsequently, the mice were returned to their home cage for a delay period of either 1 h (STM) or 24 h (LTM). The mice were then subjected to a 10 min test in which one of the objects in the arena was replaced by a novel one, evaluating the animal’s object exploration activity defined as actively sniffing and/or touching the object while maintaining their gaze on the object. Circling or sitting on top of the object was not considered exploration. Object exploration was measured as the latency to the first object, the number of interactions, percentage preference and through a Discrimination Index (DI), the latter reflecting the amount of time spent exploring the novel object relative to the total time spent exploring both objects: DI = (Tnovel—Tfamiliar)/(Tnovel + Tfamiliar) [37].
Sensory assessments
Manual von Frey test
Calibrated von Frey filaments (0.16, 0.40, 0.60, 1.0, 1.4, 2.0, 4.0, 6.0, 8.0 and 10 g: Bio-VF-M, BioSeb, France) were applied perpendicular to the plantar surface of each hind paw with just enough force to bend the filament. They were each applied 10 times to both paws, in ascending order, after a 30 min habituation in an individual plastic cage over a metal grid. The withdrawal response to the mechanical stimulus was considered as the rapid removal of the hind paw from the filament, usually followed by flinching or licking of the plantar surface. The percentage of response was derived from the number of withdrawals to each filament [38, 39].
Plantar test
Thermal thresholds were established through the Hargreaves’ method [40]. Mice were placed in individual plastic cages over an elevated glass surface and habituated for 45 min. Radiant heat was applied to the hind paw at a constant intensity using a Plantar test device (Ugo Basile, Italy), with a 30 s cut-off to prevent tissue damage. Two measurements were made and the mean latency of the paw withdrawal was considered as the thermal nociceptive threshold [41].
Tissue processing, immunohistochemistry and immunofluorescence
Perfusion and sample extraction
At the end of the behavioral tests, 10 mice per group were anesthetized with 25% sodium pentobarbital and perfused transcardially through the ascending aorta with a 0.9% saline solution using a perfusion pump, followed by a 4% paraformaldehyde (PFA) solution prepared in 0.1 M phosphate buffered saline (PBS). The animal’s brain was removed carefully, post-fixed for an additional 2 h in 4% PFA, and then transferred to a 30% sucrose solution in phosphate buffer (0.1 M) with 0.1% sodium azide and left at least overnight at 4 °C. Coronal freezing microtome Sects. (40 µm) containing the LC were collected and stored in a cryoprotective solution at 4 ºC until further processing.
Structural and morphological studies
DAB immunostaining was performed on one of four series of 40 µm thick LC sections from 5 mice per sex. LC sections were probed for two nights at 4 °C with a primary antiserum against Tyrosine Hydroxylase (TH, rabbit anti-TH, 1:1000: OPA1-04050 Millipore), and then incubated with biotinylated donkey anti-rabbit antibodies (1:200: Jackson ImmunoResearch Europe, UK). Immunodetection was achieved using the ultra-sensitive ABC peroxidase staining kit (1:1000: Thermo Scientific, Spain) and DAB [42], and the sections were then mounted on slides, cleared in xylene and coverslipped with DPX. Images were acquired at the same exposure and illumination settings on an Olympus BX60 microscope equipped with an Olympus DP74 camera.
Immunofluorescence was performed as described previously [43] on all the 40 µm thick LC sections from 5 mice per sex. The same primary antiserum was used and revealed with the appropriate fluorophore-conjugated secondary antibodies (Donkey anti-rabbit Alexa 488, 1: 1,000: A-21206 Invitrogen). Sections were mounted onto glass slides with hard-setting antifade mounting media (Dako, S3023) and TH expression was captured at the same exposure from the rostral to caudal level of the LC (about − 5.32 mm to − 5.84 mm from Bregma) using a 20X oil immersion objective on a confocal microscope (Olympus FV1000).
To analyze the total number of LC neurons, TH+ neurons were counted manually using the ImageJ Cell Counter plugin, considering only those neurons whose nuclei could be visualized in the analysis, and the mean was calculated for the left and right LC of each animal. Cavalieri’s principle was used to obtain an unbiased stereological estimation of the LC volume [44], as V = ∑A x T, where ∑A is the sum of the areas measured in all LC sections and T is the distance between sections. The parallel LC images spaced 40 µm apart were stacked and analyzed using ImageJ (National Institutes of Health, Bethesda, Maryland). The area of staining (region of interest -ROI) was automatically outlined and measured in each section with the wand tool, using the 8-connected configuration mode to find connected regions. This protocol was performed by selecting the area occupied by the somas of the noradrenergic cells, as well as the area occupied by the entire LC by delimiting the somatodendritic area. The area occupied exclusively by the dendrites was obtained by subtracting the area occupied by the somas from the area of the entire LC. The mean was calculated for the left and right LC of each animal. Resulting areas were expressed as mm2 and the volumes as mm3.
Electrophysiology
Slice preparation
Male and female mice (n = 5 per group) were sacrificed by decapitation under deep anesthesia (4% isoflurane), brains were removed and transferred to ice-cold artificial cerebrospinal fluid (ACSF, pH 7.4) equilibrated with 95% O2 and 5% CO2, and containing (in mM): 250 sucrose, 26 NaHCO3, 1.25 NaH2PO4.H2O, 0.5 CaCl2.2H2O, 10 MgSO4.7H2O, 10 D-glucose. Coronal sections of the brain containing the LC (220 µm thick) were obtained with a vibratome (VT1200S; Leica Microsystems, Germany) and slices were incubated in warmed (30–35 °C) ACSF for at least 30 min before recording, containing (in mM): 126 NaCl, 2.5 KCl, 1.25 NaH2PO4.H2O, 2 CaCl2.2H2O, 2 MgSO4.7H2O, 10 D-glucose, 26 NaHCO3, 1 sodium pyruvate and 4.9 L-glutathione [pH 7.4], gassed with 95% O2 and 5% CO2.
Whole-cell patch clamp recordings
Each slice was transferred to a recording chamber that was perfused continuously with oxygenated ACSF at 32–34 °C following our standard protocol [45, 46]. LC neurons were visualized using infrared gradient contrast video microscopy (Eclipse workstation, Nikon) and with a 60X water-immersion objective (Fluor 60X/1.00 W, Nikon). The LC was identified as a dense and compact group of cells at the lateral border of the central gray and the fourth ventricle, just anterior to the genu of the facial nucleus. Recordings from individual LC neurons were obtained with pipettes (impedance, 3–6 MΩ) prepared from borosilicate glass capillaries (G150-4: Warner Instruments, Hamdem, CR, USA). The patch pipette was filled with a KGluconate-based solution containing (in mM): 130 KGluconate, 5 NaCl, 1 MgCl2.6H2O, 10 HEPES, 1 Na4EGTA, 2 MgATP, 0.5 NaGTP, and 10 Na2PCr. The junction potential between the electrode solution and the external media (empirically estimated as 13 mV) was not corrected, and electrode signals were low-pass filtered at 4 kHz and sampled at 20 kHz. LC neurons were identified by the presence of a resting inwardly-rectifying potassium (IRK) conductance by stepping the membrane potential from − 40 to − 120 mV in − 10 mV increments (100 ms/step) [47]. In voltage clamp experiments, neurons were maintained at − 60 mV and the series resistance was monitored with steps of − 5 mV at the end of each recording. Data were discarded when the series resistance increased by > 20%. The average current response was analyzed off-line and the cell capacitance (Cm) and membrane resistance (Rm) were calculated. In the current clamp mode, incremental currents from − 150 to + 300 pA were injected in 25 pA steps to explore the subthreshold and firing properties of the neurons. Off-line analysis was performed using pClamp V9.2 (Molecular Devices, San Jose, CA, USA).
Statistical analysis
All the data are presented as the mean ± SEM and the statistical analyses were performed using GraphPad Prism software (GraphPad Software 9.0.3, La Jolla, CA). Grubbs’ test was used to identify any statistical outliers and normal distributions of the data was confirmed with the Shapiro–Wilk test. Differences between two groups were determined using unpaired Student t-tests (two-tailed) when normally distributed or the non-parametric Mann–Whitney U tests when not. For comparisons between the soma, somatodendritic and dendritic distributions, as well as in the burrowing test, the data from each group was analyzed through the area under the curve (AUC). The Chi-squared test was used to analyze frequency distributions. Differences between more than two groups were determined using one-way ANOVA followed by a Tukey post hoc test. The material burrowed along hours, the percentage of response to the mechanical stimuli, as well as the IRK currents, firing frequency and voltage response to current injections were analyzed using repeated-measures ANOVA followed by Tukey post hoc test. Significance was set at p < 0.05.
Results
Female mice exhibit more anxiety-related behaviors than male mice
Female mice spent significantly less percentage of time in the open arms of the EPM (p < 0.01: see Table 1 and Fig. 1a), and a subsequent exploration of the estrous cycle revealed that female mice in the proestrus and estrus (P/E) stages spent less time in the open arms than those in the diestrus and metestrus (D/M) stages (p < 0.05: Additional file 1: Fig. S1b). Indeed, in these latter two stages the percentage of time spent in the open arms was similar to that of males. Furthermore, similar values of total activity (Additional file 1: Fig. S1c) and a similar number of entries into the open arms were observed (Additional file 1: Fig. S1d), suggesting a comparable level of exploration between sexes. Thus, the following behavioral tests were performed on females in the P/E stages. Similar results were found in the light/dark test, where females spent less percentage of time in the lit compartment than males (p < 0.05: Fig. 1b). However, the latency of both sexes to enter the dark compartment was similar (Additional file 1: Fig. S1e), as was the number of transitions between compartments (Additional file 1: Fig. S1f), suggesting a similar index of exploration in both groups. More anxiogenic behavior in the OFT was also attributed to female mice, which spent less percentage of time in the center of the arena than males (p < 0.05: Fig. 1c) even though the total distance traveled was similar in both these groups (Additional file 1: Fig. S1g).
Evaluation of anxiety, well-being and depressive-like behavior in male and female mice. The results of anxiety-like behavior expressed as a the relative time spent in the open arms of the EPM by male and female mice. b Percentage of time spent in the lit compartment in the light–dark test. c Percentage of time spent in the central zone in the OFT, along with representative heatmaps of activity. d Graph depicting the material burrowed (in grams) and e the AUC of the material burrowed over time. f Time spent grooming in the splash test. Evaluation of the depressive-like behavior expressed as g immobility time in the TST and h FST. The data are presented as the mean ± SEM of n = 7–10 mice per group: *p < 0.05, **p < 0.01 vs male. From b to h females were in proestrus and estrus (P/E) stages. AUC, area under the curve
The burrowing test was used to evaluate the general well-being of the animals. It is based on the natural instinct of rodents to burrow, and the test involves placing a rodent in a cage with a burrow (a container filled with substrate like food pellets) and allowing them to burrow overnight. The amount of material displaced from the burrow was measured to quantify the burrowing behavior at different time points [32]. Our results showed that females were less apt to remove pellets from the burrow than males 3 and 6 h after the beginning of the test (p < 0.01: Fig. 1d). Accordingly, the AUC analysis revealed that females displaced significantly less material from the burrow than males (p < 0.01: Fig. 1e). In the splash test, the latency to grooming of females was lower than that of males (p < 0.01: Additional file 1: Fig. S1h), although the total grooming time was similar in both groups (Fig. 1f).
Behavioral despair was evaluated using the TST and FST. In the TST, there was no significant difference between the groups in the time spent immobile (Fig. 1g) but when analyzing other behaviors adopted during the test, the Chi-squared analysis revealed that the proportion of females that climbed was significantly higher than that of males (p < 0.01: Additional file 1: Fig. S1i). However, the test failed to detect differences between the sexes in swinging, curling or clasping behaviors (Additional file 1: Fig. S1i). Similar results were obtained in the FST, where mice showed no differences in the immobility time over the last 4 min of the test (Fig. 1h), or in the latency to immobility (Additional file 1: Fig. S1j).
Sex differences in novel object recognition depend on the ability to distinguish details
The NOR cognitive paradigm was used to investigate whether sex might influence learning and memory, employing two different approaches in the test phase. First the novel object was not identical in shape, texture, color or size (Fig. 2a), whereas in the second approach, the novel object had minimal differences to the familiar one (Fig. 2g). Both sexes traveled a similar distance in the 10 min habituation phase (Fig. 2b, c) and the percentage of preference in exploring the two identical objects was similar in both groups (Fig. 2d), even though females interacted less frequently with the identical objects in the 15 min training phase (p < 0.05: Fig. 2e). In the test phase, where two very different objects were used, both sexes were able to distinguish the novel object and there were no significant differences between the two groups in the STM or LTM protocols (Fig. 2f). By contrast, when the novel object had minimal differences to the familiar one (Fig. 2g), only female mice were able to recognize it in the STM protocol and they achieved a higher DI than males (p < 0.01: Fig. 2h, see Table 2).
Evaluation of the NOR paradigm and the sensorial assessment of male and female mice. a Schematic representation of the NOR experimental design to assess short-term (STM, learning index) and long-term memory (LTM, memory index), using two different objects. Graphs depicting b the total distance traveled in the habituation phase of the NOR paradigm and c its representation in 1 min intervals. Graphs showing d the percentage of preference exploring identical objects (represented as A) and e the number of interactions during the 15-min training phase of the test. f Graph representing the discrimination index (DI) between objects following the STM and LTM protocols when using a novel object (represented as B in STM protocol and C in LTM protocol) that differed drastically from the familiar object. g Schematic representation of the NOR experimental design to assess STM using objects with minimal differences between them. h Graph depicting the DI following the STM protocol and representative heatmaps showing activity around the objects, when the novel object (represented as D cross out) presented strong similarity with the familiar one (represented as D). Evaluation of the i mechanical response in the von Frey test using calibrated filaments from 0.16 to 10 g and j the paw withdrawal (in seconds) in the plantar test. The data are presented as the mean ± SEM of n = 10 mice per group: *p < 0.05, **p < 0.01 vs male; #p < 0.05 vs the first minute. Females were in proestrus and estrus (P/E) stages. DI, discrimination index; AU, arbitrary units
Female mice were more sensitive to heat stimulus
To evaluate sex-dependent pain sensitivity, mechanical and heat sensory thresholds were assessed using the von Frey filament application and the plantar test on both male and female mice. No differences in mechanical sensitivity were observed between the two groups (Fig. 2i), although female mice exhibited a shorter latency in paw withdrawal than males in terms of heat nociception (p < 0.05: Fig. 2j, see Table 2).
Structural and morphological sex differences in the mouse LC nucleus
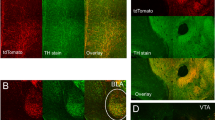
The number of TH+ cells in the LC was assessed by immunohistochemistry (Fig. 3a) and females had fewer TH+ cells in the LC than males (p < 0.05: Fig. 3b), as confirmed in immunofluorescence confocal images of the entire LC (p < 0.05: Fig. 3c, d, Additional file 2: Fig.S2a). Furthermore, along the rostrocaudal axis the difference in the number of TH+ in females relative to males was particularly pronounced in the central region of the LC (− 5.44 mm to − 5.60 mm from Bregma: Fig. 3e). The analysis of the AUC also showed significant differences between the two sexes in terms of the number of TH+ cells (p < 0.05: Fig. 3f, see Table 3).
Quantification of TH+ cells and the LC volume in male and female mice. a Representative images of the LC in male and female mice stained for TH by DAB, and b quantification of the number of TH+ cells in both groups. c Representative confocal images of the area occupied by the soma (white line) and/or dendrites (yellow line) outlined for volume estimation. d Quantification of the number of TH+ cells detected by immunofluorescence in the entire LC. e Graph depicting the distribution of TH+ cells along the rostrocaudal axis of the LC and f its representation as the AUC. g The volume occupied by the soma, h somatodendrites and i dendrites of the entire LC. j Graph depicting the distribution of TH+ dendrites along the rostrocaudal axis of the LC and k its representation as the AUC. The data are presented as the mean ± SEM of n = 5 animals per group for DAB and another 5 animals per group for inmunofluorescence: *p < 0.05, **p < 0.01 vs male. Scale bars = 100 μm. IV, fourth ventricle; TH, Tyrosine Hydroxylase; DAB, 3,3′-diaminobenzidine tetrahydrochloride; AUC, area under curve
According to the Cavalieri’s principle, the analysis of the volume occupied by noradrenergic somas did not show differences between the groups (Fig. 3g), although female mice had a significantly higher somatodendritic volume than males (p < 0.05: Fig. 3h), as a result of a higher dendritic volume in the female LC relative to that of males (p < 0.01: Fig. 3i). The somatic (Additional file 2: Fig. S2b, c) and somatodendritic (Additional file 2: Fig. S2d, e) distribution in the rostrocaudal axis was subsequently analyzed by plotting the somas and the somatodendritic occupied areas in each LC section, which failed to reveal any significant differences. However, when the dendritic distribution in the rostrocaudal axis was analyzed by plotting the area occupied by dendrites in each LC section, a larger area of the LC was occupied by dendrites in females, particularly in the rostral region of the LC nucleus (− 5.32 mm to − 5.44 mm from Bregma: Fig. 3j). The AUC when the LC dendrite distribution in the LC was plotted also revealed significant differences between the sexes (p < 0.05: Fig. 3k, see Table 3).
The passive properties and excitability of LC neurons differ between male and female mice
Since we found behavioral and structural differences in the LC, we further studied if the mice sex conditions the electrical activity of LC neurons. We used whole-cell patch clamp recordings to evaluate the intrinsic properties and excitability of LC neurons, identifying a smaller membrane capacitance (Cm, p < 0.05: Fig. 4a) and a tendency towards a higher membrane resistance (Rm, p = 0.07: Fig. 4b) in female animals, yet with a similar membrane resting potential to males (Fig. 4c). IRK currents were smaller in female animals (p < 0.01: Fig. 4d), reflecting possible differences in cell excitability. Nevertheless, several parameters related to the action potential (AP) were similar between male and female mice, such as amplitude, half-width, threshold and after hyperpolarization amplitude (AHP: Fig. 4e). In addition to the changes in the passive properties, excitability was enhanced in the female mice (Fig. 4f), which showed a smaller rheobase (p < 0.001: Fig. 4g), together with a faster firing frequency (p < 0.05: Fig. 4h) and a larger voltage-deflection in response to positive or negative current injection (25 pA steps, p < 0.001, ANOVA: Fig. 4i, see Table 4).
Evaluation of the passive properties and excitability of LC neurons in male and female mice. Population graphs depicting differences in a the membrane capacitance, b a slight difference in the membrane resistance and c similar membrane resting potentials. d Representative example and graph of the IRK currents. e Representative example of the action potential (AP) and corresponding parameters, such as the amplitude, half-width, threshold and after hyperpolarization potential (AHP). f Representative examples of the voltage responses of identified LC neurons to current injection of + 100 and − 100 pA, respectively. g Rheobase. h Graph showing the activity driven in response to the injection of positive currents (+ 25 pA steps). i Graph showing the voltage deflections in response to the injection of negative currents (− 25 pA steps). The data are presented as the mean ± SEM of n = 5 mice per group: *p < 0.05, **p < 0.01 ***p < 0.001 vs male. ANOVA (sex factor): #p < 0.05, ###p < 0.001
Discussion
Through immunohistochemistry studies, sex differences in the noradrenergic LC nucleus of C57BL/6J mice were demonstrated here. This was reflected by a relatively smaller number of TH+ cells in the central region of the female LC and a larger volume of the rostral female LC occupied by dendrites. Furthermore, whole-cell patch-clamp electrophysiology revealed that LC neurons from female mice had distinct intrinsic properties and cell excitability, summarized by a smaller membrane capacitance and enhanced excitability. In behavioral studies female mice exhibit more anxiety-related behaviors, although females and males developed similar depressive-like behaviors. Females outperformed males in memory tasks that involved distinguishing between objects with minor differences and they also exhibited greater thermal pain sensitivity, yet no differences were found when mechanical stimuli were applied.
We used different immunohistochemistry approaches to compare the number of TH+ cells along the rostrocaudal axis of the LC nucleus in female and male mice. DAB and immunofluorescence studies indicated that there were fewer TH+ cells in the LC of females, particularly in the central region of the LC. Previous studies demonstrated sex differences in LC neurons, mainly in rats, and strain and age-dependent results were reported. While there were more neurons in the LC of female Wistar rats compared to males [48, 49], this difference was not evident in Long-Evans rats [50, 51]. Although there is little data from mice, no differences in TH+ cells were found in the LC of adult female and male C57BL/6J mice [52]. However, we found here more TH+ cells in the male LC and a higher proportion of TH+ cells mainly in the central region of the LC. The differences between these two studies could be due to the methodological approaches, as the earlier study counted the number of TH+ cells in 3 serial sections spanning the rostral to caudal extent of the LC, while we counted the number of TH+ cells by immunofluorescence in all sections covering the entire LC region. Taking into account that the main differences were found in the central region of the male LC, it is plausible that data collection from serial LC sections could mask differences along the rostrocaudal axis.
Although there were fewer TH+ cells in the female mice LC, there were no differences in the volume occupied by their somas between females and males. Interestingly, when the volume occupied by TH+ soma or dendrites was analyzed separately, a higher dendritic volume was evident in the female LC, predominantly in the rostral LC. Studies in Sprague–Dawley rats indicated that the dendritic arbor of the LC is more complex and extends further in female than in male rats [25]. Moreover, synaptophysin expression was stronger in the LC of female rats, which might suggest more synaptic inputs in this region. Interestingly, hypothalamic-projecting LC neurons are located rostrally and consequently, the rostral LC presumably receives more corticotropin releasing factor (CRF), which has been reported to activate LC neurons and promote an anxiety-like behavioral phenotype [13].
LC cells were evaluated functionally by whole-cell patch-clamp electrophysiology, identifying a lower membrane capacitance and slightly higher resistance in the LC of female mice. Despite these differences, the properties of the action potential were similar in male and female mice, suggesting similar implications of ion channels in shaping the action potential [53]. Interestingly, in female mice higher excitability was seen than in males, as corroborated further through a lower rheobase and smaller IRK currents. Previous studies on C57BL/6 background mice also found enhanced LC excitability in females relative to males [54]. Females were also more sensitive to hyperpolarizing stimuli than males, suggesting that female LC cells are more excitable and functionally more sensitive to external inputs. This also agrees with previous electrophysiological findings in anesthetized animals showing that female LC neurons fire faster than those of males when exposed to hypotensive stress [55]. Importantly, our electrophysiological assessment does not make it possible to demonstrate the extensive connectivity of the LC or the consequences of the afferents it receives for the modelling of cellular activities. Indeed, it is currently known that the LC has a heterogeneous organization and function with discrete modes of activation, whereby different modules of noradrenergic neurons enter segregated operational modes [56]. Therefore, future electrophysiological experiments in combination with neural tracers of specific subpopulations are still pending.
The activity of LC-noradrenergic neurons is required to elicit acute stress-induced anxiety and indeed, optogenetic/chemogenetic activation of LC neurons is itself anxiogenic [57, 58]. Thus, sex differences in the LC might be involved in the behavioral differences found between sexes. The evaluation of anxiety-like behavior through the EPM, the light/dark and the OFT paradigms are based on unconditioned reactions, consistently showing that female mice have a higher index of anxiety. These sex differences are in agreement with reports [59] but not with others [60]. The discrepancy in the data obtained from different studies may be masked by factors that influence these unconditioned anxiety tests, such as locomotion [61]. Thus, studies reporting no differences in the time spent in the open side, classically found that female mice showed significantly higher scores in distance moved or time spent walking parameters [60] introducing a clear interpretation bias regarding anxiety state. Our results did not show sex differences in the motor activity, providing greater confidence about the anxiogenic state of females. The higher anxiety found in female mice could also be linked to the estrous cycle because estrogen up-regulates TH gene transcription [62] and mRNA expression in the LC [63, 64], as well as the noradrenaline release in multiple brain areas [65, 66]. Thus, it is likely that stronger reactivity of the LC is due to higher estrogens levels promoting an anxiogenic phenotype. In line with the anxiety assessment, we also found that females were less able to remove pellets from the burrow than males, and that they had a lower latency to groom than males, although their total grooming time was similar in the splash test. These are measurements of well-being and self-care behaviors [67] in which males outperformed females. Accordingly, other studies reported that less time is spent burrowing by female mice than males [68] and that the burrow formed by males is longer than that of females [69]. Thus, males and females perform burrowing behavior differently which may reflect differences in basal well-being.
Interestingly, males and females showed similar behavioral despair in the TST and FST tests, although adopting different active behaviors, such as females climbing more in the TST. Climbing behavior in cages is considered stereotypic-like behavior, suggesting that increased climbing by animals may reflect psychological distress and anxiety [70]. Earlier data from rodents showed females routinely adopt more “grid-climbing” activity in cages than males [71], which could be related to greater curiosity as part of the exploratory behavior of unknown environments outside the cage. Despite no sexual differences were found in response to a situation of short-term inescapable stress like the TST or FST, further studies using other stress modalities of longer duration, such as the repeated social defeat paradigm [72], where the LC might be involved, would be of great interest.
The enhanced structural and functional LC sensitivity in female rodents might also be involved in learning and memory in the NOR paradigm [73,74,75]. To test STM as a learning index and LTM as an index of memory, we adopted the NOR cognitive paradigm. We found that both males and females had similar baseline preferences for the two identical objects presented in the training phase. However, females required less interactions with identical objects to display the same preference as males, suggesting that they are more efficient in this recognition. In addition, when a novel object was presented in the test phase, the learning and memory index of males and females was similar in terms of recognition. Interestingly, when an object with minimal differences to the familiar one was presented in a STM protocol, females exhibited a higher DI. While advantages in the recognition of novel objects have been reported in female Long Evans rats [76], this is not so clear in female C57BL/6J mice. Indeed, male preference was reported for exploring a novel object [77], yet a clear advantage of females over males in the recognition of a novel object has been found [78]. Here, we reported similar preferences for a novel object in STM and LTM protocols using two very different objects, although females had a clear advantage in recognizing minimal differences between objects. These results are consistent with previous findings from humans, whereby object details went unnoticed by men but women were more adept at distinguishing such details [78,79,80,81]. Presentation of a novel stimulus can trigger an increase in corticosterone plasma levels, an index of stress [82]. Thus, the precise encoding of visual details in females could be related to their capacity to detect emotional or stressful events rapidly, as females are generally more emotional and stressed than males [83,84,85]. All these results align with the existing literature on mice and other species, highlighting the importance of controlling object characteristics when performing this type of task and encourage the evaluation of different learning modalities [60, 86].
We also found that females displayed greater thermal sensitivity in the plantar test, although no differences were found in the response when applying a mechanical stimulus. At least some important aspects of pain processing are robustly sex-dependent [87, 88] and although further research is necessary, most studies in rodents show females to be more sensitive to pain stimuli [87,88,89]. In addition, studies in humans show that women are more sensitive to pain than men, evident through greater sensitivity to the exposure for first time to a thermal stimulus [7, 90,91,92]. However, studies that employed mechanical stimuli reported lower pain thresholds for women but also found no sex differences [93,94,95]. Although there is evidence that LC projections are involved in thermal thresholds in male rodents [96, 97], to date this issue has not been addressed in females.
With the increasing interest in exploring sexual differences in rodent behaviors, some laboratories are reporting sexual differences that others do not find. Many factors may explain such inconsistencies, such as strain, estrous cycle, and animal age [59, 98, 99]. Furthermore, the environment is also known to play a crucial role, including factors like housing temperature, light intensity, handling and even the sex of the experimenter [100,101,102,103,104]. Therefore, to determine the impact of sex on rodent behaviors related to emotions, cognition, and pain, systematic reviews or meta-analyses are mandatory, like some already available [1, 3, 88]. However, our study holds the value of exploring rodent behavior in parallel with LC physiology, in a specific mice strain in our laboratory conditions. Having this in mind, we found that female mice have a higher anxiogenic profile, yet they better distinguish minimal differences in objects and have a lower thermal pain threshold than males. However, no differences were detected when assessing behavioral despair and mechanical responses. In parallel, the dendritic volume in the LC is greater in female mice, especially in regions receiving inputs from areas that process salient information. LC neurons in female mice differ in their intrinsic properties and they are more excitable than in males, which may contribute to the observed behavioral differences. Although causal experiments remain pending, and considering that the LC may act as a mediator of emotions, cognition and pain in response to stimuli, as well as dynamic environmental challenges, these structural and functional sex differences observed in the LC may lead to heightened emotional arousal in females. This arousal may be adaptive but it may also contribute to higher rates of stress-related psychiatric disorders in women, such as post-traumatic stress disorder or generalized anxiety. Based on all of these findings, it is clear that further studies are needed to decipher and understand the sexual differences in LC-related behaviors.
Perspectives and significance
Our data demonstrated pronounced sex differences in the LC nucleus and in the LC-related behavior. Here, females reported fewer noradrenergic cells and a larger volume of dendrites in the rostral region of the LC. Also, LC electrophysiology studies revealed that female mice showed an enhanced cell excitability. This higher excitability might explain why females are more sensitive to relevant sensorial inputs as shown by better performance recognizing minimal differences between objects and higher anxiety-related behaviors than males. Overall suggest the importance of knowledge of sex differences in the CNS to understand brain and related pathologies in a sex-specific manner.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35(3):303–19.
Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111(1):52–8.
Hamson DK, Roes MM, Galea LA. Sex hormones and cognition: neuroendocrine influences on memory and learning. Compr Physiol. 2016;6(3):1295–337.
Angst J, Gamma A, Gastpar M, Lépine JP, Mendlewicz J, Tylee A, et al. Gender differences in depression. Epidemiological findings from the European DEPRES I and II studies. Eur Arch Psychiatry Clin Neurosci. 2002;252(5):201–9.
Bekker MH, van Mens-Verhulst J. Anxiety disorders: sex differences in prevalence, degree, and background, but gender-neutral treatment. Gend Med. 2007;4:S178-93.
Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–6.
Fillingim RB. Sex, gender, and pain: women and men really are different. Curr Rev Pain. 2000;4(1):24–30.
Gogos A, Ney LJ, Seymour N, Van Rheenen TE, Felmingham KL. Sex differences in schizophrenia, bipolar disorder, and post-traumatic stress disorder: are gonadal hormones the link? Br J Pharmacol. 2019;176(21):4119–35.
Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, Heath AC, et al. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatry. 1995;152(6):833–42.
Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science. 2005;310(5749):819–23.
Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, Skuse D. Sex differences in autism spectrum disorder: evidence from a large sample of children and adolescents. J Autism Dev Disord. 2012;42(7):1304–13.
Rucklidge JJ. Gender differences in attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2010;33(2):357–73.
Bangasser DA, Wiersielis KR, Khantsis S. Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Res. 2016;1641(Pt B):177–88.
De Vries GJ, Simerly RB. Anatomy, development, and function of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, Arnold AP, Rubin RT, editors. Hormones, brain and behaviour. Amsterdam: Elsevier; 2002. p. 137– XXIX.
Hutton LA, Gu G, Simerly RB. Development of a sexually dimorphic projection from the bed nuclei of the stria terminalis to the anteroventral periventricular nucleus in the rat. J Neurosci. 1998;18(8):3003–13.
Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43(3):313–9.
Yagi S, Galea LAM. Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology. 2019;44(1):200–13.
Schwarz LA, Luo L. Organization of the locus coeruleus-norepinephrine system. Curr Biol. 2015;25(21):R1051–6.
Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50.
Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13(12):1526–33.
Li L, Feng X, Zhou Z, Zhang H, Shi Q, Lei Z, et al. Stress accelerates defensive responses to looming in mice and involves a locus coeruleus-superior colliculus projection. Curr Biol. 2018;28(6):859-71.e5.
McCall JG, Siuda ER, Bhatti DL, Lawson LA, McElligott ZA, Stuber GD, et al. Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. Elife. 2017;6: e18247.
Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10(3):211–23.
Waterhouse BD, Navarra RL. The locus coeruleus-norepinephrine system and sensory signal processing: a historical review and current perspectives. Brain Res. 2019;1709:1–15.
Bangasser DA, Zhang X, Garachh V, Hanhauser E, Valentino RJ. Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav. 2011;103(3–4):342–51.
Van Bockstaele EJ, Bajic D, Proudfit H, Valentino RJ. Topographic architecture of stress-related pathways targeting the noradrenergic locus coeruleus. Physiol Behav. 2001;73(3):273–83.
Mulvey B, Bhatti DL, Gyawali S, Lake AM, Kriaucionis S, Ford CP, et al. Molecular and functional sex differences of noradrenergic neurons in the mouse locus coeruleus. Cell Rep. 2018;23(8):2225–35.
McLean AC, Valenzuela N, Fai S, Bennett SA. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp. 2012;67: e4389.
Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry. 2011;70(5):479–86.
Hascoët M, Bourin M. A new approach to the light/dark test procedure in mice. Pharmacol Biochem Behav. 1998;60(3):645–53.
Suárez-Pereira I, García-Domínguez I, Bravo L, Santiago M, García-Revilla J, Espinosa-Oliva AM, et al. The absence of caspase-8 in the dopaminergic system leads to mild autism-like behavior. Front Cell Dev Biol. 2022;10: 839715.
Deacon RM. Burrowing in rodents: a sensitive method for detecting behavioral dysfunction. Nat Protoc. 2006;1(1):118–21.
Humo M, Ayazgök B, Becker LJ, Waltisperger E, Rantamäki T, Yalcin I. Ketamine induces rapid and sustained antidepressant-like effects in chronic pain induced depression: role of MAPK signaling pathway. Prog Neuropsychopharmacol Biol Psychiatry. 2020;100: 109898.
Berrocoso E, Ikeda K, Sora I, Uhl GR, Sánchez-Blázquez P, Mico JA. Active behaviours produced by antidepressants and opioids in the mouse tail suspension test. Int J Neuropsychopharmacol. 2013;16(1):151–62.
Berrocoso E, Rojas-Corrales MO, Mico JA. Differential role of 5-HT1A and 5-HT1B receptors on the antinociceptive and antidepressant effect of tramadol in mice. Psychopharmacology. 2006;188(1):111–8.
Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327–36.
Suárez-Pereira I, Canals S, Carrión AM. Adult newborn neurons are involved in learning acquisition and long-term memory formation: the distinct demands on temporal neurogenesis of different cognitive tasks. Hippocampus. 2015;25(1):51–61.
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63.
Deuis JR, Dvorakova LS, Vetter I. Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci. 2017;10:284.
Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88.
Alba-Delgado C, Borges G, Sánchez-Blázquez P, Ortega JE, Horrillo I, Mico JA, et al. The function of alpha-2-adrenoceptors in the rat locus coeruleus is preserved in the chronic constriction injury model of neuropathic pain. Psychopharmacology. 2012;221(1):53–65.
Llorca-Torralba M, Pilar-Cuéllar F, Bravo L, Bruzos-Cidon C, Torrecilla M, Mico JA, et al. Opioid activity in the locus coeruleus is modulated by chronic neuropathic pain. Mol Neurobiol. 2019;56:4135–50.
Bravo L, Mariscal P, Llorca-Torralba M, López-Cepero JM, Nacher J, Berrocoso E. Altered expression of vesicular glutamate transporter-2 and cleaved caspase-3 in the locus coeruleus of nerve-injured rats. Front Mol Neurosci. 2022;15: 918321.
Michel RP, Cruz-Orive LM. Application of the Cavalieri principle and vertical sections method to lung: estimation of volume and pleural surface area. J Microsc. 1988;150(Pt 2):117–36.
Miguelez C, Morin S, Martinez A, Goillandeau M, Bezard E, Bioulac B, et al. Altered pallido-pallidal synaptic transmission leads to aberrant firing of globus pallidus neurons in a rat model of Parkinson’s disease. J Physiol. 2012;590(22):5861–75.
Rivera A, Suárez-Boomgaard D, Miguelez C, Valderrama-Carvajal A, Baufreton J, Shumilov K, et al. Dopamine D4 receptor is a regulator of morphine-induced plasticity in the rat dorsal striatum. Cells. 2021;11(1):31.
Williams JT, North RA, Tokimasa T. Inward rectification of resting and opiate-activated potassium currents in rat locus coeruleus neurons. J Neurosci. 1988;8(11):4299–306.
Guillamón A, de Blas MR, Segovia S. Effects of sex steroids on the development of the locus coeruleus in the rat. Brain Res. 1988;468(2):306–10.
Pinos H, Collado P, Rodríguez-Zafra M, Rodríguez C, Segovia S, Guillamón A. The development of sex differences in the locus coeruleus of the rat. Brain Res Bull. 2001;56(1):73–8.
Garcia-Falgueras A, Pinos H, Collado P, Pasaro E, Fernandez R, Segovia S, et al. The expression of brain sexual dimorphism in artificial selection of rat strains. Brain Res. 2005;1052(2):130–8.
Garcia-Falgueras A, Pinos H, Fernández R, Collado P, Pasaro E, Segovia S, et al. Sexual dimorphism in hybrids rats. Brain Res. 2006;1123(1):42–50.
Pendergast JS, Tuesta LM, Bethea JR. Oestrogen receptor beta contributes to the transient sex difference in tyrosine hydroxylase expression in the mouse locus coeruleus. J Neuroendocrinol. 2008;20(10):1155–64.
Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8(6):451–65.
Wyrofsky RR, Reyes BAS, Yu D, Kirby LG, Van Bockstaele EJ. Sex differences in the effect of cannabinoid type 1 receptor deletion on locus coeruleus-norepinephrine neurons and corticotropin releasing factor-mediated responses. Eur J Neurosci. 2018;48(5):2118–38.
Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31(3):544–54.
Uematsu A, Tan BZ, Ycu EA, Cuevas JS, Koivumaa J, Junyent F, et al. Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat Neurosci. 2017;20(11):1602–11.
McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, et al. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87(3):605–20.
Sciolino NR, Plummer NW, Chen YW, Alexander GM, Robertson SD, Dudek SM, et al. Recombinase-dependent mouse lines for chemogenetic activation of genetically defined cell types. Cell Rep. 2016;15(11):2563–73.
An XL, Zou JX, Wu RY, Yang Y, Tai FD, Zeng SY, et al. Strain and sex differences in anxiety-like and social behaviors in C57BL/6J and BALB/cJ mice. Exp Anim. 2011;60(2):111–23.
Fritz AK, Amrein I, Wolfer DP. Similar reliability and equivalent performance of female and male mice in the open field and water-maze place navigation task. Am J Med Genet C Semin Med Genet. 2017;175(3):380–91.
Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4(9):775–90.
Thanky NR, Son JH, Herbison AE. Sex differences in the regulation of tyrosine hydroxylase gene transcription by estrogen in the locus coeruleus of TH9-LacZ transgenic mice. Brain Res Mol Brain Res. 2002;104(2):220–6.
Pau KY, Hess DL, Kohama S, Bao J, Pau CY, Spies HG. Oestrogen upregulates noradrenaline release in the mediobasal hypothalamus and tyrosine hydroxylase gene expression in the brainstem of ovariectomized rhesus macaques. J Neuroendocrinol. 2000;12(9):899–909.
Serova L, Rivkin M, Nakashima A, Sabban EL. Estradiol stimulates gene expression of norepinephrine biosynthetic enzymes in rat locus coeruleus. Neuroendocrinology. 2002;75(3):193–200.
Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: implications for female reproductive physiology. Horm Behav. 2001;40(2):169–77.
Karkanias GB, Morales JC, Etgen AM. Effects of diabetes and estradiol on norepinephrine release in female rat hypothalamus, preoptic area and cortex. Neuroendocrinology. 1998;68(1):30–6.
Jirkof P. Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods. 2014;234:139–46.
Pond HL, Heller AT, Gural BM, McKissick OP, Wilkinson MK, Manzini MC. Digging behavior discrimination test to probe burrowing and exploratory digging in male and female mice. J Neurosci Res. 2021;99(9):2046–58.
Bedford NL, Weber JN, Tong W, Baier F, Kam A, Greenberg RA, et al. Interspecific variation in cooperative burrowing behavior by. Evol Lett. 2022;6(4):330–40.
Garner JP, Mason GJ. Evidence for a relationship between cage stereotypies and behavioural disinhibition in laboratory rodents. Behav Brain Res. 2002;136(1):83–92.
Borbélyová V, Janišová K, Mysliveček J, Riljak V. Sex-related differences in locomotion and climbing of C57Bl/6NTac mice in a novel environment. Physiol Res. 2019;68(Suppl 3):S353–9.
Reyes BAS, Zhang XY, Dufourt EC, Bhatnagar S, Valentino RJ, Van Bockstaele EJ. Neurochemically distinct circuitry regulates locus coeruleus activity during female social stress depending on coping style. Brain Struct Funct. 2019;224(4):1429–46.
Mello-Carpes PB, da Silva de Vargas L, Gayer MC, Roehrs R, Izquierdo I. Hippocampal noradrenergic activation is necessary for object recognition memory consolidation and can promote BDNF increase and memory persistence. Neurobiol Learn Mem. 2016;127:84–92.
Mello-Carpes PB, Izquierdo I. The Nucleus of the Solitary Tract → Nucleus Paragigantocellularis → Locus Coeruleus → CA1 region of dorsal hippocampus pathway is important for consolidation of object recognition memory. Neurobiol Learn Mem. 2013;100:56–63.
Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci USA. 2016;113(51):14835–40.
Saucier DM, Shultz SR, Keller AJ, Cook CM, Binsted G. Sex differences in object location memory and spatial navigation in Long-Evans rats. Anim Cogn. 2008;11(1):129–37.
Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117(6):1283–91.
Bettis T, Jacobs LF. Sex differences in object recognition are modulated by object similarity. Behav Brain Res. 2012;233(2):288–92.
De Goede M, Postma A. Gender differences in memory for objects and their locations: a study on automatic versus controlled encoding and retrieval contexts. Brain Cogn. 2008;66(3):232–42.
Levy LJ, Astur RS, Frick KM. Men and women differ in object memory but not performance of a virtual radial maze. Behav Neurosci. 2005;119(4):853–62.
McGivern RF, Mutter KL, Anderson J, Wideman G, Bodnar M, Huston PJ. Gender differences in incidental learning and visual recognition memory: support for a sex difference in unconscious environmental awareness. Personal Individ Differ. 1998;25(2):223–32.
Bevins RA, Besheer J, Palmatier MI, Jensen HC, Pickett KS, Eurek S. Novel-object place conditioning: behavioral and dopaminergic processes in expression of novelty reward. Behav Brain Res. 2002;129(1–2):41–50.
Fugate JM, Gouzoules H, Barrett LF. Separating production from perception: perceiver-based explanations for sex differences in emotion. Behav Brain Sci. 2009;32(5):394–5.
Kring AM, Gordon AH. Sex differences in emotion: expression, experience, and physiology. J Pers Soc Psychol. 1998;74(3):686–703.
Schoofs D, Pabst S, Brand M, Wolf OT. Working memory is differentially affected by stress in men and women. Behav Brain Res. 2013;241:144–53.
Hall BJ, Abreu-Villaça Y, Cauley M, Junaid S, White H, Kiany A, et al. The ventral hippocampal muscarinic cholinergic system plays a key role in sexual dimorphisms of spatial working memory in rats. Neuropharmacology. 2017;117:106–13.
Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859–66.
Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci. 2020;21(7):353–65.
Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20(3):371–80 (discussion 435-513).
Arendt-Nielsen L, Bjerring P. Sensory and pain threshold characteristics to laser stimuli. J Neurol Neurosurg Psychiatry. 1988;51(1):35–42.
Feine JS, Bushnell CM, Miron D, Duncan GH. Sex differences in the perception of noxious heat stimuli. Pain. 1991;44(3):255–62.
Hashmi JA, Davis KD. Women experience greater heat pain adaptation and habituation than men. Pain. 2009;145(3):350–7.
Isselée H, De Laat A, Bogaerts K, Lysens R. Long-term fluctuations of pressure pain thresholds in healthy men, normally menstruating women and oral contraceptive users. Eur J Pain. 2001;5(1):27–37.
Konrad A, Kasahara K, Yoshida R, Murakami Y, Koizumi R, Nakamura M. Pain-pressure threshold changes throughout repeated assessments with no sex related differences. Healthcare (Basel). 2023;11(4):475.
Neziri AY, Scaramozzino P, Andersen OK, Dickenson AH, Arendt-Nielsen L, Curatolo M. Reference values of mechanical and thermal pain tests in a pain-free population. Eur J Pain. 2011;15(4):376–83.
Hirschberg S, Li Y, Randall A, Kremer EJ, Pickering AE. Functional dichotomy in spinal- vs prefrontal-projecting locus coeruleus modules splits descending noradrenergic analgesia from ascending aversion and anxiety in rats. Elife. 2017;6: e29808.
Howorth PW, Thornton SR, O’Brien V, Smith WD, Nikiforova N, Teschemacher AG, et al. Retrograde viral vector-mediated inhibition of pontospinal noradrenergic neurons causes hyperalgesia in rats. J Neurosci. 2009;29(41):12855–64.
Le AA, Lauterborn JC, Jia Y, Wang W, Cox CD, Gall CM, et al. Prepubescent female rodents have enhanced hippocampal LTP and learning relative to males, reversing in adulthood as inhibition increases. Nat Neurosci. 2022;25(2):180–90.
Pitzer C, Kurpiers B, Eltokhi A. Sex differences in depression-like behaviors in adult mice depend on endophenotype and strain. Front Behav Neurosci. 2022;16: 838122.
Lewejohann L, Reinhard C, Schrewe A, Brandewiede J, Haemisch A, Görtz N, et al. Environmental bias? Effects of housing conditions, laboratory environment and experimenter on behavioral tests. Genes Brain Behav. 2006;5(1):64–72.
Neuwirth LS, Verrengia MT, Harikinish-Murrary ZI, Orens JE, Lopez OE. Under or absent reporting of light stimuli in testing of anxiety-like behaviors in rodents: the need for standardization. Front Mol Neurosci. 2022;15: 912146.
Novak J, Jaric I, Rosso M, Rufener R, Touma C, Würbel H. Handling method affects measures of anxiety, but not chronic stress in mice. Sci Rep. 2022;12(1):20938.
Sensini F, Inta D, Palme R, Brandwein C, Pfeiffer N, Riva MA, et al. The impact of handling technique and handling frequency on laboratory mouse welfare is sex-specific. Sci Rep. 2020;10(1):17281.
Georgiou P, Zanos P, Mou TM, An X, Gerhard DM, Dryanovski DI, et al. Experimenters’ sex modulates mouse behaviors and neural responses to ketamine via corticotropin releasing factor. Nat Neurosci. 2022;25(9):1191–200.
Acknowledgements
We are very grateful to the Central Services of Scientific and Technological Research, Health Sciences and Animal Research from the University of Cádiz and to the PTA2021-019890-I contract financed by the “Agencia Estatal de Investigación-Ministerio de Ciencia, Innovación y Universidades and FSE+”. Figures were created with www.BioRender.com.
Funding
Funding for open access publishing: Universidad de Cádiz/CBUA This study was supported by the “Fondo Europeo de Desarrollo Regional” (FEDER)-UE “A way to build Europe” from the “Ministerio de Economía y Competitividad” (PID2022-142785OB-I00, PDC2022-133987-I00); from the “Programa Operativo de Andalucía FEDER, Iniciativa Territorial Integrada ITI 2014–2020 Consejería Salud y Familias, Junta de Andalucía” (PI-0080-2017), "Consejería de Salud y Familias, Junta de Andalucía" (PI-0134-2018); from the "Consejería de Transformación Económica, Industria, Conocimiento y Universidad, Junta de Andalucía" (P20_00958 and CTS-510); from the “Instituto de Investigación e Innovación en Ciencias Biomédicas de Cádiz-INiBICA” (LI19/06IN-CO22; IN-C09); from the “CIBERSAM”: CIBER-Consorcio Centro de Investigación Biomédica en Red (CB07/09/0033), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 955684. Grant PID2 021-126434OB-I00 funded by MCIN/AEI/10. 13039/501100011033 and ERDF A way of making Europe. It has also been funded by the Basque Government (IT1706-22 and PUE21- 03). This research was conducted in the scope of the Transborder Joint Laboratory (LTC) “non-motor Comorbidities in Parkinson’s Disease (CoMorPD)”.
Author information
Authors and Affiliations
Contributions
EB, LB and CM proposed the project and the experimental design. Behavioral studies were performed by PM with the contribution of ISP. Tissue processing, image acquisition and analysis was performed by PM and LB. Patch-clamp studies were conducted by MLLT, JR and CM. EB, LB and MLLT interpreted the results. EB, LB and PM wrote the first draft of the manuscript. All the authors reviewed and proof read the last draft of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Included in the “Materials and methods” section.
Consent for publication
Not applicable.
Competing interests
The authors have no biomedical financial interests or potential competing interests to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1. a
Representative images showing the different stages of the estrous cycle in female mice. Proestrus is characterized by nucleated epithelial cells, estrus by cornified epithelial cells, and metestrus and diestrus by the presence of leukocytes. The results of anxiety-like behavior expressed as b the percentage of time spent in the open arms of the EPM for males and females, represented by estrous cycle stages. Graphs depicting (c) the total activity in arbitrary units (AU) and d the number of entries into the open arms in the EPM. e The latency to enter the dark compartment (in seconds) and f the number of transitions between compartments in the light/dark test. g Graph showing the total distance traveled in the OFT. h Graph depicting the latency to grooming (in seconds) in the splash test, i the percentage of animals that perform clasping, curling, swinging and climbing behavior in the TST, and j the latency (in seconds) to immobility in the FST. Females were in proestrus and estrus (P/E) stages in (e) to (j) behavioral tests. The data are presented as the mean ± SEM of n = 7–10 mice per group: **p < 0.01, ***p < 0.001 vs male; #p < 0.05 vs female P/E. P/E, Proestrus/Estrus; D/M, Diestrus/Metestrus. Scale bar = 50 µm.
Additional file 2: Figure S2. a
Representative immunofluorescence confocal images of the entire LC region along the rostrocaudal axis (distances from Bregma in mm). b Graph depicting the distribution of the area occupied by the soma in the rostrocaudal axis of the LC, followed by c the AUC analysis. d Graph depicting the distribution of the area occupied by the somatodendritic region along the rostrocaudal axis of the LC, followed by e AUC analysis. The data are presented as the mean ± SEM of n = 5 animals per group. Females were in proestrus and estrus (P/E) stages. Scale bar = 100 μm. TH, Tyrosine Hydroxylase; AUC, area under the curve.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mariscal, P., Bravo, L., Llorca-Torralba, M. et al. Sexual differences in locus coeruleus neurons and related behavior in C57BL/6J mice. Biol Sex Differ 14, 64 (2023). https://doi.org/10.1186/s13293-023-00550-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13293-023-00550-7