Abstract
Sex differences in the rates of affective disorders have been recognized for decades. Studies of physiologic sex-related differences in animals and humans, however, have generally yielded little in terms of explaining these differences. Furthermore, the significance of these findings is difficult to interpret given the dynamic, integrative, and highly context-dependent nature of human physiology. In this article, we provide an overview of the current literature on sex differences as they relate to mood disorders, organizing existing findings into five levels at which sex differences conceivably influence physiology relevant to affective states. These levels include the following: brain structure, network connectivity, signal transduction, transcription/translation, and epigenesis. We then evaluate the importance and limitations of this body of work, as well as offer perspectives on the future of research into sex differences. In creating this overview, we attempt to bring perspective to a body of research that is complex, poorly synthesized, and far from complete, as well as provide a theoretical framework for thinking about the role that sex differences ultimately play in affective regulation. Despite the overall gaps regarding both the underlying pathogenesis of affective illness and the role of sex-related factors in the development of affective disorders, it is evident that sex should be considered as an important contributor to alterations in neural function giving rise to susceptibility to and expression of depression.
Similar content being viewed by others
Introduction
Sex is increasingly regarded as an important contributor to the development of mental illness, including affective disorders, neurodevelopmental disorders, and addiction. Since 2014, it has been NIH policy that grant applications must address the possible role of sex in the proposed study. With regards to affective disorders, sex differences in the prevalence and symptomatology of major depressive disorder (MDD), anxiety, and post-traumatic stress disorder (PTSD) have been known for decades, with females experiencing these disorders at approximately twice the rate as males [1]. In general, studies of sex-related differences in animals and humans have focused on specific, often single, measures that have yielded little in terms of explaining the overall sex differences in depression evident in epidemiological studies. Our current lack of understanding is not surprising, given the complexity of the relationship between sex and brain function, as well as the confusing array of evidence regarding the biological underpinnings of affective illness. The impact of sex widely ranges from direct influence on central nervous system processes (through genetic sex and gonadal steroids) to indirect effects, such as those elicited from the environment (e.g., as a result of social and cultural expectations). From a mechanistic perspective, sex encompasses enduring effects of exposure to sex hormones during critical developmental periods (organizational effects), transient effects of sex hormones (activational effects), interactions between organizational and activational effects, and genetic effects (i.e., having two X chromosomes vs. one Y). Mapping sex onto the processes underlying affective regulation, which are similarly complex and dependent on contextual factors (such as timing during the lifespan, prior experience, genetic background, species, and stimulus, all of which interact with sex), may, therefore, be best served by the organization of existing knowledge into a framework that allows us to think more broadly about the role of sex in dynamic emotional states. In this overview, we attempt to create such a framework, illustrating with examples the broad conceptual levels at which sex differences have been described in the brain and conceivably could contribute to female predominance of affective disorders. Our current knowledge can be roughly divided into several categories, with sex effects having been identified in brain structure, brain connectivity, signal transduction, transcription/translation, and epigenesis. Each of these categories will be discussed below with examples of key findings of sex differences related to affective function, though a fully comprehensive review is clearly precluded by the scope of the topic. In our discussion, we reflect on the importance and limitations of this work, as well as offer perspectives on the future of research on sex differences as they relate to affective disorders.
Though “affective disorders” encompass a broad range of illnesses, including MDD, bipolar disorder, anxiety disorders, and PTSD, this review primarily utilizes examples from depression and depressive-like behavior for the following reasons: (1) an abundance of animal models exist for depressive-like behavior [2]; (2) sex differences in prevalence are well-established [3]; (3) reproductive events during the lifespan (e.g., pregnancy, menopause) alter the expression and substrates of depression [4, 5]; (4) a comparatively large body of preclinical and clinical research exists pertaining to sex differences in depression; and (5) depression is either a core feature of or highly comorbid with the other disorders listed [6, 7].
In addition, we have chosen to view the relationship between sex and affect regulation through the lens of stress and the stress response for several reasons. First, stress is a critical factor in both the precipitation of and susceptibility to affective disorders, demonstrated in numerous epidemiologic studies (e.g., the Adverse Childhood Experiences study, which found relationships between early stress and depression, substance use, and suicide [8,9,10]). Abundant evidence also confirms early observations of depression-related disturbances in the stress axis (including cortisol hypersecretion, impaired negative feedback, and corticotropin-releasing hormone (CRH) dysregulation) [11,12,13,14,15]. Second, there is a rich literature describing sex differences in the stress response, both in animals and humans [16,17,18,19,20,21,22]. Third, stressors in animal studies are the means of inducing behaviors believed to model symptoms of affective disorders in humans, thus permitting investigation of potential biological mediators of these disorders and associated sex differences. It should be noted that context-determining factors interacting with the stress response, such as developmental stage/aging, prior experience (e.g., adversity), and sex-specific life events (e.g., pregnancy, menopause), are known to dramatically alter both physiologic and behavioral responses and may be required for expression of sexually dimorphic traits [4, 23,24,25]. Although certain examples are highlighted below, a full discussion of the critical role of context in the origin and expression of sex differences in affective dysfunction is beyond the scope of this review.
Finally, as noted above, when considering the evidence for a role of sex in the biology of affect regulation, one must keep in mind the multiple means by which sex can influence biology (e.g., is the difference hormone-dependent or not). The impact of sex chromosomes independent of hormones is not well-understood, in large part due to the difficulty in distinguishing the effects of genetic sex from those of gonadal sex [26, 27]. However, several lines of emerging research are beginning to elucidate a substantial role for genetic complement on sexual differentiation and function. Higher rates of certain neurodevelopmental and affective disorders have been observed in sex chromosome aneuploidies (in which sex chromosomes are present in abnormal quantities) [28], and heritability analyses have suggested a significant influence of sex-chromosomes on brain anatomy [29]. In addition, the four-core genotypes (FCG) model (described in detail in a later section) is a preclinical paradigm that makes possible the separation of gonadal sex and genetic sex, allowing for direct comparison of different gene complement/hormone profile combinations [26]. Within the category of hormone-dependent effects, differences may arise as a result of organizational/programming effects, acute/activational effects, or a combination of the two. Organizational effects occur consequent to exposure to sex-steroids during critical periods of development and persist irrespective of subsequent changes in hormone levels. Important demonstrations of these programming effects are illustrated in the classic studies of Phoenix et al., Gorski et al., and Arnold et al., which established that behavioral capacities in adulthood (e.g., aggression, sex behaviors) are dependent upon these perinatal exposures [30,31,32]. Organizational effects, in addition to associated effects on brain morphology [33, 34], are a product of sex-steroid regulation of many of the fundamental processes of brain development, including neuroplasticity, epigenesis, and immunoregulation [35,36,37]. Activational effects, on the other hand, comprise the immediate and reversible effects of sex hormones and are mediated largely, albeit not exclusively, through sex hormone receptors. Sex hormone receptors are ubiquitous in the central nervous system, and there is virtually no element of neural function that is not regulated by sex hormones. Sex steroids can acutely regulate neural structure, excitability, cell function, and transmission [38], effects which ultimately extend to the level of brain circuits and global brain function. At the interface of organizational and activational effects are those that cannot occur without both early exposure to and current presence of a hormone, i.e., an acute effect programmed by a developmental one. For instance, male rats castrated at birth show incomplete mating behavior upon re-exposure to testosterone in adulthood, a pattern that is not seen in males castrated in adulthood [39, 40]. The impact of organizational and activational effects and their interactions must be disentangled to understand how observed sex differences are produced. Methods employed (such as four-core genotypes) often reflect the effort to decompose the underlying mechanisms of hypothesized sex differences. Complexity of effects and mechanisms notwithstanding, the implications from the findings summarized below suggest that sex is a powerful acute and developmental context and must be considered as a critical potential contributor to alterations in neural function giving rise to susceptibility to and expression of depression.
Brain structure
Structural brain differences between males and females of various species have been described since the latter half of the twentieth century, with evidence for sex differences firmly established by the seminal discovery of sexually dimorphic brain regions responsible for vocal control in songbirds [41]. In humans, women have been observed to have a higher percentage of gray matter volume relative to white matter [42, 43], as well as greater volumes of the orbital frontal cortices [44]; men appear to have higher gray matter densities in several brain regions, including amygdala, hippocampus, insular cortex, and putamen [45]. Developmentally, men appear to obtain peak brain volumes at a later age than women, and characteristics such as brain volume, directional organization, and myelination of many regions have been shown to vary by sex in adolescents [46, 47]. In this section, we first explore how differences in affect-regulating brain areas may predispose female animals to an increased CNS response to stress. We then review clinical findings, with a focus on structural brain differences between males and females exposed to childhood trauma.
Preclinical findings
While structural changes in the brains of depressed individuals have been observed independent of sex [48,49,50], there is little conclusive evidence relating structural differences to sex differences in depression. Preclinical findings, however, suggest that regions implicated in affective processing, such as the locus coeruleus, contain sexually dimorphic features that may play a role in increased female vulnerability to depressed states. The locus coeruleus (LC) directs attention and mediates arousal via the integration and relay of stress signals to and from the HPA axis. While important for adaptive responses to environmental stimuli that threaten survival, the LC-norepinephrine system demonstrates amplified reactivity following chronic stress, resulting in pathological behaviors resembling anxiety in animal models [51]. Unsurprisingly, it has been hypothesized that dysfunction of this system underlies hyperarousal states characterizing human anxiety and trauma related disorders [52, 53]. In female rats, the LC dendritic processes synapsing on terminals (originating in the amygdala) that release corticotropin releasing factor (which both activates the HPA axis and acts as a central neuromodulator) demonstrate longer trees, with more branches and longer branch lengths, and have a greater number of synaptic contacts [54]. This increased complexity ultimately results in a framework for more emotion-related information to be transmitted by the amygdala in response to stress in females, and represents a potential link between structure, HPA-axis/arousal response, and vulnerability to affective illness. It is also important to note that the effects of stress on regional morphology in various brain regions, particularly following prenatal stress, have been shown to vary by sex in animal models [55,56,57,58,59,60].
Clinical findings
Previous studies of brain structure in major depressive disorder were mostly underpowered to detect sex differences (in those that included both men and women), and single-sex studies offer little in the way of comparative data [61, 62]. Further hampering comparisons, it is likely that individual men and women represent mosaics, where each individual brain is a composite of male-typical and female-typical features [63]. Indeed, recent MRI findings support both the extensive overlap between individuals and the emergence of sex differences only at a group level [63]. Nonetheless, recent evidence suggests that brain structure within regions implicated in depression is affected in a sexually dimorphic fashion by prenatal and childhood stress, representing a possible structural link between early exposure and subsequent susceptibility. A meta-analysis of data obtained by the Enhancing Neuroimaging Genetics through Meta-Analysis (ENIGMA) consortium demonstrated that, in cases of childhood maltreatment, greater maltreatment severity was associated with lower gray matter thickness and caudate volumes in adolescent and adult females, whereas in males, greater maltreatment severity was associated with decreased thickness of rostral anterior cingulate cortex [64]. Postnatal maternal depression has been associated with greater fractional anisotropy of the amygdala in female children [65], and several studies have demonstrated sex-specific effects of prenatal maternal stress on subsequent amygdala structure in newborns, with differential effects seen on volume [65,66,67,68] and microstructure [69]. One recent study demonstrated sex-specific effects of early perinatal stress on cortical gyrification, with young adult women who were previously exposed to stress either in-utero or during the first 18 months of life showing higher temporal gyrification and greater propensity for mood disturbance [70].
Summary
Despite the limitations described above, as well as our poor understanding of the relationship of brain structure to depression generally (and potentially limited contribution of structure to depression overall), sex differences in specific brain regions implicated in affective function provide plausible explanations for findings of differential stress processing and susceptibility to depression. For example, sex differences in brain function under stressful conditions (e.g., learning is facilitated by stress in male rodents under certain conditions and impaired in females [71]) not only represent differential activation of certain regions and circuits, but as well are associated with dimorphic microstructural differences, such as synapse concentration [71]. Similar sex-dependent morphological differences have been identified in rodent mPFC pyramidal neurons [72] following repeated stress, a model for depression. It is, therefore, conceivable that regional sex-differences in brain structure—either innate or acquired—may contribute to the well-studied effects of sex-steroids on emotion processing [73] in influencing sex-dependent susceptibility to disturbances in affective regulation.
Network connectivity
There is extensive evidence for effects of sex and sex steroids on neural processes related to network development and function [38, 74,75,76,77]. First, sex differences have been described in networks subserving emotional valence [78], pain and pain sensitivity [79], resting state function of the amygdala during adolescence [80] and in autism [81], and neurocognitive function [82]. Diffusion tensor imaging (DTI) studies have demonstrated higher fractional anisotropy and lower mean diffusivity of major white matter tracts in men [83,84,85], while studies of functional cortical connections suggest that female connectivity patterns are characterized by less laterality [86] but greater local and global connectivity [87, 88]. While the implications of these findings may be unclear, Ingalhalikar et al. postulated that, based on their analysis of the “structural connectome,” male brains are optimized for perception and coordinated action through intrahemispheric communication, while female brains are more adept at relaying information between analytical and intuitive processing modes via interhemispheric communication [89] (although it should be noted that the authors’ conclusions have raised many questions—see [90]). In addition, sex hormones are known to exert a substantial influence on network function. PET and fMRI studies in humans have shown neuroregulatory effects of estradiol on working memory [91,92,93], reward [94,95,96,97], default mode function [98,99,100], emotional processing [73, 101,102,103,104], and components of the salience network [102, 105, 106], and functional connectivity effects have more recently been demonstrated for progesterone [107], particularly with regards to network changes across the menstrual cycle [108, 109]. Comparative data (between males and females) for hormonal effects on network function is relatively limited, though one recent study noted a potentially protective effect of endogenous estradiol against the deleterious effects of visceral adipose tissue on network covariance associated with cognitive decline in aging women, but not men [110].
Depression in both sexes has been characterized by alterations in the activity and connectivity of multiple, relevant CNS networks. Increases in default mode network (DMN) activity and decreases of the salience and central executive networks have been observed, which have been suggested as physiologic substrates of the increased rumination and decreased responsiveness to external stimuli often seen in depressed states [111, 112]. Aberrations in reward circuitry have been repeatedly documented [113], bearing a plausible association to the cardinal depression symptom, anhedonia. Changes in blood flow to and from critical nodes within the corticolimbic system, such as prefrontal cortex and amygdala, have also been shown to be altered in major depression [114, 115] and following stress in animal models [116]. Below, we present preclinical and clinical evidence for sex differences in network function in stress/affective disorders. Clinical research findings suggest a particularly important role of pubertal maturation in the development of network sex differences, consistent with the aforementioned effects of sex steroids [117].
Preclinical findings
The existing preclinical evidence supports the idea that sex influences how antecedent stress shapes early organization and mature function of such networks. For instance, several studies have documented sexually dimorphic changes in functional connectivity following repeated stress between brain regions associated with the default mode network [118, 119] (e.g., between hippocampus and amygdala [119] and between prefrontal cortex and amygdala [118]). Differential circuit activation by exogenous administration of corticotropin-releasing factor has been observed in adult rats [120], suggesting one possible mechanism underlying this dimorphism. Differences in network function may also be reflected in sex-specific microstructural changes (i.e., at the neuronal level) that underlie network organization. Dendritic remodeling has been shown to occur in adult male rats, but not females, in hippocampal CA3 neurons following chronic restraint stress [121]. Adult female, but not male, rats show hormone-dependent selective dendritic remodeling in mPFC neurons projecting to basolateral amygdala in response to stress (males instead show remodeling of mPFC neurons projecting elsewhere) [122, 123]. Ovariectomy abolishes these mPFC changes, and estradiol addback to gonadectomized females increases mPFC dendritic branching, irrespective of the downstream target [122]. One study in adult rats demonstrated sex differences in several aspects of function in basolateral amygdala, including increased neuronal firing rates, more dendritic spines, and greater sensitivity/responsivity to glutamate in females [124]. Investigators noted that estrous cycle shifts in neuronal activity paralleled the rate of cued fear extinction, suggesting that activational hormonal effects produce identifiable CNS changes related to subsequent behavioral outcomes [124]. Sex-specific effects of stress on non-neuronal cell populations that affect neural circuitry, such as microglia, have also been observed [125].
Clinical findings
While there is, on the whole, a paucity of research reporting human sex differences in functional connectivity related to depression, recent evidence from specific subpopulations has suggested that depressive and anxious symptomatology may have different network correlates in men and women. Higher “internalizing” symptoms (which correlate with depressive/anxious symptomatology) in female, but not male, adolescents have been associated with greater resting-state connectivity between amygdala and regions implicated in emotional and somatosensory processing, salience detection, and action selection, including cingulate gyrus, insula, and somatosensory cortices [126]. Similarly, intrinsic functional connectivity (iFC) of the DMN appears to weaken with pubertal maturation in females (compared to strengthening in males), with decreased iFC of the anterior cingulate within the DMN predicting higher internalizing symptoms later in adolescence [127]. Connectivity may vary as a function of sex and diagnostic classification (e.g., MDD vs. control) as well, with one study of adolescents demonstrating increased connectivity strength between cerebellum and superior frontal gyrus with age in male controls, but decreased connectivity with age in males with MDD (no effects were seen in females) [128]. Major depression in adult chronic ketamine users has been shown to have sex-specific resting-state connectivity patterns, with women showing increased connectivity between subgenual anterior cingulate cortex (sgACC) and dorsomedial prefrontal cortex and men showing increased connectivity between sgACC and bilateral superior temporal gyrus [129].
Even when sex itself is not a variable, studies of the effects of hormone fluctuations, either naturally occurring or induced, are consistent with the notion that sex steroids modulate brain dynamics relevant to mood. For one, manipulations of estradiol and progesterone have been shown to induce depressed states in certain women [130,131,132]. In addition, in studies of premenstrual dysphoric disorder (PMDD), the luteal (symptomatic) phase is associated with differential task-related activation of affect-relevant brain regions (e.g., amygdala, dorsolateral prefrontal cortex, medial prefrontal cortex, insula, orbitofrontal cortex) in adult patients compared with non-PMDD controls [73, 106, 133,134,135,136]. Manipulations employing GnRH agonists (which lead to suppression of ovarian hormone release through pituitary desensitization) produce reductions in PMDD symptoms with GnRH treatment [132, 137], with subsequent addback of estrogen and progesterone producing not only recurrence of symptoms but alterations in neural activity in regions and networks subserving mood [138, 139] and cognition [93]. As an example, one recent study found decreased resting regional blood flow in subgenual cingulate, a hub for affect regulation, following both estrogen and progesterone addback after leuprolide treatment in adult PMDD participants, but not controls [138].
Summary
Given that brain regions implicated in affective disorders, such as hippocampus, amygdala, hypothalamus, and brainstem are rich in steroid hormone receptors [140], and that sex- and sex hormones exert a substantial influence on both mood and processes associated with network function, it is reasonable to infer a significant role of sex on network function related to affect. While affective disorders secondary to reproductive endocrine changes, such as postpartum depression, PMDD, and perimenopausal depression, are the most obvious examples of sex affecting neurocircuitry underlying behavioral state kinetics, reported sex differences in network function in depressed/stressed states suggest that meaningful sex effects exist beyond those generated by hormones. Sex as a whole is multifaceted and more complex than the acute effects produced by changes in hormone levels, encompassing genetic complement and organizational effects (and the interactions between them all) as well. Sex is also only one determining factor in the expression of network states, interacting with individual trait characteristics (e.g., genetic factors) to influence brain connectivity [138]. Nevertheless, the studies described clearly suggest that certain features of sex can not only be isolated and examined for their relationship to affect and neural function, but as well are likely to yield specific behavioral and neural findings that deepen our understanding of the pathogenesis of depressed states.
Signal transduction
Sex hormones affect many, if not all, neurotransmitter systems in myriad ways [140]. Both excitatory and inhibitory effects of estradiol on several neurotransmitters, including glutamatergic [141,142,143,144], GABAergic [145, 146], dopaminergic [147,148,149,150,151,152], serotonergic [153,154,155,156,157], and noradrenergic [158,159,160], have been extensively documented. These effects occur via multiple mechanisms (including synthesis [153], release [149], turnover/degradation [161], receptor trafficking [154], and transport [162]) and are dependent on contextual factors, such as receptor subtype [163], brain area [164], developmental stage [165], duration of treatment/time following exposure [166, 167], mode of administration [167], and amount of steroid present [168]. Findings from both human and animal research suggest that these hormone–neurotransmitter interactions have meaningful functional consequences. For example, in animal studies, estradiol interacts with dopamine to influence reward decision-making and memory, with high estradiol states generating bias toward smaller, more accessible rewards [169] and preferential use of certain memory strategies [170] (This relationship appears to be modulated by individual baseline dopamine processing, with evidence in humans that working memory performance following estradiol exposure is either enhanced or impaired depending on genetic background [171]). Progesterone has a similarly complex relationship with various neurotransmitter systems, with effects distinct from (and at times opposite to) estradiol [140, 168]. Of considerable interest, allopregnanolone, a neurosteroid metabolite of progesterone, is a positive allosteric modulator of GABA-A receptors and facilitator of GABA’s inhibitory action, which has been implicated in the etiology of several affective disorders, including postpartum depression (PPD) [172, 173]. The precipitous decline in progesterone/allopregnanolone levels and resultant decrease in GABAergic transmission following delivery is hypothesized to underlie decreased mood and increased anxiety experienced by women susceptible to PPD [174], a suggestion supported by the recent approval of Brexanolone, a synthetic version of allopregnanolone, for the treatment of PPD [175, 176]. The allopregnanolone withdrawal hypothesis, however, would not explain the development of depression during pregnancy. Nonetheless, the relevance to affective regulation of allopregnanolone is further suggested by studies suggesting its role in the susceptibility to developing PTSD, both in men and women [177, 178].
The role of neurotransmitters in stress and depressed states has become less clear as conceptualizations of depression have moved away from hypotheses of dysfunctional aminergic signaling toward theories of systemic dysregulation that involves and is expressed as changes in cell neurotrophic factors (e.g., BDNF), circadian physiology, immune system response, brain network function, neuroendocrine function (e.g., HPA axis), and transcriptional and epigenetic activity [179]. However, alterations in serotonergic [180], dopaminergic [181], GABAergic [182], glutamatergic [111], opioidergic [183], and noradrenergic [184] function have all been documented in depression or following stress, with their significance supported by the therapeutic effects of medications that influence these systems (SSRIs and TCAs for monoamines [185]; allopregnanolone for GABA [174]; ketamine for glutamate, and opioids [186]). This section provides examples from animal and human studies, respectively, that illustrate the myriad sex differences in signal transduction present in stress and affective disorders. Attention is given to preclinical findings of neurotransmitter function and cell-signaling differences that may underlie differences in affect regulation. In our discussion of clinical findings, we address not only male–female differences in neurotransmitter systems, but note findings related to hormonal effects as well.
Preclinical findings
With regards to sex differences, preclinical work has demonstrated that stress can differentially affect neurotransmission in key brain regions in depression. Adult female mice have been shown, to a greater degree than males, to have increased parvalbumin mRNA expression and parvalbumin-containing cells (measures of GABAergic interneurons) in prefrontal cortex following chronic stress, molecular changes that correlate strongly with behavioral endpoints reflecting anxiety and depression [187]. In CRH-receptor deficient adult mice that display increased vulnerability to stress-related behavior, females exhibit increased sensitivity to the acute modulation of serotonin (via SSRI administration) relative to males on tail suspension, elevated plus maze, and light–dark box tests (acute stressors) [188]. This effect is hypothesized to be due to serotonergic hypofunction in prefrontal cortex as well as in hippocampus in females with CRH-receptor deficiency. (It should be noted that female hippocampal serotonin function is also decreased relative to males in wild type animals) [188]. With regards to dopamine, hyperactivity in the nucleus accumbens–ventral tegmental area (NAc-VTA) reward pathway is induced by social defeat stress in adult female, but not male, mice [189, 190], and D1-receptor activation in the NAc appears to be a uniquely important mediator of the stress-induced withdrawal phenomenon in females [189, 190]. Similarly, subchronic variable stress has been observed to increase firing in neurons projecting from the lateral habenula to the VTA in adult female, but not male, rats, a finding associated with behavioral correlates of decreased reward sensitivity [191]. In a rodent experiment of excessive glucocorticoid exposure during the late gestational period (modeling prenatal stress), dimorphic effects in adult offspring were observed on structural characteristics of dopaminergic neuronal populations and factors associated with dopamine neurotransmission, such as innervation pattern, number of receptors and transporters, as well as basal and amphetamine-stimulated dopamine release in multiple brain regions [192], again suggesting an interaction between sex, stress, and development. Ketamine, a novel antidepressant, impacts the function of glutamate and GABA receptor systems. Sex differences in ketamine's antidepressant-like effects in rodents have been explored in a number of studies [186, 193, 194], with female rodents typically demonstrating greater sensitivity to rapid and sustained antidepressant effects than males based on forced swim test immobility time, an effect mediated by estrogen and progesterone [193]. Conversely, physiologic biomarkers associated with stress-related behavioral changes were reversed following higher doses of ketamine in adult male, but not female, animals in response to chronic social isolation (another form of chronic variable stress), consistent with an increase in spine density in mPFC pre-limbic pyramidal neurons of males only [194]. How these effects relate to human depression is yet to be determined.
Other animal studies have yielded intriguing evidence for sex-differences in cellular function at individual synapses within regions implicated in depressive pathology. Some of the findings represent convergent differences (i.e., different mechanisms leading to the same functional outcome). For example, Woolley et al. showed that pre and post-synaptic hippocampal glutamate receptors are regulated by completely different estrogen receptor subtypes in male and female adult rats, with no resultant difference in glutamatergic transmission [195]. Clear examples of different functional outcomes can be seen in the studies of CRH receptor signaling in the locus coeruleus, where sex differences appear to potentiate emotional arousal in female rats [54]. As described by Bangasser and Valentino, increased coupling between the corticotropin releasing hormone (CRH) receptor and its associated G protein on the membrane surface renders female LC neurons more sensitive to CRH [54, 196]. Furthermore, cellular internalization of the CRH receptor, an adaptive process that prevents adverse effects secondary to overstimulation, occurs in males but not females in response to stress [54, 196]. While the CRH receptor is able to couple with beta-arrestin 2 (a protein involved in agonist-mediated desensitization) and be internalized in males, the G-protein outcompetes beta-arrestin 2 for binding with the receptor in females, making an internalizable complex less likely to form [196,197,198].
Clinical findings
Clinical research in depression has focused primarily on sex differences in serotonin (in part due to the success of SSRIs in the treatment of mood disorders), with studies demonstrating sexual dimorphism in serotonergic function in MDD [199,200,201,202]. Positron emission tomography (PET) and single photon emission computed tomography (SPECT) studies have shown higher 5HT1A receptor concentrations, lower 5-HTT binding potentials, and decreased serotonin uptake [199, 200] in depressed adult women relative to depressed men, findings that extend across several cortical and subcortical brain regions. However, some studies have reported contrasting findings with regards to the directionality of effects (e.g., depressed men showing decreased serotonin transporter (SERT) [203]), which may reflect methodological differences, small sample sizes, or the influence of other factors affecting serotonin transmission [204]. As an example of such factors, changes in SERT in response to seasonal changes show a combined effect of both sex and genetic background, with premenopausal adult women carrying the short allele of the serotonin-transporter-linked polymorphic region (5-HTTLPR) demonstrating both higher propensity toward seasonal affective disorder and poorer ability to downregulate serotonin transporter levels during shorter photoperiods relative to men of either genotype and women with the long-allele [205]. Research on other neurotransmitter systems is fairly limited, though postmortem studies have demonstrated greater down-regulation of somatostatin, a marker of one type of GABAergic neurons, in DLPFC, anterior cingulate, and amygdala in women with MDD relative to men [206,207,208]. In addition, one study demonstrated increased expression of several glutamatergic genes in DLPFC in adult women with MDD compared to men [209]. From the perspective of therapeutic response, sex and hormonal status may play a role in the efficacy of monoaminergic antidepressant treatments. Some studies have reported that women respond better to SSRIs and MAOIs, and men to tricyclic antidepressants [210], though this has not been borne out by meta-analyses [210, 211]. In women, gonadal steroids appear to modify the response to neurotransmitter modulation, with premenopausal individuals showing a better response to SSRIs than postmenopausal individuals [210]. Results from one study also suggest that estrogen treatment may improve quality of life (but not depression scores) in postmenopausal women during SSRI treatment of depression [212].
Summary
The difficulty in making generalizable statements with regards to the effect of sex on neurotransmission (e.g., being male/female produces X effects on Y neurotransmitter, resulting in Z behavioral outcome) speaks to the highly complex, dynamic, and overall poorly understood interaction between sex and neurotransmission in depressed states. However, regardless of specific effects, it is apparent that sex/sex hormones have a broad impact on the fundamental signaling processes that ultimately contribute to higher order neural phenomena implicated in psychiatric illness. Even as theories of depression centered on neurotransmitter deficits are updated, sex effects on signal transduction remain applicable to newer, more comprehensive systems-level hypotheses. For instance, it has recently been postulated that network imbalances between excitation and inhibition (E:I) may underlie several neuropsychiatric illnesses, including major depression [182, 213]. Estradiol plays a major role in this balance, as estrogen receptors can activate metabotropic glutamate (the major excitatory neurotransmitter) receptors, even in the absence of glutamate, and can increase synaptic trafficking of ionotropic AMPA receptors [214]. Estradiol also acutely regulates excitation, inhibition, and neurosecretory coupling through direct effects on calcium and potassium channel activity [215, 216]. Other neurotransmitter systems that contribute to E:I balance, as described above, are widely influenced by sex and sex hormones as well, and bear a relevant connection to behavioral outcomes. Therefore, if depression results from E:I imbalance, then that disturbance may well reflect the effects of sex on the basic signaling processes that regulate this balance.
Transcription/translation
Animal studies have identified multiple genes and gene networks that are impacted in stress models of depression (e.g., unpredictable chronic mild stress) [26, 206, 217]. Many of these stress-related genes show marked sex-differences. For example, certain genes have been shown to be critical to behavioral dysregulation uniquely in human males and females [218], and gene networks appear to be altered in a sex-specific manner following stressful stimuli in both animals and humans [218,219,220,221]. In human studies, not only does there appear to be little overlap between the genes and gene pathways that are affected in depression in males and females, but the genes that do overlap are often regulated differently, with transcription often occurring in an opposite fashion depending on sex. In addition, studies that have attempted to correlate transcripts with physiologic function have shown that differentially expressed genes produce unique downstream effects. For example, gene associations to immune processes have been documented to a larger extent in females than males in several studies [26, 222]. Gene expression differences are, therefore, prime candidates for exploring the molecular basis of systems-level sexual dimorphism. In this section, we focus on a recent series of studies: two preclinical studies examining transcriptional profiles following stress in animals with various gene complement/sex steroid combinations; one study comparing transcriptional “signatures” in men and women with MDD, validated with a rodent model; and one study examining downstream targets of transcription in men and women with MDD.
Preclinical findings
Sex differences in transcription of depression-associated genes could reflect hormonal effects, genetic effects, or both. Two recent studies utilized the "four core genotype" paradigm in an attempt to disentangle hormonal and genetic sources of sex differences in animals, with results suggesting a combined role of genes, acute hormonal exposure (activational effects), and developmental hormonal exposure (organizational effects) to differences in stress-induced transcription. In FCG, the testes-determining SRY gene is moved to an autosome, generating animal subjects whose gonadal sex can be made independent of their genetic sex. This yields four genotypes: XX females, XX (gonadal) males, XY males, and XY (gonadal) females. Animals may be gonadectomized and provided with hormone replacement depending on the outcome of interest, yielding several genetic complement/hormonal milieu combinations. Using FCG adult mice in a chronic variable stress paradigm, Barko and colleagues [219] demonstrated more pronounced effects of stress on gene expression for several genes responsible for dopamine and glutamate metabolism in mesocorticolimbic brain regions, as well as on gene network coordination, in female conditions (XX, gonadal female, and/or no hormone replacement) than in male conditions (XY, gonadal male, and/or testosterone-treated). In a follow-up study assessing organizational hormonal effects (FCG mice were gonadectomized but did not receive hormone replacement), both hormone exposure during critical developmental periods and genetic sex were shown to contribute to differential patterns of gene expression in mesocorticolimbic brain regions under stressed conditions [220]. The investigators also identified a set of differentially expressed “hub” genes regulated in opposite directions by stress in XY males and XX females. Of note, several of the biological pathways encoded by these differentially expressed genes were related to immune function, consonant with the putative role of inflammation in MDD.
Clinical findings
Using RNA sequencing methodologies, LaBonte and colleagues [218] made several important observations related to sex differences in genetic expression of MDD. In addition to demonstrating little overlap in global and regional transcription between depressed adult men and women, they were able to identify distinct genetic “nodes” for critical gene networks implicated in male and female depression, a finding supported by direct genetic manipulation in mice. Across 6 brain regions implicated in depression, there was only 5 to 10% overlap between men and women of genes differentially expressed in depressed subjects, as well as little similarity in terms of the pattern of up/down regulation of genes across regions (i.e., when comparing transcription profiles from individual regions to one another). Similar findings were observed in adult mice subjected to chronic variable stress, and the significant number of differentially expressed genes shared between males of both species and females of both species suggests conservation of sexually dimorphic pathways of stress-induced pathology.
As part of the same study, the investigators utilized multi-brain region co-expression networks to evaluate transcriptional "signatures" associated with human MDD. Among the shared modules of gene connectivity (i.e., coordinate expression across brain regions) in depressed individuals vs. controls, the majority showed increased connectivity (association) in men compared to women. Their analysis identified genes DUSP6 and EMX1 as important nodes for gene networks implicated in MDD in women and men, respectively (DUSP6 encoding a widely prevalent phosphatase, and EMX1 encoding a similarly ubiquitous transcription factor), findings subsequently supported by gene knockout/overexpression in adult animals. DUSP6 downregulation led to depressive behavior in female, but not male, mice subjected to chronic variable stress, an effect that was reversed with subsequent vector-mediated overexpression of DUSP6. In contrast, upregulation of the EMX1 gene resulted in similar behavioral dysregulation in stressed males, without inducing stress susceptibility in females. An interesting finding, in line with the concept of sex-related physiologic convergence, was similar functional changes (increased excitatory postsynaptic currents in ventromedial prefrontal cortex) in both sexes as a result of genetic manipulation of either DUSP6 (in female mice) or EMX1 (in male mice).
A study by Seney and colleagues [223] utilized similar methodologies in a meta-analytic format to explore the differences between the male and female transcriptome in major depression in humans. Their findings replicated the minimal transcriptional overlap observed in the LaBonte study while showing a high degree of overlap in genes regulated in opposite directions in adult men and women with depression and demonstrating differences in the downstream effects of each transcriptional profile. Using gene ontology analysis, they identified genes for synapse related pathways, inner mitochondrial membrane protein complex, and G protein coupled amine receptor activity as associated with male MDD, whereas pathways related to antigen processing and mitochondrial function were associated with female MDD. In their cell-type analysis, they demonstrated that differentially expressed genes expressed in oligodendrocytes and microglia were upregulated in men with MDD but downregulated in women with MDD, while genes expressed in neurons were downregulated in men but unchanged in women. It was notable that sex differences had not been reported in the individual studies from which these data were compiled, either because sex was not considered or statistical power was not great enough in the individual studies to detect differences.
Summary
Overall, these studies suggest significant differences between males and females in terms of genetic expression underlying stress-related pathology. Additional studies are needed to characterize the functional relevance of these differences—it will be crucial to explore how molecular differences manifest themselves on a physiologic level to produce susceptible and resilient phenotypes. Experiments such as those conducted in the LaBonte study (genetic manipulation of hub-genes identified in their network analysis) and a previous study by LaPlant and colleagues, which demonstrated both an increase in transcription of genes coding for nuclear factor kappaB (a transcription factor involved in cellular protection during stress) following ovariectomy and an association of increased nuclear factor kappaB with susceptibility to stress [221], are likely to provide valuable links between gene expression, sex, and dysregulated behavior.
Epigenesis
Epigenetic mechanisms (e.g., DNA methylation; histone methylation and acetylation) serve to alter gene expression through modification of nucleosomes (DNA and histone proteins) without changing the fundamental nucleotide sequence. Methyl groups covalently linked to DNA at specific cytosine–phosphate–guanine sites (CpGs), result primarily (albeit not exclusively) in gene repression [224]. Histone acetylation serves as an opposing process, with acetyl groups added to the N-termini of histone proteins to ultimately remodel chromatin and allow for enhanced/increased DNA transcription [225]. Other epigenetic modifications include ubiquitination, phosphorylation, sumoylation, and ribosylation, as well as post-transcriptional modifications, such as those induced by microRNA and sRNA. Epigenetic sex differences have been documented across species and in multiple tissues, including blood [226, 227], placenta [228], liver [229, 230], pancreas [231], muscle [232], heart [233], and brain [234,235,236,237,238,239]. Epigenesis appears to be a critical mechanism by which sexual differentiation occurs during the neonatal period and puberty [240,241,242]. Evidence is emerging for sex differences in epigenesis for several disease conditions as well, including diabetes [243], autoimmune diseases [244], cardiovascular disease [245], and cancer [246]. Methylation and acetylation have been shown to underlie behavioral adaptations to chronic stress in animal models [247,248,249], and convergent associations between depression and epigenetic modifications have been demonstrated in human clinical studies [250], highlighting the role of these processes in dynamic emotional states. Given its reversible nature and plausible link to episodic (as opposed to continuous or progressive) dysfunction, epigenesis represents an appealing hypothesis for regulation and dysregulation of mood and behavioral states [251,252,253]. We review some of the recent clinical and preclinical evidence for epigenetic sex differences below. Particularly notable is the association between sex-specific transcriptional profiles and both DNA methylation and micro RNA networks, suggesting these processes work together as part of a coordinated response to stress. Clinical findings are limited, and we present the example of epigenetic changes in human offspring secondary to maternal behavioral characteristics during pregnancy.
Preclinical findings
Though the research is still in its nascent stages, evidence from preclinical studies supports the notion of sex-specific epigenetic adaptations to stress. One study looking at the effects of chronic variable mild stress on CRH gene methylation and epigenetic enzymes (DNA methyltransferases, histone acetyltransferases) demonstrated overall lower methylation in adult female rats following stress across several CRF-containing brain areas, though with pronounced regional effects [254]. In the paraventricular nucleus (PVN), total DNA methylation of the CRH gene was consistently higher in stressed females (consistent with decreased expression) relative to female controls, an effect not seen in males. Conversely, in the bed nucleus of the stria terminalis (BNST), CRH methylation was decreased in stressed males relative to controls, with females showing no effect. In amygdala, stress resulted in decreased total methylation for females relative to males. Following stress, CREB-binding protein, a histone acetyltransferase, was increased in female BNST, and mRNA for histone deacetylase-5 was decreased in male amygdala. All of these differences were reflected by sex-specific modifications to one or more specific CpGs, as well as differences in expression of c-Fos, FosB, CRH mRNA, and CRH peptide.
DNA methylation appears to mediate expression of sex-specific transcriptional profiles associated with susceptibility to stress-induced behavioral changes. A study by Hodes et al. [255] examined transcriptional regulation in nucleus accumbens of adult mice in response to a chronic stress paradigm. These authors demonstrated that conditional deletion of DNA methyltransferase 3a (Dmnt3a) resulted in increased behavioral resilience in female mice, defined as resisting changes in behaviors normally produced by stress, including decreased grooming time, increased latency to eating in the novelty suppressed feeding paradigm, decreased sucrose preference, and reduced active coping in the forced swim test. This effect was not noted in males, as males without the knockout were already behaviorally resilient. Using RNA sequencing, they demonstrated that Dnmt3a knockout resulted in alterations of the stress-associated transcriptional profile, creating a hybrid of male and female phenotypes associated with increased resilience in female animals. This suggests that Dnmt3a may be a more important modulator of stress susceptibility in females than in males, who appear to possess mechanisms counteracting its deleterious effects. Notably, investigators also found Dnmt3a to be increased in postmortem samples of both male and female humans diagnosed with MDD [255].
MicroRNA regulation is another epigenetic process being explored in terms of its relationship to affective disorders. MicroRNAs are small RNAs involved in the post-transcriptional regulation of mRNA, acting via base-paring with mRNA to cause cleavage, destabilization, and decreased translation. This mechanism is ubiquitous and evolutionarily conserved, as well as widely present in the central nervous system [256, 257]. Research has shown that the neonatal microRNA environment in the hypothalamus is both sexually dimorphic and dynamically responsive to estrogen, suggesting that this additional layer of gene regulation is crucial to sexual differentiation and fetal epigenetic programming [258]. Pfau and colleagues found evidence to suggest that adult mouse microRNA networks are regulated in a sex-specific way in response to stress, and that these effects are part of a larger, coordinated response involving transcriptional and post-transcriptional regulation that is unique to each sex [222]. Using genome wide analysis of sex-specific microRNA and mRNA transcriptional profiles, they demonstrated that, analogous to transcriptional profiles for other stress-related genes, microRNA transcriptional profiles induced by stress were largely non-overlapping between males and females. In addition, similar to transcriptional findings, these miRNA profiles demonstrated markedly different associations to molecular pathways and functions in each sex. Overall, males, but not females, showed a robust transcriptional and post-transcriptional response to stress, suggesting a form of “active” resistance leading to behavioral resilience [222]. Male miRNA functional pathways overlapped to a greater degree with the pathways of other differentially expressed genes involved in the stress response than did female miRNA pathways, pointing to a potentially greater role of miRNA in stress-responsive molecular processes in males. (However, because the enrichment analyses were lower powered in females due to smaller gene lists, this effect may have been exaggerated). As there was minimal overlap in genes, miRNAs, and functional processes related to the stress response between males and females, these results also support the notion that the female response to stress is unique, and not simply an attenuated version of the male response.
Clinical findings
Clinically, there has been a recent focus on ways in which prenatal experience can affect subsequent susceptibility to mental illness [259,260,261], with female offspring being more susceptible to affective dysregulation and males more likely to suffer from memory and learning impairment if exposed to prenatal stress [262]. Early DNA methylation appears to play a role in this vulnerability, with evidence suggesting that predisposition to affective disorders in children is associated with sex-specific methylation of critical HPA-axis genes. In a recent experiment [263], it was hypothesized that anxious-depressive behavior in young female, but not male, children would be accompanied by greater methylation of the NR3C1 gene, a glucocorticoid receptor gene implicated in HPA feedback mechanisms [264]. In a prior study, mood worsening in mothers following delivery (i.e., low prenatal depression followed by high postnatal depression) was associated with methylation of NR3C1 in their offspring, an effect that was reversed by early postnatal maternal stroking of the infant [265]. In addition to evidence supporting the authors' behavioral hypothesis that girls were more likely to experience depressive symptoms in the setting of mismatched maternal prenatal–postnatal depression (in this case, either low prenatal depression followed by high postnatal depression, or vice versa), the results of their follow-up study showed that prenatal–postnatal mismatch had strong effects on NR3C1 methylation in girls only, with low prenatal depression followed by high postnatal depression resulting in the largest increase [263]. Higher NR3C1 methylation predicted anxious-depressive behavior at 14 months in girls, whereas no association was seen for boys. Whether this association persists into adolescence and adulthood has not yet been determined.
Summary
Epigenetics represents a promising area of research for affective disorders. The relationship between the epigenetic response and the transcriptional response to stress suggest these processes act in a coordinated fashion to elicit broader physiologic effects. Additional clinical research is needed. Current findings are concordant with the notion that early epigenetic changes play a role in subsequent behavioral vulnerability.
Discussion
Taken as a whole, these studies provide considerable evidence for sex differences in the CNS structures and processes that contribute to affective regulation. Sex and sex steroids exert specific but wide-ranging effects on the brain (in general) and affective regulation (in particular) at virtually any level of investigation, from molecular to systems level, from synapse structure to network regulation (see Table 1). Because mood dysregulation does not reside in a specific brain region nor does it rely solely on changes at any one physiologic level, cause-and-effect with regards to these differences cannot be inferred. A change in transcription, for instance, may be compensated for by other changes resulting in no difference in the ultimate outcome measure. As such, sex differences can exist without behavioral consequence. Nonetheless, we can conclude the following: (1) sex and sex steroids create a context that determines or influences the structures and processes underlying behavior, including affective regulation and response; (2) if we are attempting to understand the physiology of behavior by studying only one sex, we are likely to fail to uncover alternate molecular pathways that would help define the most critical loci at which physiologic adaptation to stressful stimuli fails to occur; and (3) our efforts to develop new therapeutics may be advanced by exploiting the observation that manipulation of reproductive steroids can regulate mood in susceptible subgroups of women; i.e., sex steroids can serve as probes for defining the changes in cell signaling that precede and accompany changes in affective state.
Unfortunately, sex as a moderating variable has often been ignored or intentionally excluded from research studies due to concerns about added complexity (e.g., studies would need to control for factors, such as menstrual/estrous cycles) and sample size (greater numbers are needed to power studies looking at sex differences a priori). Combined with our lack of understanding about the etiology of depression in general, this has left us with little evidence for generalizable sex-dependent characteristics associated with "male" or" female" depression. As neuroscientific and genetic techniques yield a better understanding of affective physiology, and as newer, more efficacious therapeutics with unique mechanisms of action such as ketamine and neurosteroids [174, 266] become better studied, a clearer picture may emerge regarding how sex ultimately influences susceptibility, symptom expression, and treatment.
Additional considerations for future work include the following: addressing, for any sexual dimorphism, whether findings reflect organizational, activational, or genetic differences; exploring other contextual factors that interact with stress/sex differences, including point in lifespan, past experience, and the different environments (both internal and external) to which males and females are subjected; and studying the effects of different stressors, particularly in human brains that are obviously more complex than the rodent models from which much of the evidence is derived. As an aspiration, relevant sex-specific targets may lead to the development of more precise therapeutics, with requisite consideration of safety/risk, practicality, and effectiveness. The extant research makes it clear that interventions in one sex cannot be assumed to have equivalent effects in the other, requiring studies powered to detect effects in both males and females. For instance, a treatment such as allopregnanolone that has known efficacy and safety profiles in women must be rigorously studied in men if it is to be considered for use in this population.
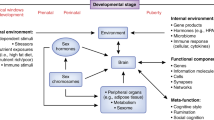
Sex and sex hormone signaling occupy a central role in the formation, programming, and functional orchestration of the brain. As such, attempting to define the specific effects of sex on the already mysterious and complex processes giving rise to affective disorders is daunting. Despite our inability to define the role of sex signaling factors in depression, their potential impact can be seen throughout the brain. Translation of described sex differences in stress-related disorders into actionable data will require a far more comprehensive picture of the link between predisposing factors and behavioral outcomes, ideally in the form of novel, predictive, biosignatures. Though not specifically focusing on sex, Hultman and colleagues [267] used machine learning to distinguish profiles of depression-like behavior from profiles of the susceptibility to depression-like behavior. With EEG, these investigators provided evidence of a network-level, spatiotemporal, dynamic signature associated with vulnerability to depression that preceded stress, and that a) was distinct from the dynamic signature associated with behavioral dysfunction following the stressor; b) differentiated susceptible mice from resilient mice; c) was present in three independent models of MDD; and d) was not affected by antidepressant manipulations. This type of mesoscopic phenotype may serve as an outcome measure that integrates sex differences at multiple levels and across developmental timepoints, facilitating the assessment of how sex differences at the genetic or cellular level influence brain dynamics associated with the vulnerability to or experience of affective dysregulation. Nonetheless, despite the impressively large number of ways in which sex may plausibly influence the development and expression of affective disorders (see Fig. 1), we must look to the future to transform the current state of isolated findings into a more coherent picture of how sex differences meaningfully impact the regulation and dysregulation of affect.
Depiction of the levels at which sex influences brain function. Sex modulates brain function and behavior through both acute effects (i.e., activational effects), as well as the programming of brain sensitivities during critical periods (i.e., organizational effects). These levels interact with one another in a dynamic fashion and include components within the central nervous system, in peripheral body systems, and external to the organism altogether (e.g., external environmental effects). Though not specifically explored in this paper, non-CNS physiologic factors including microbiome effects, immune response, and differences in peripheral organ/metabolic function are important when considering how sex influences the brain. Non-physiologic factors, such as social responses from others and meta-cognitive function, also play a significant role (from [38])
Perspectives and significance
Abundant evidence exists for biological sex differences that may contribute to both susceptibility to depression and sex-differences in its prevalence. Nonetheless, our lack of understanding of the ontogeny of depression itself precludes determination of the etiopathogenic significance of reported sex differences. To address the more general question, “Why would you think that sex would influence depression,” we present examples of the role of sex in regulating neurobiology at five related levels of observation. We believe that this framework for organizing observations from the literature may facilitate a less particularized, more integrative approach to examining the role of sex in depression and, by so doing, also generate a more comprehensive picture about the relationship between predisposing factors and behavioral outcomes in affective illness.
Availability of data and materials
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, et al. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc. 2009;18(1):23–33.
Czéh B, Fuchs E, Wiborg O, Simon M. Animal models of major depression and their clinical implications. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:293–310.
Weissman MM, Klerman GL. Sex differences and the epidemiology of depression. Arch Gen Psychiatry. 1977;34(1):98–111.
Hoekzema E, Barba-Müller E, Pozzobon C, Picado M, Lucco F, García-García D, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287–96.
Bromberger JT, Epperson CN. Depression during and after the perimenopause: impact of hormones, genetics, and environmental determinants of disease. Obstet Gynecol Clin North Am. 2018;45(4):663–78.
Tiller JWG. Depression and anxiety. Med J Aust. 2013;199(S6):S28-31.
American Psychiatric Asssociation. Diagnostic and statistical manual of mental disorders . 5th ed. Virginia: American Psychiatric Asssociation; 2013.
Anda RF, Whitfield CL, Felitti VJ, Chapman D, Edwards VJ, Dube SR, et al. Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. Psychiatr Serv. 2002;53(8):1001–9.
Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. JAMA. 2001;286(24):3089–96.
Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–58.
Kathol RG, Jaeckle RS, Lopez JF, Meller WH. Pathophysiology of HPA axis abnormalities in patients with major depression: an update. Am J Psychiatry. 1989;146(3):311–7.
Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacol . 2000;23(5):477–501.
Rubin RT, Poland RE, Lesser IM, Winston RA, Blodgett AL. Neuroendocrine aspects of primary endogenous depression. I. Cortisol secretory dynamics in patients and matched controls. Arch Gen Psychiatry. 1987;44(4):328–36.
Nemeroff CB, Widerlöv E, Bissette G, Walléus H, Karlsson I, Eklund K, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226(4680):1342–4.
Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988;45(6):577–9.
Goel N, Workman JL, Lee TT, Innala L, Viau V. Sex differences in the HPA axis. Compr Physiol. 2014;4(3):1121–55.
Oyola MG, Handa RJ. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress. 2017;20(5):476–94.
Hillerer KM, Neumann ID, Couillard-Despres S, Aigner L, Slattery DA. Sex-dependent regulation of hippocampal neurogenesis under basal and chronic stress conditions in rats. Hippocampus. 2013;23(6):476–87.
Romeo RD, Lee SJ, McEwen BS. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology. 2004;80(6):387–93.
Luo J, Wang T, Liang S, Hu X, Li W, Jin F. Experimental gastritis leads to anxiety- and depression-like behaviors in female but not male rats. Behav Brain Funct. 2013;9:46.
Jezová D, Juránková E, Mosnárová A, Kriska M, Skultétyová I. Neuroendocrine response during stress with relation to gender differences. Acta Neurobiol Exp (Wars). 1996;56(3):779–85.
Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28(4):464–76.
Workman JL, Gobinath AR, Kitay NF, Chow C, Brummelte S, Galea LAM. Parity modifies the effects of fluoxetine and corticosterone on behavior, stress reactivity, and hippocampal neurogenesis. Neuropharmacology. 2016;105:443–53.
Morrison KE, Epperson CN, Sammel MD, Ewing G, Podcasy JS, Hantsoo L, et al. Preadolescent adversity programs a disrupted maternal stress reactivity in humans and mice. Biol Psychiatry. 2017;81(8):693–701.
Morrison KE, Cole AB, Kane PJ, Meadows VE, Thompson SM, Bale TL. Pubertal adversity alters chromatin dynamics and stress circuitry in the pregnant brain. Neuropsychopharmacol . 2020;45(8):1263–71.
Seney ML, Ekong KI, Ding Y, Tseng GC, Sibille E. Sex chromosome complement regulates expression of mood-related genes. Biol Sex Differ. 2013;4(1):20.
Arnold AP. A general theory of sexual differentiation. J Neurosci Res. 2017;95(1–2):291–300.
Green T, Flash S, Reiss AL. Sex differences in psychiatric disorders: what we can learn from sex chromosome aneuploidies. Neuropsychopharmacol . 2019;44(1):9–21.
Mallard TT, Liu S, Seidlitz J, Ma Z, Moraczewski D, Thomas A, et al. X-chromosome influences on neuroanatomical variation in humans. Nat Neurosci. 2021;24(9):1216–24.
Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–82.
Barraclough CA, Gorski RA. Evidence that the hypothalamus is responsible for androgen-induced sterility in the female rat. Endocrinology. 1961;68:68–79.
Arnold AP. The effects of castration and androgen replacement on song, courtship, and aggression in zebra finches (Poephila guttata). J Exp Zool. 1975;191(3):309–26.
Pfaff DW. Morphological changes in the brains of adult male rats after neonatal castration. J Endocrinol. 1966;36(4):415–6.
Raisman G, Field PM. Sexual dimorphism in the preoptic area of the rat. Science. 1971;173(3998):731–3.
Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol. 2012;33(3):267–86.
Lenz KM, Nelson LH. Microglia and beyond: innate immune cells as regulators of brain development and behavioral function. Front Immunol. 2018;9:698.
Villa A, Gelosa P, Castiglioni L, Cimino M, Rizzi N, Pepe G, et al. Sex-specific features of microglia from adult mice. Cell Rep. 2018;23(12):3501–11.
Rubinow DR, Schmidt PJ. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacol . 2019;44(1):111–28.
Beach FA. Mating behavior in male rats castrated at various ages and injected with androgen. J Exp Zool. 1946;101:91–142.
Grady KL, Phoenix CH, Young WC. Role of the developing rat testis in differentiation of the neural tissues mediating mating behavior. J Comp Physiol Psychol. 1965;59:176–82.
Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194(4261):211–3.
Gur RC, Gur RE. Complementarity of sex differences in brain and behavior: From laterality to multimodal neuroimaging. J Neurosci Res. 2017;95(1–2):189–99.
Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, et al. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19(10):4065–72.
Gur RC, Gunning-Dixon F, Bilker WB, Gur RE. Sex differences in temporo-limbic and frontal brain volumes of healthy adults. Cereb Cortex. 2002;12(9):998–1003.
Ruigrok ANV, Salimi-Khorshidi G, Lai M-C, Baron-Cohen S, Lombardo MV, Tait RJ, et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39(100):34–50.
Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cogn. 2010;72(1):46–55.
Wierenga LM, Sexton JA, Laake P, Giedd JN, Tamnes CK. A key characteristic of sex differences in the developing brain: greater variability in brain structure of boys than girls. Cereb Cortex. 2018;28(8):2741–51.
Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49(9):741–52.
Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22(6):900–9.
Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516–8.
Morris LS, McCall JG, Charney DS, Murrough JW. The role of the locus coeruleus in the generation of pathological anxiety. Brain Neurosci Adv. 2020;4:2398212820930321.
Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, et al. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci U S A. 2000;97(1):325–30.
Southwick SM, Bremner JD, Rasmusson A, Morgan CA 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46(9):1192–204.
Valentino RJ, Bangasser DA. Sex-biased cellular signaling: molecular basis for sex differences in neuropsychiatric diseases. Dialogues Clin Neurosci. 2016;18(4):385–93.
García-Cáceres C, Lagunas N, Calmarza-Font I, Azcoitia I, Diz-Chaves Y, García-Segura LM, et al. Gender differences in the long-term effects of chronic prenatal stress on the HPA axis and hypothalamic structure in rats. Psychoneuroendocrinology. 2010;35(10):1525–35.
Del Cerro MCR, Ortega E, Gómez F, Segovia S, Pérez-Laso C. Environmental prenatal stress eliminates brain and maternal behavioral sex differences and alters hormone levels in female rats. Horm Behav. 2015;73:142–7.
Behan ÁT, van den Hove DLA, Mueller L, Jetten MJA, Steinbusch HWM, Cotter DR, et al. Evidence of female-specific glial deficits in the hippocampus in a mouse model of prenatal stress. Eur Neuropsychopharmacol. 2011;21(1):71–9.
Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci. 2006;24(5):1477–87.
Bock J, Murmu MS, Biala Y, Weinstock M, Braun K. Prenatal stress and neonatal handling induce sex-specific changes in dendritic complexity and dendritic spine density in hippocampal subregions of prepubertal rats. Neuroscience. 2011;193:34–43.
Mandyam CD, Crawford EF, Eisch AJ, Rivier CL, Richardson HN. Stress experienced in utero reduces sexual dichotomies in neurogenesis, microenvironment, and cell death in the adult rat hippocampus. Dev Neurobiol. 2008;68(5):575–89.
Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. NeuroReport. 1998;9(9):2023–8.
Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry. 2002;51(4):342–4.
Joel D, Berman Z, Tavor I, Wexler N, Gaber O, Stein Y, et al. Sex beyond the genitalia: the human brain mosaic. Proc Natl Acad Sci U S A. 2015;112(50):15468–73.
Tozzi L, Garczarek L, Janowitz D, Stein DJ, Wittfeld K, Dobrowolny H, et al. Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: mega-analytic findings from a large multi-site cohort. Psychol Med. 2020;50(6):1020–31.
Wen DJ, Poh JS, Ni SN, Chong Y-S, Chen H, Kwek K, et al. Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl Psychiatry. 2017;7(4):e1103–e1103.
Jones SL, Dufoix R, Laplante DP, Elgbeili G, Patel R, Chakravarty MM, et al. Larger amygdala volume mediates the association between prenatal maternal stress and higher levels of externalizing behaviors: sex specific effects in project ice storm. Front Hum Neurosci. 2019;13:144.
Lehtola SJ, Tuulari JJ, Scheinin NM, Karlsson L, Parkkola R, Merisaari H, et al. Newborn amygdalar volumes are associated with maternal prenatal psychological distress in a sex-dependent way. NeuroImage Clin. 2020;28:102380.
Acosta H, Tuulari JJ, Scheinin NM, Hashempour N, Rajasilta O, Lavonius TI, et al. Maternal pregnancy-related anxiety is associated with sexually dimorphic alterations in amygdala volume in 4-year-old children. Front Behav Neurosci. 2019;13:175.
Stoye DQ, Blesa M, Sullivan G, Galdi P, Lamb GJ, Black GS, et al. Maternal cortisol is associated with neonatal amygdala microstructure and connectivity in a sexually dimorphic manner. Elife. 2020. https://doi.org/10.7554/eLife.60729.
Mareckova K, Miles A, Andryskova L, Brazdil M, Nikolova YS. Temporally and sex-specific effects of maternal perinatal stress on offspring cortical gyrification and mood in young adulthood. Hum Brain Mapp. 2020;41(17):4866–75.
Shors TJ. A trip down memory lane about sex differences in the brain. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150124.
Urban KR, Geng E, Bhatnagar S, Valentino RJ. Age- and sex-dependent impact of repeated social stress on morphology of rat prefrontal cortex pyramidal neurons. Neurobiol Stress. 2019;10:100165.
Toffoletto S, Lanzenberger R, Gingnell M, Sundström-Poromaa I, Comasco E. Emotional and cognitive functional imaging of estrogen and progesterone effects in the female human brain: a systematic review. Psychoneuroendocrinology. 2014;50:28–52.
McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci. 2012;126(1):4–16.
Romeo RD, Staub D, Jasnow AM, Karatsoreos IN, Thornton JE, McEwen BS. Dihydrotestosterone increases hippocampal N-methyl-D-aspartate binding but does not affect choline acetyltransferase cell number in the forebrain or choline transporter levels in the CA1 region of adult male rats. Endocrinology. 2005;146(4):2091–7.
Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, et al. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130(1):151–63.
Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10(12):4035–9.
Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50(7):1578–93.
Kim JA, Bosma RL, Hemington KS, Rogachov A, Osborne NR, Cheng JC, et al. Sex-differences in network level brain dynamics associated with pain sensitivity and pain interference. Hum Brain Mapp. 2021;42(3):598–614.
Alarcón G, Cservenka A, Rudolph MD, Fair DA, Nagel BJ. Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage. 2015;115:235–44.
Jung M, Mody M, Saito DN, Tomoda A, Okazawa H, Wada Y, et al. Sex differences in the default mode network with regard to autism spectrum traits: a resting state fMRI study. PLoS ONE. 2015;10(11):e0143126.
de Lacy N, McCauley E, Kutz JN, Calhoun VD. Sex-related differences in intrinsic brain dynamism and their neurocognitive correlates. Neuroimage. 2019;202:116116.
Abe O, Yamasue H, Yamada H, Masutani Y, Kabasawa H, Sasaki H, et al. Sex dimorphism in gray/white matter volume and diffusion tensor during normal aging. NMR Biomed. 2010;23(5):446–58.
Lebel C, Caverhill-Godkewitsch S, Beaulieu C. Age-related regional variations of the corpus callosum identified by diffusion tensor tractography. Neuroimage. 2010;52(1):20–31.
Clayden JD, Jentschke S, Muñoz M, Cooper JM, Chadwick MJ, Banks T, et al. Normative development of white matter tracts: similarities and differences in relation to age, gender, and intelligence. Cereb Cortex. 2012;22(8):1738–47.
Tomasi D, Volkow ND. Laterality patterns of brain functional connectivity: gender effects. Cereb Cortex. 2012;22(6):1455–62.
Gong G, Rosa-Neto P, Carbonell F, Chen ZJ, He Y, Evans AC. Age- and gender-related differences in the cortical anatomical network. J Neurosci. 2009;29(50):15684–93.
Tomasi D, Volkow ND. Gender differences in brain functional connectivity density. Hum Brain Mapp. 2012;33(4):849–60.
I ngalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, et al. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A. 2014;111(2):823–8.
Joel D, Tarrasch R. On the mis-presentation and misinterpretation of gender-related data: the case of Ingalhalikar’s human connectome study. Proc Natl Acad Sci USA. 2014. https://doi.org/10.1073/pnas.1323319111.
Shanmugan S, Loughead J, Cao W, Sammel MD, Satterthwaite TD, Ruparel K, et al. Impact of tryptophan depletion on executive system function during menopause is moderated by childhood adversity. Neuropsychopharmacol . 2017;42(12):2398–406.
Berent-Spillson A, Persad CC, Love T, Tkaczyk A, Wang H, Reame NK, et al. Early menopausal hormone use influences brain regions used for visual working memory. Menopause. 2010;17(4):692–9.
Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, et al. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci U S A. 1997;94(16):8836–41.
Macoveanu J, Henningsson S, Pinborg A, Jensen P, Knudsen GM, Frokjaer VG, et al. Sex-steroid hormone manipulation reduces brain response to reward. Neuropsychopharmacol . 2016;41(4):1057–65.
Rupp HA, James TW, Ketterson ED, Sengelaub DR, Janssen E, Heiman JR. Neural activation in women in response to masculinized male faces: mediation by hormones and psychosexual factors. Evol Hum Behav. 2009;30(1):1–10.
Dreher J-C, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A. 2007;104(7):2465–70.
Bayer J, Bandurski P, Sommer T. Differential modulation of activity related to the anticipation of monetary gains and losses across the menstrual cycle. Eur J Neurosci. 2013;38(10):3519–26.
Arélin K, Mueller K, Barth C, Rekkas PV, Kratzsch J, Burmann I, et al. Progesterone mediates brain functional connectivity changes during the menstrual cycle-a pilot resting state MRI study. Front Neurosci. 2015;9:44.
Petersen N, Kilpatrick LA, Goharzad A, Cahill L. Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. Neuroimage. 2014;90:24–32.
Syan SK, Minuzzi L, Costescu D, Smith M, Allega OR, Coote M, et al. Influence of endogenous estradiol, progesterone, allopregnanolone, and dehydroepiandrosterone sulfate on brain resting state functional connectivity across the menstrual cycle. Fertil Steril. 2017;107(5):1246-1255.e4.
Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25(40):9309–16.
Henningsson S, Madsen KH, Pinborg A, Heede M, Knudsen GM, Siebner HR, et al. Role of emotional processing in depressive responses to sex-hormone manipulation: a pharmacological fMRI study. Transl Psychiatry. 2015;5(12):e688.
Protopopescu X, Pan H, Altemus M, Tuescher O, Polanecsky M, McEwen B, et al. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci U S A. 2005;102(44):16060–5.
Shafir T, Love T, Berent-Spillson A, Persad CC, Wang H, Reame NK, et al. Postmenopausal hormone use impact on emotion processing circuitry. Behav Brain Res. 2012;226(1):147–53.
Joffe H, Deckersbach T, Lin NU, Makris N, Skaar TC, Rauch SL, et al. Metabolic activity in the insular cortex and hypothalamus predicts hot flashes: an FDG-PET study. J Clin Endocrinol Metab. 2012;97(9):3207–15.
Engman J, Sundström Poromaa I, Moby L, Wikström J, Fredrikson M, Gingnell M. Hormonal cycle and contraceptive effects on amygdala and salience resting-state networks in women with previous affective side effects on the pill. Neuropsychopharmacol . 2018;43(3):555–63.
Riddle J, Ahn S, McPherson T, Girdler S, Frohlich F. Progesterone modulates theta oscillations in the frontal-parietal network. Psychophysiology. 2020;57(10):e13632.
Pritschet L, Santander T, Taylor CM, Layher E, Yu S, Miller MB, et al. Functional reorganization of brain networks across the human menstrual cycle. Neuroimage. 2020;220:117091.
Wang J-X, Zhuang J-Y, Fu L, Lei Q, Zhang W. Association of ovarian hormones with mapping concept of self and others in the brain’s default mode network. NeuroReport. 2020;31(10):717–23.
Zsido RG, Heinrich M, Slavich GM, Beyer F, Kharabian Masouleh S, Kratzsch J, et al. Association of estradiol and visceral fat with structural brain networks and memory performance in adults. JAMA Netw open. 2019;2(6):e196126.
Duman RS, Sanacora G, Krystal JH. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron. 2019;102(1):75–90.
Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106(6):1942–7.
Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, et al. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. Am J Psychiatry. 2018;175(11):1111–20.
Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacol . 2017;42(6):1210–9.
Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50(9):651–8.
Grandjean J, Azzinnari D, Seuwen A, Sigrist H, Seifritz E, Pryce CR, et al. Chronic psychosocial stress in mice leads to changes in brain functional connectivity and metabolite levels comparable to human depression. Neuroimage. 2016;142:544–52.
Peper JS, van den Heuvel MP, Mandl RCW, Hulshoff Pol HE, van Honk J. Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology. 2011;36(8):1101–13.
Honeycutt JA, Demaestri C, Peterzell S, Silveri MM, Cai X, Kulkarni P, et al. Altered corticolimbic connectivity reveals sex-specific adolescent outcomes in a rat model of early life adversity. Elife. 2020. https://doi.org/10.7554/eLife.52651.
White JD, Arefin TM, Pugliese A, Lee CH, Gassen J, Zhang J, et al. Early life stress causes sex-specific changes in adult fronto-limbic connectivity that differentially drive learning. Elife. 2020. https://doi.org/10.7554/eLife.58301.
Salvatore M, Wiersielis KR, Luz S, Waxler DE, Bhatnagar S, Bangasser DA. Sex differences in circuits activated by corticotropin releasing factor in rats. Horm Behav. 2018;97:145–53.
Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81(3):689–97.
Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex. 2010;20(11):2560–7.
Shansky RM, Morrison JH. Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res. 2009;1293:108–13.
Blume SR, Freedberg M, Vantrease JE, Chan R, Padival M, Record MJ, et al. Sex- and estrus-dependent differences in rat basolateral amygdala. J Neurosci. 2017;37(44):10567–86.
Bollinger JL, Collins KE, Patel R, Wellman CL. Behavioral stress alters corticolimbic microglia in a sex- and brain region-specific manner. PLoS ONE. 2017;12(12):e0187631.
Padgaonkar NT, Lawrence KE, Hernandez LM, Green SA, Galván A, Dapretto M. Sex differences in internalizing symptoms and amygdala functional connectivity in neurotypical youth. Dev Cogn Neurosci. 2020;44:100797.
Ernst M, Benson B, Artiges E, Gorka AX, Lemaitre H, Lago T, et al. Pubertal maturation and sex effects on the default-mode network connectivity implicated in mood dysregulation. Transl Psychiatry. 2019;9(1):103.
Chuang J-Y, Hagan CC, Murray GK, Graham JME, Ooi C, Tait R, et al. Adolescent major depressive disorder: neuroimaging evidence of sex difference during an affective Go/No-Go task. Front psychiatry. 2017;8:119.
Li C-SR, Zhang S, Hung C-C, Chen C-M, Duann J-R, Lin C-P, et al. Depression in chronic ketamine users: sex differences and neural bases. Psychiatry Res Neuroimaging. 2017;269:1–8.
Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157(6):924–30.
Schmidt PJ, Ben Dor R, Martinez PE, Guerrieri GM, Harsh VL, Thompson K, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiat. 2015;72(7):714–26.
Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209–16.
Gingnell M, Morell A, Bannbers E, Wikström J, Sundström PI. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm Behav. 2012;62(4):400–6.
Gingnell M, Bannbers E, Wikström J, Fredrikson M, Sundström-Poromaa I. Premenstrual dysphoric disorder and prefrontal reactivity during anticipation of emotional stimuli. Eur Neuropsychopharmacol. 2013;23(11):1474–83.
Comasco E, Hahn A, Ganger S, Gingnell M, Bannbers E, Oreland L, et al. Emotional fronto-cingulate cortex activation and brain derived neurotrophic factor polymorphism in premenstrual dysphoric disorder. Hum Brain Mapp. 2014;35(9):4450–8.
Gingnell M, Ahlstedt V, Bannbers E, Wikström J, Sundström-Poromaa I, Fredrikson M. Social stimulation and corticolimbic reactivity in premenstrual dysphoric disorder: a preliminary study. Biol Mood Anxiety Disord. 2014;4(1):3.
Schmidt PJ, Martinez PE, Nieman LK, Koziol DE, Thompson KD, Schenkel L, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. 2017;174(10):980–9.
Wei S-M, Baller EB, Martinez PE, Goff AC, Li HJ, Kohn PD, et al. Subgenual cingulate resting regional cerebral blood flow in premenstrual dysphoric disorder: differential regulation by ovarian steroids and preliminary evidence for an association with expression of ESC/E(Z) complex genes. Transl Psychiatry. 2021;11(1):206.
Fisher PM, Larsen CB, Beliveau V, Henningsson S, Pinborg A, Holst KK, et al. Pharmacologically induced sex hormone fluctuation effects on resting-state functional connectivity in a risk model for depression: a randomized trial. Neuropsychopharmacol . 2017;42(2):446–53.
Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015;9:37.
Yokomaku D, Numakawa T, Numakawa Y, Suzuki S, Matsumoto T, Adachi N, et al. Estrogen enhances depolarization-induced glutamate release through activation of phosphatidylinositol 3-kinase and mitogen-activated protein kinase in cultured hippocampal neurons. Mol Endocrinol. 2003;17(5):831–44.
Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, et al. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol Psychiatry. 2014;19(5):588–98.
Bethea CL, Reddy AP. Ovarian steroids increase glutamatergic related gene expression in serotonin neurons of macaques. Mol Cell Neurosci. 2012;49(3):251–62.
Smith SS, Woolley CS. Cellular and molecular effects of steroid hormones on CNS excitability. Cleve Clin J Med. 2004;71(Suppl 2):S4-10.
Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18(7):2550–9.
Herbison AE. Estrogen regulation of GABA transmission in rat preoptic area. Brain Res Bull. 1997;44(4):321–6.
Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem. 1994;62(5):1750–6.
Becker JB. Sex differences in addiction. Dialogues Clin Neurosci. 2016;18(4):395–402.
Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett. 1990;118(2):169–71.
Disshon KA, Boja JW, Dluzen DE. Inhibition of striatal dopamine transporter activity by 17beta-estradiol. Eur J Pharmacol. 1998;345(2):207–11.
Watson CS, Alyea RA, Hawkins BE, Thomas ML, Cunningham KA, Jakubas AA. Estradiol effects on the dopamine transporter—protein levels, subcellular location, and function. J Mol Signal. 2006;1:5.
Morel GR, Carón RW, Cónsole GM, Soaje M, Sosa YE, Rodríguez SS, et al. Estrogen inhibits tuberoinfundibular dopaminergic neurons but does not cause irreversible damage. Brain Res Bull. 2009;80(6):347–52.
Lu NZ, Shlaes TA, Gundlah C, Dziennis SE, Lyle RE, Bethea CL. Ovarian steroid action on tryptophan hydroxylase protein and serotonin compared to localization of ovarian steroid receptors in midbrain of guinea pigs. Endocrine. 1999;11(3):257–67.
Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23(1):41–100.
Pecins-Thompson M, Brown NA, Kohama SG, Bethea CL. Ovarian steroid regulation of tryptophan hydroxylase mRNA expression in rhesus macaques. J Neurosci. 1996;16(21):7021–9.
Sumner BE, Fink G. Testosterone as well as estrogen increases serotonin2A receptor mRNA and binding site densities in the male rat brain. Brain Res Mol Brain Res. 1998;59(2):205–14.
Hiroi R, Neumaier JF. Estrogen decreases 5-HT1B autoreceptor mRNA in selective subregion of rat dorsal raphe nucleus: inverse association between gene expression and anxiety behavior in the open field. Neuroscience. 2009;158(2):456–64.
Shansky RM, Bender G, Arnsten AFT. Estrogen prevents norepinephrine alpha-2a receptor reversal of stress-induced working memory impairment. Stress. 2009;12(5):457–63.
Mahmood ASMH, Uddin MM, Ibrahim MMH, Briski KP. Norepinephrine regulation of ventromedial hypothalamic nucleus metabolic-sensory neuron 5’-AMP-activated protein kinase activity: impact of estradiol. Int J Mol Sci. 2020;21(6):2013.
Wang W, Bai W, Cui G, Jin B, Wang K, Jia J, et al. Effects of estradiol valerate and remifemin on norepinephrine signaling in the brain of ovariectomized rats. Neuroendocrinology. 2015;101(2):120–32.
Luine VN, McEwen BS. Effect of oestradiol on turnover of type A monoamine oxidase in brain. J Neurochem. 1977;28(6):1221–7.
Sánchez MG, Morissette M, Di Paolo T. Oestradiol modulation of serotonin reuptake transporter and serotonin metabolism in the brain of monkeys. J Neuroendocrinol. 2013;25(6):560–9.
Sumner BE, Fink G. Effects of acute estradiol on 5-hydroxytryptamine and dopamine receptor subtype mRNA expression in female rat brain. Mol Cell Neurosci. 1993;4(1):83–92.
Zheng P. Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Prog Neurobiol. 2009;89(2):134–52.
Juraska JM, Sisk CL, DonCarlos LL. Sexual differentiation of the adolescent rodent brain: hormonal influences and developmental mechanisms. Horm Behav. 2013;64(2):203–10.
Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336(2):293–306.
Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994;5(1):27–41.
Smith SS, Waterhouse BD, Chapin JK, Woodward DJ. Progesterone alters GABA and glutamate responsiveness: a possible mechanism for its anxiolytic action. Brain Res. 1987;400(2):353–9.
Uban KA, Rummel J, Floresco SB, Galea LAM. Estradiol modulates effort-based decision making in female rats. Neuropsychopharmacol . 2012;37(2):390–401.
Quinlan MG, Almey A, Caissie M, LaChappelle I, Radiotis G, Brake WG. Estradiol and striatal dopamine receptor antagonism influence memory system bias in the female rat. Neurobiol Learn Mem. 2013;106:221–9.
Jacobs E, D’Esposito M. Estrogen shapes dopamine-dependent cognitive processes: implications for women’s health. J Neurosci. 2011;31(14):5286–93.
Deligiannidis KM, Sikoglu EM, Shaffer SA, Frederick B, Svenson AE, Kopoyan A, et al. GABAergic neuroactive steroids and resting-state functional connectivity in postpartum depression: a preliminary study. J Psychiatr Res. 2013;47(6):816–28.
Pinna G. Allopregnanolone, the neuromodulator turned therapeutic agent: thank you, next? Front Endocrinol (Lausanne). 2020;11:236.
Meltzer-Brody S, Kanes SJ. Allopregnanolone in postpartum depression: Role in pathophysiology and treatment. Neurobiol Stress. 2020;12:100212.
Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet (London, England). 2017;390(10093):480–9.
Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet (London, England). 2018;392(10152):1058–70.
Pineles SL, Nillni YI, Pinna G, Irvine J, Webb A, Arditte Hall KA, et al. PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology. 2018;93:133–41.
Rasmusson AM, King MW, Valovski I, Gregor K, Scioli-Salter E, Pineles SL, et al. Relationships between cerebrospinal fluid GABAergic neurosteroid levels and symptom severity in men with PTSD. Psychoneuroendocrinology. 2019;102:95–104.
Pitsillou E, Bresnehan SM, Kagarakis EA, Wijoyo SJ, Liang J, Hung A, et al. The cellular and molecular basis of major depressive disorder: towards a unified model for understanding clinical depression. Mol Biol Rep. 2020;47(1):753–70.
Dell’Osso L, Carmassi C, Mucci F, Marazziti D. Depression, serotonin and tryptophan. Curr Pharm Des. 2016;22(8):949–54.
Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai H-C, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493(7433):537–41.
Fogaça MV, Duman RS. Cortical GABaergic dysfunction in stress and depression: new insights for therapeutic interventions. Front Cell Neurosci. 2019;13:87.
Browne CA, Jacobson ML, Lucki I. Novel targets to treat depression: opioid-based therapeutics. Harv Rev Psychiatry. 2020;28(1):40–59.
Leonard BE. Stress, norepinephrine and depression. J Psychiatry Neurosci. 2001;26:S11–6.
Undurraga J, Baldessarini RJ. Direct comparison of tricyclic and serotonin-reuptake inhibitor antidepressants in randomized head-to-head trials in acute major depression: Systematic review and meta-analysis. J Psychopharmacol. 2017;31(9):1184–9.
Rincón-Cortés M, Grace AA. Antidepressant effects of ketamine on depression-related phenotypes and dopamine dysfunction in rodent models of stress. Behav Brain Res. 2020;379:112367.
Shepard R, Page CE, Coutellier L. Sensitivity of the prefrontal GABAergic system to chronic stress in male and female mice: Relevance for sex differences in stress-related disorders. Neuroscience. 2016;332:1–12.
McEuen JG, Semsar KA, Lim MA, Bale TL. Influence of sex and corticotropin-releasing factor pathways as determinants in serotonin sensitivity. Endocrinology. 2009;150(8):3709–16.
Campi KL, Greenberg GD, Kapoor A, Ziegler TE, Trainor BC. Sex differences in effects of dopamine D1 receptors on social withdrawal. Neuropharmacology. 2014;77:208–16.
Laman-Maharg A, Trainor BC. Stress, sex, and motivated behaviors. J Neurosci Res. 2017;95(1–2):83–92.
Zhang S, Zhang H, Ku SM, Juarez B, Morel C, Tzavaras N, et al. Sex differences in the neuroadaptations of reward-related circuits in response to subchronic variable stress. Neuroscience. 2018;376:108–16.
Gillies GE, Virdee K, Pienaar I, Al-Zaid F, Dalley JW. Enduring, sexually dimorphic impact of in utero exposure to elevated levels of glucocorticoids on midbrain dopaminergic populations. Brain Sci. 2016. https://doi.org/10.3390/brainsci7010005.
Carrier N, Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013;70:27–34.
Sarkar A, Kabbaj M. Sex differences in effects of ketamine on behavior, spine density, and synaptic proteins in socially isolated rats. Biol Psychiatry. 2016;80(6):448–56.
Oberlander JG, Woolley CS. 17β-Estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J Neurosci. 2016;36(9):2677–90.
Bangasser DA, Curtis A, Reyes BAS, Bethea TT, Parastatidis I, Ischiropoulos H, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15(9):896–904.
Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308(5721):512–7.
Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279(34):35518–25.
Jovanovic H, Lundberg J, Karlsson P, Cerin A, Saijo T, Varrone A, et al. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage. 2008;39(3):1408–19.
Staley JK, Sanacora G, Tamagnan G, Maciejewski PK, Malison RT, Berman RM, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry. 2006;59(1):40–7.
Veldman ER, Svedberg MM, Svenningsson P, Lundberg J. Distribution and levels of 5-HT(1B) receptors in anterior cingulate cortex of patients with bipolar disorder, major depressive disorder and schizophrenia—An autoradiography study. Eur Neuropsychopharmacol . 2017;27(5):504–14.
Arango V, Underwood MD, Mann JJ. Postmortem findings in suicide victims. Implications for in vivo imaging studies. Ann N Y Acad Sci. 1997;836:269–87.
Ruhé HG, Booij J, Reitsma JB, Schene AH. Serotonin transporter binding with [123I]beta-CIT SPECT in major depressive disorder versus controls: effect of season and gender. Eur J Nucl Med Mol Imaging. 2009;36(5):841–9.
Spies M, Handschuh PA, Lanzenberger R, Kranz GS. Sex and the serotonergic underpinnings of depression and migraine. Handb Clin Neurol. 2020;175:117–40.
Mc Mahon B, Andersen SB, Madsen MK, Hjordt LV, Hageman I, Dam H, et al. Seasonal difference in brain serotonin transporter binding predicts symptom severity in patients with seasonal affective disorder. Brain. 2016;139(Pt 5):1605–14.
Seney ML, Chang L-C, Oh H, Wang X, Tseng GC, Lewis DA, et al. The role of genetic sex in affect regulation and expression of GABA-related genes across species. Front psychiatry. 2013;4:104.
Seney ML, Tripp A, McCune S, Lewis DA, Sibille E. Laminar and cellular analyses of reduced somatostatin gene expression in the subgenual anterior cingulate cortex in major depression. Neurobiol Dis. 2015;73:213–9.
Tripp A, Kota RS, Lewis DA, Sibille E. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis. 2011;42(1):116–24.
Gray AL, Hyde TM, Deep-soboslay A, Kleinman JE, Sodhi MS. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol Psychiatry. 2015;20(9):1057–68.
Sramek JJ, Murphy MF, Cutler NR. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin Neurosci. 2016;18(4):447–57.
Wohlfarth T, Storosum JG, Elferink AJA, van Zwieten BJ, Fouwels A, van den Brink W. Response to tricyclic antidepressants: independent of gender? Am J Psychiatry. 2004;161(2):370–2.
Schnieder LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9(4):393–9.
Foss-Feig JH, Adkinson BD, Ji JL, Yang G, Srihari VH, McPartland JC, et al. Searching for cross-diagnostic convergence: neural mechanisms governing excitation and inhibition balance in schizophrenia and autism spectrum disorders. Biol Psychiatry. 2017;81(10):848–61.
Srivastava DP, Waters EM, Mermelstein PG, Kramár EA, Shors TJ, Liu F. Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry. J Neurosci. 2011;31(45):16056–63.
Zhang L, Sukhareva M, Barker JL, Maric D, Hao Y, Chang YH, et al. Direct binding of estradiol enhances Slack (sequence like a calcium-activated potassium channel) channels’ activity. Neuroscience. 2005;131(2):275–82.
Zup SL, Madden AMK. Gonadal hormone modulation of intracellular calcium as a mechanism of neuroprotection. Front Neuroendocrinol. 2016;42:40–52.
Puralewski R, Vasilakis G, Seney ML. Sex-related factors influence expression of mood-related genes in the basolateral amygdala differentially depending on age and stress exposure. Biol Sex Differ. 2016;7:50.
Labonté B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. Sex-specific transcriptional signatures in human depression. Nat Med. 2017;23(9):1102–11.
Barko K, Paden W, Cahill KM, Seney ML, Logan RW. Sex-specific effects of stress on mood-related gene expression. Mol neuropsychiatry. 2019;5(3):162–75.
Paden W, Barko K, Puralewski R, Cahill KM, Huo Z, Shelton MA, et al. Sex differences in adult mood and in stress-induced transcriptional coherence across mesocorticolimbic circuitry. Transl Psychiatry. 2020;10(1):59.
LaPlant Q, Chakravarty S, Vialou V, Mukherjee S, Koo JW, Kalahasti G, et al. Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol Psychiatry. 2009;65(10):874–80.
Pfau ML, Purushothaman I, Feng J, Golden SA, Aleyasin H, Lorsch ZS, et al. Integrative analysis of sex-specific microrna networks following stress in mouse nucleus accumbens. Front Mol Neurosci. 2016;9:144.
Seney ML, Huo Z, Cahill K, French L, Puralewski R, Zhang J, et al. Opposite molecular signatures of depression in men and women. Biol Psychiatry. 2018;84(1):18–27.
Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacoln. 2013;38(1):23–38.
Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705.
Liu J, Morgan M, Hutchison K, Calhoun VD. A study of the influence of sex on genome wide methylation. PLoS ONE. 2010;5(4):e10028.
Yousefi P, Huen K, Davé V, Barcellos L, Eskenazi B, Holland N. Sex differences in DNA methylation assessed by 450 K BeadChip in newborns. BMC Genomics. 2015;16:911.
Martin E, Smeester L, Bommarito PA, Grace MR, Boggess K, Kuban K, et al. Sexual epigenetic dimorphism in the human placenta: implications for susceptibility during the prenatal period. Epigenomics. 2017;9(3):267–78.
García-Calzón S, Perfilyev A, de Mello VD, Pihlajamäki J, Ling C. Sex differences in the methylome and transcriptome of the human liver and circulating HDL-cholesterol levels. J Clin Endocrinol Metab. 2018;103(12):4395–408.
Reizel Y, Spiro A, Sabag O, Skversky Y, Hecht M, Keshet I, et al. Gender-specific postnatal demethylation and establishment of epigenetic memory. Genes Dev. 2015;29(9):923–33.
Hall E, Volkov P, Dayeh T, Esguerra JLS, Salö S, Eliasson L, et al. Sex differences in the genome-wide DNA methylation pattern and impact on gene expression, microRNA levels and insulin secretion in human pancreatic islets. Genome Biol. 2014;15(12):522.
Davegårdh C, Hall Wedin E, Broholm C, Henriksen TI, Pedersen M, Pedersen BK, et al. Sex influences DNA methylation and gene expression in human skeletal muscle myoblasts and myotubes. Stem Cell Res Ther. 2019;10(1):26.
McCormick H, Young PE, Hur SSJ, Booher K, Chung H, Cropley JE, et al. Isogenic mice exhibit sexually-dimorphic DNA methylation patterns across multiple tissues. BMC Genomics. 2017;18(1):966.
Ghahramani NM, Ngun TC, Chen P-Y, Tian Y, Krishnan S, Muir S, et al. The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biol Sex Differ. 2014;5:8.
Xu H, Wang F, Liu Y, Yu Y, Gelernter J, Zhang H. Sex-biased methylome and transcriptome in human prefrontal cortex. Hum Mol Genet. 2014;23(5):1260–70.
Gross JA, Pacis A, Chen GG, Barreiro LB, Ernst C, Turecki G. Characterizing 5-hydroxymethylcytosine in human prefrontal cortex at single base resolution. BMC Genomics. 2015;16(1):672.
Spiers H, Hannon E, Schalkwyk LC, Smith R, Wong CCY, O’Donovan MC, et al. Methylomic trajectories across human fetal brain development. Genome Res. 2015;25(3):338–52.
Spiers H, Hannon E, Schalkwyk LC, Bray NJ, Mill J. 5-hydroxymethylcytosine is highly dynamic across human fetal brain development. BMC Genomics. 2017;18(1):738.
Tsai H-W, Grant PA, Rissman EF. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics. 2009;4(1):47–53.
Lomniczi A, Ojeda SR. The emerging role of epigenetics in the regulation of female puberty. Endocr Dev. 2016;29:1–16.
Lomniczi A, Wright H, Ojeda SR. Epigenetic regulation of female puberty. Front Neuroendocrinol. 2015;36:90–107.
Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, et al. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18(5):690–7.
Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37(3):278–316.
Ortona E, Pierdominici M, Maselli A, Veroni C, Aloisi F, Shoenfeld Y. Sex-based differences in autoimmune diseases. Ann Ist Super Sanita. 2016;52(2):205–12.
Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev. 2017;97(1):1–37.
Rubin JB, Lagas JS, Broestl L, Sponagel J, Rockwell N, Rhee G, et al. Sex differences in cancer mechanisms. Biol Sex Differ. 2020;11(1):17.
Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci. 2010;13(11):1351–3.
Renthal W, Maze I, Krishnan V, Covington HE 3rd, Xiao G, Kumar A, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56(3):517–29.
Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8(5):355–67.
Park C, Rosenblat JD, Brietzke E, Pan Z, Lee Y, Cao B, et al. Stress, epigenetics and depression: a systematic review. Neurosci Biobehav Rev. 2019;102:139–52.
Nestler EJ, Peña CJ, Kundakovic M, Mitchell A, Akbarian S. Epigenetic basis of mental illness. Neuroscientist . 2016;22(5):447–63.
Bagot RC, Labonté B, Peña CJ, Nestler EJ. Epigenetic signaling in psychiatric disorders: stress and depression. Dialogues Clin Neurosci. 2014;16(3):281–95.
Peña CJ, Nestler EJ. Progress in epigenetics of depression. Prog Mol Biol Transl Sci. 2018;157:41–66.
Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Elliott E, et al. Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PLoS ONE. 2011;6(11):e28128.
Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, et al. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J Neurosci. 2015;35(50):16362–76.
O’Connor RM, Dinan TG, Cryan JF. Little things on which happiness depends: microRNAs as novel therapeutic targets for the treatment of anxiety and depression. Mol Psychiatry. 2012;17(4):359–76.
O’Carroll D, Schaefer A. General principals of miRNA biogenesis and regulation in the brain. Neuropsychopharmacol . 2013;38(1):39–54.
Morgan CP, Bale TL. Sex differences in microRNA-mRNA networks: examination of novel epigenetic programming mechanisms in the sexually dimorphic neonatal hypothalamus. Biol Sex Differ. 2017;8(1):27.
Kim DR, Bale TL, Epperson CN. Prenatal programming of mental illness: current understanding of relationship and mechanisms. Curr Psychiatry Rep. 2015;17(2):5.
Al-Haddad BJS, Oler E, Armistead B, Elsayed NA, Weinberger DR, Bernier R, et al. The fetal origins of mental illness. Am J Obstet Gynecol. 2019;221(6):549–62.
Flinkkilä E, Keski-Rahkonen A, Marttunen M, Raevuori A. Prenatal inflammation, infections and mental disorders. Psychopathology. 2016;49(5):317–33. https://doi.org/10.1159/000448054.
Glover V, Hill J. Sex differences in the programming effects of prenatal stress on psychopathology and stress responses: an evolutionary perspective. Physiol Behav. 2012;106(5):736–40.
Hill J, Pickles A, Wright N, Quinn JP, Murgatroyd C, Sharp H. Mismatched prenatal and postnatal maternal depressive symptoms and child behaviours: a sex-dependent role for NR3C1 DNA methylation in the wirral child health and development study. Cells. 2019;8(9):943.
Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 2007;13(7):269–77.
Murgatroyd C, Quinn JP, Sharp HM, Pickles A, Hill J. Effects of prenatal and postnatal depression, and maternal stroking, at the glucocorticoid receptor gene. Transl Psychiatry. 2015;5(5):e560.
Corriger A, Pickering G. Ketamine and depression: a narrative review. Drug Des Devel Ther. 2019;13:3051–67.
Hultman R, Ulrich K, Sachs BD, Blount C, Carlson DE, Ndubuizu N, et al. Brain-wide electrical spatiotemporal dynamics encode depression vulnerability. Cell. 2018;173(1):166-180.e14.
Bleier R, Byne W, Siggelkow I. Cytoarchitectonic sexual dimorphisms of the medial preoptic and anterior hypothalamic areas in guinea pig, rat, hamster, and mouse. J Comp Neurol. 1982;212(2):118–30.
McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88(1):91–124.
Green PS, Simpkins JW. Neuroprotective effects of estrogens: potential mechanisms of action. Int J Dev Neurosci Off J Int Soc Dev Neurosci. 2000;18(4–5):347–58.
Fang Y-Y, Zeng P, Qu N, Ning L-N, Chu J, Zhang T, et al. Evidence of altered depression and dementia-related proteins in the brains of young rats after ovariectomy. J Neurochem. 2018;146(6):703–21.
Herting MM, Sowell ER. Puberty and structural brain development in humans. Front Neuroendocrinol. 2017;44:122–37.
Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YSK, Knickmeyer RC, et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 2007;27(6):1255–60.
Pletzer B, Harris T, Hidalgo-Lopez E. Subcortical structural changes along the menstrual cycle: beyond the hippocampus. Sci Rep. 2018;8(1):16042.
Catenaccio E, Mu W, Lipton ML. Estrogen- and progesterone-mediated structural neuroplasticity in women: evidence from neuroimaging. Brain Struct Funct. 2016;221(8):3845–67.
Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, et al. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008;18(10):985–8.
Kohl J, Babayan BM, Rubinstein ND, Autry AE, Marin-Rodriguez B, Kapoor V, et al. Functional circuit architecture underlying parental behaviour. Nature. 2018;556(7701):326–31.
Da Silva JT, Tricou C, Zhang Y, Seminowicz DA, Ro JY. Brain networks and endogenous pain inhibition are modulated by age and sex in healthy rats. Pain. 2020;161(6):1371–80.
Da Silva JT, Zhang Y, Asgar J, Ro JY, Seminowicz DA. Diffuse noxious inhibitory controls and brain networks are modulated in a testosterone-dependent manner in Sprague Dawley rats. Behav Brain Res. 2018;349:91–7.
Wang Z, Guo Y, Mayer EA, Holschneider DP. Sex differences in insular functional connectivity in response to noxious visceral stimulation in rats. Brain Res. 2019;1717:15–26.
Tang S, Xu S, Zhu W, Gullapalli RP, Mooney SM. Alterations in the whole brain network organization after prenatal ethanol exposure. Eur J Neurosci. 2020;51(10):2110–8.
McHenry JA, Otis JM, Rossi MA, Robinson JE, Kosyk O, Miller NW, et al. Hormonal gain control of a medial preoptic area social reward circuit. Nat Neurosci. 2017;20(3):449–58.
Dumais KM, Chernyak S, Nickerson LD, Janes AC. Sex differences in default mode and dorsal attention network engagement. PLoS ONE. 2018;13(6):e0199049.
Zorn JV, Schür RR, Boks MP, Kahn RS, Joëls M, Vinkers CH. Cortisol stress reactivity across psychiatric disorders: a systematic review and meta-analysis. Psychoneuroendocrinology. 2017;77:25–36.
Ter Horst GJ, Wichmann R, Gerrits M, Westenbroek C, Lin Y. Sex differences in stress responses: focus on ovarian hormones. Physiol Behav. 2009;97(2):239–49.
Zhang L, Li BS, Zhao W, Chang YH, Ma W, Dragan M, et al. Sex-related differences in MAPKs activation in rat astrocytes: effects of estrogen on cell death. Brain Res Mol Brain Res. 2002;103(1–2):1–11.
Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159(2):883–95.
Bangasser DA, Wicks B. Sex-specific mechanisms for responding to stress. J Neurosci Res. 2017;95(1–2):75–82.
Pandya M, Palpagama TH, Turner C, Waldvogel HJ, Faull RL, Kwakowsky A. Sex- and age-related changes in GABA signaling components in the human cortex. Biol Sex Differ. 2019;10(1):5.
Li HJ, Goff A, Rudzinskas SA, Jung Y, Dubey N, Hoffman J, et al. Altered estradiol-dependent cellular Ca(2+) homeostasis and endoplasmic reticulum stress response in Premenstrual Dysphoric Disorder. Mol Psychiatry. 2021. https://doi.org/10.1038/s41380-021-01144-8.
Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240(4854):889–95.
Hah N, Kraus WL. Hormone-regulated transcriptomes: Lessons learned from estrogen signaling pathways in breast cancer cells. Mol Cell Endocrinol. 2014;382(1):652–64.
Dubey N, Hoffman JF, Schuebel K, Yuan Q, Martinez PE, Nieman LK, et al. The ESC/E(Z) complex, an effector of response to ovarian steroids, manifests an intrinsic difference in cells from women with premenstrual dysphoric disorder. Mol Psychiatry. 2017;22(8):1172–84.
Kovács T, Szabó-Meleg E, Ábrahám IM. Estradiol-induced epigenetically mediated mechanisms and regulation of gene expression. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21093177.
Hodes GE, Epperson CN. Sex differences in vulnerability and resilience to stress across the life span. Biol Psychiatry. 2019. https://doi.org/10.1016/j.biopsych.2019.04.028.
Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28(36):9055–65.
Bessa DS, Maschietto M, Aylwin CF, Canton APM, Brito VN, Macedo DB, et al. Methylome profiling of healthy and central precocious puberty girls. Clin Epigenetics. 2018;10(1):146.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
CSK and DRR were both major contributors to the preparation of the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable; this article is a review only and did not involve human participants, human data, human tissue, or animal subjects.
Consent for publication.
Not applicable.
Competing interests
Dr. Rubinow is on the Clinical Advisory Board of (and has received honoraria and stock options from) Sage Therapeutics. He is also on the Scientific Advisory Boards of Terran BioSciences and Sensorium Therapeutics. Dr. Sikes-Kelp reports no conflicts of interest. The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sikes-Keilp, C., Rubinow, D.R. In search of sex-related mediators of affective illness. Biol Sex Differ 12, 55 (2021). https://doi.org/10.1186/s13293-021-00400-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13293-021-00400-4