Abstract
Androgenetic alopecia (AGA) is the most prevalent type of hair loss. Its morbility is mainly psychological although an increased incidence in melanoma has also been observed in affected subjects. Current drug based therapies and physical treatments are either unsuccessful in the long term or have relevant side effects that limit their application. Therefore, a new therapeutic approach is needed to promote regenerative enhancement alternatives. These treatment options, focused on the cellular niche restoration, could be the solution to the impact of dihydrotestosterone in the hair follicle microenvironment. In this context emerging regenerative therapies such as Platelet-rich plasma or Platelet-rich fibrine as well as hair follicle stem cells and mesenchymal stem cell based therapies and their derivatives (conditioned medium CM or exoxomes) are highlighting in the evolving landscape of hair restoration. Nanotechnology is also leading the way in AGA treatment through the design of bioinks and nanobiomaterials whose structures are being configuring in a huge range of cases by means of 3D bioprinting. Due to the increasing number and the rapid creation of new advanced therapies alternatives in the AGA field, an extended review of the current state of art is needed. In addition this review provides a general insight in current and emerging AGA therapies which is intented to be a guidance for researchers highlighting the cutting edge treatments which are recently gaining ground.
Similar content being viewed by others
Introduction
AGA is a dynamic and progressive hair loss disorder which affects men and women around the world. The incidence of AGA increases with age, affecting 80% of Caucasian men population and 30–50% bellow the age of 50 years old [1, 2]. A similar prevalence is observed in elderly women [3]. Although AGA is often considered a minor dermatological condition, hair loss has a huge impact on self-esteem and quality of life, hence its frequent association with anxiety and depression [4].
The etiology of AGA entails an intricate interplay among different genetic and hormonal factors, resulting in the miniaturization of hair follicles and alterations in the dynamics of the hair growth cycle, specifically the shortening of the anagen phase. AGA hormonal etiology is caused by DHT, an androgen derived from testosterone by 5-alpha reductase enzyme action. This hormone has a higher affinity towards androgen receptors (ARs) in the hair follicles. In fact, individuals with AGA have an overexpressed AR gene compared to controls [5, 6]. This situation leads to follicle miniaturisation after the expression of senescence genes [6, 7].
AR locus is located in the X cromosome hence it shows a X-linked inheritance. In addition several AR polimorfisms are known to be linked to a higher probability to suffer AGA [8]. Although 5α-reductase enzime is also a key factor in AGA development, SRD5A1 and SRD5A2 (5-αreductase genes) association studies do not showed any relation between them and AGA [9].
Although male AGA etiology is well known to be caused by DHT action in hair follicles, female AGA is related to a huge range of trigger factors, hence observed clinical differences between both sexes. For example women have a diffuse hair loss patron whereas all men keep their hair density in occipital areas. Female AGA cases are in many cases linked to hirsutism patients and in menopause period [10, 11] Estrogens exerts a protective impact probably due to their capacity to participate in androgen metabolism in the dermal papilla cells (DPCs).
Hair cycle includes three phases: hair growth phase (anagen) [12], regression phase (catagen) [13] and relative rest phase (telogen) [14]. In AGA patients, a shortening of the anagen phase is observed, so that the telogen phase sets in progressively. Hair becomes thinner and eventually the anagen phase turns so short that hair is not long enough to reach the skin surface [15] Testosterone and DHT act on the ARs in the DPCs by negatively modulating growth factors genes transcription and positively growth factor suppressors such as Transforming Growth Factor Beta (TGF-β) and Dickkopf-1 (DKK-1), both inducers of the catagen phase [16, 17]. It is well known that anagen phase is characterised by the proliferation of follicular cells, mainly epithelial cells and DPCs. The latter, together with bulge stem cells (BSCs), are the two main types of hair follicle stem cells involved in hair growth. Their importance has been documented detecting changes in their functionality in AGA patients [18, 19]. Inflammation is also one of the pathophysiological characteristics of AGA, as evidenced by the lymphocytes and mast cells infiltration around the bulge area [20,21,22].
Material and methods
A systematic clinical trials review was conducted in ClinicalTrials.gov (https://clinicaltrials.gov/). The keywords “AGA” as Condition or disease and “CELL THERAPY” were used. A literature search about preclinical studies was conducted using Pubmed (https://pubmed.ncbi.nlm.nih.gov/) and introducing various combinations of the following terms: FINASTERIDE, AGA, CELL THERAPY, ASCs (Adipose derived Stem Cells), ASCs-CM (ASCs Conditioned Medium), MESOTHERAPY, PRP, MINOXIDIL, KETOKENAZOL, BICALUTAMIDE, CORTEXOLONE 17A-PROPIONATE, LASER THERAPY, HAIR TRANSPLANT, MURINE MODEL, MICE, MICRONEEDLING, WNT PATHWAY, JAK-STAT PATHWAY, MSCs and SVF (Stromal Vascular Fraction), REGENERATIVE THERAPIES, NANOPARTICLES, BIOINKS, GREEN NANOMATERIALS, HERBAL EXTRACTS, PHYTOMEDICINE amd 3D BIOPRINTING. The electronic databases were systematically searched until May, 2024.
Conventional therapies
Antiandrogens
Finasteride 1 mg/day is the only one oral FDA and EMA approved drugs for AGA treatment. This drug is a potent and specific inhibitor of the 5-alpha reductase (type II) which shows benefitial effects stopping hair loss in the 80% of the patients after one year-treatment [23]. Its primary action, which involves halting DHT production is responsible of adverse effects such as reduced eyaculation volume, loss of libido and erectile dysfunction. Furthermore, although prolonged treatments with Finasteride do not produce drastic semen alterations in healthy men, it may impact patients experiencing infertility symptoms, leading to controversial prescription in young men [24]. In general Finasteride levels are very low in semen hence its use is not restricted [25]. Moreover, Finasteride is also contraindicated in women due to its teratogenic effects during pregnancy and its potential risk of developing breast cancer [26].
Thus, efforts have been led to develop various topical formulations of Finasteride to mitigate systemic side effects associated with oral administration, such as sprays or nano-transferosomal gels. Studies indicate that topical Finasteride increases hair count, is well tolerated and is as effective as oral Finasteride [27,28,29,30].
Nevertheless, despite its effectiveness and minimal adverse effects, further research is essential to evaluate the long-term efficacy of hair regrowth, therapeutic safety, cost-effectiveness, patient tolerability and satisfaction with topical Finasteride among individuals with androgenetic alopecia. Although oral is the only Finateride formulation approved for AGA treatment in Europe, also recommmended as concomitant therapy after follicular unit transplantation procedure, topical solution is expected to be approved proximately due to the numerous ongoing and completed phase III clinical trials (NCT03004469/EUDRACT2015-002877–40).
Concomitantly Ketoconazole, a topical antifungal shampoo for the treatment of seborrhoeic dermatitis can be applied. Its anti-inflammatory and anti-androgenic properties by affecting steroid genesis enhance Finasteride effect by decreasing DHT levels in the male scalp [31, 32]. An improvement in the progression of AGA and hirsutism conditions in women was also observed [33].
On the other hand, Dutasteride, another selective inhibitor of both type-1 and type-2 5-alpha reductase enzymes was assessed as Dutasteride 0,5 mg showing superior outcomes in terms of hair density and hair width compared to Finasteride 1 mg treatment [34]. However, its clinical use remains limited as it is only approved in Mexico and Korea [26]. Currently its topical formulation is being tested in Europe (EUDRACT2022-001802–23).
A topical application of these inhibitor drugs is expected to be approved proximately if clinical trials continue showing as effective results as oral ones.
Other drugs that avoid AR activation are its antagonists such as Cortexolone 17a-propionate, Spironolactone or Cyproterone. In general these drugs do not achieve better results than approved ones (oral Finasteride and topical Minoxidil) and cause several adverse effects.
Minoxidil
As aforementioned, Minoxidil is currently the only topical drug approved for AGA. Its application for hair growth was discovered as an adverse effect of hypertension treatment due to its vasodilator action by opening potassium channels. Its oral formulation is not prescribed for AGA because it promotes a systemic arterial hypotension caused by vascular smooth muscle relaxation. Its topical application is completely safe and it was approved with the aim of acting only on the scalp vessels. It increases blood flow thus an extra nutrients and oxygen supply to the hair follicle. Moreover, one study found cytoprotective activity resulting from the activation of prostaglandin synthase-1, the main isoform in DPCs [35]. Doses range from 2 to 5%, and like other hair growth stimulators, Minoxidil treatment can cause telogen follicles to fall out and be replaced by new ones [36]. Nevertheless, the efficacy in the population is still low, not exceeding 40% of treated patients after 24 weeks of treatment [23].
In order to improve Minoxidil outcomes several oral doses and sulphate compositions are being tested. Recently, a clinical study of 30 male participants has shown efficacy and safety of 5 mg daily dose of oral Minoxidil after being administered to patients for 24 weeks [37]. Lower oral doses such as 1.25 mg/day for 24 weeks, daily capsules containing minoxidil 0.25 mg and spironolactone 25 mg as well as a dose range of 0.25–2.5 mg in female hair loss also showed a clinical improvement and a hair shedding reduction in AGA patients [38,39,40] as well as the sublingual daily dose of 0.45 mg, tested in female and male AGA subjects with an acceptable safety and efficacy profile [41]. The most common adverse effects are irritant and allergic contact dermatitis on the scalp and facial hypertrichosis [26].
Throughout the years, several studies have pointed out the importance of sulphate as an effective supplement to Minoxidil treatment. In particular Minoxidil sulphate is known to be fourteen times more potent than Minoxidil tested in vitro follicles [42]. These results have been supported over the years. In 2019, Maekawa et al. reported hair growth promoting effects in an in vivo murine study after treatment with sodium thiosulphate without significant adverse effects [43]. These results were tested as a single treatment and in combination therapy with Minoxidil. They supported the additional effect of sulphate in Minoxidil therapy due to the cysteine supply. Minoxidil bio-activation by sulfotransferase enzymes has also been highlighted as an important clinical outcome predictor in female hair loss [44]. With the aim of improving topical Minoxidil penetrability, tissue retention and a side effects reduction lecithin-based microparticles has been tested as a vehicle in combination to sulphate [45].
Nowadays, different minoxidil formulations have not showed enough efficacy in all patients and this is the principal cause of the ongoing new therapies research. Specially since there is only indicated treatment for females (topical Minoxidil), nowadays, efforts are being undertaken in order to promote other alternative therapies. Other clinical trials performed with prostaglandin F2 analogues and Cetirizine, that we will address, has the same purpose. AR receptor antagonists outcomes such as from Bicalutamide or Flutamide applications have also been assessed showing mild favourable results in females and hepatotoxicity risk hence its not recomended use [26, 46].
Prostagladins analogues and antagonists receptors
In general in order to avoid Finasteride and Minoxidil side effects, other topical drugs have been tested (Table 1). Prostaglandin F2 analogues such as Bimatoprost, approved for eyelashes hypotrichosis and Latanoprost, known to induce anagen phase in hair follicles, have been tested. Clinical trials reported are both effective comparing to placebo [47,48,49,50,51,52].
Setipiprant is also an oral prostaglandin D2 (PG D2) receptor antagonist which is overexpressed in AGA patients and related to follicle miniaturization [53]. A clinical trial testing a Setipiprant dose of 2000 mg/day was also conducted with slightly better results in hair density versus control [54]. In order to inhibite PG D2 receptor activation, topical Cetirizine, H1 antihistaminic and PG D2 production reducer, was also tested in a clinical study of 60 subjects. Experimental group showed significantly higher results in hair growth and patient satisfaction than control [55]. Recently Bassiouny et al. (2023) had performed a clinical trial testing topical Cetirizine as a concomitant medication with topical Minoxidil therapy in the treatment of female AGA. Results showed an increase in the hair shaft thickness and a higher clinical improvement. Its anti-inflammatory action can be also responsible of an improvement in AGA conditions [56].
Although prostaglandin F2 analogues are known to cause hair lightening and prostaglandin D2 antagonists to be related to atrophy and alopecia conditions, none of them achieve enough efficacy to be considered as promising therapeutical options.
Wnt and JAK-STAT regulators
Other pathways such as Wnt and JAK-STAT are known to regulate the hair cycle. Wnt activator and JAK-STAT inhibitor drugs have been tested in the light of in vivo and in vitro experimental studies with favourable results in hair growth [57, 58]. Current clinical trials results are positive for SM04554, a Wnt pathway activator, promoting hair growth and Ki67 expression in the hair bulb [47, 59, 60]. Other such as Valproic acid and Ciclosporine A have also shown an enhancer hair growth effect. Regarding JAK-STAT inhibitor drugs, a JAK inhibitor 1/3 was clinically tested but no significant differences were observed [61].
Physical treatments
Laser therapy and hair transplant hightlight among physical treatments. In order to stop hair loss in AGA, a wide range of wavelengths has been studied in laser therapy which could stimulate angiogenesis and inflammation possibly mediated by HSP27 leading to follicular stem cell activation [62].
Damage and hair follicle scarring derived from the difficulty in defining adjusted energy parameters are some of the laser therapy limitations [63]. Lower wavelengths are considered as optimal ones around 655 nm [64]. In 2007, Low-Level Laser (Light) Therapy was approved by the FDA as a treatment for hair loss [26, 65, 66]. Several devices using this technology have been applied such as the HairMax Laser Comb (Lexington International LLC, Boca Raton, FL, United States) in 2011 or iRestore Light Therapy Apparatus, tested in a clinical trial in 2015 for male and female AGA [26, 62, 65]. Several randomised, controlled, double-blinded studies results have shown an increased self-rated questionnaire and total hair score in daily and weekly irradiated individuals [67, 68].
Fractionated Erbium laser studies in women with AGA have also reported an improvement in hair density [69,70,71]. A trial combining 1550 nm fractionated Erbium laser treatment with topical Minoxidil 5% compared to Minoxidil treatment was conducted in 2019. Combined treatment results were significantly higher than Minoxidil alone [72]. Another study tested 1550 nm fractionated Erbium laser and PRP alone and in combination. A synergistic effect was showed in the combinated therapy with the greatest outcome [73].
As European guidelines report, laser can be applied as an ancillary therapy with Finasteride or Minoxidil therefore single laser therapy outcomes does not show efficacy by itself.
Hair transplantation as a surgical technique is based on the sensitivity and distribution of the ARs in different scalp areas. Thus, follicles are extracted from a donor area which is not sensitive to androgen action and then are inserted into the affected scalp. In general, transplantation involves a lasting impact resulting in natural hair growth after 6 months and in a patient's self-esteem significant improvement. Low density of the donor area [74], reduced viability of the cells obtained [75] or curly hair are limitations in the process [76]. Transplantation success depends on the technique, surgeon`s skills and individual characteristics [77]. Follicular unit transplantation is the gold standard technique with a low frequency of complications [26, 78] however AGA progression continues so adjuvant therapies are necessary hence its use in combination to oral Finasteride [1, 74]. One of its limitations is that it provides a partial solution since the other nontransplanted follicles, which remains in the frontal and vertex area, still are contingent to DHT atrophic action.
Apparently Finasteride should show a 100% of successfull outcomes because it acts exactly in the etiologic target. However this is not a fact. Althought DHT production will stop after blocking 5-α reductase by antiandrogen therapies, minituarization process would have been triggered, afecting numerous hair follicles becoming fibrotic stellae. It is seemed that this phenomena will be mostly irreversible although DHT levels were low as clinical evidence shows. Other injury factors such as inflammation or fibrosis are needed to be treated as well. These are the reasons why new regenerative therapies are necessary in order to restore damaged follicular mechanisms caused by a prolonged DHT action. The angiogenic and anti-inflammatory growth factors release, PRP functions and the supply of different cell populations have shown to be promising therapies in a near future.
Microneedling
On one hand, microneedling is a widely used technique in dermatology in which a large number of microneedles positioned on a dermatological roller on the skin activates the healing process and thus triggers angiogenesis, platelets mobilisation and growth factors, collagen and elastin synthesis [79]. The mechanism of action is based on the aforementioned regenerative activation, the BSCs stimulation caused by scarring, and growth-related genes overexpression such as Vascular Endothelial Growth Factor (VEGF), β-catenin or Wnt pathway products [80]. Hundred cases of mild to moderate AGA were recruited and it was observed that Minoxidil microneedling was more effective than Minoxidil alone. Since then, numerous trials have supported the microneedling sessions efficacy as an adjuvant technique to pharmacological therapy with Minoxidil or Finasteride and after PRP and PRF administration procedure. In 2018, Kumar et al. [81] compared weekly microneedling plus twice-daily application of topical Minoxidil to Minoxidil-treated group. Hair density increased and patient satisfaction score was higher in the former group, although response was not macroscopically significant. Later these results were supported by Bao et al. [82]. Yu et al. [83] also tested an AGA treatment based on a topical fibroblast growth factor (FGF) solution sprayed before microneedling and topical Minoxidil. They observed in this group the most satisfactory results comparing to Minoxidil, FGF or saline alone.
Mesotherapy
On the other hand, mesotherapy is an intradermal technique which consists of administering pharmacological substances and natural active ingredients diluted in small doses at specific points on the affected scalp. Melo et al. [84] reported a case whose results showed a considerable increase in hair density after 20 treatment sessions using a mixture composed of 1 ml of Minoxidil 0.5%, 1 ml of Finasteride 0.05%, 2 ml of biotin 5 mg/ml and 2 ml of D-panthenol 50 mg/ml. Mesotherapy with natural compounds seemed to be clinically effective as an adjunctive treatment to Minoxidil and Finasteride. Although it is a minimally invasive technique adverse events may include burning, erythema, headaches, subcutaneous necrosis, scalp abscesses and edema [85].
Gajjar et al. [86] conducted a clinical trial to evaluate safety and efficacy of an amino acids, vitamins and other nutritional compounds solution, and compared it to the group treated with topical Minoxidil 5%. They observed no significant differences between groups. Recently Nassar et al. [87] compared LC hair essence serum (formulated with hyaluronic acid, stem cell extract peptides, and zinc arginine and red clover extract) and botulinum toxin A administration. Better results were obtained in LC treated group than in botox treated one although both showed a significant improvement in hair growth.
Jung et al. [88] obtained favourable results in a pilot animal study whereby botox was subcutaneously administered in a stress model mice, and Zhou et al. [89] showed promising results with an favourable safety and efficacy profiles alone and in combination with Finasteride in a clinical study of 63 patients. Aforementioned, Nassar et al. [87] also obtained significant results. Currently a clinical trial is recruiting male and female participants in order to assess its effect in mild to moderate AGA subjects [90]. It has also been studied as a preventive drug by intramuscular injection for the progressive hair loss in AGA men [91].
Simultaneously microcirculation is enhanced by both methods and consequently additional benefits, besides the active ingredient of the solution applied, are provided to the hair growth and a faster and more effective absortion as well. The needles damage along the surface promotes the activation of skin regenerative mechanisms along with angiogenesis.
Emerging therapies
The number of innovative therapies increases constantly improving or incorporating new physical techniques and active pharmaceutical or living beings extracts ingredients. Among them the most of AGA emerging therapies are regenerative-based whose main favourable actions are angiogenesis activation, growth factors supply and antiinflamatory action (Table 2).
Phytomedicine
Traditionally phytomedicine has been applied in AGA treatment for many years. Topical and subcutaneous administration of plant extracts has been extended in experimental studies showing a favourable hair promoting effect [92, 93].
Nowadays some clinical trials are been performed in order to validate these preliminar results. Among them, niacin, ascorbic acid, vitamin B complex, tocopherol, grape seed, rosemary oil, sage, nettles and Hibiscus rosasinensis are used because of their capacity to improve blood supply [94]. Serenoa repens extract prevents TGF-β induction, caused by DHT, and interacts with mithocondrial signaling pathway contributing to its protective action [92]. Other are antioxidants which can act against microinflammation (grape seed) or actively inhibits 5α-reductase (green tea [95], ginkgo biloba [94], gingenoside ro [93] or curcumin [96]). Other tea extracts such as Chinese black tea has shown a higher affinity to estrogen receptors promoting also a hair growth enhancer effect [97].
Neurotoxines
In the last years the study of neurotoxines in disorders like AGA has been documented.
The mechanism of action of Botulinum toxin A particularly is based in its relaxation effect. It is known that a turgency loss can enhance hair growth. Hair follicle is considered as a mechanosensitive organ which can be affected by an increase of occipitofrontal muscle tightening which reduce blood flow. This fact is considered as a hair loss promoter but not a trigger itself [87]. Another suggested Botulinum toxin A function is the inhibition of TGF-β1 released by hair follicles and related to AGA fibrosis and which is considered as a supressor factor of the follicular keratinocyte growth [98].
Specially Botulinum toxin application has recently been considered as an effective and safe therapeutical option for AGA treatment which improves Finasteride and Finasteride plus Minoxidil outcomes as a supplement of these standard therapies [99].
All the clinical trials performed have reported an increase of hair count, clinical response or patient satisfaction [98,99,100,101,102]. Today further neurotoxins implications in AGA clinical improvement are needed to dilucidate. The heterogenous methodology applied and the absence of control studies are some of the weakest points to be improved [103].
Nanotechnology
Nanotechnology offers innovative solutions in several areas of biomedicine. They have been widely used in wound healing, tissue regeneration, drug delivery systems and personalised medicine [104,105,106].
Its use in AGA is growing rapidly and it is establishing itself as a promising new therapeutic option. Diverse nanosystems including nanoparticles, nanostructured lipid carriers and nanotransferosomes have been proposed for the treatment of hair follicle disorders.
These systems share a common strategy: achieving a more precise control over drug release and enhancing the efficacy of drug delivery to the target niche as biocompatible complexes. Their size and design facilitate the accumulation of these nanoparticles in follicle casts, effectively serving as drug reservoirs, thereby increasing local drug concentration at the target site while minimising systemic side effects [107].
The topical use of Minoxidil using lecithin based nanoestructures has been used to enhance a percutaneous delivery and to avoid skin side effects showing yielding comparable efficacy with a reduced incidence of skin issues [45]. Polymeric nanoparticles has also been explored as carriers of topical Finasteride [108]. Whereas the reported lecithin based nanoparticles benefits were associated with an enhanced safety profile, the polymeric Finasteride carriers were found to yield a prolongued Finasteride release thereby increasing its time of residence onto the skin [108, 109].
Other nanoparticles conformed by Molybdenum has been also applied. Molybdelum inhibits oxidative stress due to the presence in its composition of transition metal elements with rapid electron transfer. It is suggested to be a promising therapeutic approach alone and in combination with Minoxidil [110].
Additionally, other formulations based on nano-transferosomes, widely used as drug nanocarriers across the skin [111], were also applied for Finasteride administration as a gel form [28].
A recent study has illustrated an increased efficacy of Aminexil, a keratin fibers stimulator and hair growth promoter, loaded in a nanostructured lipid carrier (NLC) in chemotherapy-induced alopecia rats showing an increase in hair growth promotion compared to the use of the commercial product alone [112].
In the context of phytomedicine, green nanomaterials such as Poly-γ-glutamic acid (PGA) nanoparticles have been widely used as delivery agents due to a high biocompatibility and a good safety profile [113]. In particular it has been shown to be an excellent carrier for an herbal mixture consisting of Phellinus linteus, Cordyceps militaris, Polygonum multiflorum, Ficus carica, and Cocos nucifera oil. PGA as a vehicle of the herbal mixture also promoted a higher hair length, an earlier anagen initiation and a more prolonged anagen phase in C57BL/6N mice. An increase in β-catenin protein expression, a stimulator of the anagen phase, was reported to improve the effect of the herbal mixture alone [113]. This herbal extract has been also carried by PGA in combination to Chitosan Hydrogel supporting these results and reporting an induction of changes in DPCs to a polygonal shape which is associated with an enlargement of the hair bulbs [114].
PGA has also been proven as a curcumic-zinc framework carrier through a microneedle patch with promising results in hair growth [115]. Therefore, nanoparticles offer an innovative approach for treating AGA, through a targeted delivery, which could potentially improve hair growth outcomes. Nevertheless, further research in this area is needed.
Hydradermabrasion
Other therapies such as hydradermabrasion, an extended method in the aesthetic field, is being clinically assessed for improving AGA outcomes and for enhancing hair quality through Hydraderm and Hydrafacial in combination to Keravive Peptide spray, an hyperconcentrated solution of biomimetic growth factors and dermal proteins. Results are not available yet [116, 117]. This tecnique is also led to enhance microcirculation in affected scalps.
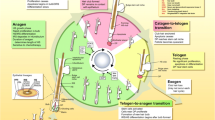
Regenerative therapies
Regenerative therapies for AGA offer a new perspective, against traditional treatments limitations, with potential long-term solutions and fewer side effects.
These regenerative therapies encompass various alternatives such as PRP and its newly generation of products called PRF, SVF, and stem cell-based therapies, including MSCs condicionated medium (MSCs-CM) and extracellular vesicles application.
Tissue engineering is also gaining ground through the development of new 3D cell structures composed by HFSCs and DPCs embebbed in specific scaffolds which pretend to evolve to functional hair follicles after being transplanted into the bald scalp.
PRP
Platelet-rich plasma (PRP) represents the main autologous alternative currently utilized in the treatment of AGA by subcutaneous administration. Although it was initially used for connective tissue regeneration in the field of orthopaedics demonstrating its efficacy in varios conditions such as bone breaks [118], ligament tears [119], osteoarthritis [120] and arthritis [121], its application in the AGA field is widespread with a primary function centered on the restoration of the niche environment. Different growth factors contained in alpha granules of platelets, such as VEGF or Plaque Derived Growth Factor (PDGF) stimulate hair regrowth by inducing the activation of genes associated to various biological processes leading to anagen phase start and proliferation, elastin and collagen synthesis and to an extracellular matrix development [122]. Additionally, its effects includes hypoxia reduction, vasoconstriction and inflammation in bald areas while promoting neoangiogenesis [123,124,125].
In 2014, Khatu et al. [126] conducted a clinical study on 11 AGA non-responders to treatment with Minoxidil or Finasteride patients for 6 months. Each dose was injected twice a month in 4 sessions. Results were evaluated after 3 months macroscopically based on clinical criteria and photography, hair pull test and satisfaction questionnaire. A significant hair density increase was observed.
In 2018, a prospective and comparative study in AGA young men obtained favourable results with an improvement of hair growth in 16 out of 20 participants [127] and in 2019 another prospective and comparative study between PRP and Minoxidil was also conducted [128]. Both groups were treated for 6 months. Standardised tests data, satisfaction surveys and correlation index between platelet concentration and clinical improvement were collected. It was concluded that PRP treatment was more effective than Minoxidil and that platelet count was proportional to hair density increase. The same year a clinical trial with 19 patients, in which PRP plasma was administered every 4 months in a total of 3 times, reported an increase in the number of hair follicles before the second session [129].
Later Pakhomova & Smirnova [130] tested PRP and Minoxidil combination obtaining promising results in male subjects. Recently Qu et al. [131] have proven the therapeutic PRP effect monthly administrated in a total of 3 times in 32 men. Results revealed that PRP treatment produced a significant increase in hair density, hair diameter and anagen hair ratio at month 6 compared to control.
PRP administration has also shown efficacy in female AGA although it is less effective than Minoxidil [132, 133].
PRF
In spite of the extensive use of PRP, in the management of AGA its use presents important limitations. There is not an established protocol for PRP preparation and effectiveness varies due to different preparation methods so the optimal concentration of platelets, the relative centrifugal force and the possible benefits or not of the presence of leukocytes in the final composition of the PRP remains unknown. In addition, there is a restricted long term efficacy due to the relatively short half-lives of growth factors and prompt release following PRP activation.
Therefore, in an attempt to overcome these limitations, second-generation platelet concentrates, called platelet-rich fibrin (PRF), was developed. PRF is similar to PRP except that PRF naturally contains fibrin for clot scaffolding which allows the retention of small biomolecules, stem cells and high concentrations of host immune cells contributing to tissue healing and regeneration. Its formulation is entirely autologous since anticoagulants are no needed in the preparation and present a much longer release of growth factors due to its 3D scaffold structure [134].
Since its gel-based consistency nature limited its application, in 2014 an injectable generation of PRF was developed. This new injectable formulation obtained by reduction of the speed centrifugation based on the low speed centrifugation concept [135] presented the advantage of use a liquid form before being converted to a fibrin matrix (clot), which allowed a slower and more gradual release of the growth factors [136]. Also an increase release of growth factors when compared to traditional PRF was observed [137].
Numerous studies have investigated the clinical applications of PRF in different regenerative fields including odontology [136], surgery [138], traumatology [139], wound healing [140], facial esthetics [141] and in hair regrowth [142,143,144,145].
Different comparative studies have reported PRF to be more effective in improving fat grafting than PRP [141], when fat graft was combined with either PRP or PRF during facial lipostructure surgery or in the treatment of acne scars [146].
In regard to hair regrowth, there is a growing interest in the application of PRF. In 2021, a study conducted by Lu et al. indicated the role of PRF in promoting hair follicule regeneration through the enhancement of cell proliferation, migration and trichogenic inductivity [142].
In addition, recent clinical studies have highlighted the beneficial effect of PRF in the treatment of AGA. Arora et al. (2019), including three patients between 35 and 40 years of age with a varying degree of hair loss, reported an increase in hair density when treated with injectable PRF [143]. In another study conducted by Shashank et al. (2020), a 34-year-old male with hair thinning diagnosed with grade 4, experimented an increase in hair density after PRF sessions [147] and Bhoite et al. (2022) reported a clinically noticeable improvement in the hair growth in 11 out of 15 patients after receiving PRF and microneedling treatment for 4 sessions along with Minoxidil, Finasteride and multivitamin supplements [144].
To date, the largest human clinical trial (168 patients) was conducted by Schiavone et al. reporting a clinical improvement of AGA parameters at month 6 in all the participants after platelet concentrates administration sessions [145].
Currently, PRF therapy represents an effective, safe and inexpensive innovative treatment for AGA. Neverheless further investigation is required in order to optimize preparation protocols for a more effective composition.
HFSCs, DPCs and MSCs and their derivatives
MSCs has been widely used in the field of regenerative medicine and their secretoma. Derivative products such as MSCs condicionated medium (MSC-CM) and extracellular vesicles are progressively being more investigated in different pathologies. Both MSCs-CM and exosomes (nanomembranous vesicles) contain different biomolecules capable of restoring physiological conditions.
Since most of the beneficial effects associated with the utilization of MSCs arise from their paracrine action, facilitated by different components present in their secretome such as growth factors, cytokines or chemokines, there is a growing interest in investigating MSCs-CM and exosomes. Extracellular vesicles are secreted through paracrine signaling including microRNAs, mRNAs, metabolites, second messengers, adhesion proteins, growth factor receptors, ligands and long-coding RNAs [148]. Among these components, RNA and proteins are the functional ones implicated in tissue regeneration [149].
The effectiveness of MSCs derivatives treatment is atributed to their main function: organs and tissues homeostasis. Additionally, they exhibit the ability to secrete growth factors and anti-inflammatory cytokines thereby participating in inmunomodulation within the niche and in lymphocyte infiltration reduction generally observed in these patients [150, 151]. Although MSCs are found in different anatomical locations such as periosteum, bone trabeculae, synovial membrane, muscle tissue, dermis and bone marrow [152, 153]; it is from adipose tissue where extraction is less complex through a non-invasive process with a high cell yield [154].
For this reason, adipose-derived stem cells (ASCs) are being widely used in the dermatological field, particularly in tissue regeneration, psoriasis and alopecia. In AGA treatment, SVF, derived from adipose tissue, and stem cells (SCs) or their derivative products have demonstrated significant improvement in hair density and diameter according to the lastest reported outcomes.
The most of the publications are refered to its conditioned medium as a growth factors enriched secretome produced by MSCs metabolism but also to SVF as an easily extractable heterogenous adipose cell population composed of adipocytes, preadipocytes, adipose stem cells, endotelial progenitor cells, hematopoietic progenitors, monocytes, leukocytes and pericytes [155,156,157].
Currently, HFSCs and MSCs derivatives administration is considered as the main focus in numerous lines of hair regrowth research.
Experimental regeneration based-studies
In 2019 Gentile et al. [158] collected every hair follicle cell function, interactions among them as well as MSCs signalling impact at follicular level, extracted from in vitro experiments to date. These experiments have elucidated the mechanisms which are involved after supplying MSCs to the hair follicle and about their role as intrinsic populations.
In this context ASCs-CM is known to specifically trigger DPCs replication and hair shafts lengthening in isolated hair follicles [159]. Park et al. [155] evaluated enriched medium effects. ASCs-CM promoted DPCs differentiation and epidermal keratinocytes. They differentiated between normoxic and hypoxic conditions and unexpestedly, after 100 µl injection in each group, faster hair activation was detected in the latter.
Studies with ASCs in murids were applied by intradermal administration in the order of 106 cells. Positive results were obtained in 7-week-old C3H/HeN mice after 12 weeks in the 1.5· 106 ASCs intradermally administered and in the 1 ml of ASCs-CM topically administered groups [150]. Results suggested that ASCs and ASCs-CM promote hair growth by increasing DPCs through cell cycle modulation and anagen phase activation. In 2011, Festa et al. [160] indicated that preadipocytes played an important role in hair growth by activating the SCs follicular activity, whereas mature adipocytes did not show this capacity. They administered SVF and isolated adipogenic precursors in 7-week-old mice. This study confirmed the importance of ASCs. Hair growth was observed when isolated adipocyte precursors were administered while no such effect occurred when SVF was applied. Experimental results indicate that mature adipocytes are not the primary adipogenic cell type involved in the induction of stem cell activity in hair follicles and that adipocyte precursor cells are essential for skin epithelial stem cells activation.
Results obtained by He et al. [161] also supported this conclusion. They evaluated the puripotency of CD34 + , CD34- and SVF cells from adipose tissue in a nude mouse model. Results showed that CD34 + cells administration resulted in a higher number of hair follicles than CD34- and SVF groups. These progenitor cells would participate in hair morphogenesis by integrating into the dermal sheath. On the other hand, differentiation to blood vessel endothelial cells was observed in CD34 + and SVF cells. The former group was shown to have a high differentiation potential in skin development.
These studies were supported by other ones based on tissue regeneration such as the one performed by Zografou et al. [162] in which 106ASCs, distributed in 10 spots, were administered in diabetic Spargue Dawley rats. They observed a survival and angiogenesis increase in the grafted areas. In other pathologies such as psoriasis Rokunohe et al. [163] and Lee et al. [164] applied 3·106 ASCs and 4·106 human umbilical cord-derived mesenchymal stem cells (hUCB-MSCs) respectively in the dorsal area of diseased mice and obtained favourable results in terms of expected immunosuppressive activity.
In regard to the use of exosomes in AGA although most of the investigation is still in a preclinical setting. Several case reports has shown promising results. Instead of MSC-CM use, exosomes are more resistant to degradation, citokines and growth factors half-lives are larger and according to Wu et al. (2021) exosomes show a safer and more efficient profile [165]. In hair regeneration preclinical studies, different sources of exosomes have been explored including exosomes derived from dermal papilla cells (DPCs) [166], from adipose-derived stem cells [165], from hair outer root sheath cells [167] or immune cell-derived exosomes such as macrophage extracellular vesicles [168]. In fact perifollicular macrophages are known to activate DPCs promoting anagen phase.
Commonly, results show an enhancement of hair follicle proliferation and migration, an increase of β-catenin expression via Wnt pathway and an aceleration of the anagen onset [168]. In addition exosomes reduce proinflammatory levels [169] and accelerate re-ephitelialization [170].
Clinical regeneration based-studies
Cell therapy in AGA has been applied by different procedures such as subcutaneous or intradermal administration from different types of sources in the human body, including, autologous HFSCs [171], ASCs extracted from the occipital region for hair transplantation [172] or obtained from the abdominal area, as part of autologous SVF [173].
In regard to ASCs-CM, Fukuoka and Suga [156] proved its efficacy in a clinical trial involving 22 patients, resulting in a significant increase in hair count after treatment in both male and female subjets. Positive results in hair density and thickness parameters were also observed by Shin et al. in a female cohort [174]. At present, a study including 37 participants using two different ASCs-CM concentrations has been completed with pending results [175].
The efficacy of ASCs-CM was further evaluated in a study with 38 participants over 16 weeks, showing a significant increase in hair count and thickness compared to placebo within 8 weeks of application [176]. Moreover, it was also tested female hair loss with the Hair Stimulating Complex, a derivative solution of ASCs-CM enriched with growth factors [177]. The use of HUCB-MSCs conditioned medium with paracrine factors in a experimental solution called NGF-574H was tested by a twice a day topical administration [178]. In addition, small-scale clinical studies were conducted using SVF [179, 180]. A recent clinical trial comparing PRP versus mesotherapy containing the ASCs-CM and a mixture of recombinant growth factors in 100 participans is still awaiting results [181].
ASCs-CM has also been applied in transplantation areas [172] and formulated with several growth factors, interleukin 6 as AAPE Prostemics commercial product via microneedling administration [174, 182].
Zanzottera et al. [172], in order to optimized the SVF extraction process, used the Rigenera® system to obtain a heterogeneous solution of the hypodermis with autologous SCs from the donor occipital area during hair transplantation procedure. The suspension was applied to the scalp areas undergoing hair transplantation in 3 AGA subjects. Monthly follow-up revealed faster healing after transplantation and improved hair growth after two months. Subsequently Gentile et al. [183] isolated HFSCs using Rigenera® Securdrill resulting in a 29% higher density in the treatment area compared to placebo.
Other researchers have suggested that autologous ASCs-enriched fat grafting could be a promising alternative for treating AGA. Hamed Kadry et al. [173] conducted a study comparing PRP and intradermal SVF treatment, showing that SVF-treated patients exhibited more marked improvements in hair count and hair thickness in both sexes. Both treatments has also been compared in a clinical trial of 22 participants [184]. Ghazally et al. also compared PRP and ASCs suspension vs PRP application in the recipient site during follicular unit extraction [185].
On the other hand, Stevens et al. [186] tested a combined treatment of PRP and SVF, resulting in a significant increase of hair density at 6 and 12 weeks after a single injection in 10 AGA subjects. In this context, safety and efficacy of using a biocellular mixture consisting of emulsified adipose-derived tissue SVF and high density PRP concentrate group is under evaluation in comparison to other groups in 60 female subjects. Experimental groups includes adipose-derived cell-enriched SVF, SVF, high density PRP concentrate and high density PRP concentrate alone [187].
Recently El-Khalawany et al. [188] conducted a clinical study with a single administration of autologous SVF in 30 patients, with positive results in terms of hair density, hair thickness, global photography and patient satisfaction.
Since 2008, researchers have showed a growing interest exploring the efficacy and safety of ex vivo-cultured, expanded and autologous cells. These isolated cells include dermal cells from the occipital region [189,190,191,192] or in combination with epidermal cells [193,194,195,196,197,198,199]. Currently, a clinical trial is going using autologous HFSCs extracted from occipital area [200] with a previous similar one performed using isolating and replicating HFSCs from scalp biopsies [201]. Results from both are pending. Elmaadawi et al. [202] used autologous bone marrow mononuclear cells and HFSCs in different groups to treat refractory alopecia areata and AGA. A significant improvement was observed in all treatment groups after the administration of a solution containing a total of 105 cells. Despite their different origins, both therapies exhibited similar safety and efficacy, presenting a higher efficacy in females.
To date, the only clinical trial detailing a clinical dose of ASCs in AGA treatment is the STYLE Transplantation [203]. They conducted a randomized study including 71 subjects in 4 groups, two of which received SCs enriched population from SVF (high dose- 1·106 ASCs/cm2 and low dose 0.5· 106 ASCs/cm2) while the other received Puregraft fat graft and saline respectively. Fat and enriched SVF were administered in the subdermal layer and at a rate of 0.1 ml/cm2 over a total area of 40 cm2. Follow-up was performed at weeks 6, 12, 24 and 52 revealing an increase of 16 hairs in hair count at weeks 12, 24 and 52 in the low-dose group compared to baseline. In addition, more participants in this group showed a higher number of positive responses on the hair satisfaction questionnaire at week 24, followed by those who received only SVF. No severe adverse effects were reported.
According to exosomes, one clinical trial is only ongoing in order to assess eficacy and safety of exosomes versus PRP in AGA treatment [204].
Tissue engineering and 3D bioprinting techniques
Nowadays tissue engineering development is making a good progress but the construction of a functional hair follicle is still a huge challenge due to the complexe mesenchymal-epithelial interactions [205]. Three are the aims which are pursued in the context of tissue regeneration: inductive signals intake, sustitution of damaged cells or onstruction of 3D structures composed by cells on synthetic or collagen matrix [206].
Currently the cutting edge advances are based on bioinks constituted by isolated and expanded autologous HFCs and DPCs in vitro extracted from a follicular unit extraction, the construction of spheroid cultures of both lines together and the incorporation into in a biomaterial scaffold which are contigent upon a modulation signaling [106, 207]. An appropiate design, the use of a biocompatible material and the viability of the cells are essential points in order to achieve complete and functional hair follicles after transplantation.
3D bioprinting techniques, such as the refered one, offers the possibility of manufacturing constructs that mimic a particular tissue architecture, regardless of its complexity, facilitating the hierarchical arrangement of cells within intricate 3D biomaterials while promoting tissue regeneration. As a consequence, nanomaterials can also be integrated, creating complex biological structures with enchanced properties including biocompatibility and regeneration action [106].
Currently, 3D printing techniques have been used for the regeneration of different types of tissues, including skin [208], cartilage [209], vascular networks [210] or organs such a bioprosthetic ovary [211]. The potential use of this technology in clinical settings addressing hair loss like AGA seems promising.
In this context, a recent study using 3D printing technique incorporating magnesium silicate nanomaterials, manufactured a multicellular micropattern constituded by hair follicle cells and a vascular network which leaded to hair regrowth in an AGA immunodeficient mouse model [212].
Additionally, 3D skin equivalents with hair follicle structures and epidermal-papillary-dermal layers has been developed using skin tissue equivalents [213].
Nevertheless to success in AGA using bioprinting techniques it will be neccesary to develop biomaterials capable of mimicking the intricate structure of the hair follicle and its surrounding microenvironment, while being biocompatible, bioactive and non immunogenic. Due to the complex structure of the hair follicle considered as a dynamic miniorgan, an improved bioprinting method is required in order to replicate as similarly as possible the hair biological conformation. The presence of factors to be incorporated to the nanostructure providing to the hair follicle microenvironment the functional activity of the absent sebaceous gland is also a challenge.
Conclusion
Although topical Minoxidil and oral Finasteride are the only approved drugs for AGA, numerous adverse events are associated with their administration. Hair transplant is an effective option with favourable results, however long-term efficacy may diminish due to progressive miniaturization and loss of preexisting nontransplanted hairs induced by DHT chronic action. The elucidation of strategies to ameliorate the AGA hindered microenvironment is a complex challenge. Recurrent local hormonal action can jeopardize follicle funcionality and regular cycling processes. Early therapeutic intervention is crucial in order to preserve follicles and prevent irreversible damage and fibrosis.
Currently, different strategies aiming to restore physiological conditions or the hair follicle are being under investigation. Allogenic and autologous stem cells administration, along with their derivatives including ASCs-CM or exosomes, are well known for supplying essencial growth factors pivotal for the niche restoration. In addition, their immunomodulatory role plays a crucial role to ameliorate microinflammation associated with AGA and despite of the numerous advantages potentially offered by these therapies their widespread application hinders their implementation. Additionally, to support the clinical use of MSC derivatives, there is a need to standardise and optimise preparation protocols to tackle issues related to donor variability or tissue origin that influence the secretome composition and thereby their therapeutic action.
In addition, contemporary literature highlights research studies on bioinks and bionanomaterial scaffolds for 3D bioprinting techniques across different fields. Despite being in the early stages of exploration, these techniques show considerable potential and offer significant promise for the treatment of AGA. The intricate nature of biological systems, exemplified by the dynamic life cycle of the hair follicle, presents pivotal considerations in the assembly of multi-layered scaffolds. The construction of spheroidal mix cultures of HFSCs and DPCs embebbed in scaffolds has replaced isolated and expanded HFCS administration alone. The DPCs and HFCs interaction is the basis for the onset of anagen phase so an adecuate choice of trigger factors which would induce bioink grafting and transformation into a functional biological struture is essential. The adecuate scaffold biomaterial should be also biocompatible and resilient enough to persist in the dermal layers until progression to become a functional hair follicle analogue structure.
The aforementioned regenerative approaches are leading to two different pursuits. One focuses on the restoration of miniaturized hair follicles and the altered microenviroment surrounding them to rekindle inherent selfrenewal capacities whereas the other one aims to recreate from scratch a structure as complex as such as the hair follicle. It would be necessary to assess patient clinical characteristics carefully according to AGA severity and the onset of alopecic conditions in order to set a treatment which pretends to revive his own follicular regenerative mechanisms or to create de novo lost hair follicles which became fibrotic stellae.
Further investigation is needed in order to define the exogen factors that could lead to functional hair follicle development from bioinks.
Availability of data and materials
All references are included in this review.
Abbreviations
- AGA:
-
androgenetic alopecia
- AR:
-
androgen receptor
- ASCs:
-
adipose derived stem cells
- ASCs-CM:
-
adipose derived stem cells conditioned medium
- DHT:
-
dihydrotestosterone
- DPCs:
-
dermal papilla cells
- FGF:
-
fibroblast growth factor
- HFSCs:
-
hair follicle stem cells
- HUCB-MSCs:
-
human umbilical cord blood-derived mesenchymal stem cells
- LLLT:
-
low-level laser therapy
- MSCs:
-
mesenchimal stem cells
- PDGF:
-
plaque derived growth factor
- PRP:
-
platelet rich plasma
- PRF:
-
platelet rich fibrin
- TGF:
-
transforming growth factor
- VEGF:
-
vascular endotelial growth factor
References
Blumeyer A, Tosti A, Messenger A, Reygagne P, del Marmol V, Spuls PI, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. JDDG J der Dtsch Dermatologischen Gesellschaft. 2011;9:1–57. https://doi.org/10.1111/j.1610-0379.2011.07802.x.
Hamilton JB. Patterned loss of hair in man: types and incidence. Ann N Y Acad Sci. 1951;53(3):708–28. https://doi.org/10.1111/j.1749-6632.1951.tb31971.x.
Ramos PM, Miot HA. Female pattern hair loss: a clinical and pathophysiological review. An Bras Dermatol. 2015;90(4):529–43.
Gentile G. Advances in regenerative stem cell therapy in androgenic alopecia and hair loss: wnt pathway, growth-factor, and mesenchymal stem cell signaling impact analysis on cell growth and hair follicle development. Cells. 2019;8(5):466.
Ellis JA, Sinclair R, Harrap SB. Androgenetic alopecia: pathogenesis and potential for therapy. Expert Rev Mol Med. 2002;4(22):1–11.
Urysiak-Czubatka I, Kmieć ML, Broniarczyk-Dyła G. Assessment of the usefulness of dihydrotestosterone in the diagnostics of patients with androgenetic alopecia. Postep dermatologii i Alergol. 2014;31(4):207–15.
Otberg N, Finner AM, Shapiro J. Androgenetic Alopecia. Endocrinol Metab Clin North Am. 2007;36(2):379–98.
Ellis JA, Stebbing M, Harrap SB. Polymorphism of the androgen receptor gene is associated with male pattern baldness. J Invest Dermatol. 2001;116(3):452–5.
Ellis JA, Stebbing M, Harrap SB. Genetic analysis of male pattern baldness and the 5α-reductase genes. J Invest Dermatol. 1998;110(6):849–53.
Mirmirani P. Hormones and clocks: Do they disrupt the locks? Fluctuating estrogen levels during menopausal transition may influence clock genes and trigger chronic telogen effluvium. Dermatol Online J. 2016;22(5). https://pubmed.ncbi.nlm.nih.gov/27617515/
Bienenfeld A, Azarchi S, Lo Sicco K, Marchbein S, Shapiro J, Nagler AR. Androgens in women: Androgen-mediated skin disease and patient evaluation. J Am Acad Dermatol. 2019;80(6):1497–506. https://doi.org/10.1016/j.jaad.2018.08.062.
Rompolas P, Greco V. Stem cell dynamics in the hair follicle niche. Semin Cell Dev Biol. 2014;25–26:34–42.
Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451(7176):340–4.
Wang AB, Jain P, Tumbar T. The hair follicle stem cell niche: the bulge and its environment. Tissue-Specific Stem Cell Niche. 2015. https://doi.org/10.1007/978-3-319-21705-5_1.
Tsuboi R, Niiyama S, Irisawa R, Harada K, Nakazawa Y, Kishimoto J. Autologous cell–based therapy for male and female pattern hair loss using dermal sheath cup cells: a randomized placebo-controlled double-blinded dose-finding clinical study. J Am Acad Dermatol. 2020;83(1):109–16.
Kwack MH, Sung YK, Chung EJ, Im SU, Ahn JS, Kim MK, et al. Dihydrotestosterone-inducible Dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J Invest Dermatol. 2008;128(2):262–9.
Kitagawa T, Matsuda K-I, Inui S, Takenaka H, Katoh N, Itami S, et al. Keratinocyte growth inhibition through the modification of Wnt signaling by androgen in balding dermal papilla cells. J Clin Endocrinol Metab. 2009;94(4):1288–94.
Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006;116(1):249–60.
Mohammadi P, Youssef KK, Abbasalizadeh S, Baharvand H, Aghdami N. Human hair reconstruction: close, but yet so far. Stem Cells Dev. 2016;25(23):1767–79.
Cervelli V, Garcovich S, Bielli A, Cervelli G, Curcio BC, Scioli MG, et al. The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: clinical and histomorphometric evaluation. Biomed Res Int. 2014;2014:760709.
Jaworsky C, Kligman AM, Murphy GF. Characterization of inflammatory infiltrates in male pattern alopecia: implications for pathogenesis. Br J Dermatol. 1992;127(3):239–46.
Houschyar KS, Borrelli MR, Tapking C, Popp D, Puladi B, Ooms M, et al. Molecular mechanisms of hair growth and regeneration: current understanding and novel paradigms. Dermatology. 2020;236(4):271–80.
Andy G, John M, Mirna S, Rachita D, Michael K, Maja K, et al. Controversies in the treatment of androgenetic alopecia: The history of finasteride. Dermatol Ther. 2019;32(2):e12647.
Overstreet JW, Fuh VL, Gould J, Howards SS, Lieber MM, Hellstrom W, et al. Re: Chronic treatment with finasteride daily does not affect spermatogenesis or semen production in young men [5]. J Urol. 2000;164(4):1319–20.
Kanti V, Messenger A, Dobos G, Reygagne P, Finner A, Blumeyer A, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men – short version. J Eur Acad Dermatology Venereol. 2018;32(1):11–22.
Kelly Y, Blanco A, Tosti A. Androgenetic Alopecia: An Update of Treatment Options. Vol. 76, Drugs. Springer International Publishing; 2016. p. 1349–64
Study to Evaluate the Efficacy and Safety of P-3074 Topical Solution in the Treatment of Androgenetic Alopecia. 2023: https://www.clinicaltrials.gov/search?term=NCT03004469
Ahmed OAA, Rizq WY. Finasteride nano-transferosomal gel formula for management of androgenetic alopecia: ex vivo investigational approach. Drug Des Devel Ther. 2018;12:2259–65.
Piraccini BM, Blume-Peytavi U, Scarci F, Jansat JM, Falqu M, Otero R, et al. Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. 2021; https://doi.org/10.1111/jdv.17738
Gupta AK, Talukder M. Topical finasteride for male and female pattern hair loss: is it a safe and effective alternative? J Cosmet Dermatol. 2022;21(5):1841–8. https://doi.org/10.1111/jocd.14895.
Inui S, Itami S. Reversal of androgenetic alopecia by topical ketoconzole: Relevance of anti-androgenic activity. J Dermatol Sci. 2007;45(1):66–8.
Hugo Perez BS. Ketocazole as an adjunct to finasteride in the treatment of androgenetic alopecia in men. Med Hypotheses. 2004;62(1):112–5.
Sonino N, Scaroni C, Biason A, Boscaro M, Mantero F. Low-dose ketoconazole treatment in hirsute women. J Endocrinol Investig Off J Ital Soc Endocrinol. 1990;13(1):35–40.
Gubelin Harcha W, Barboza Martínez J, Tsai TF, Katsuoka K, Kawashima M, Tsuboi R, et al. A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. J Am Acad Dermatol. 2014;70(3):489–98.
Mahe YF, et al. Androgenetic alopecia and microinflammation. Int J Dermatol. 2000;39:576–84.
Orasan MS, Roman II, Coneac A, Muresan A, Orasan RI. Hair loss and regeneration performed on animal models. Med Pharm Reports. 2015;89(3):327–34.
Panchaprateep R, Lueangarun S. Efficacy and safety of oral minoxidil 5 mg once daily in the treatment of male patients with androgenetic alopecia: an open-label and global photographic assessment. Dermatol Ther (Heidelb). 2020;10(6):1345–57.
Jha A, Sonthalia S, Zeeshan MD. Efficacy and safety of very-low-dose oral minoxidil 1.25 mg in male androgenetic alopecia. J Am Acad Dermatology. 2020;83(5):1491–3.
Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57(1):104–9.
Sinclair R, Perera E. Treatment of chronic telogen effluvium with oral minoxidil: A retrospective study. F1000Research 2017 61650. 2017;6:1650.: https://f1000research.com/articles/6-1650
Sinclair R, Trindade de Carvalho L, Ferial Ismail F, Meah N. Treatment of male and female pattern hair loss with sublingual minoxidil: a retrospective case-series of 64 patients. J Eur Acad Dermatology Venereol. 2020;34(12):795–6.
Buhl AE, Waldon DJ, Baker CA, Johnson GA. Minoxidil sulfate is the active metabolite that stimulates hair follicles. J Invest Dermatol. 1990;95(5):553–7.
Maekawa M, Ohnishi T, Balan S, Hisano Y, Nozaki Y, Ohba H, et al. Thiosulfate promotes hair growth in mouse model. Biosci Biotechnol Biochem. 2019;83(1):114–22.
Ramos PM, Goren A, Sinclair R, Miot HA. Oral minoxidil bio-activation by hair follicle outer root sheath cell sulfotransferase enzymes predicts clinical efficacy in female pattern hair loss. J Eur Acad Dermatology Venereol. 2020;34(1):e40-1.
Lee HJ, Oh DW, Na MJ, Kim DW, Yuk DY, Choi HC, et al. Preparation and in vivo evaluation of lecithin-based microparticles for topical delivery of minoxidil. Arch Pharm Res. 2017;40(8):943–51.
Carvalho R de M, Santos LDN, Ramos PM, Machado CJ, Acioly P, Frattini SC, et al. Bicalutamide and the new perspectives for female pattern hair loss treatment: What dermatologists should know. J Cosmet Dermatol. 2022;21(10):4171–5
Ocampo-Garza J, Griggs J, Tosti A. New drugs under investigation for the treatment of alopecias. Expert Opin Investig Drugs. 2019;28(3):275–84.
NCT01904721. A Safety and Efficacy Study of Bimatoprost in Men With Androgenic Alopecia (AGA). 2016.: https://www.clinicaltrials.gov/study/NCT01904721?term=NCT01904721&rank=1&limit=10
NCT01325337. Safety and Efficacy Study of Bimatoprost in the Treatment of Men With Androgenic Alopecia. 2014: https://www.clinicaltrials.gov/study/NCT01325337?term=NCT01325337&rank=1
NCT0132535. Safety and Efficacy Study of Bimatoprost in the Treatment of Women With Female Pattern Hair Loss. 2014: https://www.clinicaltrials.gov/study/NCT01325350?term=NCT01325350&rank=1
Topical Bimatoprost Effect on Androgen Dependent Hair Follicles. [cited 2023 Jul 31].: https://www.clinicaltrials.gov/study/NCT02170662?term=NCT02170662&rank=1
Blume-Peytavi U, Hillmann K, Garcia Bartels N. A randomized double-blind placebo-controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia. https://pubmed.ncbi.nlm.nih.gov/21875758
Nieves A, Garza LA. Does prostaglandin D2 hold the cure to male pattern baldness? Exp Dermatol. 2014 Apr [cited 2019 Jul 29];23(4):224–7.: http://www.ncbi.nlm.nih.gov/pubmed/24521203
NCT02781311. A Safety and Efficacy Study of Setipiprant Tablets in Androgenetic Alopecia in Males. clinicaltrials.gov. 2019: https://www.clinicaltrials.gov/study/NCT02781311?term=NCT02781311&rank=1
Mohamed S. Zaky MD1 | Hassan Abo Khodeir MD1 | Hebat-Allah Ahmed MD1 |, Mohamed L. Elsaie MD. Therapeutic implications of topical cetirizine 1% in treatment of male androgenetic alopecia: A case-controlled study. 2020: https://onlinelibrary.wiley.com/doi/pdfdirect/https://doi.org/10.1111/jocd.13940
Bassiouny EA, Solwan, El-Samanoudy I, Abbassi MM, Nada HR, Samar , et al. Comparison between topical cetirizine with minoxidil versus topical placebo with minoxidil in female androgenetic alopecia: a randomized, double-blind, placebo-controlled study. 2023;315:1293–304.: https://doi.org/10.1007/s00403-022-02512-2
Lee S-H, Yoon J, Shin SH, Zahoor M, Kim HJ, Park PJ, et al. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. 2012: https://pubmed.ncbi.nlm.nih.gov/22506014/
Harel S, Higgins CA, Cerise JE, Dai Z, Chen JC, Clynes R, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1(9).: https://pubmed.ncbi.nlm.nih.gov/26601320/
A Study of the Safety, Tolerability, and Efficacy of Topical SM04554 Solution in Male Subjects With Androgenetic Alopecia (AGA) - Full Text View - ClinicalTrials.gov. [cited 2022 Sep 26].: https://clinicaltrials.gov/ct2/show/NCT02275351
A Study of SM04554 Applied Topically to the Scalp of Male Subjects With Androgenetic Alopecia Analyzed by Biopsy of the Scalp Prior To and Post Dosing - Full Text View - ClinicalTrials.gov. [cited 2022 Sep 26].: https://clinicaltrials.gov/ct2/show/NCT02503137?cond=NCT02503137&draw=2&rank=1
A Study in Male and Female Subjects With Androgenetic Alopecia Treated With ATI-50002 Topical Solution - Full Text View - ClinicalTrials.gov. [cited 2023 Mar 27].: https://clinicaltrials.gov/ct2/show/NCT03495817?term=NCT03495817&draw=2&rank=1
“iRestore” Light Therapy Apparatus - Full Text View - ClinicalTrials.gov. [cited 2023 Mar 27].: https://clinicaltrials.gov/ct2/show/NCT03331003?term=CELL+THERAPY&cond=aga&draw=2&rank=25
Wu Y-F, Wang S-H, Wu P-S, Fan SM-Y, Chiu H-Y, Tsai T-H, et al. Enhancing hair follicle regeneration by nonablative fractional laser: assessment of irradiation parameters and tissue response. Lasers Surg Med. 2015;47(4):331–41
Avci P, Gupta GK, Clark J, Wikonkal N, Hamblin MR. Low-level laser (Light) therapy (LLLT) for treatment of hair loss. Lasers Surg Med. 2014;46:144–51.
Darwin E, Heyes A, Hirt PA, Wikramanayake TC, Jimenez JJ. Low-level laser therapy for the treatment of androgenic alopecia: a review. Lasers Med Sci. 2018;33(2):425–34.
Pillai J, Mysore V. Role of low-level light therapy (LLLT) in androgenetic alopecia. Vol. 14, Journal of Cutaneous and Aesthetic Surgery. 2021. p. 385. https://pubmed.ncbi.nlm.nih.gov/35283601
Kim H, Choi JW, Kim JY, Shin JW, Lee SJ, Huh CH. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39(8):1177–83.
Lanzafame RJ, Blanche RR, Bodian AB, Chiacchierini RP, Fernandez-Obregon A, Kazmirek ER. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45(8):487–95.
Lee GY, Lee SJ, Kim WS. The effect of a 1550 nm fractional erbium-glass laser in female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25(12):1450–4.
Meephansan J, Ungpraphakorn N, Ponnikorn S, Suchonwanit P, Poovorawan Y. Efficacy of 1,550-nm erbium-glass fractional laser treatment and its effect on the expression of insulin-like growth factor 1 and Wnt/β-catenin in androgenetic alopecia. Dermatol Surg. 2018;44(10):1295–303.
Alhattab MK, AL Abdullah MJ, Al-Janabi MH, Aljanaby WA, Alwakeel HA. The effect of 1540-nm fractional erbium-glass laser in the treatment of androgenic alopecia. J Cosmet Dermatol. 2020;19(4):878–83.
Suchonwanit P, Rojhirunsakool S, Khunkhet S. A randomized, investigator-blinded, controlled, split-scalp study of the efficacy and safety of a 1550-nm fractional erbium-glass laser, used in combination with topical 5% minoxidil versus 5% minoxidil alone, for the treatment of androgenetic alopecia. Lasers Med Sci. 2019;34(9):1857–64.
Haddad N, Arruda S, Sadick N. Evaluating the efficacy of platelet rich plasma and 1550 nm fractional laser in combination and alone for the management of androgenetic alopecia. J Drugs Dermatol. 2022;21(11):1166–9.
Leavitt M, Perez-Meza D, Rao NA, Barusco M, Kaufman KD, Ziering C. Effects of finasteride (1 mg) on hair transplant. Dermatol Surg. 2005;31(10):1268–76.
Cole JP. An analysis of follicular punches, mechanics, and dynamics in follicular unit extraction. Facial Plast Surg Clin North Am. 2013;21(3):437–47.
Rogers NE, Callender VD. Advances and challenges in hair restoration of curly Afrocentric hair. Dermatol Clin. 2014;32(2):163–71.
Rose P. The latest innovations in hair transplantation. Facial Plast Surg. 2011;27(04):366–77.
Caroli S, Pathomvanich D, Amonpattana K, Kumar A. Current status of hair restoration surgery. Int Surg. 2011;96(4):345–51.
Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7(4):244.
Dhurat R, Sukesh M, Avhad G, Dandale A, Pal A, Pund P. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5(1):6.
Kumar M, Inamadar A, Palit A. A randomized controlled, single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11(4):211–6.
Bao L, Fang H, Zheng LLY. Randomized trial of electrodynamic microneedling combined with 5% minoxidil topical solution for treating androgenetic alopecia in Chinese males and molecular mechanistic study of the involvement of the Wnt/β-catenin signaling pathway. 2020. p. 483–93
Yu C-Q, Zhang H, Guo M-E, Li X-K, Chen H-D, Li Y-H, et al. Combination therapy with topical minoxidil and nano-microneedle-assisted fibroblast growth factor for male androgenetic alopecia: a randomized controlled trial in Chinese patients. 2021. http://links.lww.com/CM9/A373
Melo DF, de Mattos Barreto T, Plata GT, Araujo LR, Tortelly VD. Excellent response to mesotherapy as adjunctive treatment in male androgenetic alopecia. J Cosmet Dermatol. 2019; 2019; https://pubmed.ncbi.nlm.nih.gov/ 31066492/
Melo DF, Saceda-Corralo D, Tosti A, Weffort F, Carla Jorge M, de Barros CC, et al. Frontal edema due to mesotherapy for androgenetic alopecia: a case series. Dermatol Ther. 2022;35(2):8–10.
Gajjar P, Mehta H, Barvaliya M, Sonagra B. Comparative study between mesotherapy and topical 5% minoxidil by dermoscopic evaluation for androgenic alopecia in male: a randomized controlled trial. Int J Trichol. 2019;11(2):58–67.
Nassar A, Abdel-Aleem H, Samir M, Khattab FM. Efficacy of botulinum toxin A injection in the treatment of androgenic alopecia: a comparative controlled study. J Cosmet Dermatol. 2022;21(10):4261–8.
Jung BH, Song SH, Yoon SJ, Koo JH, Yoo KY. The effect of botulinum toxin on hair follicle cell regeneration under continuous stress conditions: a pilot animal study. Neurotox Res. 2022;40(1):103–10. https://doi.org/10.1007/s12640-021-00453-8.
Zhou Y, Yu S, Zhao J, Feng X, Zhang M, Zhao Z. Effectiveness and safety of botulinum toxin type a in the treatment of androgenetic alopecia. Biomed Res Int. 2020;2020.
Use of Botulinum Toxin in the Treatment of Androgenic Alopecia. 2022 [cited 2023 Aug 2]. https://www.clinicaltrials.gov/study/NCT05456087?term=botulinum toxin&cond=aga&rank=2
Treatment of Male Pattern Baldness With Botulinum Toxin. 2009. https://www.clinicaltrials.gov/study/NCT00965640?term=botulinum toxin&cond=aga&rank=1
Zhu H-L, Gao Y-H, Yang J-Q, Li J-B, Gao J. Serenoa repens extracts promote hair regeneration and repair of hair loss mouse models by activating TGF-β and mitochondrial signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22(12):4000–8.
Murata K, Takeshita F, Samukawa K, Tani T, Matsuda H. Effects of Ginseng Rhizome and Ginsenoside Ro on Testosterone 5α-Reductase and Hair Re-growth in Testosterone-treated Mice. 2011; https://pubmed.ncbi.nlm.nih.gov/21538628
Wall D, Meah N, Fagan N, York K, Sinclair R. Advances in hair growth. Fac Rev. 2022;11. https://pubmed.ncbi.nlm.nih.gov/35156098/
Kwon OS, Han JH, Yoo HG, Chung JH, Cho KH, Eun HC, et al. Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). Phytomedicine. 2007;14(7–8):551–5.
Srivilai J, Rabgay K, Khorana N, Waranuch N, Nuengchamnong N, Wisuitiprot W, et al. Anti-androgenic curcumin analogues as steroid 5-alpha reductase inhibitors. Med Chem Res. 2017. https://doi.org/10.1007/s00044-017-1869-y.
Hou IC, Oi Y, Fujita H, Yano Y, Fukami H, Yoshikawa M. A hair growth-promoting effect of Chinese black tea extract in mice. Biosci Biotechnol Biochem. 2013;77(7):1606–7.
Uri Shon MD, Myung Hwa Kim MD, Dong Yoon Lee MD SHKP y BCPM. The effect of intradermal botulinum toxin on androgenetic alopecia and its possible mechanism. J Am Acad Dermatol. 2020;83(6):1838–9
Zhou Y, Yu S, Zhao J, Feng X, Zhang M, Zhao Z. Effectiveness and safety of botulinum toxin type a in the treatment of androgenetic alopecia. 2020: https://doi.org/10.1155/2020/1501893
Zhang L, Yu Q, Wang Y, Ma Y, Shi Y, Li X. A small dose of botulinum toxin A is effective for treating androgenetic alopecia in Chinese patients. Dermatol Ther. 2019;32(4).: https://pubmed.ncbi.nlm.nih.gov/30566260/
Singh S, Neema S, Vasudevan B. A pilot study to evaluate effectiveness of botulinum toxin in treatment of androgenetic alopecia in males. J Cutan Aesthet Surg. 2017;10(3):163–7.
Freund BJ, Schwartz M. Treatment of male pattern baldness with botulinum toxin: A pilot study. Plast Reconstr Surg. 2010 Nov [cited 2024 May 27];126(5).: https://journals.lww.com/plasreconsurg/fulltext/2010/11000/treatment_of_male_pattern_baldness_with_botulinum.79.aspx
English Jr Sophia Ruiz RS. Systematic Review and Meta-Analysis Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review. 2021: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8928186/
Khalilov R, Bakishzade A, Nasibova A. Future prospects of biomaterials in nanomedicine. Adv Biol Earth Sci. 2024;9:5–10.
Huseynov E, Khalilov R, Mohamed AJ. Novel nanomaterials for hepatobiliary diseases treatment and future perspectives. Adv Biol Earth Sci. 2024;9:81–91.
Salahshour P, Abdolmaleki S, Monemizadeh S, Gholizadeh S, Khaksar S. Nanobiomaterials/bioinks based scaffolds in 3d bioprinting for tissue engineering and artificial human organs. Adv Biol Earth Sci. 2024;9:97–104.
Saghir Z, Shaikh A, Ahmed B, Patel A, Patil SG, Raheem A, et al. Nanotechnology-based strategies for hair follicle regeneration in androgenetic alopecia. Mater Proc. 2023;14:57.
Roque LV, Dias IS, Cruz N, Rebelo A, Roberto A, Rijo P, et al. Design of finasteride-loaded nanoparticles for potential treatment of alopecia. Skin Pharmacol Physiol. 2017;30(4):197–204.
Kim JH, Na J, Bak DH, Lee BC, Lee E, Choi MJ, et al. Development of finasteride polymer microspheres for systemic application in androgenic alopecia. Int J Mol Med. 2019;43(6):2409–19.
Xiao Q, Lu Y, Yao W, Gong CC, Jia C, Gao J, et al. Molybdenum nanoparticles as a potential topical medication for alopecia treatment through antioxidant pathways that differ from minoxidil. J Trace Elem Med Biol. 2024;82. https://pubmed.ncbi.nlm.nih.gov/38150949/
Fernández-García R, Lalatsa A, Statts L, Bolás-Fernández F, Ballesteros MP, Serrano DR. Transferosomes as nanocarriers for drugs across the skin: quality by design from lab to industrial scale. Int J Pharm. 2020;573.: https://pubmed.ncbi.nlm.nih.gov/31678520/
Makky AMA, El-leithy E, Hussein DG, Khattab A. A full factorial design to optimize aminexil nano lipid formulation to improve skin permeation and efficacy against alopecia. AAPS PharmSciTech. 2023;24(1):1–19.
Lee HJ, Kwon HK, Kim HS, Kim M Il, Park HJ. Hair growth promoting effect of 4HGF encapsulated with PGA nanoparticles (PGA-4HGF) by β-catenin activation and its related cell cycle molecules. Int J Mol Sci. 2019;20(14). https://www.researchgate.net/publication/334461649_Hair_Growth_Promoting_Effect_of_4HGF_Encapsulated_with_PGA_Nanoparticles_PGA-4HGF_by_b-Catenin_Activation_and_Its_Related_Cell_Cycle_Molecules
Monti D, Tampucci S, Burgalassi S, Chetoni P, Lenzi C, Pirone A, et al. Topical formulations containing finasteride Part I: In vitro permeation/penetration study and in vivo pharmacokinetics in hairless rat. J Pharm Sci. 2014;103(8):2307–14.
Yang Y, Wang P, Gong Y, Yu Z, Gan Y, Li P, et al. Curcumin-zinc framework encapsulated microneedle patch for promoting hair growth. Theranostics. 2023;13(11):3675–88.
Hydraderm for Androgenic Alopecia - Full Text View - ClinicalTrials.gov. [cited 2023 Mar 27].: https://clinicaltrials.gov/ct2/show/NCT05426629?term=CELL+THERAPY&cond=aga&draw=2&rank=24
Keravive by Hydrafacial for Scalp Health and Enhanced Hair Quality - Full Text View - ClinicalTrials.gov. [cited 2024 May 31].: https://classic.clinicaltrials.gov/ct2/show/NCT06112782?term=hydradermabrasion&cond=androgenetic+alopecia&draw=2&rank=2
Utomo DN, Hernugrahanto KD, Edward M, Widhiyanto L, Mahyudin F. Combination of bone marrow aspirate, cancellous bone allograft, and platelet-rich plasma as an alternative solution to critical-sized diaphyseal bone defect: A case series. Int J Surg Case Rep. 2019;58:178–85.
Kunze KN, Pakanati JJ, Vadhera AS, Polce EM, Williams BT, Parvaresh KC, et al. The efficacy of platelet-rich plasma for ligament injuries a systematic review of basic science literature with protocol quality assessment. 2019. http://www.sagepub.com/journals-permissions.
Kon E, Buda R, Filardo G, Di Martino A, Timoncini A, Cenacchi A, et al. Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surgery, Sport Traumatol Arthrosc. 2010;18(4):472–9.
Filardo G, Previtali D, Napoli F, Candrian C, Zaffagnini S, Grassi A. PRP injections for the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Cartilage. 2021;13(1):364S-375S.
Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44(9):1191–200.
Gupta AK, Cole J, Deutsch DP, Everts PA, Niedbalski RP, Panchaprateep R, et al. Platelet-rich plasma as a treatment for androgenetic alopecia. Dermatologic Surg. 2019;1.: http://insights.ovid.com/crossref?an=00042728-900000000-98404
Gentile P, Scioli MG, Bielli A, De Angelis B, De Sio C, De Fazio D, et al. Platelet-rich plasma and micrografts enriched with autologous human follicle mesenchymal stem cells improve hair re-growth in androgenetic alopecia. biomolecular pathway analysis and clinical evaluation. Biomedicines. 2019;7(2):27
Gupta AK, Versteeg SG, Rapaport J, Hausauer AK, Shear NH, Piguet V. The efficacy of platelet-rich plasma in the field of hair restoration and facial aesthetics—a systematic review and meta-analysis. J Cutan Med Surg. 2019;23(2):185–203.
Khatu SS, More YE, Gokhale NR, Chavhan DC, Bendsure N. Platelet-rich plasma in androgenic alopecia: myth or an effective tool. J Cutan Aesthet Surg. 2014;7(2):107–10.
Shetty VH, Goel S. Dermoscopic pre‐ and posttreatment evaluation in patients with androgenetic alopecia on platelet‐rich plasma—A prospective study. J Cosmet Dermatol. 2018;jocd.12845.
Verma K, Tegta GR, Verma G, Gupta M, Negi A, Sharma R. A study to compare the efficacy of platelet-rich plasma and minoxidil therapy for the treatment of androgenetic alopecia. Int J Trichology. 2019;11(2):68–79.
Bayat M, Yazdanpanah MJ, Hamidi Alamdari D, Banihashemi M, Salehi M. The effect of platelet-rich plasma injection in the treatment of androgenetic alopecia. J Cosmet Dermatol. 2019. https://doi.org/10.1111/jocd.12907.
Pakhomova EE, Smirnova IO. Comparative evaluation of the clinical efficacy of prp-therapy, minoxidil, and their combination with immunohistochemical study of the dynamics of cell proliferation in the treatment of men with androgenetic alopecia. Int J Mol Sci. 2020;21(18):1–16.
Qu Q, Zhou Y, Shi P, Du L, Fan Z, Wang J, et al. Platelet-rich plasma for androgenic alopecia: a randomized, placebo-controlled, double-blind study and combined mice model experiment. J Cosmet Dermatol. 2021;20(10):3227–35.
Bruce AJ, Pincelli TP, Heckman MG, Desmond CM, Arthurs JR, Diehl NN, et al. A randomized, controlled pilot trial comparing platelet-rich plasma to topical minoxidil foam for treatment of androgenic alopecia in women. Dermatol Surg. 2020;46(6):826–32.
Dubin DP, Lin MJ, Leight HM, Farberg AS, Torbeck RL, Burton WB, et al. The effect of platelet-rich plasma on female androgenetic alopecia: a randomized controlled trial. J Am Acad Dermatol. 2020;83(5):1294–7.
Pavlovic V, Ciric M, Jovanovic V, Trandafilovic M, Stojanovic P. Platelet-rich fibrin: Basics of biological actions and protocol modifications. Open Med (Warsaw, Poland). 2021;16(1):446–54.
Choukroun J, Ghanaati S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. Eur J Trauma Emerg Surg. 2018;44:87–95.
Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, Ghanaati S, et al. Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? 2017; https://pubmed.ncbi.nlm.nih.gov/28154995/
Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20(9):2353–60.
Egierska D, Perszke M, Mazur M, Duś-Ilnicka I. Platelet-rich plasma and platelet-rich fibrin in oral surgery: a narrative review. Dent Med Probl. 2023;60(1):177–86.
Celikten M, Sahin H, Senturk GE, Bilsel K, Pulatkan A, Kapicioglu M, et al. The effect of platelet-rich fibrin, platelet-rich plasma, and concentrated growth factor in the repair of full thickness rotator cuff tears. J shoulder Elb Surg. 2024;33(5):e261-77.
Domingues RB, von Rautenfeld M, Kavalco CM, Caliari C, Dellagiustina C, da Fonseca LF, et al. The role of orthobiologics in chronic wound healing. Int Wound J. 2024;21(4):e14854
Omid Keyhan S, Hemmat S, Ali Badri A, Abdeshahzadeh A, Khiabani K. Use of platelet-rich fibrin and platelet-rich plasma in combination with fat graft: which is more effective during facial lipostructure? Oral Maxillofac Surg J Oral Maxillofac Surg. 2013;71:610–21. https://doi.org/10.1016/j.joms.2012.06.176.
Lu K, Han Q, Ma Z, Yan Q, Pei Y, Shi P, et al. Injectable platelet rich fibrin facilitates hair follicle regeneration by promoting human dermal papilla cell proliferation, migration, and trichogenic inductivity. Exp Cell Res. 2021;409(1):112888. https://doi.org/10.1016/j.yexcr.2021.112888.
Arora R, Shukla S. Injectable-platelet-rich fibrin-smart blood with stem cells for the treatment of alopecia: a report of three patients. Int J Trichol. 2019;11(3):128–31.
Bhoite KS, Chikhalkar SB, Mishra SN, Kharkar VD. Injectable platelet rich fibrin therapy for androgenetic alopecia: a series of 15 cases. Int J Res Dermatology. 2022;8(4):398–402.
Schiavone G, Paradisi A, Ricci F, Abeni D. Injectable platelet-, leukocyte-, and fibrin-rich plasma (Il-PRF) in the management of androgenetic alopecia. Dermatol Surg. 2018;44(9):1183–90.
Ali N, Diab F, Al-Shimaa ·, Ibrahim M, Aya ·, Abdallah M. Fluid platelet-rich fibrin (PRF) versus platelet-rich plasma (PRP) in the Treatment of Atrophic Acne Scars: A Comparative Study. 2023;315:1249–55. https://doi.org/10.1007/s00403-022-02511-3
Shashank B, Bhushan M. Injectable platelet-rich fibrin (prf): the newest biomaterial and its use in various dermatological conditions in our practice: a case series. J Cosmet Dermatol. 2021;20(5):1421–6.
Shimizu Y, Ntege EH, Sunami H, Inoue Y. Regenerative medicine strategies for hair growth and regeneration: a narrative review of literature. Regen Ther. 2022;21:527–39. https://doi.org/10.1016/j.reth.2022.10.005.
Nepal S, Venkataram A, Mysore V. The role of adipose tissue in hair regeneration: a potential tool for management? J Cutan Aesthet Surg. 2021;14(3):295–304.
Won CH, Yoo HG, Kwon OS, Sung MY, Kang YJ, Chung JH, et al. Hair growth promoting effects of adipose tissue-derived stem cells. Vol. 57, J. Dermatol Sci. 2010. p. 134–7. https://pubmed.ncbi.nlm.nih.gov/19963355/
Fukuoka H, Narita K, Suga H. Hair regeneration therapy: application of adipose-derived stem cells. Curr Stem Cell Res Ther. 2017;12(7). https://pubmed.ncbi.nlm.nih.gov/28530535/
Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyvk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17(4):331–40.
Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5(1):1–14.
Schäffler A, Büchler C. Concise review: adipose tissue-derived stromal cells-basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25(4):818–27.
Park B-S, Kim W-S, Choi J-S, Kim H-K, Won J-H, Ohkubo F, et al. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomed Res. 2010;31(1):27–34.
Fukuoka H, Suga H. Hair regeneration treatment using adipose-derived stem cell conditioned medium: follow-up with trichograms. Eplasty. 2015;15:e10.
Owczarczyk-Saczonek A, Wociór A, Placek W, Maksymowicz W, Wojtkiewicz J. The use of adipose-derived stem cells in selected skin diseases (vitiligo, alopecia, and nonhealing wounds). Stem Cells Int. 2017 Aug 21;2017:1–11. https://pubmed.ncbi.nlm.nih.gov/28904532/
Gentile P, Garcovich S. Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development. Cells. 2019;8(5). : http://www.ncbi.nlm.nih.gov/pubmed/31100937
Zhang P, Kling RE, Ravuri SK, Kokai LE, Rubin JP, Chai JK, et al. A review of adipocyte lineage cells and dermal papilla cells in hair follicle regeneration. J Tissue Eng. 2014;5. http://www.uk.sagepub.com/aboutus/openaccess.htm
Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146(5):761–71.
He J, Duan H, Xiong Y, Zhang W, Zhou G, Cao Y, et al. Participation of CD34-enriched mouse adipose cells in hair morphogenesis. Mol Med Rep. 2013;7(4):1111–6.
Zografou A, Papadopoulos O, Tsigris C, Kavantzas N, Michalopoulos E, Chatzistamatiou T, et al. Autologous transplantation of adipose-derived stem cells enhances skin graft survival and wound healing in diabetic rats. Ann Plast Surg. 2013;71(2):225–32.
Rokunohe A, Matsuzaki Y, Rokunohe D, Sakuraba Y, Fukui T, Nakano H, et al. Immunosuppressive effect of adipose-derived stromal cells on imiquimod-induced psoriasis in mice. J Dermatol Sci. 2016;82(1):50–3.
Lee YS, Sah SK, Lee JH, Seo KW, Kang KS, Kim TY. Human umbilical cord blood-derived mesenchymal stem cells ameliorate psoriasis-like skin inflammation in mice. Biochem Biophys Reports. 2016;2017(9):281–8.
Wu J, Yang Q, Wu S, Yuan R, Zhao X, Li Y, et al. Adipose-derived stem cell exosomes promoted hair regeneration. Tissue Eng Regen Med. 2021;18(4):685–91. https://doi.org/10.1007/s13770-021-00347-y.
Zhou L, Wang H, Jing J, Yu L, Wu X, Lu Z. Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem Biophys Res Commun. 2018;500(2):325–32. https://doi.org/10.1016/j.bbrc.2018.04.067.
Nilforoushzadeh MA, Aghdami N, Taghiabadi E. Human hair outer root sheath cells and platelet-lysis exosomes promote hair inductivity of dermal papilla cell. Tissue Eng Regen Med. https://doi.org/10.1007/s13770-020-00266-4
Rajendran RL, Gangadaran P, Seo CH, Kwack MH, Oh JM, Lee HW, et al. Macrophage-derived extracellular vesicle promotes hair growth. Cells. 2020;9(4):856.
Riazifar M, Mohammadi MR, Pone EJ, Yeri A, Lasser C, Segaliny AI, et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano. 2019;13(6):6670–88.
Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33(7):2158–68.
Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem cell Investig. 2017;4(7):58–58.
Zanzottera F, Lavezzari E, Trovato L, Icardi A, Graziano A. Adipose derived stem cells and growth factors applied on hair transplantation. follow-up of clinical outcome. J Cosmet Dermatological Sci Appl. 2014;4(4):268–74
Hamed Kadry M, Kheir WA El, Sayed Shalaby M El, El Shahid AR, Metwally HG. Autologous Adipose Derived Stem Cell versus Platelet Rich Plasma Injection in the Treatment of Androgentic Alopecia: Efficacy, Side Effects and Safety. J Clin Exp Dermatol Res. 2018;09(03). : https://www.omicsonline.org/open-access/autologous-adipose-derived-stem-cell-versus-platelet-rich-plasma-injection-in-the-treatment-of-androgentic-alopecia-efficacy-side-2155-9570-1000447-102382.html
Shin H, Ryu HH, Kwon O, Park BS, Jo SJ. Clinical use of conditioned media of adipose tissue-derived stem cells in female pattern hair loss: A retrospective case series study. Int J Dermatol. 2015;54(6):730–5.
Adipose-derived Stem Cell Conditioned Media as a Novel Approach for Hair Regrowth in Male Androgenetic Alopecia - Full Text View - ClinicalTrials.gov. [cited 2023 Mar 31]. : https://clinicaltrials.gov/ct2/show/NCT05296863?term=NCT05296863&draw=2&rank=1
Tak YJ, Lee SY, Cho AR, Kim YS. A randomized, double-blind, vehicle-controlled clinical study of hair regeneration using adipose-derived stem cell constituent extract in androgenetic alopecia. Stem Cells Transl Med. 2020;9(8):839–49.
Safety and Tolerability of Hair Stimulating Complex (HSC) in Female Pattern Hair Loss - Full Text View - ClinicalTrials.gov. 2018. https://clinicaltrials.gov/ct2/show/NCT03662854?term=NCT03662854&draw=2&rank=1
Hair Growth Efficacy and Safety of NGF-574H in Adult With Androgenic Alopecia - Full Text View - ClinicalTrials.gov. 2018: https://clinicaltrials.gov/ct2/show/NCT03676400?term=NCT03676400&draw=2&rank=1
Adipose-derived SVF for Treatment of Alopecia - Full Text View - ClinicalTrials.gov. 2016. https://clinicaltrials.gov/ct2/show/NCT02626780?term=adipose&cond=AGA&draw=2&rank=4
Point-of-Care Adipose-derived Cells for Hair Growth - Full Text View - ClinicalTrials.gov. 2016. https://clinicaltrials.gov/ct2/show/NCT02729415?term=NCT02729415&draw=2&rank=1
Efficacy of Platelet-Rich Plasma Versus Mesotherapy in Androgenetic Alopecia: A Retrospective Study - Full Text View - ClinicalTrials.gov. [cited 2023 Mar 27]. : https://clinicaltrials.gov/ct2/show/NCT05129800?term=CELL+THERAPY&cond=aga&draw=2&rank=27
Fukuoka H, Suga H, Narita K, Watanabe R, Shintani S. The latest advance in hair regeneration therapy using proteins secreted by adipose-derived stem cells. Am J Cosmet Surg. 2015;29(4):273–82.
Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4(7):58–58.
Mahmood S Choudhery APKEMU. Adipose tissue derived stem cell based hair restoration therapy for androgenetic alopecia - ClinicalTrials.gov. 2018. https://clinicaltrials.gov/ct2/show/NCT02865421?term=NCT02865421&draw=2&rank=1
Adipose Derived Stem Cells Versus Platelet Rich Plasma on Follicular Unit Extraction - Full Text View - ClinicalTrials.gov. [cited 2023 Mar 27]. : https://clinicaltrials.gov/ct2/show/NCT03388840?term=NCT03388840&draw=2&rank=1
Stevens HP, Donners S, De Bruijn J. Introducing platelet-rich stroma: platelet-rich plasma (PRP) and stromal vascular fraction (SVF) combined for the treatment of androgenetic alopecia. Aesthetic Surg J. 2018;38(8):811–22.
Kenneth Williams. AGA Biocellular Stem/Stromal Hair Regenerative Study - Full Text View - ClinicalTrials.gov. 2021. : https://clinicaltrials.gov/ct2/show/NCT02849470?term=NCT02849470&draw=2&rank=1
El-Khalawany M, Rageh MA, Elnokrashy I, Ibrahim SMA. Efficacy of autologous stromal vascular fraction injection in the treatment of androgenic alopecia. Arch Dermatol Res. 2022;0123456789. https://doi.org/10.1007/s00403-022-02501-5
A Study to Evaluate and Compare Injections of Autologous Mixed Population of Dermal Cells Cells Into the Balding Scalp of Subjects With Hair Loss (CA-0006931) - Full Text View - ClinicalTrials.gov. [cited 2023 Mar 27]. : https://clinicaltrials.gov/ct2/show/NCT01669746?term=NCT01669746&draw=2&rank=1
A Study to Evaluate and Compare Injections of Autologous Mixed Population of Dermal Cells Into the Balding Scalp of Subjects With Hair Loss (CA-0004542) - Full Text View - ClinicalTrials.gov. [cited 2023 Mar 27]. : https://clinicaltrials.gov/ct2/show/NCT01451151?term=CELL+THERAPY&cond=aga&draw=2&rank=17
A Study to Evaluate and Compare Injections of Autologous Dermal Cells Into the Balding Scalp of Subjects With Hair Loss - Full Text View - ClinicalTrials.gov. [cited 2023 Mar 27]. : https://clinicaltrials.gov/ct2/show/NCT01451099?term=CELL+THERAPY&cond=aga&draw=2&rank=16
A Study to Evaluate and Compare Injections of Autologous Mixed Population of Dermal Cells Into the Balding Scalp of Subjects With Hair Loss (CA-0005995) - Full Text View - ClinicalTrials.gov. [cited 2023 Mar 27]. : https://clinicaltrials.gov/ct2/show/NCT01451190?term=CELL+THERAPY&cond=aga&draw=2&rank=20
A Study to Evaluate and Compare Injections of Autologous Dermal and Epidermal Cells Into the Balding Scalp of Subjects With Hair Loss (CA-0004669) - Full Text View - ClinicalTrials.gov. [cited 2023 Mar 27]. : https://clinicaltrials.gov/ct2/show/NCT01451177?term=NCT01451177&draw=2&rank=1
A Study to Evaluate and Compare Injections of Autologous Dermal and Epidermal Cells Into the Balding Scalp of Subjects With Hair Loss (CA-0004512) - Full Text View - ClinicalTrials.gov. [cited 2023 Mar 27]. : https://clinicaltrials.gov/ct2/show/NCT01451125?term=CELL+THERAPY&cond=aga&draw=2&rank=15
A Study to Evaluate and Compare Injections of Autologous Dermal and Epidermal Cells Into the Balding Scalp of Subjects With Hair Loss (CA-0004541) - Full Text View - ClinicalTrials.gov. [cited 2023 Mar 27]. : https://clinicaltrials.gov/ct2/show/NCT01451138?term=CELL+THERAPY&cond=aga&draw=2&rank=18
A Study to Evaluate and Compare Injections of Autologous Dermal and Epidermal Cells Into the Balding Scalp of Subjects With Hair Loss (CA-0002899) - Full Text View - ClinicalTrials.gov. 2009. : https://clinicaltrials.gov/ct2/show/NCT01451112?term=NCT01451112&draw=2&rank=1
A Study to Evaluate and Compare Injections of Autologous Dermal and Epidermal Cells Into the Balding Scalp of Subjects With Hair Loss (CA-0002012) - Full Text View - ClinicalTrials.gov. [cited 2023 Mar 27]. : https://clinicaltrials.gov/ct2/show/NCT01451047?term=CELL+THERAPY&cond=aga&draw=2&rank=21
A Study to Evaluate and Compare Injections of Autologous Dermal and Epidermal Cells Into the Balding Scalp of Subjects With Hair Loss - Full Text View - ClinicalTrials.gov. [cited 2023 Mar 27]. : https://clinicaltrials.gov/ct2/show/NCT01451021?term=CELL+THERAPY&cond=aga&draw=2&rank=22
A Study to Evaluate and Compare Injections of Autologous Dermal and Epidermal Cells Into the Balding Scalp of Subjects With Hair Loss (CA-0002013) - Full Text View - ClinicalTrials.gov. [cited 2023 Mar 27]. : https://clinicaltrials.gov/ct2/show/NCT01451073?term=CELL+THERAPY&cond=aga&draw=2&rank=23
Autologous Hair Follicle Derived Mesenchymal Stem Cell Suspension to Treat AGA - Full Text View - ClinicalTrials.gov. [cited 2023 Mar 31]. : https://clinicaltrials.gov/ct2/show/NCT05659095?term=NCT05659095&draw=1&rank=1
Safety and Efficacy Study of Human Autologous Hair Follicle Cells to Treat Androgenetic Alopecia - Full Text View - ClinicalTrials.gov. 2021. p. ClinicalTrials.gov. : https://clinicaltrials.gov/ct2/show/NCT01286649?term=CELL+THERAPY&cond=AGA&draw=2&rank=4
Elmaadawi IH, Mohamed BM, Ibrahim ZAS, Abdou SM, El Attar YA, Youssef A, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia. J Dermatolog Treat. 2018;29(5):431–40. https://pubmed.ncbi.nlm.nih.gov/27553744/
STYLE -- A Trial of Cell Enriched Adipose For Androgenetic Alopecia. 2020. : https://www.clinicaltrials.gov/ct2/show/NCT02503852?term=kerastem&cond=androgenetic+alopecia&draw=2&rank=1
Efficacy and Safety of Exosomes Versus Platelet Rich Plasma in Patients of Androgenetic Alopecia | ClinicalTrials.gov. 2024. https://clinicaltrials.gov/study/NCT06239207?cond=androgenetic alopecia &term=Exosomes&rank=1
Llamas-Molina JM, Carrero-Castaño A, Ruiz-Villaverde R, Campos A. Tissue engineering and regeneration of the human hair follicle in androgenetic alopecia. Literature Review. 2022. https://doi.org/10.3390/life12010117.
Fisher JP, Mikos AG, Bronzino JD. Tissue engineering. Science (80- ). 1993. https://doi.org/10.1126/science.8493529
Castro AR, Logarinho E. Tissue engineering strategies for human hair follicle regeneration: How far from a hairy goal? 2019; https://pubmed.ncbi.nlm.nih.gov/31876379/
Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Tran TN, et al. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C Methods. 2014;20(6):473.
Markstedt K, Mantas A, Tournier I, Martínez Ávila H, Hägg D, Gatenholm P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules. 2015;16(5):1489–96.
Cui H, Zhu W, Nowicki M, Zhou X, Khademhosseini A, Grace Zhang L, et al. Hierarchical fabrication of engineered vascularized bone biphasic constructs via dual 3D bioprinting: integrating regional bioactive factors into architectural design. Adv Healthc Mater. 2016;5(17):2174–81. https://doi.org/10.1002/adhm.201600505.
Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017;8(1):1–10.
Ma J, Qin C, Wu J, Zhuang H, Du L, Xu J, et al. 3D multicellular micropatterning biomaterials for hair regeneration and vascularization. Mater Horizons. 2023;10(9):3773–84.
Kang MS, Kwon M, Lee SH, Kim WH, Lee GW, Jo HJ, et al. 3D printing of skin equivalents with hair follicle structures and epidermal-papillary-dermal layers using gelatin/hyaluronic acid hydrogels. Chem – An Asian J. 2022;17(18):202200620. https://doi.org/10.1002/asia.202200620
Acknowledgements
Not applicable.
Funding
No funding was requested for this review.
Author information
Authors and Affiliations
Contributions
LLPP conducted the literature research and articles collection, wrote the review and elaborated tables. PPTE collaborated in the literature research, writing and reviewing the paper. EELB designed, reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
They authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pozo-Pérez, L., Tornero-Esteban, P. & López-Bran, E. Clinical and preclinical approach in AGA treatment: a review of current and new therapies in the regenerative field. Stem Cell Res Ther 15, 260 (2024). https://doi.org/10.1186/s13287-024-03801-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-024-03801-5