Abstract
Background
Chronic kidney disease (CKD) is linked to an increased cardiovascular disease (CVD) burden. Albeit underappreciated, sex differences are evident in CKD with females being more prone to CKD development, but males progressing more rapidly to kidney failure (KF). Cardiovascular remodelling is a hallmark of CKD with increased arterial and valvular calcification contributing to CKD. However, little is known regarding sex differences in calcific cardiovascular remodelling in KF patients. Thus, we hypothesise that sex differences are present in coronary artery calcification (CAC) and aortic valve calcification (AVC) in patients with KF.
Methods
KF patients, males (n = 214) and females (n = 107), that had undergone computer tomography (CT) assessment for CAC and AVC were selected from three CKD cohorts. All patients underwent non-contrast multi-detector cardiac CT scanning, with CAC and AVC scoring based on the Agatston method. Baseline biochemical measurements were retrieved from cohort databases, including plasma analyses for inflammation markers (IL-6, TNF, hsCRP) and oxidative stress by skin autofluorescence measuring advanced glycation end-products (AGE), amongst other variables.
Results
Sex-disaggregated analyses revealed that CAC score was associated with age in both males and females (both p < 0.001). Age-adjusted analyses revealed that in males CAC was associated with diabetes mellitus (DM) (p = 0.018) and CVD (p = 0.011). Additionally, for females CAC associated with IL-6 (p = 0.005) and TNF (p = 0.004). In both females and males CAC associated with AGE (p = 0.042 and p = 0.05, respectively). CAC was associated with mortality for females (p = 0.015) independent of age. AVC in females was not reviewed due to low AVC-positive samples (n = 14). In males, in multivariable regression AVC was associated with age (p < 0.001) and inflammation, as measured by IL-6 (p = 0.010).
Conclusions
In female KF patients inflammatory burden and oxidative stress were associated with CAC. Whereas in male KF patients oxidative stress and inflammation were associated with CAC and AVC, respectively. Our findings suggest a sex-specific biomarker signature for cardiovascular calcification that may affect the development of cardiovascular complications in males and females with KF.
Plain language summary
Chronic kidney disease (CKD) is a condition that affects the kidneys and increases the risk of heart problems. Males and females may experience CKD differently, and our study aimed to understand the differences in the development of calcification in the blood vessels of the heart (coronary artery calcification, or CAC) and the heart valves (aortic valve calcification, or AVC) between males and females with CKD.
We analysed 214 males and 107 females with CKD who had undergone a heart scan (computer tomography, or CT) to measure CAC and AVC. We collected information on age, diabetes, cardiovascular disease, and markers of inflammation and oxidative stress.
Our results showed that in both males and females CAC was associated with age. In males, CAC was associated with diabetes and cardiovascular disease, while in females, it was linked to markers of inflammation. In females, CAC was also associated with mortality regardless of age. Unfortunately, we had insufficient samples of females with AVC for analysis. However, in males AVC was associated with age and inflammation.
Overall, our study indicates sex-specific differences in the development of calcification in the blood vessels and heart valves of CKD patients. In females, inflammation and oxidative stress are associated with CAC, while in males, oxidative stress and inflammation are associated with CAC and AVC, respectively. These findings underscore the importance of considering these differences when assessing cardiovascular complications in CKD patients. It may help in developing personalised treatment approaches for both males and females with CKD.
Highlights
-
The gold standard technique of computer-tomography was used to measure coronary artery calcification (CAC) and aortic valve calcification (AVC) scores in males and females with kidney failure.
-
In male kidney failure patients age and inflammatory burden was significantly associated with AVC. In addition, cardiovascular disease, diabetes, and oxidative stress were significantly associated with CAC score.
-
In female kidney failure patients age, oxidative stress, and inflammation were significantly associated with CAC score.
-
Cardiovascular calcification presents with a sex-specific biomarker signature that may affect cardiovascular complications in males and females with kidney failure.
Similar content being viewed by others
Background
Chronic kidney disease (CKD) is a debilitating disease that has become a worldwide health care burden affecting foremost individuals with burden of lifestyle diseases, such as diabetes and hypertension [1]. CKD is linked to an increased risk for cardiovascular disease (CVD) and mortality, with the elderly and females more prone to CKD development [2]. However, CKD in males tends to progress more rapidly toward kidney failure (KF), which highlights the important influence of sex on CKD phenotype.
Cardiovascular remodelling via vascular and/or valvular calcification is a key hallmark of CKD-mineral and bone disorders (CKD-MBD) [3]. CKD-MBD contributes to accelerated uraemia-induced vascular ageing [4] by potentiating cellular senescence [5], modifying the function and structure of vascular endothelial and vascular smooth muscle cells (VSMC), which includes effects on innate immunity [5].
Vascular calcification, per se, is an active process that manifests as early as kidney function starts to deteriorate [6] and is influenced by both traditional and non-traditional cardiovascular risk factors [7]. Potential CKD-related mechanisms behind the simultaneous tunica intima (atherosclerosis) and tunica media (arteriosclerosis) calcification, alongside calcific aortic valve disease (CAVD), have been described in previous studies [8,9,10]. However, the identification of biological sex-related factors in this population is still lacking. This is of importance, as sex as a biological variable could modulate several triggering processes associated with vascular calcification, including inflammation, imbalanced calcium-phosphate metabolism, VSMC damage and its associated phenotypic switch towards the activation of bone formation pathways.
Thus, the rationale behind our study was to assess sex-specific differences in cardiovascular calcification measured at the different sites. We aimed to analyse associations between CAC and AVC, evaluated via computed tomography (CT) scans, with comorbidities and cardiovascular, glycaemic, and oxidative stress biomarkers in a population of males and females with kidney failure. The hypothesis is that males with kidney failure will have greater levels of CAC and AVC compared to that of females with kidney failure.
Methods
Study population
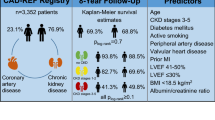
For the current investigation post hoc analyses of data collected from 321 KF patients that had undergone CT scans to assess CAC and AVC, were included. Patient data were included from three prospective CKD cohort databases from the Division of Renal Medicine, Karolinska University Hospital, Sweden. Patients with KF were included from either an incident CKD cohort [11], a prevalent CKD cohort with peritoneal dialysis (PD) treatment [12], or a living-donor kidney transplantation (LD-Tx) cohort [9]. The incident CKD cohort is a prospective cohort, with ongoing recruitment since 1994, of patients with kidney failure (CKD stage 5, GFR < 15 ml/min) with baseline sampling occurring close to the initiation of renal replacement therapy (RRT), with the vast majority having started haemodialysis or PD [11]. For the current study, data were collated for patients included between February 2006 and December 2014 from the incident KF cohort, where CT scans were performed. The prevalent CKD cohort was a prospective cohort, with recruitment between March 2008 and April 2011, including CKD patients undergoing PD. The LD-Tx cohort is a prospective cohort, with ongoing recruitment since March 2009, of kidney failure patients undergoing kidney transplantation. The available study population consisted of 214 males and 107 females, and all data previously included in the cohort databases, either derived from patient records or prior experimental results, were made available for analysis in relation to CAC and AVC.
The Regional Ethics Committee (EPN), Stockholm, Sweden, approved the study protocols that were performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients involved in the study.
Clinical data were recorded at baseline, during the first visit, and included information on demographics, medications, comorbidities (CVD and diabetes mellitus), smoking status, and malnutrition that was assessed by subjective global assessment (SGA). eGFR collected for this study was calculated using the creatinine-based CKD-EPI 2009 Equation [13], validated in the European population. Mortality was documented over a five year follow up period among KF patients. Death cases were retrieved either from patient’s medical journal or the National cause of death register.
Biochemical measurements
Baseline data for biochemicals were retrieved from the cohort databases for all patients included in the current investigation, where available. Biochemical measurements were recorded using overnight fasting blood samples that were collected in the morning, with serum isolated for necessary analyses, and samples were either immediately analysed or stored at -70 °C for future analyses. Analyses for blood lipids, haemoglobins, albumin, creatinine, and inflammation markers (IL-6, hsCRP, TNF) were performed via routine clinical laboratory techniques. Cardiovascular disease (CVD) risk markers (GDF-15, MMP-9, YKL-40) were analysed using commercial enzyme-linked immunosorbent (ELISA) kits, previously described [14]. Skin autofluorescence was measured as a proxy of advanced glycation end-products (AGE), using an Autofluorescence AGE reader (DiagnOptics Technologies BV, the Netherlands) as previously described [15].
Coronary artery calcification and aortic valve calcification
All patients underwent non-contrast multi-detector cardiac CT (LightSpeed VCT or Revolution CT; GE Healthcare, WI, USA) scanning with standard ECG-gated protocol, to evaluate AVC and CAC scores. We used semi-automatic software (syngo.via CT Ca Scoring, Siemens Healthcare, Germany). CAC score was assessed as a lesion with an area > 1 mm2and peak intensity > 130 Hounsfield units (HU) based on the Agatston method and expressed in Agatston units (AU) [16]. AVC scores were computed using the Agatston CAC-scoring method from non-contrast cardiac CT scans. AVC score was determined as the sum of total calcifications in the aortic valve area including calcifications within the valve leaflets as well as in the aortic wall immediately connected to the leaflets.
Statistical analyses
Continuous data are expressed as median ± interquartile range, and categorical data are presented as the frequency with percentage. Individual counts for analytes are provided to present the number of patients with available data. Baseline comparisons between females and males, for continuous data Wilcoxon sum rank test was performed, and for categorical data either Fisher’s exact test or Pearson’s Chi-squared test were performed.
This investigation was designed as a sex-disaggregated study, therefore further analysis was performed after the sub-setting the data set by sex, one for males and the other for females. First, all analytes were separately put in the sex-disaggregated univariate Gamma regression by using CAC or AVC scores as dependent variables. Gamma regression, using the identity link, analyses were performed to assess associations between independent variables and CAC scores. The Variance Inflation Factors (VIF) tool was used to address multicollinearity within multivariable regression analyses assessing CAC scores. Herein, multivariable models that resulted in variables with a VIF greater then 2, indicating a high collinear relationship to other variables, were not interpreted. Multivariable models were reduced in terms of number of variables included so that collinearity was not present in accordance with VIF.
For analysing AVC score we had to perform Gamma regression analyses using the log link to solve convergence issues. The values were displayed as relative rates using inverse transformation of each value. Then, multivariable regression was performed by adjusting only significantly associated variables (p-value < 0.05).
After performing sex-disaggregated logistic regression analyses, all significant variables were put into multivariate regression to assess associations between mortality and CAC scores. The best model was selected using the Bayesian Information Criterion and included age, systolic blood pressure, plasma albumin, CAC score and GDF-15 (only in males). All statistical analyses were carried out using R (v4.1.3) in the RStudio environment (RStudio, MA, USA).
Results
Study population and baseline characteristics
Patients with KF enrolled in the study included 214 males and 107 females with baseline characteristics presented in Table 1. As expected, males presented with a greater frequency of cardiovascular disease comorbidity, and this is presumed why males were more frequently treated with beta-blockers when compared to females (Table 1). Females had significantly elevated cholesterol, high-density lipoprotein (HDL), lipoprotein(a), and apolipoprotein-A1 when compared to males (Table 1). Additionally, females presented with a higher frequency of protein energy wasting, assessed via SGA, and lower albumin levels than males (Table 1). As expected, males had higher creatinine levels than females, likely a generalised reflection of greater muscle mass (Table 1).
Sex-specific CAC score associations with age, comorbidities, inflammation, and oxidative stress
In linear regression analyses, age was significantly associated with CAC scores in both males and females (Table 2). Age-adjusted linear regressions were performed for all other available variables, with age remaining significantly associated with CAC score in all models for both males and females. More extensive multivariable linear regressions were not possible due to increasing collinearity, as tested via the VIF tool, between additional variables that were included in models. For example, when for analysing predictive factors for CAC scores in males, we could not interpret a model including age (VIF = 54), CVD (VIF = 1), DM (VIF = 1), use of statins (VIF = 44), cholesterol (VIF = 9), AGE (VIF = 2) and homocysteine (VIF = 9) due to the high VIFs. In females, all VIFs were above 2 in multivariate Gamma regression.
Therefore, any significant biomarker correlation is not an independent association, but coincides with the significant association of age. DM and CVD were associated with CAC scores in males, with HbA1c only being associated with CAC scores in females (Table 2). Other predictive parameters for CAC score in females were body mass index (BMI), systolic blood pressure (SBP), RRT, malnutrition, use of beta-blockers, and ACE inhibitors/ARBs; whereas in males—only with the use of statins. Inflammatory markers hs-CRP, IL-6 and TNF were associated with CAC score in females only, whereas oxidative stress marker AGE was associated with CAC score in both males and females. Lipid profile was significantly correlated to CAC score only in females, alongside additional cardiovascular biomarkers GDF-15, MMP-9, and YKL-40.
AVC score associations with age and IL-6 in male patients with KF
Gaussian regression analyses for possible associations with AVC score were also performed. Regression models for females were not performed due to the low number of females with an AVC score > 0 (n = 14). In males, the AVC score was significantly associated with age, haemodynamic parameters, lipid status, antihypertensive medication, inflammatory and cardiovascular biomarkers (Table 3). However, after multiple adjustments, the AVC score remained significantly associated with IL-6 and age only.
CAC score is associated with mortality in females with KF
In multivariate logistics regression analyses, CAC score was shown to be associated with mortality in female patients only, independent of age. Moreover, in multivariable analyses a significant association with albumin was also present for female patients. In males, CAC score was associated with mortality in univariate analyses, however, this association was lost in multivariate analyses. GDF-15 was the only marker to remain significantly associated with mortality in males in the multivariate model. Other significant variables that predicted mortality are listed in Table 4.
Discussion
The calcification process may have differing impacts on CVD outcomes due to specific pathways related to the progression of disease or calcification per se. Therefore, understanding sex differences may have implications for potential therapeutic targets and interventions. We report that in males oxidative stress (measured as AGEs in the skin) seems to be predominantly related to CAC when compared with females. However, in females, both oxidative stress and inflammation are associated with CAC. In addition, AVC seems to be driven by chronological age and inflammation in males.
AVC and CAC are both associated with increased risk for cardiovascular events [17]. Examination of the link between AVC and CAC in the Multi-Ethnic Study of Atherosclerosis (MESA) study [17] revealed that AVC increased as CAC severity increases, and that AVC is independently correlated with increased coronary atherosclerosis as assessed by CAC. Aortic sclerosis and atherosclerosis share risk factors such as hypertension, diabetes mellitus, hypercholesterolaemia, and an elevated inflammatory profile [17]. Thus, AVC may be indicative of atherosclerotic burden. CAC has been reported to correlate with aortic root calcification based on shared arterial tissue, which could indicate diffuse arterial disease, whilst the absence of a link between AVC and CAC may reflect a distinct disease [18]. Conversely, a study by Dai et al. [19], observed that there is an overlap of AVC and CAC in the KF population and AVC was associated with increased all-cause mortality regardless of the presence of CAC, conventional risk factors or inflammation. The same group also noted that the amount of AVC and CAC in various vascular calcification groups varied significantly, indicating that despite comparable risk profiles the diverse vascular beds may have distinct underlying mechanism of calcification [19]. There is still much to understand regarding the shared or differing pathophysiology of AVC and CAC.
Coronary artery calcification (CAC) is a proxy of vascular lesions that predicts atherosclerotic CVD in both CKD and non-CKD populations [20, 21]. The pooled prevalence of CAC among CKD patients has been reported to be as high as 60%, whilst in haemodialysis and renal transplant patients a pooled prevalence of 65% and 51% have been reported, respectively [22]. In addition, CAC was positively associated with an increased risk of all-cause and cardiovascular mortality [22]. Meanwhile, aortic valve calcification (AVC) has a detrimental effect on aortic valve function and gives rise to stenosis [23] that contributes to left ventricular hypertrophy and increased morbidity and mortality in CKD [10]. A recent review indicated inconsistencies in the prevalence and risk factors for vascular calcification in CKD that might be sex-dependent [24]. One study reported that males not requiring dialysis treatment have predominantly higher CAC scores than females, while other studies report a higher prevalence of aortic and carotid artery calcification in females [24]. Thus, the impact of sex influence on AVC in CKD is underrepresented.
Males presented with more prevalent CVD and greater AVC, compared to females, although the CAC score was similar in both sexes. Dyslipidaemia, with lower HDL, lipoprotein(a), and apolipoprotein A1, might explain the higher prevalence of CVD in males since it contributes to atherosclerosis [25] and higher CV event risk [26]. Additionally, the higher prescription of beta-blockers in males may also reflect a higher cardiovascular risk. Previous reports in the community-based non-CKD population showed that the choice of antihypertensive medication is sex-dependent [27]. Additionally, pharmacokinetics may play an essential role as females has lower oral beta-blocker (metoprolol) clearance and higher bioavailability [27]. Thus, clinicians may be reluctant to prescribe these medications to females.
Cardiovascular calcification is an umbrella term that includes accelerated vascular ageing typical to CKD [5, 28, 29]. In this process inflammation and oxidative stress are the common triggers for vascular dysfunction and remodeling [29,30,31]. In our study, we find that CAC-related biomarkers had a sex-specific signature. Females with CAC were prone to be more often inflamed, with higher IL-6 and TNF levels alongside increased GDF-15 and YKL-40. These findings concur with our previous report [30] and Wang et al. [31] suggesting that chronic inflammation among CKD patients is linked to CAC. Conversely, males seemed to be subjected to more pronounced oxidative stress, measured by AGE. Whilst accumulation of AGEs are a hallmark of normal ageing, the overproduction of AGEs may lead to vascular dysfunction and structural changes via the activation of nuclear factors kappa B (NFκB) signalling [32]. This can result in coronary artery disease, even in the absence of diabetes [33, 34]. Animal studies reported higher levels of AGEs in male rats than in female rats [35]. AGE accumulation in humans may also be associated with non-traditional variables for CKD and CVD progression [36] such as cholesterol, lipoprotein(a), albuminuria, Apo-A1, and protein energy wasting as observed in females of the current study. Tanaka et al. [36] claims that racial disparities in AGE accumulation may also occur, perhaps owing to darker skin's increased absorption grade of excitation and emission light. Therefore, further investigations are required to determine if ethnic disparities or disparities in reference values between ethnicities exist among our patients, in addition to sex differences.
Despite the higher prevalence of CVD in males in the present study, the CAC score was not significantly different from that of females. RRT might obliviate the impact of sex-related factors on the vascular calcification process. The lack of a sex difference in CAC scores in our study population suggest that vascular calcification progresses occur regardless of RRT modality [37]. Owing to the small sample size, data from Jansz et al. [38] on the influence of different RRT on CAC development remain ambiguous. Niu et al. [39] also reported no significant differences in CAC development between haemodialysis and peritoneal dialysis patients. Although no sex-disaggregated analysis was undertaken their findings confirm that age and diabetes were substantially linked with CAC. However, we could see that in females RRT was associated with higher CAC score. This could also be related to the observations that haemodialysis and peritoneal dialysis contribute to sex hormone imbalance [40] by causing premature menopause in females and a decline in testosterone in males [41]. Further examination of the impact of various RRT on vascular calcification in different sexes is warranted.
It is known that the CAC score predicts all-cause mortality in CKD. CAC score was also found to be dependent on age, but not sex, in studies of CKD stages 3 to 5 [42] and advanced CKD [43]. Herein, we observe a relationship between CAC score and mortality in both males and females. After multiple adjustments this relationship of CAC score and mortality was found to be independent of age in females, in addition to hypoalbuminemia being identified as mortality predictors. However, in males after multiple adjustments, CAC score was found not to be associated with mortality and GDF-15 was identified as a potential mortality predictor, and observation we have previously reported [14]. The association of vascular calcification and KF patient mortality may be associated with a cumulative interaction of hazards such as vascular stiffness, left ventricular hypotrophy, myocardial fibrosis, and/or conductive anomalies [38]. More studies are required in larger cohorts to assess the associations more accurately between CAC and mortality in males and females.
The higher life expectancy of females than males, may be partially attributable to a lower frequency of CVD [44]. In contrast, females and males with CKD have similar life expectancies. After the initiation of RRT the sexes die at the same rate and the “cancelled survival advantage” in females is not regained even after renal transplantation [44]. This phenomenon may explain why despite male participants having more CVD than females, there was no observed difference in CAC score between the sexes. Moreover, the CAC score was associated with mortality in females in our study. It might be important to note that mortality can result from the complex interplay of other factors, such as protein energy wasting, the type and duration of RRT therapy, variation in types of surgical vascular access, as well as response and compliance to therapy.
Computed tomography is the standard technique in assessing AVC, while echocardiography (ECG) evaluates valve function. Several studies addressing AVC in CKD have used ECG for estimating calcification load and aortic valve stenosis [22, 45]. Neither a haemodialysis cohort [45] nor CKD stages 1 to 4 cohort [22] reported sex differences in AVC. In contrast, studies performed in the general population shows that severe aortic valve stenosis is more prevalent in males than females and that males are exposed to a faster progression of valvular calcification [46]. Additionally, less calcification but more fibrosis in valve biopsies is seen in female vs male valves [47, 48]. As females with AVC were underrepresented (only 14 cases), we only conducted analysis of males in whom we observed that inflammatory markers were associated with AVC. Our findings are in accordance with those reported in subjects without overt CVD [49].
The main strength of our study is a sex-disaggregated approach to the cardiovascular calcification profile in KF patients. Additionally, that we used the gold standard technique–computed tomography—to evaluate CAC and AVC. Some limitations should also be considered. At first, the low number of females with positive AVC precludes sex-stratified statistics. Moreover, we did not collect data on sex hormone status. Future investigations should include sex hormone levels or menopausal status as well as information on racial or ethnic representation. In addition, available oxidative stress markers were limited to only AGE and homocysteine in the present cohort, expansion with a wider panel of oxidative stress markers would be preferential for future investigations to properly assess the associations between oxidative stress and calcification. These may provide additional insights into the disparities or heterogeneity of findings on uremic vascular calcification.
Perspectives and significance
In summary, we report a sex-specific signature of CAC and AVC-related biomarkers. Herein, CAC was found to be associated with oxidative stress in male KF patients, and oxidative stress and inflammation in female KF patients. In addition, in male KF patients AVC presented with an inflammatory phenotype. Our study highlights a sex-specific signature of CAC-related biomarkers that may have a potential effect on vascular complications associated with KF in males and females. Moreover, CAC was associated with mortality in females, although larger studies are warranted. Future directions include the assessment of RRT outcome concerning the sex origin of the donated kidney that may affect amelioration of vascular calcification and mortality. In addition, the impact of biological vs chronological age on the vascular calcification process needs attention. Finally, CVD phenotyping, particularly in males, should be accompanied by more frequent testing to screen for early vascular ageing and calcification.
Availability of data and materials
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022;2011(12):7–11.
Bikbov B, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2020;395:709–33.
Düsing P, et al. Vascular pathologies in chronic kidney disease: pathophysiological mechanisms and novel therapeutic approaches. J Mol Med (Berl). 2021;99:335–48.
Dai L, Schurgers LJ, Shiels PG, Stenvinkel P. Early vascular ageing in chronic kidney disease: Impact of inflammation, Vitamin K, senescence and genomic damage. Nephrol Dial Transplant. 2020. https://doi.org/10.1093/ndt/gfaa006.
Hobson S, Arefin S, Kublickiene K, Shiels PG, Stenvinkel P. Senescent cells in early vascular ageing and bone disease of chronic kidney disease—a novel target for treatment. Toxins (Basel). 2019;11:82.
Hruska KA, Sugatani T, Agapova O, Fang Y. The chronic kidney disease—Mineral bone disorder (CKD-MBD): advances in pathophysiology. Bone. 2017;100:80–6.
Veit Barreto D, et al. Coronary calcification in hemodialysis patients: the contribution of traditional and uremia-related risk factors. Kidney Int. 2005;67:1576–82.
Byon CH, Chen Y. Molecular mechanisms of vascular calcification in chronic kidney disease: the link between bone and the vasculature. Curr Osteoporos Rep. 2015. https://doi.org/10.1007/s11914-015-0270-3.
Jaminon AMG, et al. Matrix Gla protein is an independent predictor of both intimal and medial vascular calcification in chronic kidney disease. Sci Rep. 2020;10:6586.
Brandenburg VM, Schuh A, Kramann R. Valvular calcification in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26:464–71.
Stenvinkel P, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–911.
Xu H, et al. Oxidative DNA damage and mortality in hemodialysis and peritoneal dialysis patients. Perit Dial Int. 2015;35:206.
KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease.
Laucyte-Cibulskiene A, et al. Role of GDF-15, YKL-40 and MMP 9 in patients with end-stage kidney disease: focus on sex-specific associations with vascular outcomes and all-cause mortality. Biol Sex Differ. 2021;12:1–11.
Mukai H, et al. Skin autofluorescence, arterial stiffness and Framingham risk score as predictors of clinical outcome in chronic kidney disease patients: a cohort study. Nephrol Dial Transplant. 2019;34:442–8.
Agatston AS, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32.
Nasir K, et al. Relationship of aortic valve calcification with coronary artery calcium severity: the Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomogr. 2010;4:41–6.
Henein M, et al. Aortic root, not valve, calcification correlates with coronary artery calcification in patients with severe aortic stenosis: a two-center study. Atherosclerosis. 2015;243:631–7.
Dai L, et al. Aortic valve calcium associates with all-cause mortality independent of coronary artery calcium and inflammation in patients with end-stage renal disease. J Clin Med. 2020;9:607.
Hermann DM, et al. Coronary artery calcification is an independent stroke predictor in the general population. Stroke. 2013;44:1008–13.
Folsom AR, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2008;168:1333–9.
Wang L, et al. Prevalence and correlates of cardiovascular calcification and its prognostic effects among patients with chronic kidney disease: results from the C-STRIDE study. Front Public Health. 2022;9: 762370.
Lerman DA, Prasad S, Alotti N. Calcific aortic valve disease: molecular mechanisms and therapeutic approaches. Eur Cardiol. 2015;10:108–12.
Wu PY, Lee SY, Chang KV, ter Chao C, Huang JW. Gender-related differences in chronic kidney disease-associated vascular calcification risk and potential risk mediators: a scoping review. Healthcare (Basel). 2021;9:979.
Ceponiene I, et al. Association of coronary calcium, carotid wall thickness, and carotid plaque progression with low-density lipoprotein and high-density lipoprotein particle concentration measured by ion mobility (From Multiethnic Study of Atherosclerosis [MESA]). Am J Cardiol. 2021;142:52–8.
Tatematsu M, Inaguma D, Yamada T, Sakamoto I, Sakakibara M. The impact of gender difference on the relationship between serum high-density lipoprotein level and cardiovascular events in incident dialysis patients: a multicenter prospective cohort study. Int Urol Nephrol. 2020;52:1357–65.
Kalibala J, Pechère-Bertschi A, Desmeules J. Gender differences in cardiovascular pharmacotherapy—the example of hypertension: a mini review. Front Pharmacol. 2020;11:564.
Dai L, Qureshi AR, Witasp A, Lindholm B, Stenvinkel P. Early vascular ageing and cellular senescence in chronic kidney disease. Comput Struct Biotechnol J. 2019;17:721.
Cunha PG, Boutouyrie P, Nilsson PM, Laurent S. Early vascular ageing (EVA): definitions and clinical applicability. Curr Hypertens Rev. 2017;13:8–15.
Laucyte-Cibulskiene A, et al. Role of GDF-15, YKL-40 and MMP 9 in patients with end-stage kidney disease: focus on sex-specific associations with vascular outcomes and all-cause mortality. Biol Sex Differ. 2021;12:50.
Wang XR, Zhang JJ, Xu XX, Wu YG. Prevalence of coronary artery calcification and its association with mortality, cardiovascular events in patients with chronic kidney disease: a systematic review and meta-analysis. Ren Fail. 2019;41:244–56.
Fishman SL, Sonmez H, Basman C, Singh V, Poretsky L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: a review. Mol Med. 2018;24(1):59. https://doi.org/10.1186/s10020-018-0060-3.
Kanauchi M, Tsujimoto N, Hashimoto T. Advanced glycation end products in nondiabetic patients with coronary artery disease. Diabetes Care. 2001;24:1620–3.
Kerkeni M, et al. Increased serum concentrations of pentosidine are related to presence and severity of coronary artery disease. Thromb Res. 2014;134:633–8.
Wang X, Desai K, Juurlink BHJ, de Champlain J, Wu L. Gender-related differences in advanced glycation endproducts, oxidative stress markers and nitric oxide synthases in rats. Kidney Int. 2006;69:281–7.
Tanaka K, et al. Skin autofluorescence is associated with the progression of chronic kidney disease: a prospective observational study. PLoS ONE. 2013;8:e83799.
Jansz TT, et al. Coronary artery calcification in hemodialysis and peritoneal dialysis. Am J Nephrol. 2018;48:369–77.
Jansz TT, Verhaar MC, London GM, van Jaarsveld BC. Is progression of coronary artery calcification influenced by modality of renal replacement therapy? A systematic review. Clin Kidney J. 2018;11:353–61.
Niu Q, Zhao H, Zuo L, Wang M, Gan L. The effects of dialysis modalities on the progression of coronary artery calcification in dialysis patients. BMC Nephrol. 2020;21:1–8.
Chou J, Kiebalo T, Jagiello P, Pawlaczyk K. Multifaceted sexual dysfunction in dialyzing men and women: pathophysiology, diagnostics, and therapeutics. Life. 2021;11:311.
Eckersten D, et al. MicroRNA-155 and anti-müllerian hormone: new potential markers of subfertility in men with chronic kidney disease. Nephron Extra. 2017;7:33–41.
Cano-Megías M, et al. Coronary calcification as a predictor of cardiovascular mortality in advanced chronic kidney disease: a prospective long-term follow-up study. BMC Nephrol. 2019;20:1–9.
Lamarche MC, Hopman WM, Garland JS, White CA, Holden RM. Relationship of coronary artery calcification with renal function decline and mortality in predialysis chronic kidney disease patients. Nephrol Dial Transplant. 2019;34:1715–22.
Cobo G, et al. Sex and gender differences in chronic kidney disease: Progression to end-stage renal disease and haemodialysis. Clin Sci. 2016;130:1147–63.
Strózecki P, Odrowa̧z-Sypniewska G, Manitius J. Cardiac valve calcifications and left ventricular hypertrophy in hemodialysis patients. Ren Fail. 2005;27:733–8.
Peeters FECM, et al. Sex differences in valve-calcification activity and calcification progression in aortic stenosis. JACC Cardiovasc Imaging. 2020;13:2045–6.
Myasoedova VA, et al. Sex-specific differences in age-related aortic valve calcium load: a systematic review and meta-analysis. Ageing Res Rev. 2020;61:101077.
Voisine M, et al. Age, sex, and valve phenotype differences in fibro-calcific remodeling of calcified aortic valve. J Am Heart Assoc. 2020;9:e015610.
Peeters FECM, et al. Biomarkers associated with aortic valve calcification: should we focus on sex specific processes? Front Cell Dev Biol. 2020;8:604.
Acknowledgements
We are grateful to all research personnel at Renal Medicine Research and Transplantation Units at Karolinska hospital with the help of the inclusion of patients, as well as a comprehensive collection of samples in addition to all the logistics required for additional investigations related to different biochemical markers. We thank the radiology department for performing CT scans, analyzing coronary artery calcification scores, and measuring aortic valve calcification.
GOING-FWD Collaborators: Louise Pilote (McGill University Health Center and McGill University, Canada); Colleen M. Norris (University of Alberta, Canada); Valeria Raparelli (University of Ferrara, Italy); Alexandra Kautzky-Willer (Medical University of Vienna, Austria); Karolina Kublickiene (Karolinska Institutet, Sweden); Maria Trinidad Herrero (Universidad de Murcia, Spain).
Louise Pilote, Colleen M. Norris, Valeria Raparelli, Alexandra Kautzky-Willer, Karolina Kublickiene, Maria Trinidad Herrero
Funding
Open access funding provided by Karolinska Institute. Karolinska Institute provides open access funding. The GOING-FWD Consortium belongs to GENDER-NET Plus ERANET Initiative (Project Ref. No. GNP-78), which is supported by the individual country foundations: La Caixa Foundation (ID 100010434, with code LCF/PR/DE18/52010001); the Canadian Institutes of Health Research (GNP161904); the Swedish Research Council (2018-00932); the Austrian Science Fund (FWF, I 4209). In addition, support from Njurfonden (Swedish Renal foundation) and Swedish Heart and Lung Foundation (No 20160384) is appreciated.
Author information
Authors and Affiliations
Consortia
Contributions
LJW, AL-C and KK contributed to the study design. LJW, LH and JR curated data. JR was responsible for CT imaging and assessments for calcification scores. LJW and AL-C performed statistical analyses. LJW, AL-C, LH, JR and KK drafted the manuscript. PS and KK critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Regional Ethics Committee (EPN), Stockholm, Sweden, approved the study protocols that were performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients involved in the study.
Consent for publication
Not applicable.
Competing interests
All authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ward, L.J., Laucyte-Cibulskiene, A., Hernandez, L. et al. Coronary artery calcification and aortic valve calcification in patients with kidney failure: a sex-disaggregated study. Biol Sex Differ 14, 48 (2023). https://doi.org/10.1186/s13293-023-00530-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13293-023-00530-x