Abstract
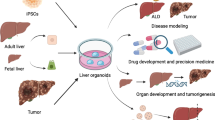
The liver is the most important metabolic organ in the body. While mouse models and cell lines have further deepened our understanding of liver biology and related diseases, they are flawed in replicating key aspects of human liver tissue, particularly its complex structure and metabolic functions. The organoid model represents a major breakthrough in cell biology that revolutionized biomedical research. Organoids are in vitro three-dimensional (3D) physiological structures that recapitulate the morphological and functional characteristics of tissues in vivo, and have significant advantages over traditional cell culture methods. In this review, we discuss the generation strategies and current advances in the field focusing on their application in regenerative medicine, drug discovery and modeling diseases.
Similar content being viewed by others
Introduction
The liver is a complex organ with heterogeneous structure and function, including different cell types, including epithelial cells (i.e., hepatocytes and cholangiocytes), kupffer cells, circulating monocytes, mesenchymal cells (i.e., hepatic stellate cells), and sinus endothelial cells [1]. The main epithelial component is hepatocytes, which account for more than 60% of the total liver cells, which are mainly responsible for the metabolic activities, detoxification, protein secretion and bile production of the liver. Another major epithelial component is the bile duct epithelial cells (cholangiocytes), which line the intrahepatic and extrahepatic bile ducts and are involved in the transport and modification of primary bile produced by hepatocytes [1, 2].
So far, the lack of suitable models to accurately simulate physiological conditions have been major obstacles hindering progress in chronic liver disease research [3]. Animal models cannot accurately reflect the metabolic reactions of drugs in the human body, and high costs and ethical issues also limit the application of animal models [4]. Immortalized cell lines are the most common models in the laboratory, but they are limited by many genetic and functional changes that can impair the authenticity of liver disease models [5]. Primary human hepatocytes (PHH) are considered the gold standard for evaluating liver metabolism, but are limited due to their loss of in vitro proliferation ability [6, 7]. Organoids represent a promising model system that can bridge the gap between 2D culture and in vivo mice/human models. Here, we endorse the definition of organoids by the hepatic, pancreatic, and biliary (HPB) Organoid Consortium composed of more than 60 experts from 16 countries around the world, defining organoids as a three-dimensional structure derived from (pluripotent) stem cells, progenitor, and/or differentiated cells that self-organize through cell-cell and cell-matrix interactions to recapitulate aspects of the native tissue architecture and function in vitro [8]. Although early reports on the establishment of organoid systems indicated that organoids are exclusively derived from stem cells [9], it is now clear that organoids can also be initiated from differentiated cells, such as cholangiocytes [10]. Organoids were named “Method of the Year 2017” by Nature Methods, reflecting the excitement and promise of this rapidly evolving field that provides new experimental ease of handling, physiologically relevant organ development, models of human pathology, and paves the way for therapeutic applications.
Organoids from (pluripotent) stem cells and progenitor cells have been used to mimic many organs from the endoderm, mesoderm, and ectoderm [5]. Briefly, we provide a non-exhaustive list of organoid systems with dates: endoderm-derived organoids: thyroid [11, 12], lung [13,14,15,16], stomach [17,18,19], liver [20,21,22,23], pancreas [24,25,26,27], small intestine [28, 29] and colon [30, 31]; mesoderm-derived organoids: kidney [32,33,34,35,36,37], bone [38], fallopian tubes [39], and endometrium [40, 41]; and ectoderm-derived organoids: breast [42, 43], retina [44,45,46], brain [47,48,49], inner ear [50] and salivary glands [51].
In this review, we provide a detailed overview of the recent progress in the culture and applications of organoids derived from the liver tissue, as well as pluripotent stem cells (PSC)-derived organoids.
Generation of liver organoids
We divide organoids into distinct groups based on defining characteristics. These include epithelial organoids, multi-tissue organoids and bioengineered liver organoids (Fig. 1) (Table 1).
Tissue-derived liver epithelial organoids
Primary liver cells have now become one of the main sources of liver organoids. As we all know, the discovery and in vitro culture of Lgr5+ stem cell of the intestinal crypts marked the beginning of the organoid research field [28, 52]. In the liver, the existence of a specialised population of stem cells has been controversial. The source of regenerating hepatocytes in the liver has been attributed to different cell populations. By using culture conditions similar to those for intestinal organoids, Huch et al. reported the first cholangiocyte organoids system [20]. Lgr5+ cells can be isolated from both damaged and healthy liver tissue, which can be clonally expanded as organoids in R-spondin 1-based culture medium over multiple months. Such clonal organoids can be induced to differentiate in vitro and to generate functional hepatocytes upon transplantation into FAH−/− mice [20]. Two years later, the first 3D culture of human cholangiocyte organoids was obtained by slightly adjusting the conditions used for murine organoids [21]. Specific inhibition of TGF-β receptors Alk4/5/7 by the small molecule inhibitor A83-01 extended the time in culture, and enhanced colony-forming efficiency. Forskolin addition upregulated Lgr5 and the ductal marker CK19. Thus, Wnt signals, cAMP activation, and TGF-β inhibition were essential for long-term expansion. The cells can readily be converted into functional hepatocytes in vitro and upon transplantation in vivo. Moreover, for the first time, organoids from alpha 1-antitrypsin (A1AT) deficiency and Alagille syndrome patients mirror the in vivo pathology [21].
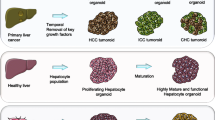
While the cholangiocyte organoids could potentially generate large quantities of hepatocytes in culture, these organoids of biliary origin appear to be recalcitrant in differentiating to hepatocytes in vitro or when engrafted into mice [22]. Over 80% of the mass of the liver is comprised of hepatocytes. When liver is damaged, quiescent hepatocytes re-enter the cell cycle and proliferate to achieve liver regeneration [53]. A study on murine liver regeneration reports that only hepatocytes are involved in liver regeneration after liver damage [54]. Hu et al. further supplemented cytokines that promote hepatocyte cell fate into the cholangiocyte organoid culture media to formulate new culture condition that enabled the expansion of mouse and human hepatocytes in similar 3D culture approach. Analysis showed that hepatocyte organoids had a ‘‘bunch-of-grapes’’ appearance and ALB secretion was only two-four folds lower compared with that of PHHs. Hepatocyte organoids had compact structure and retained key functions and gene expression profiles of hepatocytes compared with cholangiocyte organoids [22]. In 2018, Peng et al. reported that TNFa, an injury-induced inflammatory cytokine, may be implicated in expansion of hepatocyte organoids. Isolated mice hepatocytes were embedded in growth-factor reduced Matrigel with expansion medium containing EGF, HGF and TNFa. At 2 weeks after seeding, organoids were formed. The organoids exhibited stable characteristics, and could serially be passaged for over 6 months [23]. Recently, Liu et al. reported the development of novel culture conditions containing optimized levels of triiodothyronine (T3) and removal of growth factors that enable the successful generation of mature hepatocyte organoids with metabolic features characteristic of adult liver of mouse and human origin [55]. Compared to previous hepatic organoids, transcriptome analysis and a range of different functional assays demonstrated that MHOs exhibited improved metabolic functional features of adult mouse or human liver, including bile acid production, urea production, detoxification, and lipid and glucose metabolism.
PSC-derived liver epithelial organoids
PSCs have infinite proliferation potential and can differentiate into all three embryonic germ layers (endoderm, mesoderm, and ectoderm), which allows them to form well-functioning liver organoids. The ability to produce differentiated functional hepatocytes from hiPSCs presents a major opportunity to directly study mechanisms of human liver disease, to perform high-throughput drug screening for new therapies, and to facilitate hepatocyte transplantation. Currently, a three-step strategy recapitulating ontogenetic liver development is shared by the most commonly employed protocols for hepatocyte differentiation: definitive endoderm (DE) induction, hepatic endoderm (HE), and hepatocyte-like cells (HLCs) [56, 57]. (Fig. 1).
The common liver organoids produced in most studies can be divided into liver epithelial organoids and multi-tissue liver organoids according to their cell origin [8]. Typically, liver epithelial organoids are produced by the expansion of liver intermediate progenitor cells seeded in a medium rich in Matrigel. EpCAM has been identified as a surface marker on human hepatic stem/progenitor cells [58,59,60]. Akbari et al. generated and characterized the hepatic organoids culture system using hiPSC-derived EpCAM+ endodermal cells as an intermediate. The organoids could be produced within 14 days and expanded for more than 1 year without any loss in culture efficiency [61]. Notably, modification of the endoderm induction medium by adding R-spondin1, a well-known Wnt signal potentiator, caused a 15–20% increase in the number of EpCAM+ cells. Moreover, there were no morphological changes among these EpCAM+ cell populations [61]. Thus, the protocol allows for efficient generation of endodermal progenitor populations. hiPSCs can be generated from various tissue sources, including fibroblasts from skin [62, 63] and hematopoietic cells [64,65,66] or lymphocytes [67] from peripheral blood, and have been used in disease modeling. Human iPSC lines derived from peripheral blood T lymphocytes reportedly differentiate into hepatocytes more efficiently than derived from adult dermal fibroblasts [68]. Kulkeaw et al. developed a simple and fast protocol to generate a functioning human liver organoid from PSCs derived from peripheral blood CD34+ cells [69]. The level of ALB synthesis in hepatic endoderm-derived organoids is higher than that in 2D culture, indicating a higher maturity of hepatocytes in liver organoids.
EpCAM+ HE cells can also produce expandable organoids. Wang et al. developed a new method that relies on uniquely defined culture media to make a successful shift from 2D to 3D cultures for generating human embryonic stem cell (hESC)-derived expandable hepatic organoids. The organoids exhibit bipotential traits for lineage restriction into functional hepatocytes and cholangiocytes and also show significant expansion potential for 20 passages [70]. A stepwise differentiation of hESCs into hepatocytes was induced through mimicking embryonic liver development. After 3 days of exposing cells to Activin A with Wnt3a for DE formation, the cells were further treated with BMP4 and FGF2 for another 5 days for their restriction into a hepatic specification lineage under conditions of monolayer cultures. At the end of the induction stage for hepatic stem cells, it was confirmed that most of the cells were positive for the well-known hepatic stem/progenitor cell markers including HNF4A, CK19, EpCAM, SOX9, AFP. After screening the culture conditions for formation of hepatic organoids, they were subjected to treatments with the combination of N2, B27, Nicotinamide, Gastrin, N-Acetylcysteine, EGF, Wnt3a, R-spondin 1, A83-01 and Forskolin. This resulted in the generation of organoids. Because of the Lgr5 independence of HE, R-spondin 1 may be dispensable. EGF and Noggin may interfere with HE during organoid generation by promoting cholangiocyte specification during the fate determination of adult hepatic progenitor cells [71] and exert an inhibitory effect on hepatic specification during hPSC differentiation in vitro [72, 73]. Kim et al. showed that hPSC-derived HE organoid can be efficiently produced without EGF, Noggin, R-spondin 1 and proliferated in the absence of R-spondin 1 and EGF. In differentiated hPSC-derived hepatic organoids, discontinuing these factors can significantly improve liver function, especially the expression and activity of CYP450 [74]. Mun et al. developed a novel hPSC-derived HLCs organoid that is critically advanced in terms of its generation method, functional performance, and applications [75]. In brief, fully characterized and integration-free hiPSCs or hESCs were differentiated in a stepwise fashion into DE-, HE-differentiated cells, immature hepatocytes, and mature hepatocytes. In genomic and functional analyses, the HLCs organoids manifested more mature phenotypes compared with those of 2D differentiated hepatocytes and tissue-derived liver epithelial organoids.
The following studies are concerned with the formation of hepatobiliary organoids (hHBOs). Guan et al. developed an in vitro model system where iPSCs differentiate into 3D hHBOs through stages that resemble human liver during its embryonic development. The organoids consist of hepatocytes, and cholangiocytes, which are organized into epithelia that surround the lumina of bile duct–like structures [76]. In brief, iPSCs were first dissociated into single cells and induced to form endoderm spheres in response to growth factors and chemicals added to the culture according to a modified protocol. The endodermal spheres then formed posterior foregut-like structures when cultured in the presence of a low concentration (1–2%) of a Matrigel scaffold that supports the formation of 3D structures from stem cells, and by addition of various concentrations of FGF10, which is known to promote the differentiation of foregut endoderm into hepatic and gallbladder cells during organogenesis. Organoids were self-renewing and could be matured to perform some hepatic functions, including glycogen storage, liver-specific drug metabolism, as well as albumin and bile secretion. Wu et al. reported a method to generate functional hHBOs from hiPSCs. To achieve this goal, they learned from early hepatogenesis and simultaneously induced endoderm and a small part of mesoderm by the inclusion of 25% mTeSR into hepatic differentiation medium [77]. Ramli et al. developed a protocol for generating hHBOs from hESCs by using developmental cues for gut and hepatoblasts formation [78]. The Organoids contain a functional biliary system that is disrupted by cholestasis inducing drugs such as troglitazone. Shinozawa et al. developed a reproducible liver organoid protocol using stably expandable foregut cells from hPSCs [79]. They reported the high-throughput system to measure bile transport activity by live fluorescent imaging in the presence of testing compounds. And they found the informed sensitivity and specificity of the compounds was comparable to previous studies [80,81,82,83]. Recently, Wang et al. generated hiPSC-derived hHBOs composed of hepatocytes and bile ducts. They demonstrate that hHBOs recapitulate the flow of bile containing fluorescent bile acid analogs or drugs from hepatocytes into bile ducts via bile canaliculi [84]. In addition, hHBOs exhibit pathophysiological responses to troglitazone, such as cholestasis and cytotoxicity. Because hHBOs can recapitulate the function of bile ducts in hepatic bile duct clearance, they are suitable as liver disease models and will be novel in vitro platform systems for drug research use.
Schematic representation of the generation of liver organoids. Epithelial progenitor derived from resected normal liver tissue can be stimulated to form liver organoids. iPSCs/ESCs require a 3-stage differentiation protocol to generate HLCs. Small molecules and cytokines were added to enhance the generation of organoids
Multi-tissue liver organoids
Multi-tissue organoids are established through the co-culture of cells derived from at least two germ layers or the co-differentiation of PSCs. During early liver organogenesis, newly specified hepatic cells delaminate from the foregut endodermal sheet and form a liver bud, a condensed tissue mass that is soon vascularized [85]. Such large-scale morphogenetic changes depend on the exquisite orchestration of signals between endodermal epithelial, mesenchymal and endothelial progenitors before blood perfusion [86]. Based on the above theory, Takebe et al. proposed that three-dimensional liver-bud formation can be recapitulated in vitro by culturing hepatic endoderm cells with endothelial and mesenchymal lineages [87]. They were able to obtain vascularized liver bud organoids that, after transplantation in mice, gave rise to mature hepatocytes. It suggested that the endothelial populations, have important roles in the successful maturation of the derived hepatocytes [88]. Among the paracrine soluble factors known to induce hepatic differentiation is HGF, which is produced by mesenchymal stem cells (MSCs) and human umbilical vein endothelial cells (HUVECs) [89,90,91]. Notably, when MSCs and HUVECs are cultured together, they specifically expressed a protein signature of hypoxic responses, including TGF-β-related factors. TGF-β stimulation of human hepatocytes has been reported to reduce albumin production and to interfere with hepatocyte maturation [92,93,94]. But the above study ignored that Kupffer cells and other non-parenchymal cells also could play a role in liver-bud formation.
Other approaches attempted the generation of liver organoids entirely from iPSCs-derived cells. Ouchi et al. developed a new organoid culture method by co-differentiating epithelial and stromal lineages from PSCs. These multi-cellular human liver organoids coupled with free fatty acid (FFA) treatment recapitulate the progressive, step-wise nature of steatohepatitis-like pathology including steatosis, inflammation and fibrosis, and could potentially be used for drug screening by analysis of organoid stiffness [95]. Kim et al. reported that multilineage liver organoids with luminal blood vessels and bile ducts were generated by assembling hepatic endoderm, hepatic stellate cell-like cells, and endothelial cells derived entirely from hPSCs [96]. Previous studies reported that the presence of mesodermal progenitor [97,98,99] or pericytes [100, 101] is critical for vasculature formation in various organoids. In particular, recent organoid studies have begun to use assembloids that are produced by combining multiple organoids, each resembling different tissues, to obtain a better understanding of the crosstalk between different tissues in pathological conditions [102,103,104,105,106,107]. Therefore, in vitro generation of luminal vascular structures is crucial for producing usable liver organoids from both biological and technical aspects.
Bioengineered liver organoids
Liver bioengineering stands as a prominent alternative to conventional hepatic transplantation. At present, the main methods used are employing different sources of liver cell types (primary cells, immortalized cell lines and liver cells-derived stem cell) to create different bioengineered 3D liver culture system.
Liver-on-a-Chip
The basic materials of liver-on-chips include synthetic polymers and hydrogels. usually require an appropriate flow rate, pH, and temperature. These parameters depend on many auxiliary controls for regulation. Auxiliary controls include biosensors, micropumps, scaffolds, bionic membranes, and electrodes [108]. Liver-on-a-Chip has been successfully applied in liver toxicity research, disease modeling. Liver-related drug metabolism is a key aspect of pharmacokinetics and possible toxicity. Fanizza et al. developed a liver chip based on hiPSCs [109]. It interconnects iPSC-derived liver cells (iHep) and endothelial cells (iEndo) for cultivation to simulate the structure of liver sinuses. It is used for the evaluation of donepezil as a drug for Alzheimer’s disease and liver toxicity screening. Wang et al. proposed a new strategy to establish a human non-alcoholic fatty liver disease (NAFLD) model based on a hiPSC-derived liver organoids-on-a-chip system [110]. The chip can characterize the pathological features of NAFLD in liver organoids by exposure to FFA. After FFA induction, liver lipid droplets and triglycerides form, and the expression of genes related to lipid metabolism is upregulated. Furthermore, other studies showed that microfluidic devices and tissue-derived ECM promoted structural and functional maturation of organoids that recapitulate key features of the liver [111,112,113,114,115,116]. They show that the potential of liver-on-chips as an advanced in vitro model that holds promise for accurately studying in vivo biological processes.
3D bioprinting technology
3D bioprinting provides an effective strategy for replicating the native liver microenvironment at both structural and functional levels, allowing for the recreation of liver lobule microstructures and enhancing the metabolism of HLCs [117]. At present, 3D printing technology is developing rapidly, making it convenient for the production of 3D structures, especially for the research of liver diseases and drug screening [118,119,120,121,122]. Several authors reported the generation of hepatic spheroids and shown sustained and enhanced cellular phenotype, metabolism and prolonged survival in culture in comparison with conventional bidimensional cultured hepatocytes [123,124,125,126]. Goulart et al. successfully bioprinted iPSC-derived liver spheroids using bioink extrusion of an alginate/pluronic blend [127]. This resulted in increased urea synthesis, extended albumin secretion, and elevated gene expression of phase one metabolism enzymes. Sphabmixay et al. reported the first long-term culture of primary hepatocytes at mesoscale in an 3D printed scaffold [128]. Koch et al. pioneered the laser printing of HLCs, underscoring the relevance of suitable hydrogels and sols for cell maintenance and function [129]. Recently, Shrestha et al. developed a unique microarray 3D bioprinting solutions, place the progenitor cells in the bionic hydrogels with sidewalls and plate slit, and is equipped with a transparent at the bottom of the 384 deep hole plate, in order to expand the human liver organoids production [130]. Microarray 3D bioprinting is a drop-based printing technique for generating large quantities of small organoids on pillar plates for predictive hepatotoxicity analysis. Despite great potential, bioprinting hiPSC-derived hepatocytes is still a major challenge due to difficulties in preserving parenchymal epithelial phenotype in the 3D neo-environment post-printing. Given the early stage of this technology in 3D bioprinting, addressing the challenges of vascularization, reproducibility, and scalability requires further model optimization.
Applications of liver organoids
The self-renewing properties of organoids and the possibility to expand iPSCs and cells differentiated from iPSCs make organoids a promising model for basic and translational research. They have been used for the study of regenerative medicine, drug screening, toxicology and disease modeling. Moreover, it is possible to generate biobanks of healthy or diseased human organoids making this technology a significant source for future studies (Fig. 2).
Regenerative medicine
Currently orthotopic liver transplantation (OLT) is the only effective treatment for end-stage hepatic failure [131]. However, organ shortage remains as the major shortcoming for transplantation globally. Because of graft shortages, alternative treatments for OLT have received significant research attention. The expansion and differentiation potential of liver organoids makes these an alternative source of functionally mature and easily expandable cells for transplantation, overcoming the current limitations (Table 2).
The first evidence of the applicability of liver organoids goes back to 2013, when Huch et al. reported that mice Lgr5+ liver stem cell-derived organoids can be transplanted into Fah−/− mutant mice (tyrosinemia type I liver disease models) to explore the therapeutic effect of organoids. The survival of mice has increased 2 months after transplantation [20]. Further, Huch et al. transplanted human EpCAM+ ductal cell-derived organoids into Balb/c nude mice treated with CCl4 to induce acute liver damage. Human ALB and A1AT were found in serum of recipient mice within 7–14 days at a level that remained stable for more than 120 days. Notably, the levels of ALB produced by PHHs transplantation were approximated compared with human organoids transplantation within one month [21]. Hu et al. established PHH-derived organoids which were transplanted into immunodeficient Fah−/− NOD Rag1−/−Il2rg−/− mice by splenic injection. For the first 30 days after transplantation human ALB in mouse circulation remained stable. 90 days after transplantation, serum ALB had risen 200-fold to more than 200 µg/ml on average [22]. Peng et al. recently reported that mice PHH-derived organoids have high engraftment capacity. The mice were sacrificed at 103 days after transplant. Immunohistochemistry and immunofluorescence for FAH showed significant engraftment (up to 80%) in the Fah−/− mutant mice [23]. Takebe et al. showed the generation of vascularized and functional human liver from hiPSCs by transplantation of liver buds created in vitro. Transplanted hiPSC- liver buds began producing albumin at approximately day 10 and produced up to 1,983 ng/ ml by day 45. Notably, transplanted conventional hiPSC-derived organoids produced much less albumin, suggesting the importance of 3D and vascularized tissue formation for successful engraftment and maturation at ectopic sites [87]. These findings showed that liver organoids have potential uses in regenerative medicine (Table 2).
Toxicity test
Drugs or their intermediary metabolites can have a toxic effect on the liver, making drug induced liver injury the leading cause of acute liver failure in the United States alone and the major reason for drug withdrawal from the market [132]. The search of in vitro models that could predict liver primary toxicity has been so far a challenge. PHHS are considered the “gold standard” for drug metabolism and drug toxicity screening, but their availability still depends on the human donor and whether their phenotype and metabolic functions are unstable under long-term culture conditions, which varies from batch to batch. The emergence of liver organoids partially solves the above problems. Shinozawa et al. successfully developed an organoid based assay with multiplexed readouts measuring viability, cholestatic and/or mitochondrial toxicity with high predictive values for 238 marketed drugs at 4 different concentrations (Sensitivity: 88.7%, Specificity: 88.9%). Liver organoid-based toxicity screen positively predicts genomic predisposition (CYP2C9*2) for Bosentan-induced cholestasis [79]. Moreover, “liver-on-a-chip” can represent a platform for drug development and toxicology tests also allowing for pharmacokinetic and pharmacodynamic studies. Wang et al. present a new strategy for engineering liver organoids derived from hiPSCs in a 3D perfusable chip system by combining stem cell biology with microengineering technology. The liver organoids exhibited hepatotoxic response after exposure to acetaminophen in a dose- and time-dependent manner [133]. Recently, Kim et al. described a method of optimizing the cultivation of this method can significantly increase the expression and activity of CYP450s in hepatic organoids, especially the CYP3A4, CYP2C9 and CYP2C19 [134]. In addition, they proposed a simple protocol for the assessment of hepatic organoids cytotoxicity, one of the symbols of drug-induced acute hepatotoxicity. Circadian clocks coordinate the daily rhythmicity of biological processes, and their dysregulation has been implicated in various human diseases. Zhou et al. utilized microfluidics and 3D printing to construct a “Chronotoxici-plate”: a 96-well plate filled with rhythmically synchronized droplet-engineered primary liver organoids [135]. This system enables the assessment of oxaliplatin chrono-therapy toxicity within a week.
Drug screening and personalized therapies
The tumor organoid culture preserves intratumor heterogeneity [136] and the tumor microenvironment [137]. Consequently, organoids may be better suited for drug screening than cancer cell lines; in addition, organoids are significantly cheaper and more efficient than murine tumor models. Organoids have also been applied as the platform to evaluate drug efficacy, opening the doors for precision oncology (Table 2).
Broutier et al. demonstrate primary liver cancer (PLC) -derived organoids are amenable for drug screening using 29 drugs and lead to the identification of the ERK inhibitor SCH772984 as a potential therapeutic agent for PLC, which was validated in xenograft models [138]. Intratumor genetic heterogeneity has been demonstrated in several cancers [139,140,141,142,143]. Li et al. first report intratumor drug response heterogeneity in any solid human cancer. They evaluated the functional heterogeneity in a cohort of PLC organoids. For this purpose, a total of 27 liver cancer organoids were established and tested with 129 cancer drugs, generating 3,483 cell survival data points [144]. Saito et al. successfully screened 22 compounds that were able to significantly suppress intrahepatic cholangiocarcinoma (IHCC) organoids from a group of 339 medicines already in clinical use. Their results indicated that nutlin-3a, an inhibitor of MDM2, could be a potential therapeutic drug for refractory cancers harboring wild-type TP53. Their results also suggested that antifungal drugs such as amorolfine and fenticonazole could be promising therapeutic drugs against IHCC [145]. Inter-and intra-tumor heterogeneity is a major obstacle to the precision treatment of PLC. Recently, Yang et al. generated a PLC biobanking consisting of 399 tumor organoids from 144 patients that recapitalized the histopathology and genomic landscape of parental tumors and could be used for drug susceptibility screening, as demonstrated by both in vivo models and patient responses [146]. This study warrants future clinical studies to accelerate precision medicine for HCC.
Disease model
The possibility of obtaining liver organoids from adult tissues and cell reprogramming through patient-derived cells has led to their application in disease model. Patient-derived organoids preserve an individual’s genetic background, including disease-causing mutations, allowing them to be applied in personalized medicine and drug efficiency studies. In addition, by using gene editing, it is possible to introduce pathological mutations in healthy samples to assess their role in pathogenesis and responsiveness to treatment. All of these disease models are ultimately compatible with the generation of liver organoids, paving the way for several further genetic disease applications. In any case, it is conceivable that certain disease gene mutations may interfere with organoid development. Below we will present advances in liver disease model with organoid culture systems (Table 3).
Monogenic diseases
While the prevalence of most diseases caused by single-gene mutations is low and defined as rare, single-gene diseases account for about 10 per 1,000 births, according to the World Health Organization [147].
Cystic fibrosis (CF) is a genetic disease caused by mutation in the gene encoding the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR). Mutations in the CFTR gene impair cholangiocyte chloride transport, leading to a lack of alkalinisation and subsequent blockage of biliary ducts in the liver [148]. Ogawa et al. showed that functionally impaired hPSC-derived cholangiocytes from cystic fibrosis patients are rescued by CFTR correctors [149]. In cholangiocytes, CFTR is expressed at the apical membrane and responds to hormone stimulation by increasing cAMP intracellular levels resulting in chloride ions efflux in the bile duct lumen. Cholangiocyte organoids generated from iPSCs of patients with CF carrying the most common mutation in CFTR (ΔF508) could also be used to model CF in vitro. Sampaziotis et al. also showed that the experimental CF drug VX809 rescues the disease phenotype of CF cholangiopathy in vitro [150].
A1AT deficiency is caused by mutation in SERPINE1 which lead to the accumulation of misfolded A1AT in hepatocytes, endoplasmic reticulum stress, low circulating levels of A1AT and liver disease. A1AT is mainly produced by the liver and has a crucial role in protecting the lungs from proteolytic damage by regulating the elastase activity of macrophages. Its deficiency results in pulmonary and liver diseases [151]. Functional tests revealed that the differentiated cells from A1AT patients secreted high levels of Albumin and take up LDL similar to that of healthy donor-derived organoid cultures and A1AT protein aggregates were readily observed within the cells of the differentiated organoids, similar to what was found in the original biopsy [21].
Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1 [152]. Andersson et al. demonstrated that expression of a missense mutant of Jag1 disrupts bile duct development and recapitulates Alagille syndrome phenotypes in heart, eye, and craniofacial dysmorphology in mice [153]. Guan et al. demonstrated that the mutations (Cys829X and ALGS2) found in subjects with severe liver disease impaired organoid development, when different JAG1 mutations were engineered onto the same genetic background [76]. The results indicate how this organoid system and genome editing can be jointly used to determine how human disease–causing mutations affect organ development and the pathogenesis of human genetic diseases.
Wilson’s disease (WD) is an inherited, autosomal recessive disorder of copper metabolism, originating from a genetic defect in the copper-transporting ATPase ATP7B, that is required for biliary copper excretion and loading of ceruloplasmin and other cupro-enzymes with copper [154]. The worldwide most common mutation, found in the majority of WD carriers in Europe and USA, is H1069Q. Nantasanti et al. demonstrated that successful gene supplementation in hepatic organoids of COMMD1-deficient dogs restores function and can be an effective means to cure copper storage disease [155]. Kruitwagen et al. provided preclinical proof of concept of the potential of cell transplantation in a large animal model of inherited copper toxicosis, such as Wilson’s disease. This preclinical study confirms the survival of genetically corrected autologous organoid-derived HLCs in vivo and warrants further optimization of organoid engraftment and functional recovery in a large animal model of human liver disease [156].
Citrullinemia type 1 (CTLN1), also known as Arginosuccinate Synthetase Deficiency, is a genetic disease caused by mutations in the enzyme Arginosuccinate synthetase (ASS1) [157]. In patients, ASS1 mutation causes accumulation of ammonia and decreases ureagenesis. The healthy donor-derived organoids had significantly less ammonia compared with CTLN patient organoids while re-expression of wild-type ASS1 in CTLN organoids rescued this defect. These data indicate that hepatic organoids can faithfully recapitulate the urea cycle-related disease phenotype, and restoration of gene function can be carried out in the organoids model [61].
Steatohepatitis
NAFLD has emerged as the most common cause of chronic liver disease [158, 159]. NAFLD encompasses a spectrum of disease ranging from simple steatosis, to NASH, through to the development of cirrhosis and HCC [160,161,162]. Recently, Wang et al. developed a derivative model by incorporating human fetal liver mesenchymal cells into the expandable hepatic organoids, which can model alcoholic liver disease-associated pathophysiologic changes, including oxidative stress generation, steatosis, inflammatory mediators release and fibrosis, under ethanol treatment [70].
Typically, in the literature, the most common FFA used to induce steatosis in vitro are either oleate (18:1) and/or palmitate (16:0) which are used alone or in combination [163,164,165,166]. In vitro models offer the ability to perform investigations at a cellular level, aiding in elucidating the molecular mechanisms of NAFLD. However, a number of current models do not closely resemble the human condition [167]. Ouchi et al. used 11 different healthy and diseased PSC lines to develop a reproducible method to derive multi-cellular human liver organoids composed of hepatocyte-, stellate- and Kupffer-like cells that exhibit transcriptomic resemblance to in vivo derived tissues. Under FFA treatment, organoids, but not reaggregated cocultured spheroids, recapitulated key features of steatohepatitis including steatosis, inflammation and fibrosis phenotypes in a successive manner [95].
Liver infections
Therapies against viral hepatitis have improved in recent decades; however, the development of individualized treatments has been limited by the lack of individualized infection models. Nie et al. demonstrated that HBV infection in hiPSC-liver organoid could recapitulate virus life cycle and virus induced hepatic dysfunction, suggesting that hiPSC-liver organoid may provide a promising individualized infection model for the development of individualized treatment for hepatitis [168]. Baktash et al. have showed differentiated liver organoids retain the innate immune responses and maintain cell polarity of hepatocytes, recapitulating the natural entry of HCV and allowing their cell-to-cell transmission [169]. Furthermore, hPSC-derived cells/organoids provide valuable models for understanding the cellular responses of human tissues to SARS-CoV-2 infection and for disease model of COVID-19 [170].
Liver cancer organoids
PLC is the sixth most commonly diagnosed cancer and the third leading cause of cancer death worldwide in 2020, with approximately 906,000 new cases and 830,000 deaths. PLC includes HCC (comprising 75-85% of cases) and intrahepatic cholangiocarcinoma (10-15%), as well as other rare types [171]. For decades, research into PLCs depended on 2D cell culture systems and transgenic mouse models. While these have proven useful to advance our understanding of the disease, both approaches are markedly limited. Despite their broad utility, they suffer from significant shortcomings such as the lack of 3D growth and the absence of genetic heterogeneity. A better representation of human HCC features could be achieved with the generation of patient-derived xenograft (PDX) models following transplantation of HCC tissue into immunodeficient mice [172]. These models offer great advantages as they preserve the genetic and histologic features of the primary tumor as well as tumor-stroma interactions, making them promising tools for preclinical drug development and evaluation [173]. However, while PDXs show great translational potential to direct treatment in a patient-tailored manner, this strategy has several drawbacks: PDXs are not amenable to large-scale drug screens, are costly and can take a considerable amount of time to establish. The organoid technology could overcome limitations of cancer cell lines and PDX models because it combines the advantages of both systems. Indeed, the generation of patient-derived cancer organoids has been a major breakthrough in cancer biology.
Tumor organoids can be grown with high efficiency from patient-derived tumor tissues, potentially enabling patient-specific drug testing and the development of individualized treatment regimens [174]. Broutier et al. demonstrated that PLC-derived organoids are amenable for biomarker identification and drug screening testing and lead to the identification of the ERK inhibitor SCH772984 as a potential therapeutic agent for PLC [138]. In a parallel study, Nuciforo et al. showed that organoid models can be derived from needle biopsies of liver cancers and provide a tool for developing tailored therapies [175]. Similarly, Cao et al. successfully used the generated organoids to assess anticancer drug responses, showing again that liver cancer organoids recapitulate the heterogeneous therapeutic responses that are observed in patients [176]. PDO liver tumoroids are also widely used to characterize the molecular mechanisms leading to tumor progression. For instance, Chan et al. demonstrated that silencing of protein methyltransferase 6 (PRMT6) induced cancer stemness in HCC-derived PDOs. Further analysis suggested that PRMT6 functions via CRAF-ERK signaling [177]. In a follow-up study, Wong et al. indicated that the PRMT6-ERK-PKM2 regulatory axis is an important determinant of the Warburg effect in tumor cells, and provide a mechanistic link among tumorigenicity, sorafenib resistance, and glucose metabolism [178]. Zhao et al. generated seven hepatobiliary tumor organoids to explore heterogeneity and evolution via single-cell RNA sequencing. The delineates heterogeneity of hepatobiliary tumor organoids and proposes that the collaboration of intratumoral heterogenic subpopulations renders malignant phenotypes and drug resistance [179].
To model cancer, organoids derived from healthy iPSCs or normal tissues can be used, and cancer gene mutations can be induced by CRISPR/Cas9 system. In particular, a recent study has demonstrated that the combination of BAP1 loss-of-function mutation and cholangiocarcinoma mutations (TP53, PTEN, SMAD4, and NF1), induced by CRISPR/Cas9 in normal liver organoids, can affect epithelial tissue organization and cell-to-cell junctions, resulting in the acquisition of malignant features [180]. Therefore, organoid technology combined with CRISPR/Cas9 provides an experimental platform for mechanistic studies of cancer gene function in a human context.
Conclusions
Liver organoid models are increasingly being incorporated into biomedical research due to their many advantages. While significant progress has been made in this area, some limitations remain. First, the differentiation protocol needs to be improved to achieve higher cell maturity and correct representation of hepatocyte types, thus increasing the complexity level to more accurately mimic pathophysiological processes. Second, develop new bioengineering methods to ensure the reproducibility of liver organoid composition and function. This goal is fundamental to advancing the liver organoid drug discovery and preclinical testing pipeline. Finally develop improved synthetic biomaterials to obtain animal-free culture systems that can be safely applied in regenerative medicine in the future. One limitation is that classical organoids are produced by primary tissue biopsies, so the main proliferating cell type is tissue-derived epithelial cells. Tissue-derived organoids are simpler in structure and consist primarily of epithelial cells. However, these organoids rely heavily on access to target tissues, which are often scarce and therefore an unreliable source of large-scale organoid research. Although hepatocytes are the main building blocks of the liver, it is important not to forget the other cells that contribute to the complex structure of the liver.
Data availability
This review article involves no experimental data. References are listed in the end of this article.
References
Malarkey DE, Johnson K, Ryan L, et al. New insights into functional aspects of liver morphology. Toxicol Pathol. 2005;33(1):27–34.
Gerets HH, Tilmant K, Gerin B, et al. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol Toxicol. 2012;28(2):69–87.
Akbari S, Arslan N, Senturk S, et al. Next-Generation Liver Medicine using Organoid models. Front Cell Dev Biol. 2019;7:345.
Koo BK, Huch M, Organoids. A new in vitro model system for biomedical science and disease modelling and promising source for cell-based transplantation. Dev Biol. 2016;420(2):197–98.
Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research. Nat Rev Genet. 2018;19(11):671–87.
Xiang C, Du Y, Meng G, et al. Long-term functional maintenance of primary human hepatocytes in vitro. Science. 2019;364(6438):399–402.
Xia Y, Carpentier A, Cheng X, et al. Human stem cell-derived hepatocytes as a model for hepatitis B virus infection, spreading and virus-host interactions. J Hepatol. 2017;66(3):494–503.
Marsee A, Roos FJM, Verstegen MMA, et al. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell. 2021;28(5):816–32.
Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125.
Aloia L, McKie MA, Vernaz G, et al. Epigenetic remodelling licences adult cholangiocytes for organoid formation and liver regeneration. Nat Cell Biol. 2019;21(11):1321–33.
Antonica F, Kasprzyk DF, Opitz R, et al. Generation of functional thyroid from embryonic stem cells. Nature. 2012;491(7422):66–71.
Kurmann AA, Serra M, Hawkins F, et al. Regeneration of thyroid function by transplantation of differentiated pluripotent stem cells. Cell Stem Cell. 2015;17(5):527–42.
Lee JH, Bhang DH, Beede A, et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156(3):440–55.
Nikolić MZ, Caritg O, Jeng Q et al. Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. Elife 2017;6.
Sachs N, Papaspyropoulos A, Zomer-van Ommen DD et al. Long-term expanding human airway organoids for disease modeling. Embo j 2019;38(4).
Miller AJ, Dye BR, Ferrer-Torres D, et al. Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc. 2019;14(2):518–40.
Barker N, Huch M, Kujala P, et al. Lgr5(+ ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6(1):25–36.
Bartfeld S, Bayram T, van de Wetering M, et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 2015;148(1):126–e366.
Stange DE, Koo BK, Huch M, et al. Differentiated Troy + chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155(2):357–68.
Huch M, Dorrell C, Boj SF, et al. In vitro expansion of single Lgr5 + liver stem cells induced by wnt-driven regeneration. Nature. 2013;494(7436):247–50.
Huch M, Gehart H, van Boxtel R, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160(1–2):299–312.
Hu H, Gehart H, Artegiani B et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell. 2018;175(6):1591 – 606.e19.
Peng WC, Logan CY, Fish M et al. Inflammatory Cytokine TNFα Promotes the Long-Term Expansion of Primary Hepatocytes in 3D Culture. Cell. 2018;175(6):1607-19.e15.
Huch M, Bonfanti P, Boj SF, et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. Embo j. 2013;32(20):2708–21.
Greggio C, De Franceschi F, Figueiredo-Larsen M, et al. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development. 2013;140(21):4452–62.
Boj SF, Hwang CI, Baker LA, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160(1–2):324–38.
Loomans CJM, Williams Giuliani N, Balak J, et al. Expansion of Adult Human pancreatic tissue yields Organoids harboring progenitor cells with endocrine differentiation potential. Stem Cell Rep. 2018;10(3):712–24.
Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–5.
Ootani A, Li X, Sangiorgi E, et al. Sustained in vitro intestinal epithelial culture within a wnt-dependent stem cell niche. Nat Med. 2009;15(6):701–6.
Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141(5):1762–72.
Jung P, Sato T, Merlos-Suárez A, et al. Isolation and in vitro expansion of human colonic stem cells. Nat Med. 2011;17(10):1225–7.
Takasato M, Er PX, Chiu HS, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526(7574):564–8.
Freedman BS, Brooks CR, Lam AQ, et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun. 2015;6:8715.
Xia Y, Sancho-Martinez I, Nivet E, et al. The generation of kidney organoids by differentiation of human pluripotent cells to ureteric bud progenitor-like cells. Nat Protoc. 2014;9(11):2693–704.
Morizane R, Lam AQ, Freedman BS, et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol. 2015;33(11):1193–200.
Schutgens F, Rookmaaker MB, Margaritis T, et al. Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat Biotechnol. 2019;37(3):303–13.
Gijzen L, Yousef Yengej FA, Schutgens F, et al. Culture and analysis of kidney tubuloids and perfused tubuloid cells-on-a-chip. Nat Protoc. 2021;16(4):2023–50.
Kale S, Biermann S, Edwards C, et al. Three-dimensional cellular development is essential for ex vivo formation of human bone. Nat Biotechnol. 2000;18(9):954–8.
Kessler M, Hoffmann K, Brinkmann V, et al. The notch and wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun. 2015;6:8989.
Turco MY, Gardner L, Hughes J, et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 2017;19(5):568–77.
Boretto M, Cox B, Noben M, et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development. 2017;144(10):1775–86.
Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17(10):1253–70.
Linnemann JR, Miura H, Meixner LK, et al. Quantification of regenerative potential in primary human mammary epithelial cells. Development. 2015;142(18):3239–51.
Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51–6.
Nakano T, Ando S, Takata N, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10(6):771–85.
Wahlin KJ, Maruotti JA, Sripathi SR, et al. Photoreceptor outer segment-like structures in long-term 3D retinas from human pluripotent stem cells. Sci Rep. 2017;7(1):766.
Lancaster MA, Renner M, Martin CA, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373–9.
Birey F, Andersen J, Makinson CD, et al. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545(7652):54–9.
Bagley JA, Reumann D, Bian S, et al. Fused cerebral organoids model interactions between brain regions. Nat Methods. 2017;14(7):743–51.
Koehler KR, Nie J, Longworth-Mills E, et al. Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat Biotechnol. 2017;35(6):583–89.
Pringle S, Maimets M, van der Zwaag M, et al. Human salivary gland stem cells functionally restore Radiation damaged salivary glands. Stem Cells. 2016;34(3):640–52.
Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–7.
Wu HH, Lee OK. Exosomes from mesenchymal stem cells induce the conversion of hepatocytes into progenitor oval cells. Stem Cell Res Ther. 2017;8(1):117.
Grompe M. Liver stem cells, where art thou? Cell Stem Cell. 2014;15(3):257–58.
Liu Y, Zhou Y, Ahodantin J et al. Generation and characterization of mature hepatocyte organoids for liver metabolic studies. J Cell Sci 2024;137(10).
Palakkan AA, Nanda J, Ross JA. Pluripotent stem cells to hepatocytes, the journey so far. Biomed Rep. 2017;6(4):367–73.
Calabrese D, Roma G, Bergling S, et al. Liver biopsy derived induced pluripotent stem cells provide unlimited supply for the generation of hepatocyte-like cells. PLoS ONE. 2019;14(8):e0221762.
Dollé L, Theise ND, Schmelzer E, et al. EpCAM and the biology of hepatic stem/progenitor cells. Am J Physiol Gastrointest Liver Physiol. 2015;308(4):G233–50.
Schmelzer E, Wauthier E, Reid LM. The phenotypes of pluripotent human hepatic progenitors. Stem Cells. 2006;24(8):1852–8.
Yoon SM, Gerasimidou D, Kuwahara R, et al. Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology. 2011;53(3):964–73.
Akbari S, Sevinç GG, Ersoy N, et al. Robust, long-term culture of endoderm-derived hepatic organoids for Disease modeling. Stem Cell Rep. 2019;13(4):627–41.
Alawad A, Alhazzaa O, Altuwaijri S, et al. Generation of human iPS cell line SKiPSc1 from healthy human neonatal foreskin fibroblast cells. Stem Cell Res. 2016;17(1):158–60.
Du SH, Tay JC, Chen C, et al. Human iPS cell-derived fibroblast-like cells as feeder layers for iPS cell derivation and expansion. J Biosci Bioeng. 2015;120(2):210–7.
Haase A, Göhring G, Martin U. Generation of non-transgenic iPS cells from human cord blood CD34(+) cells under animal component-free conditions. Stem Cell Res. 2017;21:71–3.
Takenaka C, Nishishita N, Takada N, et al. Effective generation of iPS cells from CD34 + cord blood cells by inhibition of p53. Exp Hematol. 2010;38(2):154–62.
Kim EM, Manzar G, Zavazava N. Human iPS cell-derived hematopoietic progenitor cells induce T-cell anergy in in vitro-generated alloreactive CD8(+) T cells. Blood. 2013;121(26):5167–75.
Phillips MJ, Wallace KA, Dickerson SJ, et al. Blood-derived human iPS cells generate optic vesicle-like structures with the capacity to form retinal laminae and develop synapses. Invest Ophthalmol Vis Sci. 2012;53(4):2007–19.
Kajiwara M, Aoi T, Okita K, et al. Donor-dependent variations in hepatic differentiation from human-induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109(31):12538–43.
Kulkeaw K, Tubsuwan A, Tongkrajang N, et al. Generation of human liver organoids from pluripotent stem cell-derived hepatic endoderms. PeerJ. 2020;8:e9968.
Wang S, Wang X, Tan Z, et al. Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Res. 2019;29(12):1009–26.
Kitade M, Factor VM, Andersen JB, et al. Specific fate decisions in adult hepatic progenitor cells driven by MET and EGFR signaling. Genes Dev. 2013;27(15):1706–17.
Duncan SA, Watt AJ. BMPs on the road to hepatogenesis. Genes Dev. 2001;15(15):1879–84.
Mfopou JK, Chen B, Mateizel I, et al. Noggin, retinoids, and fibroblast growth factor regulate hepatic or pancreatic fate of human embryonic stem cells. Gastroenterology. 2010;138(7):2233–45. 45.e1-14.
Kim H, Im I, Jeon JS, et al. Development of human pluripotent stem cell-derived hepatic organoids as an alternative model for drug safety assessment. Biomaterials. 2022;286:121575.
Mun SJ, Ryu JS, Lee MO, et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. 2019;71(5):970–85.
Guan Y, Xu D, Garfin PM et al. Human hepatic organoids for the analysis of human genetic diseases. JCI Insight 2017;2(17).
Wu F, Wu D, Ren Y, et al. Generation of hepatobiliary organoids from human induced pluripotent stem cells. J Hepatol. 2019;70(6):1145–58.
Ramli MNB, Lim YS, Koe CT, et al. Human pluripotent stem cell-derived Organoids as models of Liver Disease. Gastroenterology. 2020;159(4):1471–e8612.
Shinozawa T, Kimura M, Cai Y, et al. High-Fidelity Drug-Induced Liver Injury screen using human pluripotent stem cell-derived Organoids. Gastroenterology. 2021;160(3):831–e4610.
O’Brien PJ, Irwin W, Diaz D, et al. High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch Toxicol. 2006;80(9):580–604.
Vorrink SU, Zhou Y, Ingelman-Sundberg M, et al. Prediction of Drug-Induced Hepatotoxicity using long-term stable primary hepatic 3D spheroid cultures in chemically defined conditions. Toxicol Sci. 2018;163(2):655–65.
Proctor WR, Foster AJ, Vogt J, et al. Utility of spherical human liver microtissues for prediction of clinical drug-induced liver injury. Arch Toxicol. 2017;91(8):2849–63.
Xu JJ, Henstock PV, Dunn MC, et al. Cellular imaging predictions of clinical drug-induced liver injury. Toxicol Sci. 2008;105(1):97–105.
Wang L, Koui Y, Kanegae K, et al. Establishment of human induced pluripotent stem cell-derived hepatobiliary organoid with bile duct for pharmaceutical research use. Biomaterials. 2024;310:122621.
Zhao R, Duncan SA. Embryonic development of the liver. Hepatology. 2005;41(5):956–67.
Matsumoto K, Yoshitomi H, Rossant J, et al. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294(5542):559–63.
Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499(7459):481–4.
Takebe T, Zhang RR, Koike H, et al. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc. 2014;9(2):396–409.
Ehashi T, Koyama T, Ookawa K, et al. Effects of oncostatin M on secretion of vascular endothelial growth factor and reconstruction of liver-like structure by fetal liver cells in monolayer and three-dimensional cultures. J Biomed Mater Res A. 2007;82(1):73–9.
Kamiya A, Kinoshita T, Miyajima A. Oncostatin M and hepatocyte growth factor induce hepatic maturation via distinct signaling pathways. FEBS Lett. 2001;492(1–2):90–4.
Kamiya A, Kojima N, Kinoshita T, et al. Maturation of fetal hepatocytes in vitro by extracellular matrices and oncostatin M: induction of tryptophan oxygenase. Hepatology. 2002;35(6):1351–9.
Busso N, Chesne C, Delers F, et al. Transforming growth-factor-beta (TGF-beta) inhibits albumin synthesis in normal human hepatocytes and in hepatoma HepG2 cells. Biochem Biophys Res Commun. 1990;171(2):647–54.
Clotman F, Lemaigre FP. Control of hepatic differentiation by activin/TGFbeta signaling. Cell Cycle. 2006;5(2):168–71.
Touboul T, Chen S, To CC, et al. Stage-specific regulation of the WNT/β-catenin pathway enhances differentiation of hESCs into hepatocytes. J Hepatol. 2016;64(6):1315–26.
Ouchi R, Togo S, Kimura M, et al. Modeling steatohepatitis in humans with pluripotent stem cell-derived Organoids. Cell Metab. 2019;30(2):374–. – 84.e6.
Kim HJ, Kim G, Chi KY, et al. Generation of multilineage liver organoids with luminal vasculature and bile ducts from human pluripotent stem cells via modulation of notch signaling. Stem Cell Res Ther. 2023;14(1):19.
Wörsdörfer P, Dalda N, Kern A, et al. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci Rep. 2019;9(1):15663.
Wörsdörfer P, Rockel A, Alt Y, et al. Generation of vascularized neural organoids by co-culturing with mesodermal progenitor cells. STAR Protoc. 2020;1(1):100041.
Dogan L, Scheuring R, Wagner N et al. Human iPSC-derived mesodermal progenitor cells preserve their vasculogenesis potential after extrusion and form hierarchically organized blood vessels. Biofabrication 2021;13(4).
Wimmer RA, Leopoldi A, Aichinger M, et al. Generation of blood vessel organoids from human pluripotent stem cells. Nat Protoc. 2019;14(11):3082–100.
Wimmer RA, Leopoldi A, Aichinger M, et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature. 2019;565(7740):505–10.
Hofer M, Lutolf MP. Engineering organoids. Nat Rev Mater. 2021;6(5):402–20.
Yin F, Zhang X, Wang L, et al. HiPSC-derived multi-organoids-on-chip system for safety assessment of antidepressant drugs. Lab Chip. 2021;21(3):571–81.
Tao T, Deng P, Wang Y, et al. Microengineered Multi-organoid System from hiPSCs to recapitulate Human Liver-Islet Axis in Normal and Type 2 diabetes. Adv Sci (Weinh). 2022;9(5):e2103495.
Kothari A, Rajagopalan P. The assembly of integrated rat intestinal-hepatocyte cultures. Bioeng Transl Med. 2020;5(1):e10146.
Skardal A, Devarasetty M, Rodman C, et al. Liver-tumor hybrid organoids for modeling Tumor Growth and Drug Response in Vitro. Ann Biomed Eng. 2015;43(10):2361–73.
Devarasetty M, Wang E, Soker S, et al. Mesenchymal stem cells support growth and organization of host-liver colorectal-tumor organoids and possibly resistance to chemotherapy. Biofabrication. 2017;9(2):021002.
Liu J, Du Y, Xiao X, et al. Construction of in vitro liver-on-a-chip models and application progress. Biomed Eng Online. 2024;23(1):33.
Fanizza F, Boeri L, Donnaloja F, et al. Development of an Induced Pluripotent Stem Cell-based liver-on-a-Chip assessed with an Alzheimer’s Disease Drug. ACS Biomater Sci Eng. 2023;9(7):4415–30.
Wang Y, Wang H, Deng P, et al. Modeling human nonalcoholic fatty liver Disease (NAFLD) with an Organoids-on-a-Chip system. ACS Biomater Sci Eng. 2020;6(10):5734–43.
Vyas D, Baptista PM, Brovold M, et al. Self-assembled liver organoids recapitulate hepatobiliary organogenesis in vitro. Hepatology. 2018;67(2):750–61.
Kim SK, Kim YH, Park S, et al. Organoid engineering with microfluidics and biomaterials for liver, lung disease, and cancer modeling. Acta Biomater. 2021;132:37–51.
Lee JS, Shin J, Park HM, et al. Liver extracellular matrix providing dual functions of two-dimensional substrate coating and three-dimensional injectable hydrogel platform for liver tissue engineering. Biomacromolecules. 2014;15(1):206–18.
Baptista PM, Siddiqui MM, Lozier G, et al. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53(2):604–17.
Baptista PM, Moran EC, Vyas D, et al. Fluid Flow Regulation of Revascularization and Cellular Organization in a Bioengineered Liver platform. Tissue Eng Part C Methods. 2016;22(3):199–207.
Bhushan A, Senutovitch N, Bale SS, et al. Towards a three-dimensional microfluidic liver platform for predicting drug efficacy and toxicity in humans. Stem Cell Res Ther. 2013;4(Suppl 1):S16.
Agarwal T, Banerjee D, Konwarh R, et al. Recent advances in bioprinting technologies for engineering hepatic tissue. Mater Sci Eng C Mater Biol Appl. 2021;123:112013.
Norona LM, Nguyen DG, Gerber DA, et al. editors. ‘s Highlight: Modeling Compound-Induced Fibrogenesis In Vitro Using Three-Dimensional Bioprinted Human Liver Tissues. Toxicol Sci 2016;154(2):354 – 67.
Nguyen DG, Funk J, Robbins JB, et al. Bioprinted 3D primary liver tissues allow Assessment of Organ-Level response to Clinical Drug Induced Toxicity in Vitro. PLoS ONE. 2016;11(7):e0158674.
Bhise NS, Manoharan V, Massa S, et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication. 2016;8(1):014101.
Moya A, Ortega-Ribera M, Guimerà X, et al. Online oxygen monitoring using integrated inkjet-printed sensors in a liver-on-a-chip system. Lab Chip. 2018;18(14):2023–35.
Grix T, Ruppelt A, Thomas A et al. Bioprinting perfusion-enabled liver equivalents for Advanced Organ-on-a-Chip applications. Genes (Basel) 2018;9(4).
Faulkner-Jones A, Fyfe C, Cornelissen DJ, et al. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication. 2015;7(4):044102.
Pettinato G, Ramanathan R, Fisher RA, et al. Scalable differentiation of human iPSCs in a multicellular spheroid-based 3D culture into hepatocyte-like cells through direct Wnt/β-catenin pathway inhibition. Sci Rep. 2016;6:32888.
Meier F, Freyer N, Brzeszczynska J, et al. Hepatic differentiation of human iPSCs in different 3D models: a comparative study. Int J Mol Med. 2017;40(6):1759–71.
Kamei KI, Yoshioka M, Terada S, et al. Three-dimensional cultured liver-on-a-Chip with mature hepatocyte-like cells derived from human pluripotent stem cells. Biomed Microdevices. 2019;21(3):73.
Goulart E, de Caires-Junior LC, Telles-Silva KA, et al. 3D bioprinting of liver spheroids derived from human induced pluripotent stem cells sustain liver function and viability in vitro. Biofabrication. 2019;12(1):015010.
Sphabmixay P, Raredon MSB, Wang AJ et al. High resolution stereolithography fabrication of perfusable scaffolds to enable long-term meso-scale hepatic culture for disease modeling. Biofabrication 2021;13(4).
Koch L, Deiwick A, Franke A, et al. Laser bioprinting of human induced pluripotent stem cells-the effect of printing and biomaterials on cell survival, pluripotency, and differentiation. Biofabrication. 2018;10(3):035005.
Shrestha S, Lekkala VKR, Acharya P, et al. Reproducible generation of human liver organoids (HLOs) on a pillar plate platform via microarray 3D bioprinting. Lab Chip. 2024;24(10):2747–61.
Furuta T, Furuya K, Zheng YW, et al. Novel alternative transplantation therapy for orthotopic liver transplantation in liver failure: a systematic review. World J Transpl. 2020;10(3):64–78.
Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005;4(6):489–99.
Wang Y, Wang H, Deng P, et al. In situ differentiation and generation of functional liver organoids from human iPSCs in a 3D perfusable chip system. Lab Chip. 2018;18(23):3606–16.
Kim H, Kim SK, Oelgeschläger M, et al. Prediction of Acute Hepatotoxicity with Human pluripotent stem cell-derived hepatic organoids. Curr Protoc. 2024;4(4):e1015.
Zhou J, Huang YC, Wang W et al. Chronotoxici-plate containing Droplet-Engineered Rhythmic Liver Organoids for Drug Toxicity evaluation. Adv Sci (Weinh) 2024:e2305925.
Bu L, Baba H, Yoshida N, et al. Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment. Oncogene. 2019;38(25):4887–901.
Yuan Y, Jiang YC, Sun CK, et al. Role of the tumor microenvironment in tumor progression and the clinical applications (review). Oncol Rep. 2016;35(5):2499–515.
Broutier L, Mastrogiovanni G, Verstegen MM, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23(12):1424–35.
Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–92.
Kalasekar SM, VanSant-Webb CH, Evason KJ. Intratumor Heterogeneity in Hepatocellular Carcinoma: challenges and opportunities. Cancers (Basel) 2021;13(21).
Gao Q, Wang ZC, Duan M, et al. Cell Culture System for Analysis of Genetic Heterogeneity within Hepatocellular Carcinomas and Response to Pharmacologic agents. Gastroenterology. 2017;152(1):232–e424.
Xue R, Li R, Guo H, et al. Variable Intra-tumor genomic heterogeneity of multiple lesions in patients with Hepatocellular Carcinoma. Gastroenterology. 2016;150(4):998–1008.
Friemel J, Rechsteiner M, Frick L, et al. Intratumor heterogeneity in hepatocellular carcinoma. Clin Cancer Res. 2015;21(8):1951–61.
Li L, Knutsdottir H, Hui K et al. Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity. JCI Insight 2019;4(2).
Saito Y, Muramatsu T, Kanai Y, et al. Establishment of patient-derived organoids and drug screening for biliary tract carcinoma. Cell Rep. 2019;27(4):1265–e764.
Yang H, Cheng J, Zhuang H, et al. Pharmacogenomic profiling of intra-tumor heterogeneity using a large organoid biobank of liver cancer. Cancer Cell. 2024;42(4):535–e518.
Fagiuoli S, Daina E, D’Antiga L, et al. Monogenic diseases that can be cured by liver transplantation. J Hepatol. 2013;59(3):595–612.
Kobelska-Dubiel N, Klincewicz B, Cichy W. Liver disease in cystic fibrosis. Prz Gastroenterol. 2014;9(3):136–41.
Ogawa M, Ogawa S, Bear CE, et al. Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat Biotechnol. 2015;33(8):853–61.
Sampaziotis F, de Brito MC, Madrigal P, et al. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat Biotechnol. 2015;33(8):845–52.
Greene CM, Marciniak SJ, Teckman J, et al. α1-Antitrypsin deficiency. Nat Rev Dis Primers. 2016;2:16051.
Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16(3):243–51.
Andersson ER, Chivukula IV, Hankeova S, et al. Mouse model of Alagille Syndrome and mechanisms of Jagged1 missense mutations. Gastroenterology. 2018;154(4):1080–95.
Scheiber IF, Brůha R, Dušek P. Pathogenesis of Wilson disease. Handb Clin Neurol. 2017;142:43–55.
Nantasanti S, Spee B, Kruitwagen HS, et al. Disease modeling and Gene Therapy of Copper Storage Disease in Canine hepatic organoids. Stem Cell Rep. 2015;5(5):895–907.
Kruitwagen HS, Oosterhoff LA, van Wolferen ME et al. Long-term survival of transplanted autologous canine liver organoids in a COMMD1-Deficient dog model of metabolic liver disease. Cells 2020;9(2).
Nuciforo S, Heim MH. Organoids to model liver disease. JHEP Rep. 2021;3(1):100198.
Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84.
EASL-EASD-EASO. Clinical practice guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59(6):1121–40.
Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–73.
Adams LA, Sanderson S, Lindor KD, et al. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42(1):132–8.
Angulo P. Long-term mortality in nonalcoholic fatty liver disease: is liver histology of any prognostic significance? Hepatology. 2010;51(2):373–5.
Kostrzewski T, Cornforth T, Snow SA, et al. Three-dimensional perfused human in vitro model of non-alcoholic fatty liver disease. World J Gastroenterol. 2017;23(2):204–15.
Green CJ, Johnson D, Amin HD, et al. Characterization of lipid metabolism in a novel immortalized human hepatocyte cell line. Am J Physiol Endocrinol Metab. 2015;309(6):E511–22.
Gómez-Lechón MJ, Donato MT, Martínez-Romero A, et al. A human hepatocellular in vitro model to investigate steatosis. Chem Biol Interact. 2007;165(2):106–16.
Dave T, Tilles AW, Vemula M. A cell-based assay to Investigate Hypolipidemic effects of nonalcoholic fatty liver disease therapeutics. SLAS Discov. 2018;23(3):274–82.
Green CJ, Parry SA, Gunn PJ et al. Studying non-alcoholic fatty liver disease: the ins and outs of in vivo, ex vivo and in vitro human models. Horm Mol Biol Clin Investig 2018;41(1).
Nie YZ, Zheng YW, Miyakawa K, et al. Recapitulation of hepatitis B virus-host interactions in liver organoids from human induced pluripotent stem cells. EBioMedicine. 2018;35:114–23.
Baktash Y, Madhav A, Coller KE, et al. Single particle imaging of polarized Hepatoma Organoids upon Hepatitis C virus infection reveals an ordered and sequential entry process. Cell Host Microbe. 2018;23(3):382–e945.
Yang L, Han Y, Nilsson-Payant BE, et al. A human pluripotent stem cell-based platform to Study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27(1):125–e367.
Sung H, Ferlay J, Siegel RL, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
He S, Hu B, Li C, et al. PDXliver: a database of liver cancer patient derived xenograft mouse models. BMC Cancer. 2018;18(1):550.
Hidalgo M, Amant F, Biankin AV, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013.
Drost J, Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018;18(7):407–18.
Nuciforo S, Fofana I, Matter MS, et al. Organoid models of Human Liver cancers derived from Tumor needle biopsies. Cell Rep. 2018;24(5):1363–76.
Cao W, Liu J, Wang L, et al. Modeling liver cancer and therapy responsiveness using organoids derived from primary mouse liver tumors. Carcinogenesis. 2019;40(1):145–54.
Chan LH, Zhou L, Ng KY, et al. PRMT6 regulates RAS/RAF binding and MEK/ERK-Mediated Cancer Stemness activities in Hepatocellular Carcinoma through CRAF methylation. Cell Rep. 2018;25(3):690–e7018.
Wong TL, Ng KY, Tan KV, et al. CRAF methylation by PRMT6 regulates aerobic glycolysis-driven Hepatocarcinogenesis via ERK-Dependent PKM2 Nuclear relocalization and activation. Hepatology. 2020;71(4):1279–96.
Zhao Y, Li ZX, Zhu YJ, et al. Single-cell transcriptome analysis uncovers Intratumoral Heterogeneity and underlying mechanisms for Drug Resistance in Hepatobiliary Tumor Organoids. Adv Sci (Weinh). 2021;8(11):e2003897.
Artegiani B, van Voorthuijsen L, Lindeboom RGH, et al. Probing the tumor suppressor function of BAP1 in CRISPR-Engineered Human Liver Organoids. Cell Stem Cell. 2019;24(6):927–e436.
Acknowledgements
This study was supported by CAMS Innovation Fund for Medical Sciences (2019-I2M-5-020), Tianjin Key Training Project for ‘Project + Team’ (XC202030) and Research on Cell product Development and Clinical Application of Tianjin Science and Technology Plan Project (22ZYJDSY00150). We express gratitude to the funding sponsors.
Funding
This study was supported by CAMS Innovation Fund for Medical Sciences (2019-I2M-5-020), Tianjin Key Training Project for ‘Project + Team’ (XC202030) and Research on Cell product Development and Clinical Application of Tianjin Science and Technology Plan Project (22ZYJDSY00150).
Author information
Authors and Affiliations
Contributions
Sen Liu wrote the manuscript and Liuyang Zhu, Tianyu Zhao, Ze Wang, Xiulin Yi, Fengying Yan contributed to the literature search. Chuanliang Cheng drew the picture involved in this manuscript. Xiaoliang Wang, Baofeng Yang, Tao Cui and Chunli Li revised the manuscript. All of the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This paper does not involve animal experiments or human participants.
Consent for publication
The authors consent to publish the paper.
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, S., Cheng, C., Zhu, L. et al. Liver organoids: updates on generation strategies and biomedical applications. Stem Cell Res Ther 15, 244 (2024). https://doi.org/10.1186/s13287-024-03865-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-024-03865-3